Abstract
Purpose
To evaluate whether pretreatment combined endorectal magnetic resonance imaging (MRI) and magnetic resonance spectroscopic imaging (MRSI) findings are predictive of outcome in patients who undergo external beam radiotherapy for prostate cancer.
Methods and Materials
We retrospectively identified 67 men with biopsy-proven prostate cancer who underwent combined endorectal MRI and MRSI at our institution between January 1998 and October 2003 before whole-pelvis external beam radiotherapy. A single reader recorded tumor presence, stage, and metabolic abnormality at combined MRI and MRSI. Kaplan-Meier survival and Cox univariate and multivariate analyses explored the relationship between clinical and imaging variables and outcome, using biochemical or metastatic failure as endpoints.
Results
After a mean follow-up of 44 months (range, 3–96), 6 patients developed both metastatic and biochemical failure, with an additional 13 patients developing biochemical failure alone. Multivariate Cox analysis demonstrated that the only independent predictor of biochemical failure was the volume of malignant metabolism on MRSI (hazard ratio [HR] 1.63, 95% confidence interval [CI] 1.29–2.06; p < 0.0001). The two independent predictors of metastatic failure were MRI tumor size (HR 1.34, 95% CI 1.03–1.73; p = 0.028) and the finding of seminal vesicle invasion on MRI (HR 28.05, 95% CI 3.96–198.67; p = 0.0008).
Conclusions
In multivariate analysis, MRI and MRSI findings before EBRT in patients with prostate cancer are more accurate independent predictors of outcome than clinical variables, and in particular, the findings of seminal vesicle invasion and extensive tumor predict a worse prognosis.
Keywords: Cancer-prostate, Spectroscopy-prostate, Radiotherapy, Magnetic resonance imaging, Outcome analysis
INTRODUCTION
The management of early-stage prostate cancer is controversial because we cannot reliably distinguish patients whose disease is indolent and incidental from those whose disease is progressive and life threatening. Traditional methods of prostate cancer evaluation by digital rectal examination, transrectal ultrasound, sextant biopsy results, and serum prostatic-specific antigen (PSA) assay can generally only predict behavior for very indolent or aggressive cancers. Most patients fall between these extremes, where these techniques are of limited accuracy, either alone or in combination (1–6). Given these limitations, many investigators have studied the value of endorectal magnetic resonance imaging (MRI) in assessing the local extent of prostate cancer, with good results (7, 8). Other studies have indicated that magnetic resonance spectroscopic imaging (MRSI) added additional useful information based on the metabolic changes associated with the disease (9–12). Most studies of prostate MRI and MRSI have used radical prostatectomy specimens as the standard of reference. This seems reasonable, but the approach has two disadvantages. First, evidence suggests that MRI and MRSI may be of most benefit in patients with larger and higher-risk tumors (11, 13), but such patients will be un-derrepresented in surgical series (14) so that the true benefit of imaging may be underestimated. Second, dependence on correlation with histopathology may actually overlook what really matters for the patient, which is clinical outcome. For these reasons, examining the relationship between MR findings and outcome in patients selecting radiotherapy for management is an important research direction. In a prior study, extracapsular extension at pretreatment endorectal MRI was shown to predict metastatic failure in patients undergoing radiotherapy, but the influence of MRSI was not examined, and the population was heterogeneous (15). Therefore, we undertook this study to evaluate whether pretreatment combined endorectal MRI and MRSI findings are predictive of outcome in patients who undergo external beam radiotherapy for prostate cancer.
METHODS AND MATERIALS
Subjects
Using our Departmental Prostate Cancer Database, we retrospectively identified 67 men with biopsy-proven untreated prostate cancer who underwent combined endorectal MRI and MRSI at our institution between January 1998 and October 2003 (this interval was chosen to include patients who were studied using contemporary MR technology and who also had a reasonably long period of follow-up), who subsequently underwent whole-pelvis external beam radiotherapy and who had follow-up at our institution. Of note, our database includes all patients referred for prostate MRI and MRSI at our institution, but the systematic indications for referral, if any, are unknown to us (though our impression is that patients who express an interest or who are seen by physicians supportive of the technology form our primary referral base). Sixty-two of the men were included in a prior preliminary study investigating the predictive value of MRI alone (15). The study was approved by our institutional Committee on Human Research, with a waiver of the requirement for written consent. The study was compliant with the Health Insurance Portability and Accountability Act. Baseline and follow-up clinical data were compiled by the primary investigator (T.J.). The mean age of the 67 men was 63 years (range, 49–78 years). Clinical tumor stage, as established by digital rectal examination, was T1, T2A, T2B, T2C, and T3 in 17, 18, 2, 1, and 29 men, respectively. The median tumor Gleason score was 7 (range, 2–9). The mean serum PSA level before hormonal or radiation treatment was 9.3 ng/mL (range, 1.7–36.8 ng/mL). The percentage of positive biopsies was defined as the number of positive biopsy cores over the total number of cores obtained in each patient (13). The average percentage of positive biopsies was 37% (range, 8–83%). Using the risk stratification method described by D’Amico et al. (13), clinical tumor stage, Gleason score, and percentage of positive biopsies were used to assign a score from 1 (low risk) to 9 (high risk). The median D’Amico risk stratification score was 3 (range, 1–9).
MRI technique
All imaging studies were performed on a 1.5-Tesla whole-body MR scanner (Signa; GE Medical Systems, Milwaukee, WI). Patients were positioned supine with a body coil for excitation and a pelvic phased array coil (GE Medical Systems) in combination with a commercially available balloon-covered expandable endorectal coil (Medrad, Pittsburgh, PA) for signal reception. Magnetic resonance imaging sequences acquired included thin-section high spatial resolution axial and coronal T2-weighted fast spin-echo images of the prostate and seminal vesicles with the following parameters: time to repetition (TR)/effective time to echo (TE) = 5000 ms/96 ms, echo train length = 16, slice thickness = 3 mm, interslice gap = 0 mm, field of view = 14 cm, matrix 256 × 192, anteroposterior frequency encoding (to prevent obscuration of the prostate by endorectal coil motion artifact), and 3 excitations. After review of the axial T2-weighted images, a volume of prostate tissue was selected to maximize coverage of the gland without including the adjacent rectum and periprostatic fat. Spectroscopic data were acquired using a water- and lipid-suppressed double spin-echo point-resolved spectroscopy sequence that used spectral–spatial pulses for the two 180° excitation pulses. The influence of chemical shift on the apparent location of the selected volume was also reduced by the higher spectral bandwidth of the spectral–spatial pulses (16, 17). Outer voxel saturation pulses were also used to further sharpen volume selection and conform the selected volume to the shape of the prostate to eliminate susceptibility artifacts from periprostatic fat and rectal air (18). Data sets were acquired as 16 × 8 × 8 phase-encoded spectral arrays, with a TR/TE of 1000 ms/130 ms and a 17-min acquisition time. The spectroscopic imaging data were zero-filled from 8 to 16 in both the anteroposterior and craniocaudal directions to increase the likelihood of optimal alignment between spectroscopic voxels and the peripheral zone, and yielding a nominal voxel size of 0.09 cm3. The final combined images consisted of axial T2-weighted images with an overlaid grid showing the corresponding spectra (Fig. 1).
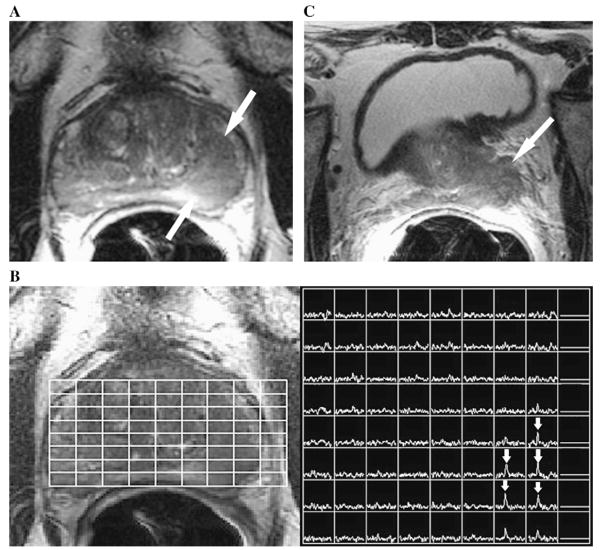
Fig. 1.
(A) Axial T2-weighted magnetic resonance imaging (MRI) section in a 68-year-old man with newly diagnosed Gleason 6 prostate cancer, serum prostate-specific antigen level of 4.6 ng/mL, and clinical stage of T2B. A large focus (arrows) of reduced T2 signal intensity is seen in the left peripheral zone of the prostate. (B) Photomontage showing the axial T2-weighted image on the left side with an overlaid grid that corresponds to the magnetic resonance spectroscopic imaging (MRSI) spectral array on the right side. The voxels that correspond to the focus of reduced T2 signal intensity show high choline peaks (arrows), consistent with prostate cancer. (C) Axial T2-weighted MRI section through the base of the prostate (at a more superior level than in panel a) shows gross extracapsular extension of tumor, with seminal vesicle invasion (Stage T3B). The patient developed metastatic recurrence 21 months after external beam radiotherapy.
MRI and MRSI interpretation
A single reader with more than 10 years of experience in the interpretation of endorectal MRI and MRSI of the prostate (F.V.C) independently reviewed all MR studies at a picture archiving and communication system workstation (Impax; Agfa, Mortsel, Belgium). The reader knew that the patients had biopsy-proven prostate cancer that was subsequently treated by external beam radiotherapy but was unaware of all other clinical, histopathologic, and outcome findings. The following MRI and MRSI findings were recorded.
Stage
A radiologic T stage was assigned according to established morphologic and metabolic criteria for MRI and MRSI of prostate cancer, in conjunction with the American Joint Committee on Cancer staging system (19–21). T1 tumors were not apparent on MRI or MRSI; T2 tumors were visible on MRI or MRSI but were organ confined; T3 tumors extended outside the capsule or into the seminal vesicles; and T4 tumors invaded adjacent structures, such as the bladder or rectum. Tumor was characterized morphologically as an ovoid masslike or crescentic subcapsular focus of reduced T2 signal intensity and metabolically by the presence of one or more voxels of suspicious metabolism (elevation of the choline peak or reduction of the citrate peak such that the two peaks were of similar height or the choline peak was higher than the citrate peak). Extracapsular extension was considered present if tumor abutted the prostate capsule and demonstrated an irregular margin with the adjacent periprostatic tissue, or if frank extension of tumor outside the confines of the prostatic capsule was present. Seminal vesical, bladder, or rectal invasion was considered present if tumor was seen to extend into any part of the respective structure.
MRI tumor size
When tumor was considered present, size was recorded as the long and short axis diameters on axial T2-weighted images. The size of peripheral and central gland tumors was recorded separately. The histopathologic volume of cancer was calculated from the long and short axis diameters using this formula: Volume = (4/3) π (D/2)3, where D is the average of the long and short diameters (22).
MRSI tumor size
The MRSI tumor size was recorded as the volume of tissue with unequivocal malignant metabolism (i.e., choline peak clearly greater than citrate peak), because this pattern has been shown to be strongly associated with malignancy (20). The volume of peripheral and central gland tumors was recorded separately. The MRSI tumor volume was calculated by multiplying the number of such voxels by the nominal voxel size after zero-filling (0.09 cm3). For example, if 10 voxels were considered to display unequivocal malignant metabolism, the calculated MRSI tumor size would be 0.9 cm3.
Patient treatment and outcome
The primary investigator reviewed all available clinical, radiologic, and laboratory results to establish the details of patient treatment and outcome. All patients underwent definitive external beam radiotherapy after MRI, with a mean interval of 5.4 months (range, 1–23 months). The mean EBRT dose was 74.2 Gy (range, 68–76 Gy). Supplementary hormonal therapy was administered to 39 patients. Nine patients received neoadjuvant hormonal therapy for a mean duration of 34 months (range, 4–83 months). Nine patients received adjuvant hormonal therapy for a mean duration of 29 months (range, 11–56 months). Twenty-one patients received both neoadjuvant and adjuvant hormonal therapy for a mean duration of 35 months (range, 13–77 months). None of the patients had begun hormonal therapy at the time of MRI and MRSI. With respect to outcome, both metastatic and biochemical recurrences were recorded. Metastatic recurrence was considered present when this diagnosis was documented in the clinical record and there was appropriately supportive evidence, such as histopathologic confirmation or development of a new lesion with characteristics consistent with prostate cancer metastasis on bone scintigraphy or cross-sectional imaging. Biochemical recurrence (also known as biochemical or PSA failure) was defined as three consecutive rises in serum PSA level after a nadir level had been reached or ≥2 ng/mL above the nadir PSA level (23).
Statistical analysis
Descriptive statistics of mean and range were used to summarize the patient cohort with respect to clinical, imaging, and outcome variables. Univariate and multivariate Cox analyses were performed to assess whether clinical or imaging features could identify which patients were more likely to develop treatment failure as identified by biochemical or metastatic recurrence when the proportional assumptions were appropriate. Kaplan-Meier actuarial survival estimates were used to calculate the biochemical and metastatic freedom from progression for variables when the Cox model was not appropriate. The relationship between the assigned T stage at imaging before external beam radiotherapy and patient outcome was evaluated using the log–rank test statistics from the Kaplan-Meier actuarial survival estimates for the freedom from treatment failure. Cox proportional hazards models were used to analyze the variables of clinical stage, Gleason score, baseline serum PSA level, D’Amico risk stratification score, months of hormonal therapy, and MR findings of tumor size and stage. Multivariate stepwise Cox regression analysis was used to identify the independently predictive variables. Relative hazards ratios (HRs) and the corresponding 95% confidence intervals (CIs) were reported. All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC). A p value of ≤0.05 was used to define statistical significance.
RESULTS
MRI and MRSI findings
On the basis of combined MRI and MRSI interpretation, 8, 30, 21, and 8 patients had stage T1, T2, and T3A, and T3B disease, respectively. In the 54 patients with visible tumor in the peripheral zone, the mean MRI tumor volume was 1.76 cm3 (range, 0.04–13.48 cm3). In the 12 patients with visible tumor in the central gland, the mean MRI tumor volume was 1.89 cm3 (range, 0.38–4.86 cm3). The overall mean MRI tumor volume per patient was 1.75 cm3 (range, 0–13.48 cm3). The mean volume of malignant-appearing metabolism in the 26 patients with visible tumor in the peripheral zone at MRSI was 1.60 cm3 (range, 0.12–8.46 cm3). The mean volume of malignant-appearing metabolism in the 6 patients with visible tumor in the central gland at MRSI was 1.25 cm3 (range, 0.45–3.57 cm3). The overall mean volume of malignant-appearing metabolism per patient at MRSI was 0.69 cm3 (range, 0–8.46 cm3).
Predictors of outcome
After a mean follow-up of 44 months (range, 3–96 months), 6 patients developed both metastasis and biochemical failure, with an additional 13 patients developing biochemical failure alone (the follow-up interval was calculated from the completion of radiotherapy). Univariate analysis showed that several clinical and MR variables were significantly predictive of both biochemical and metastatic recurrence (Table 1). Kaplan-Meier outcome curves for metastatic failure as a function of combined MRI and MRSI staging are shown in Fig. 2. Multivariate Cox analysis demonstrated that the only independent predictor of biochemical failure was the volume of malignant metabolism on MRSI (HR 1.63, 95% CI 1.29–2.06; p < 0.0001). The two independent predictors of metastatic failure were MRI tumor size (HR 1.34, 95% CI 1.03–1.73; p = 0.028) and the finding of seminal vesicle invasion on MRI (HR 28.05, 95% CI 3.96–198.67; p = 0.0008). In particular, with respect to the three variables that were predictive of outcome in multivariate analysis, (1) 10 of 17 patients with a volume of malignant metabolism >1.0 cm3 developed biochemical failure, compared with 8 of 50 with a volume of malignant metabolism <1.0 cm3; (2) 5 of 16 patients with an MRI tumor volume >2.5 cm3 developed metastases, compared with 1 of 51 with an MRI tumor volume <2.5 cm3; and (3) 4 of 8 patients with seminal vesicle invasion on pretreatment MRI developed metastases, compared with 2 of 59 without seminal vesicle invasion.
Table 1.
Results of univariate analysis for clinical and MRI/MRSI predictors of biochemical or metastatic recurrence of prostate cancer after external beam radiotherapy in a group of 67 men
| Biochemical recurrence |
Metastatic failure* |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Clinical | ||||
| Pretreatment PSA value | 1.03 (0.98–1.07) | 0.25 | 1.06 (0.99–1.13) | 0.12 |
| Gleason score | 0.84 (0.48–1.47) | 0.53 | 1.14 (0.45–2.87) | 0.78 |
| Percentage of positive biopsies | 1.98 (1.06–3.67) | 0.03† | 1.08 (0.98–1.19) | 0.10 |
| D’Amico risk category | 1.19 (0.96–1.46) | 0.11 | 1.02 (0.99–1.04) | 0.23 |
| MRI/MRSI | ||||
| MRI tumor size | 1.04 (0.98–1.10) | 0.25 | 1.12 (1.02–1.2) | 0.01† |
| MRI tumor stage* | 3.57 (1.16–11.03) | 0.03† | 0.34 (0.24–0.49) | 0.99 |
| Seminal vesicle invasion at MRI | 5.27 (2.17–12.82) | 0.0002† | 11.49 (3.23–40.88) | 0.0002† |
| Volume of malignant metabolism at MRSI | 1.54 (1.20–1.97) | 0.0007† | 1.53 (1.08–2.16) | 0.02† |
Abbreviations: MRI = magnetic resonance imaging; MRSI = magnetic resonance spectroscopic imaging; HR = hazard ratio; PSA = prostate-specific antigen.
P value calculated by log–rank test of Kaplan-Meier survival analysis; in this specific analysis, the Cox model was inappropriate because none of the patients with organ-confined disease developed metastatic failure (zero events) and so the logit method was used with a logit estimator correction of 0.5 in the cells of the outcome table that contains a zero.
p < 0.05.
Fig. 2.
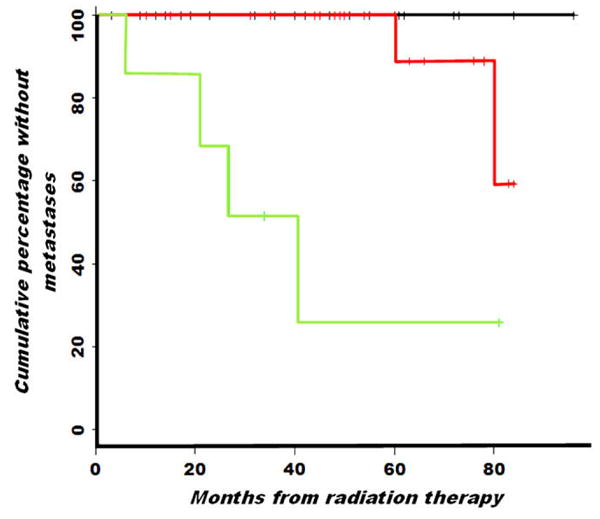
Graph showing the cumulative percentage of patients without metastatic recurrence of prostate cancer after external beam radiotherapy, when stratified by stage assigned by combined endorectal magnetic resonance imaging and magnetic resonance spectroscopic imaging. Kaplan-Meier analysis shows a significant difference (p value of log–rank test to compare three stages, <0.001) between those with organ-confined (T1 or T2) tumor (n = 38; upper line), extracapsular extension (T3A, n = 21; middle line), and seminal vesical invasion (T3B, n = 8; lower line).
DISCUSSION
On the basis of multivariate analysis, our study suggests that pretreatment MRI and MRSI findings related to tumor size and extent outperform standard clinical variables in predicting prognosis after external beam radiotherapy of prostate cancer. In particular, the findings of seminal vesicle invasion at MRI, an MRI tumor volume >2.5 cm3, or a volume of malignant metabolism at MRSI of >1.0 cm3 are associated with worse outcomes. Although this might be seen as a substantial achievement for MRI and MRSI, it is arguably unsurprising that direct evaluation of tumor morphology and metabolism would be more informative with respect to biologic tumor characterization than the relatively crude techniques of digital rectal examination, PSA measurement, and biopsy—all of which have well-described limitations (1–6). The more important issues raised by our results are related to their possible meaning and implications for patient care. The finding that large or advanced tumors are more likely to recur could be a reflection of local treatment failure, either because such tumors are less radiosensitive or because parts of the tumors that have spread outside the prostatic capsule receive a lower dose due to peripheral fall off in the radiation field gradient (24, 25). However, we suspect that these findings are more likely a marker of microscopic systemic spread, particularly given that seminal vesicle invasion is associated with microscopic nodal metastases in up to 80% of cases (26). Irrespective of the underlying pathophysiology, patients with adverse features at pretreatment MRI and MRSI may be candidates for more aggressive supplementary local or systemic treatment, such as radiation dose escalation or extended androgen deprivation therapy (26). Another important question raised by our study is whether every patient planning to undergo external beam radiotherapy for prostate cancer should have endorectal MRI and MRSI. Although there is a good argument that the information provided by MRI and MRSI is prognostically useful and may influence therapy, the reality is that the technology and interpretative expertise required is still not widely available, and reimbursement polices by third-party payers are variable. Accordingly, in our opinion, the decision to use such advanced imaging should be discussed with the patient by his treating physicians, with acknowledgment of local practice and insurance coverage issues.
It is instructive to compare our findings with those from other studies that have examined the relationship between MRI and MRSI, radiotherapy, and prognosis in patients with prostate cancer. Clarke et al. (27) showed that endorectal MRI results seem to positively influence radiation treatment planning. A more recent study showed that the presence and degree of extracapsular extension were important predictors of outcome after radiotherapy for prostate cancer (15). D’Amico et al. (28) have previously demonstrated the benefits of MRI in evaluating patients before radical prostatectomy—multivariate analysis showed that endorectal MRI was a better predictor of extracapsular extension, seminal vesicle invasion, and positive surgical margins than Gleason score and PSA level. A follow-up study (29) demonstrated that although a PSA level of >20 ng/mL was the most accurate predictor for post–radical prostatectomy PSA failure, MRI findings of extracapsular extension could also predict PSA failure. An additional study by the same investigators (30) showed that seminal vesicle invasion detected on endorectal MRI in patients with otherwise clinically localized prostate cancer was associated with an increased risk of biochemical failure and might be an indication for supplementary hormonal therapy. The common theme of these studies is that endorectal MRI and MRSI can provide information that is incrementally useful in the evaluation of prostate cancer and can positively influence both treatment planning and prediction of prognosis. Arguably, MRI and MRSI findings, if available, should be incorporated into current algorithms and nomograms for prostate cancer assessment and management.
Our study has a number of limitations. It was a retrospective study performed at a single institution, with a relatively small number of patients and only a single reader. It is likely that there is selection bias in our study design; for example, the unusually long duration of hormone treatment in the patient population suggests that these may have been patients with clinically more aggressive or concerning disease. Given the long natural history of prostate cancer, the mean follow-up of 44 months was short. Only 6 of 67 patients developed metastases, and an additional 13 developed PSA failure alone during this period. This is a relatively small number of endpoint events. We plan to extend the study, with inclusion of more patients and longer follow-up, to further investigate and confirm our results. It is possible that different trends will emerge over time. The MRI findings related to tumor volume, extracapsular extension, and seminal vesicle invasion used in this study were not validated against an objective histopathologic reference standard. Instead, their importance was judged by their relationship to outcome. It could be argued that these features are unreliable, given the lack of validation and given that MRI has generally performed with modest accuracy in studies in which these findings are compared with histopathology (8, 11). However, accuracy of up to 93% has been reported for the detection of seminal vesicle invasion (31), and ultimately outcome is a more important endpoint for the patient than correlation with histopathologic findings. In this sense, histopathology can be regarded as a surrogate marker for outcome. Additionally, surgical studies will be skewed to patients with lower risk and less-extensive tumors and presumably less-frequent and less-marked extracapsular extension and seminal vesicle invasion. Local extraprostatic spread of cancer is not a simple binary observation but rather has a quantitative component—for example, it is known that the degree of extracapsular extension affects detection by MRI (32), that microscopic bladder neck invasion is of little negative prognostic importance (33), and that extracapsular extension >5 mm in radial diameter indicates a particularly poor outcome in patients undergoing radiotherapy (15). It is plausible that greater degrees of extracapsular extension and seminal vesicle invasion are present in patients selecting external beam radiotherapy and that MRI detection and quantification of these changes will provide useful prognostic information. We did not include transrectal ultrasound findings in our study because findings from systematically performed transrectal ultrasound were not available to us. It is possible that ultrasound can provide similarly useful prognostic information based on morphologic evaluation of tumor size and stage. Finally, the MRI and MRSI tumor volumes reported in our study should be interpreted with caution because MRI and MRSI are known to be of limited accuracy for measurement of tumor volumes <0.5 cm3 (11), our study lacked any reference standard for these volumes, and the volume of frankly malignant metabolism at MRSI is likely to be an underestimation of true tumor volume (20). The latter measurement more likely reflects the volume of aggressive tumor in the gland and may be more analogous to the measurement of Gleason Grade 4 and 5 cancer, as used in the Stanford modified Gleason scale (34).
In conclusion, in multivariate analysis, MRI and MRSI findings before EBRT in patients with prostate cancer are more accurate independent predictors of outcome than clinical variables, and in particular, the findings of seminal vesicle invasion and extensive tumor predict a worse prognosis.
Acknowledgments
Supported by National Institutes of Health grant R01 CA079980.
Footnotes
Conflict of interest: none.
References
- 1.Obek C, Louis P, Civantos F, et al. Comparison of digital rectal examination and biopsy results with the radical prostatectomy specimen. J Urol. 1999;161:494–498. [PubMed] [Google Scholar]
- 2.Mettlin CJ, Black B, Lee F, et al. Workgroup 2. Screening and detection: Reference range/clinical issues of PSA. Cancer. 1993;71:2679–2680. doi: 10.1002/1097-0142(19930415)71:8<2679::aid-cncr2820710840>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Salomon L, Colombel M, Patard JJ, et al. Value of ultrasound-guided systematic sextant biopsies in prostate tumor mapping. Eur Urol. 1999;35:289–293. doi: 10.1159/000019863. [DOI] [PubMed] [Google Scholar]
- 4.Smith JA, Scardino PT, Resnick MI, et al. Transrectal ultrasound versus digital rectal examination for the staging of carcinoma of the prostate: Results of a prospective multi-institutional trial. J Urol. 1997;157:902–906. [PubMed] [Google Scholar]
- 5.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer: A multi-institutional update. JAMA. 1997;277:1445–1451. [PubMed] [Google Scholar]
- 6.Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 7.Huch Boni RA, Boner JA, Debatin JF, et al. Optimization of prostate carcinoma staging: Comparison of imaging and clinical methods. Clin Radiol. 1995;50:593–600. doi: 10.1016/s0009-9260(05)83287-8. [DOI] [PubMed] [Google Scholar]
- 8.Yu KK, Hricak H, Alagappan R, et al. Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: Multivariate feature analysis. Radiology. 1997;202:697–702. doi: 10.1148/radiology.202.3.9051019. [DOI] [PubMed] [Google Scholar]
- 9.Kurhanewicz J, Vigneron DB, Hricak H, et al. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7cm3) spatial resolution. Radiology. 1996;198:795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]
- 10.Scheidler J, Hricak H, Vigneron DB, et al. Prostate cancer: Localization with three-dimensional proton MR spectroscopic imaging-clinicopathologic study. Radiology. 1999;213:473–480. doi: 10.1148/radiology.213.2.r99nv23473. [DOI] [PubMed] [Google Scholar]
- 11.Coakley FV, Kurhanewicz J, Lu Y, et al. Prostate cancer tumor volume: Measurement with endorectal MR and MR spectroscopic imaging. Radiology. 2002;223:91–97. doi: 10.1148/radiol.2231010575. [DOI] [PubMed] [Google Scholar]
- 12.Coakley FV, Qayyum A, Kurhanewicz J. Magnetic resonance imaging and spectroscopic imaging of prostate cancer. J Urol. 2003;170:S69–S75. doi: 10.1097/01.ju.0000094958.23276.c4. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico AV, Whittington R, Malkowicz B, et al. Endorectal magnetic resonance imaging as a predictor of biochemical outcome after radical prostatectomy in men with clinically localized prostate cancer. J Urol. 2000;164:759–763. doi: 10.1097/00005392-200009010-00032. [DOI] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna DA, Coakley FV, Westphalen AC, et al. Prostate cancer: Role of pretreatment MR in predicting outcome after external-beam radiation therapy—initial experience. Radiology. 2008;247:141–146. doi: 10.1148/radiol.2471061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Star-Lack J, Vigneron DB, Pauly J, et al. Improved solvent suppression and increased spatial excitation bandwidths for three-dimensional PRESS CSI using phase-compensating spectral/spatial spin-echo pulses. J Magn Reson Imaging. 1997;7:745–757. doi: 10.1002/jmri.1880070422. [DOI] [PubMed] [Google Scholar]
- 17.Schricker AA, Pauly JM, Kurhanewicz J, et al. Dualband spectral-spatial RF pulses for prostate MR spectroscopic imaging. Magn Reson Med. 2001;46:1079–1087. doi: 10.1002/mrm.1302. [DOI] [PubMed] [Google Scholar]
- 18.Tran T-KC, Vigneron DB, Sailasuta N, et al. Very selective suppression pulses for clinical MRSI studies of brain and prostate cancer. Magn Reson Med. 2000;43:23–33. doi: 10.1002/(sici)1522-2594(200001)43:1<23::aid-mrm4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Cabrera AR, Coakley FV, Westphalen AC, et al. Is inapparent tumor at baseline endorectal MR imaging and MR spectroscopic imaging a positive prognostic finding in prostate cancer patients on active surveillance? Radiology. 2008;247:444–450. doi: 10.1148/radiol.2472070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung JA, Coakley FV, Vigneron DB, et al. Prostate depiction at endorectal MR spectroscopic imaging: Investigation of a standardized evaluation system. Radiology. 2004;233:701–708. doi: 10.1148/radiol.2333030672. [DOI] [PubMed] [Google Scholar]
- 21.American Joint Committee on Cancer. Prostate. In: Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6. New York: Springer; 2002. pp. 309–316. [Google Scholar]
- 22.Sadek AG, Mitchell DG, Siegelman ES, et al. Early hepatocellular carcinoma that develops within macroregenerative nodules: Growth rate depicted at serial MR imaging. Radiology. 1995;195:753–756. doi: 10.1148/radiology.195.3.7754006. [DOI] [PubMed] [Google Scholar]
- 23.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized with cancer: Recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Stromberg J, Martinez A, Benson R, et al. Improved local control and survival for surgically staged patients with locally advanced prostate cancer treatment with up-front low dose rate iridium-192 prostate implantation and external beam irradiation. Int J Radiat Oncol Biol Phys. 1994;28:67–75. doi: 10.1016/0360-3016(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 25.Pickles T, Pollack A. The case for dose escalation versus adjuvant androgen deprivation therapy for intermediate risk prostate cancer. Can J Urol. 2006;13(Suppl 2):68–71. [PubMed] [Google Scholar]
- 26.Epstein JI, Carmichael M, Walsh PC. Adenocarcinoma of the prostate invading the seminal vesicle: Definition and relation of tumor volume, grade, and margins of resection to disease prognosis. J Urol. 1993;149:1040–1045. doi: 10.1016/s0022-5347(17)36291-2. [DOI] [PubMed] [Google Scholar]
- 27.Clarke DH, Banks SJ, Wiederhorn AR, et al. The role of endorectal coil MRI in patient selection and treatment planning for prostate seed implants. Int J Radiat Oncol Biol Phys. 2002;52:903–910. doi: 10.1016/s0360-3016(01)02736-5. [DOI] [PubMed] [Google Scholar]
- 28.D’Amico AV, Whittington R, Malkowicz SB, et al. Critical analysis of the ability of the endorectal coil magnetic resonance imaging scan to predict pathologic stage, margin status and postoperative prostate-specific antigen failure in patients with clinically organ-confined prostate cancer. J Clin Oncol. 1995;14:1770–1777. doi: 10.1200/JCO.1996.14.6.1770. [DOI] [PubMed] [Google Scholar]
- 29.D’Amico AV, Whittington R, Malkowicz SB, et al. A multivariate analysis of clinical and pathological factors that predict for prostate specific antigen failure after radical prostatectomy for prostate cancer. J Urol. 1995;154:131–138. [PubMed] [Google Scholar]
- 30.Nguyen PL, Whittington R, Koo S, et al. Quantifying the impact of seminal vesicle invasion identified using endorectal magnetic resonance imaging on PSA outcome after radiation therapy for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:400–405. doi: 10.1016/j.ijrobp.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Sala E, Akin O, Moskowitz CS, et al. Endorectal MR imaging in the evaluation of seminal vesicle invasion: Diagnostic accuracy and multivariate feature analysis. Radiology. 2006;238:929–937. doi: 10.1148/radiol.2383050657. [DOI] [PubMed] [Google Scholar]
- 32.Jager GJ, Ruijter ET, van de Kaa CA, et al. Local staging of prostate cancer with endorectal MR imaging: Correlation with histopathology. AJR Am J Roentgenol. 1996;166:845–852. doi: 10.2214/ajr.166.4.8610561. [DOI] [PubMed] [Google Scholar]
- 33.Yossepowitch O, Sircar K, Scardino PT, et al. Bladder neck involvement in pathological stage pT4 radical prostatectomy specimens is not an independent prognostic factor. J Urol. 2002;168:2011–2015. doi: 10.1016/S0022-5347(05)64284-X. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi M, Stamey TA, McNeal JE, et al. Preoperative serum prostate specific antigen does not reflect biochemical failure rates after radical prostatectomy in men with large volume cancers. J Urol. 2000;164:1596–1600. [PubMed] [Google Scholar]