Abstract
To examine any potential role for 1,25-dihydroxyvitamin D (1,25(OH)2D) in inflammation associated with chronic kidney disease we measured vitamin D metabolites, markers of inflammation and gene expression in 174 patients with a variety of kidney diseases. Urinary MCP-1 protein and renal macrophage infiltration were each significantly but inversely correlated with serum 1,25(OH)2D levels. Logistic regression analysis with urinary MCP-1 as binary outcome showed that a 10-unit increase in serum 1,25(OH)2D or 25OHD resulted in lower renal inflammation. Analysis of 111 renal biopsies found that renal injury was not associated with a compensatory increase in mRNA for the vitamin D-activating enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1), its catabolic counterpart 24-hydroxylase, or the vitamin D receptor. There was, however, a significant association between tissue MCP-1 and CYP27B1. Patients with acute renal inflammation had a significant increase in urinary and tissue MCP-1, macrophage infiltration, and macrophage and renal epithelial CYP27B1 expression but significantly lower levels of serum 1,25(OH)2D in comparison to patients with chronic ischemic disease despite similar levels of renal damage. In vitro, 1,25(OH)2D attenuated TNFα-induced MCP-1 expression by human proximal tubule cells. Our study indicates that renal inflammation is associated with decreased serum vitamin D metabolites and involves activation of the paracrine/autocrine vitamin D system.
Keywords: VDR, inflammation, vitamin D, CYP27B1, CYP24A1, chronic kidney disease
The importance of vitamin D to renal physiology has been recognized for many years. Synthesis of the active, hormonal form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D), from its inactive precursor (25-hydroxyvitamin D, 25OHD) occurs primarily in the proximal tubules of the kidney1,2 and supports the key endocrine actions of vitamin D.3 Recently, it has become clear that 1,25(OH)2D has potent immunomodulatory and antiproliferative properties, suggesting a role for vitamin D in the pathophysiology of autoimmune disease,4 common cancers,5 hypertension,6,7 renal inflammation,8–10 and cardiovascular disease.6,11–13 Most of these nonclassical actions have been attributed to autocrine synthesis of 1,25(OH)2D, without changes in serum 1,25(OH)2D levels. Nevertheless, the kidney remains an integral component of normal vitamin D metabolism and function, with the vitamin D-activating enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1), its catabolic counterpart 24-hydroxylase (CYP24A1), and the nuclear vitamin D receptor (VDR), involving in determining the circulating levels of 1,25(OH)2D.1,14
In chronic kidney disease (CKD), perturbation of the vitamin D hormonal system is associated with progressive loss of renal function and may lead to skeletal,15 cardiovascular, 12,13 and renal9 complications, contributing directly to premature death.12,13 Although serum 1,25(OH)2D levels decline early in CKD,16,17 mainly as a consequence of decreased renal synthesis of the hormone, the predominant defect that underlies this remains unclear. Abnormal CYP27B1 activity may be due to: decreased kidney mass,14,16–18 inhibition of CYP27B1 expression,19,20 dysregulated CYP27B1 expression,21 or decreased availability of substrate for CYP27B1.17 Circulating levels of 1,25(OH)2D may also reflect aberrant activity of CYP24A1, which is abundantly expressed in the kidney and catalyses conversion of 1,25(OH)2D to inactive metabolites.22,23 Another facet of vitamin D that may impact significantly on CKD concerns is its potent antiproliferative and immunomodulatory actions. For example, 1,25(OH)2D has been shown to modulate renal inflammation and fibrosis.8–10,24 These processes have been primarily attributed to the paracrine/autocrine effects of vitamin D.10,25,26 However, other studies using vitamin D analogs have indicated that the endocrine vitamin D system may also contribute to immunomodulatory responses.8,9,24
Macrophage chemoattractant protein-1 (MCP-1) is expressed in injury and inflammation to direct macrophage recruitment.27,28 1,25(OH)2D has been shown to be able to inhibit this MCP-1 driven inflammatory process by blocking nuclear factor-κB activation, potentially protecting the kidney.29
In this work, we have used a cohort of kidney disease patients to show for the first time a link between vitamin D metabolism and renal inflammation.
RESULTS
Markers of renal impairment in a kidney disease population undergoing renal biopsy
A total of 174 kidney disease patients (99 men, 75 women; 81.6% white, 10.9% Asian, 6.9% Afro-Caribbean) were studied. Of these, 111 had frozen kidney biopsy tissue available for PCR analysis. Data in Table 1 show clinical parameters and Table 2 shows pathological diagnoses, creatinine clearance (CrCl), and index of chronic damage. The degree of renal impairment depended on disease subclassification, with thin glomerular basement membrane disease (TBMD) patients (CrCl: 103.4±34.2 ml/min; index of chronic damage 2.2±3.5%) showing relatively intact renal parenchyma (Table 2, Figure 3a and b) when compared with renal vasculitis (25.9±39.4 ml/min; index of chronic damage 46.7±34.4%) or interstitial nephritis patients (CrCl: 11.3±12.0 ml/min; index of chronic damage 70.3±40.4%). Bivariate analyses showed that CrCl correlated negatively with the index of chronic renal damage (r=0.665, P<0.001), macrophage infiltration (r=0.583, P<0.001), and urinary MCP-1 concentration (r=0.372, P<0.001) (data not shown).
Table 1.
Study population, n=174: clinical and pathological characteristics
| Median | Range | |
|---|---|---|
| Age (years) | 52 | 18–89 |
| Body mass index (kg/m2) | 26.3 | 16.6–50.4 |
| Systolic blood pressure (mm Hg) | 137 | 90–200 |
| Diastolic blood pressure (mm Hg) | 75 | 45–106 |
| Serum albumin (g/l) | 40 | 10–50 |
| Urea (mmol/l) | 7.2 | 2.2–52.0 |
| Serum creatinine (µmol/l) | 126 | 51–1110 |
| Hemoglobin (g/100 ml) | 12.9 | 6.1–17.7 |
| Leucocytes (×106/ml) | 6.8 | 2.3–35.4 |
| Creatinine clearance (ml/min) | 71.5 | 2–159 |
| Index of chronic damage (%) | 12.5 | 0–98 |
| Urinary protein excretion (g/24 h) | 0.12 | 0–18 |
Table 2.
Study population: pathological diagnosis on the renal biopsy, creatinine clearance, and index of chronic renal damage
| N | Creatinine clearance (ml/min); mean (s.d.) |
Index of chronic damage (% area); mean (s.d.) |
|
|---|---|---|---|
| Chronic ischemic renal damage | 44 | 50.1 (34.0) | 38.1 (28.1) |
| Thin glomerular basement membrane disease | 35 | 103.4 (34.2) | 2.2 (3.5) |
| IgA nephropathy | 27 | 80.2 (38.7) | 19 (27.7) |
| Focal segmental sclerosing glomerulonephritis | 14 | 81.2 (36.0) | 17.3 (14.3) |
| Diabetic nephropathy | 10 | 66.2 (46.6) | 34.1 (28.4) |
| Renal vasculitis | 9 | 25.9 (39.4) | 46.7 (34.4) |
| Membranous nephropathy | 7 | 76.9 (36.9) | 40.5 (29.1) |
| Interstitial nephritis | 6 | 11.3 (12.0) | 70.3 (40.4) |
| Lupus nephritis | 5 | 76.8 (47.8) | 14.5 (17.2) |
| Nodular light-chain glomerulopathy with myeloma | 4 | 39.1 (27.6) | 52.0 (38.4) |
| Mesangioproliferative glomerulonephritis | 3 | 93.7 (29.3) | 0.0 (0.0) |
| Minimal change nephropathy | 3 | 101.0 (25.5) | — |
| Amyloidosis | 2 | 38.0 (4.2) | 49.5 (30.4) |
| Othera | 5 | 35.9 (52.1) | 47.5 (67.2) |
| Total | 174 | 69.7 (42.5) | 24.2 (28.4) |
IgA, immunoglobulin A.
One iron overload, one metastatic carcinoma, two acute renal failure secondary to cardio-respiratory failure, and one histological diagnosis not made.
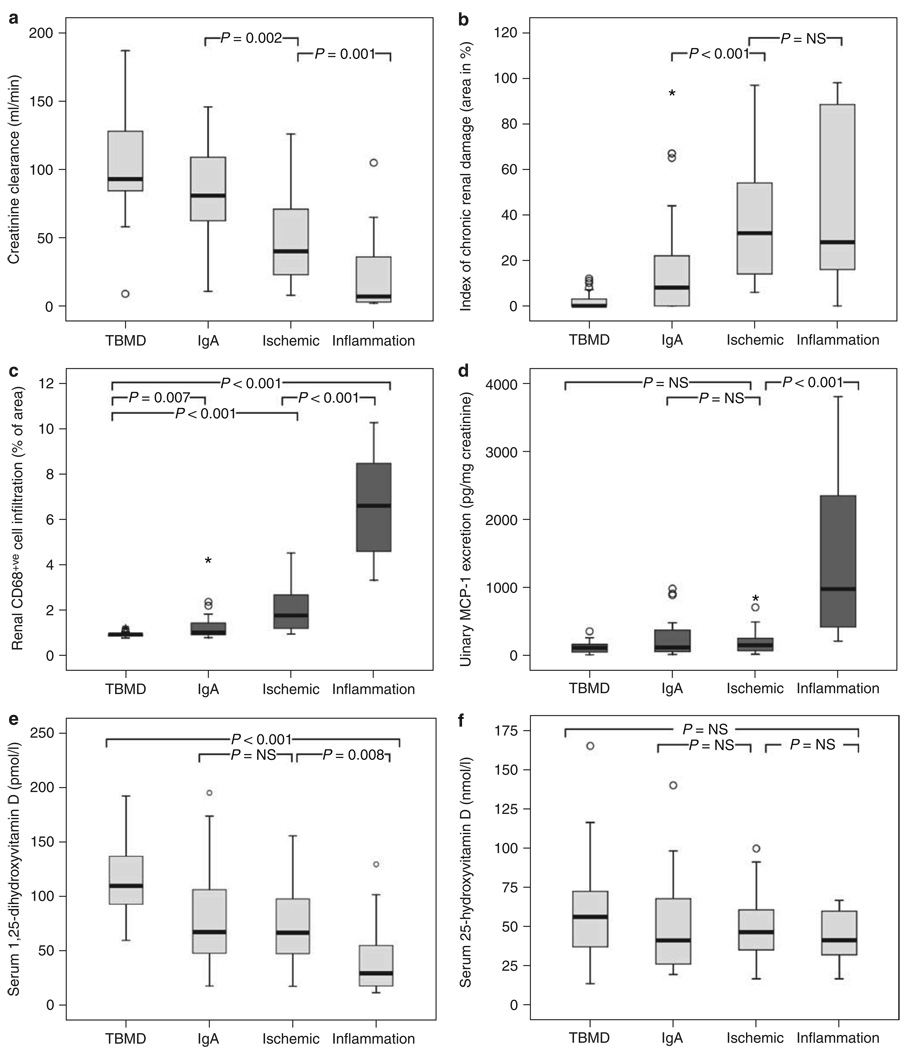
Figure 3. Analysis of renal biopsy patients according to disease diagnostic groups.
Patients were grouped according to the pathological diagnosis on renal biopsy (thin glomerular basement membrane disease (TBMD), n=35; immunoglobulin A (IgA) nephropathy, n=27; chronic ischemic renal damage, n=44 (ischemic); acute renal inflammation including the diagnosis of renal vasculitis and interstitial nephritis, n=15 (inflammation), and assessed for: (a) creatinine clearance (ml/min) following a 24-h urine collection, (b) index of chronic renal damage (area in %), (c) histological quantification of interstitial macrophage infiltration (% of area of CD68+VE cells per area assessed), (d) urinary MCP-1 protein levels (pg/mg creatinine), (e) serum 1,25(OH)2D (pmol/l), and (f) serum 25OHD (nmol/l) levels. Statistical significance between groups is shown as P-values.
Renal impairment, renal inflammation, and the vitamin D hormonal system
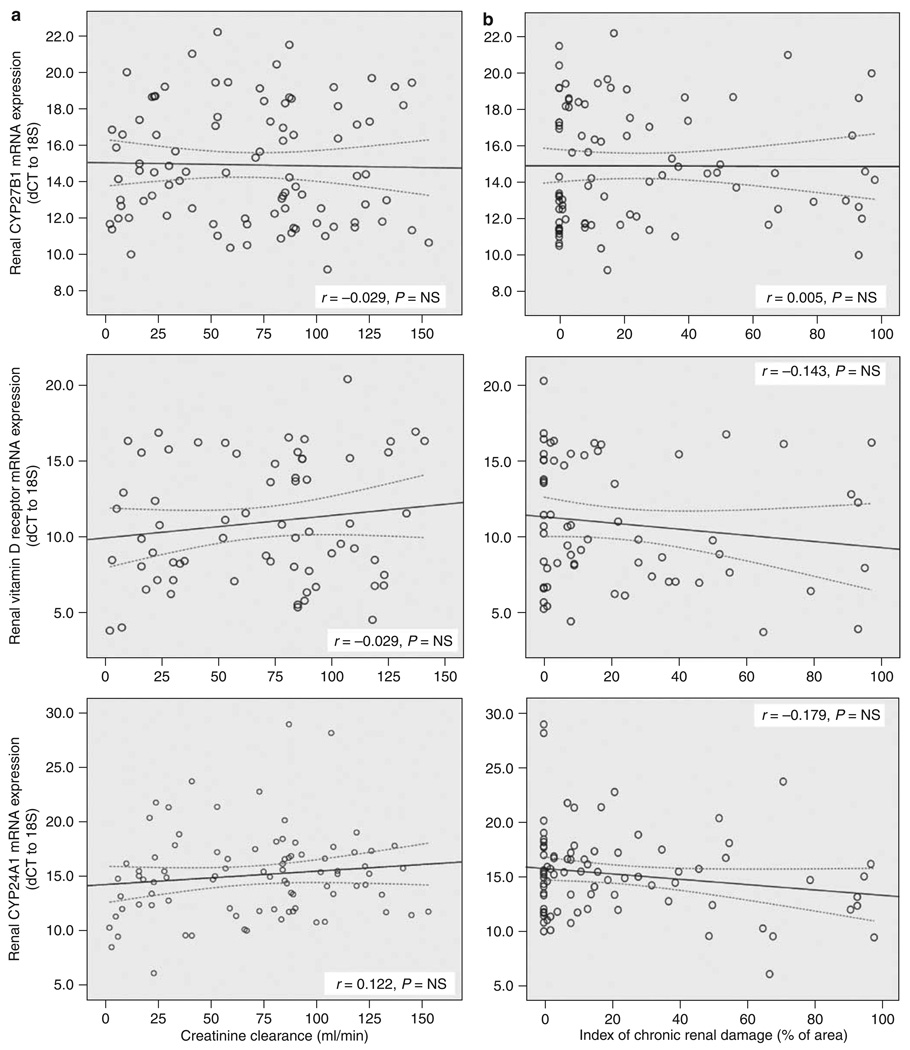
To relate the different markers of renal impairment outlined in Table 1 and Table 2, with serum and RNA measures of vitamin D metabolism and function, we used several different methods of comparison. With multiple linear regression analysis, a negative correlation was observed between serum 1,25(OH)2D and renal scarring (β=−0.430, P<0.001) and urinary MCP-1 levels (β=−0.179, P=0.026). By contrast, no correlation was observed between serum 1,25(OH)2D and serum intact parathyroid hormone, a known regulator of the renal vitamin D endocrine system. Serum 1,25(OH)2D also correlated with serum 25OHD (β=0.272, P=0.001) (Table 3a), and, as expected, renal CYP27B1 mRNA correlated positively with serum 1,25(OH)2D (β=−0.272, P=0.012), and showed no correlation with serum intact parathyroid hormone (Table 3b). Although serum 1,25(OH)2D levels correlated with renal impairment (Table 3a), no similar association was observed for CYP27B1 mRNA and chronic renal scarring (Table 3b) or CrCl (data not shown). CYP27B1 mRNA expression was negatively correlated with 24-h urinary protein excretion (β=0.349, P=0.013), whereas MCP-1 mRNA expression correlated positively with CYP27B1 mRNA (β=0.529, P=0.002). To investigate further, the effect of CKD on the expression of genes associated with vitamin D metabolism and function, linear regression analyses were carried out for CYP27B1, CYP24A1, and VDR mRNAs. Data indicated that loss of renal mass, declining serum 1,25(OH)2D levels, and deteriorating renal function, as defined by CrCl and increasing chronic renal damage, was not associated with altered expression of mRNAs for CYP27B1, CYP24A1, or VDR (Figure 1).
Table 3.
Correlates of serum 1,25(OH)2D and renal CYP27B1 mRNA expression in renal biopsy patients
| Linear regression analysis (adjusted R2=0.377) | |||
|---|---|---|---|
| β | P-values | ||
| (a) | Serum 1,25-dihydroxyvitamin D | ||
| Constant | NS | ||
| Index of chronic renal damage | −0.430 | <0.001 | |
| Serum 25(OH)D | 0.272 | 0.001 | |
| Urinary MCP-1 protein | −0.179 | 0.026 | |
| Intact PTH | 0.045 | NS | |
| Linear regression analysis (adjusted R2=0.305) | |||
| (b) | Renal CYP27B1 mRNA levels | ||
| Constant | NS | ||
| Renal MCP-1 mRNA | 0.529 | 0.002 | |
| Serum 1,25(OH)2D | −0.408 | 0.012 | |
| Urinary protein in 24 h | 0.349 | 0.013 | |
| Index of chronic renal damage | −0.247 | NS | |
| Intact PTH | 0.185 | NS | |
1,25(OH)2D, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; MCP-1, macrophage chemoattractant protein-1; NS, not significant; PTH, parathyroid hormone. Linear regression analysis of correlation between serum 1,25(OH)2D (a) or CYP27B1 (b) and: (1) index of chronic renal damage (area in %); (2) serum 25-hydroxyvitamin D (25OHD) (nmol/l); (3) serum 1,25(OH)2D (pmol/l); (4) urinary MCP-1 protein levels (pg/mg creatinine); (4) renal tissue MCP-1 mRNA levels (dCT to 18S rRNA); (5) intact PTH (pmol/l); (6) 24-h urinary protein (g/24 h). Results are presented for natural log 25OHD, natural log urinary MCP-1, natural log MCP-1 mRNA, natural log PTH, and natural log 24-h urinary protein corrected serum calcium. Low mRNA tissue expression is observed with increased dCT and vice versa. Significance of regression is shown by P<0.05.
Figure 1. Effects of CKD on the expression of vitamin D-associated genes in renal biopsy tissue.
Linear correlation between (a) creatinine clearance (ml/min) and (b) histological measurement of an index of chronic renal damage within the renal biopsy specimen (area in %) with renal expression of mRNAs for: CYP27B1, vitamin D receptor (VDR), and 24-hydroxylase (CYP24A1). All expression data are shown as dCT values with respect to 18S rRNA. Low mRNA tissue expression is observed with increased dCT and vice versa. Correlation coefficient is represented by r-value and statistical significance by P-value, with dotted lines representing 95% confidence intervals.
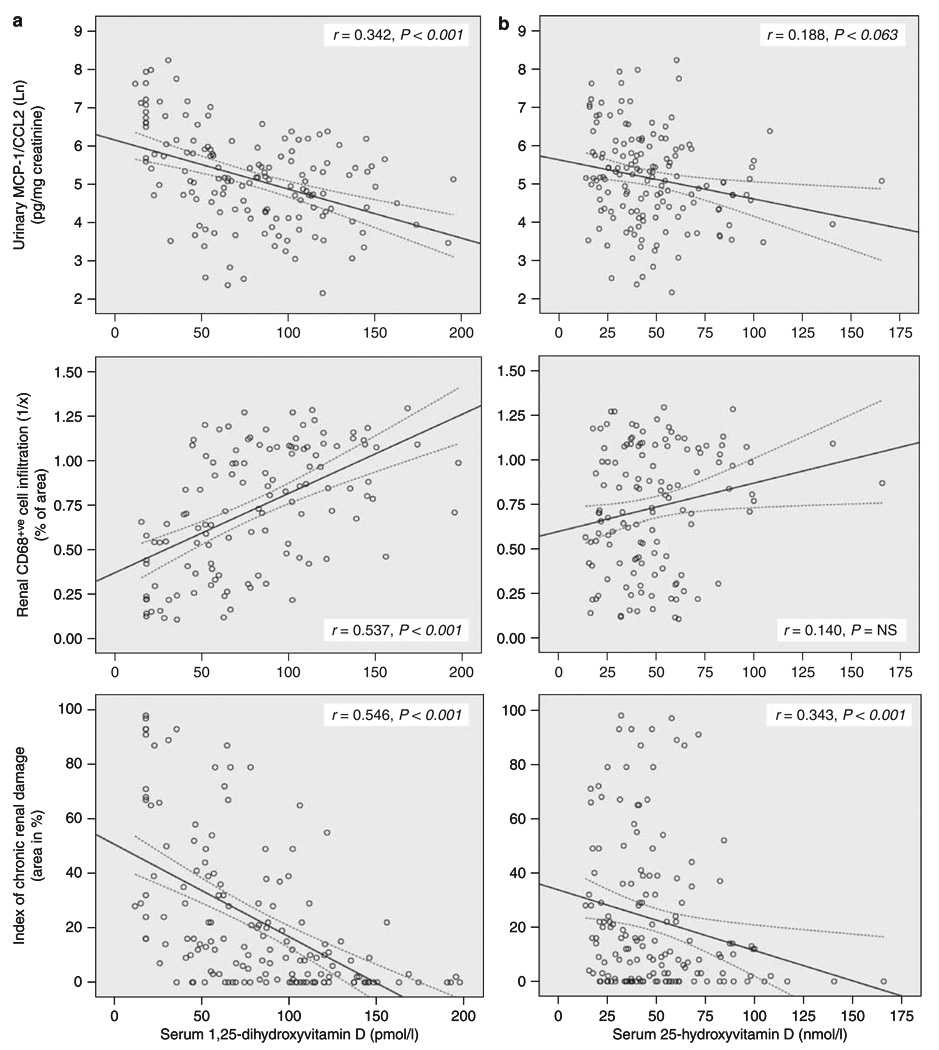
Linear regression was also used to characterize further the relationship between renal impairment and inflammation with serum concentrations of 25OHD and 1,25(OH)2D (Figure 2). The latter confirmed linear regression data in Table 3a by showing a negative correlation with urinary MCP-1 (r=0.342, P<0.001), macrophage infiltration (r=0.537, P<0.001), renal scarring (r=0.546, P<0.001) (Figure 2a), and renal MCP-1 mRNA (r=0.342, P=0.003) (data not shown). Serum 25OHD showed similar correlation trends to 1,25(OH)2D but this was only statistically significant for renal scarring (r=0.343, P<0.001) (Figure 2b) and renal MCP-1 mRNA (r=0.260, P=0.024) (data not shown). As an alternative model for correlation between renal inflammation and serum vitamin D, logistic regression was used, with urinary MCP-1 as a binary outcome variable (Table 4). Previous studies from our group reported a mean urinary MCP-1 of 245±243 pg/mg creatinine in a similar CKD patient population with chronic inflammation.27 Therefore, to assess the potential role of vitamin D in a more acute inflammatory environment, a ‘cut-off ’ value of 300 pg/mg creatinine for urinary MCP-1 was selected. Comparing urinary MCP-1 ≤300 with >300 mg/mg creatinine, a 10 unit increase in serum 1,25(OH)2D reduced the odds ratio (OR) to 0.75 (95% CI 0.65–0.88) for patients being in the group with higher urinary MCP-1, after adjusting for age, gender, ethnicity, and index of chronic renal damage (Table 4). The same observation was made for serum 25OHD (OR 0.71; 95% CI 0.57–0. 90).
Figure 2. Relationship between serum vitamin D metabolites and markers of renal inflammation and CKD.
Linear correlation between (a) serum 1,25(OH)2D (pmol/l) and (b) serum 25OHD (nmol/l) with: measurement of urinary MCP-1 protein levels (pg/mg creatinine, expressed as natural log Ln); histological quantification of macrophage infiltration (% of area of CD68+ve cells per area assessed, expressed as 1/% of area); histological measurement of an index of chronic renal damage within the renal biopsy specimen (area in %). Correlation coefficient is represented by r-value and statistical significance by P-value, with dotted lines representing 95% confidence intervals.
Table 4.
Logistic regression model for urinary MCP-1
| Unadjusted B | Unadjusted OR | Adjusted* B | Adjusted OR for a 10 unit increase in vitamin D | |
|---|---|---|---|---|
| Serum 25OHD | −0.024 | 0.98 (0.95–0.99) | −0.034 | 0.71 (0.57–0.90) |
| Serum 1,25(OH)2D | −0.028 | 0.97 (0.968–0.99) | −0.028 | 0.75 (0.65–0.88) |
1,25(OH)2D, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; MCP-1, macrophage chemoattractant protein-1; OR, odds ratio.
Urinary MCP-1 as binary outcome, comparing MCP-1 ≤300 with >300 pg/mg creatinine for 25OHD (nmol/l) and 1,25(OH)2D (pmol/l).
The model was adjusted for age, gender, ethnicity, index of chronic renal damage. OR with 95% confidence limits.
Dysregulation of the vitamin D hormonal system with different forms of kidney disease
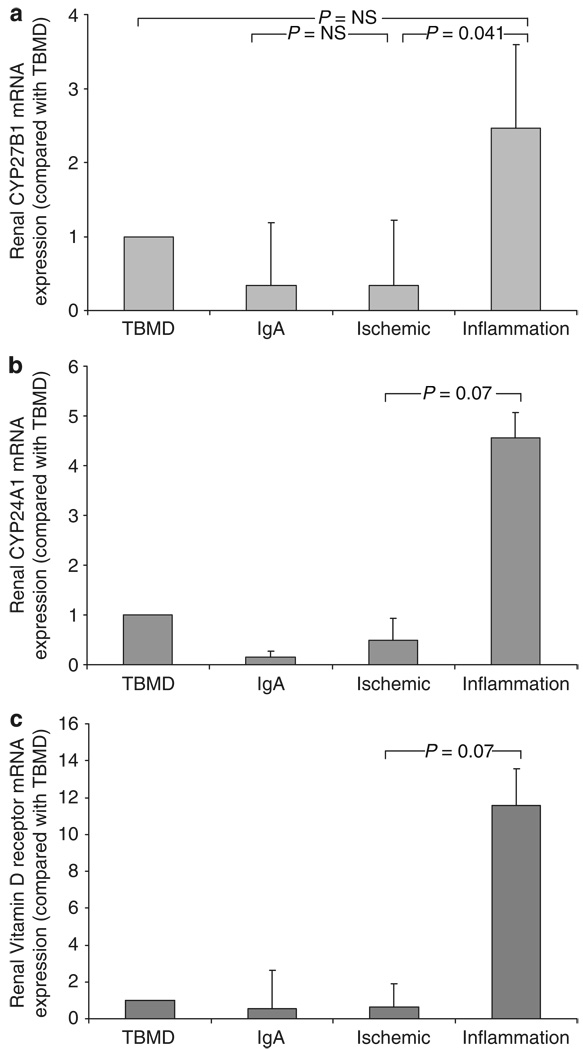
To provide an additional perspective on the vitamin D hormonal system in CKD, data were also analyzed on the basis of patient pathological/diagnostic groupings. The TBMD group was selected as a control population with minimal impaired renal function, scarring, and renal inflammation. Patients with renal vasculitis or interstitial nephritis were referred to as the acute inflammation group (inflammation) and compared with the other three groups. Patients in the acute inflammation had similar chronic renal damage to the ischemia group (28 vs 32% of area, P=NS) (Figure 3b), but much lower levels of CrCl (7 vs 40 ml/min, P=0.001) (Figure 3a), higher macrophage infiltration (6.6 vs 1.8% of area, P<0.001) (Figure 3c), and higher urinary MCP-1 (976.8 vs 143.9 pg/mg creatinine, P<0.001) (Figure 3d), reflecting the more acute, inflammatory nature of CKD in these patients. Consistent with this, serum 1,25(OH)2D levels were much lower in the inflammation group compared with the ischemia patients (29.6 vs 67.1 pmol/l, P=0.008) (Figure 3e). However, there was no significant difference in serum 25OHD levels between the groups studied (Figure 3f). Analysis of biopsy tissue mRNA expression showed that levels of renal CYP27B1 and at the same time probably also of CYP24A1 and VDR were increased in acute renal inflammation (Figure 4).
Figure 4. Analysis of renal biopsy patients according to disease diagnostic groups.
Patients were grouped according to pathological diagnosis on renal biopsy (thin glomerular basement membrane disease (TBMD), n=35; immunoglobulin A (IgA) nephropathy, n=27; chronic ischemic renal damage, n=44 (ischemic); acute renal inflammation including the diagnosis of renal vasculitis and interstitial nephritis, n=9 (inflammation) and assessed for renal biopsy expression of: (a) CYP27B1 mRNA, (b) 24-hydroxylase (CYP24A1) mRNA, and (c) vitamin D receptor (VDR) mRNA. All expression data are shown as fold change in levels of mRNA with respect to TBMD values. Statistical significance between groups is shown as P-values.
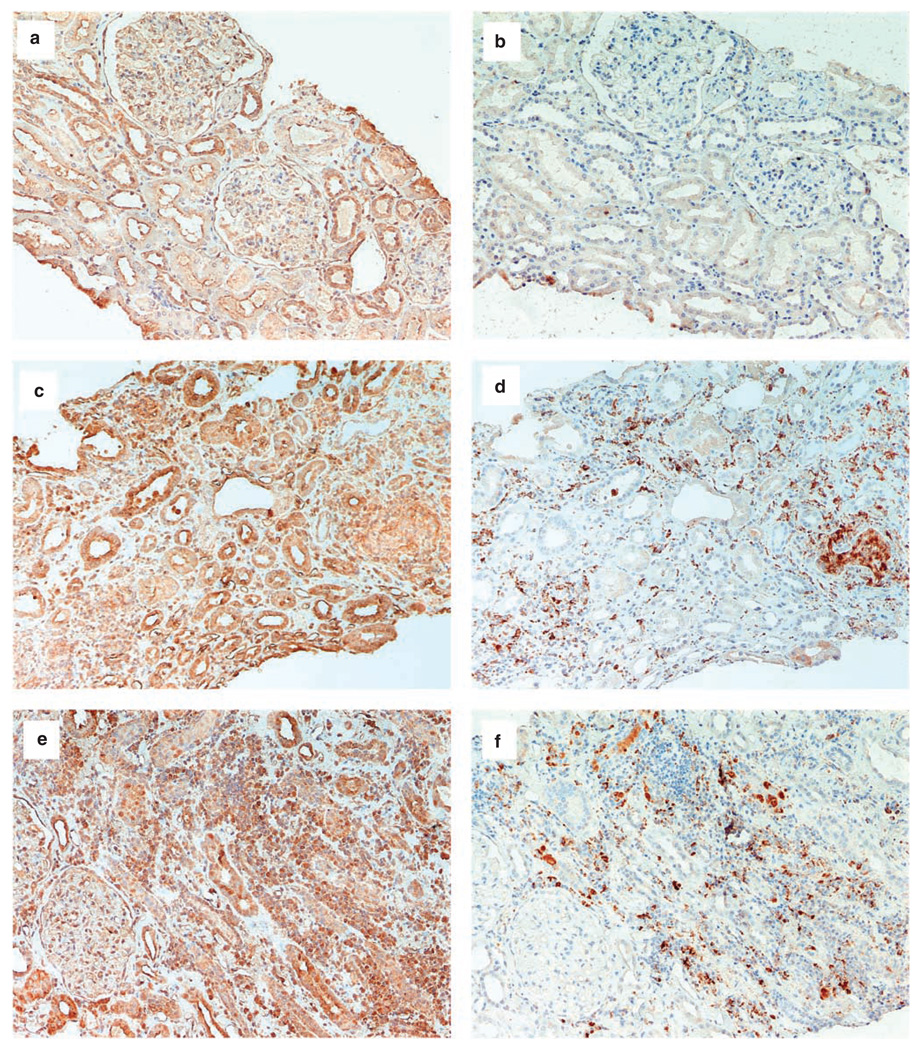
In human kidney biopsies from patients with interstitial nephritis, CYP27B1 protein expression was localized to areas with immune cell infiltrate. Staining of consecutive sections confirmed CYP27B1 protein to be colocalized to macrophages (CD68+VE cells) (Figure 5c–f). Strong CYP27B1 protein staining was also found in renal tubular cells near to immune cell infiltration (Figure 5c,e). Normal kidney expressed CYP27B1 protein along the tubules with almost no immune cells in the renal interstitium (Figure 5a and b).
Figure 5. Localization of CYP27B1 protein and macrophages by immunohistochemistry in paraffin sections from human kidney biopsies.
Immunohistochemistry (positive staining brown) for CYP27B1, (a) and macrophages (CD68+VE), (b) (consecutive sections) for normal kidney; for CYP27B1, (c, e) and CD68+VE, (d, f) (consecutive sections) of kidneys from two different patients with interstitial nephritis. Increased expression for CYP27B1 protein is found in areas of immune cell, particularly macrophage (CD68+VE) infiltration. Significant CYP27B1 protein expression is also found in remnants of renal tubular cells. Magnification (a–d: ×40, e–f: ×80).
Regulation of MCP-1 expression by vitamin D in vitro
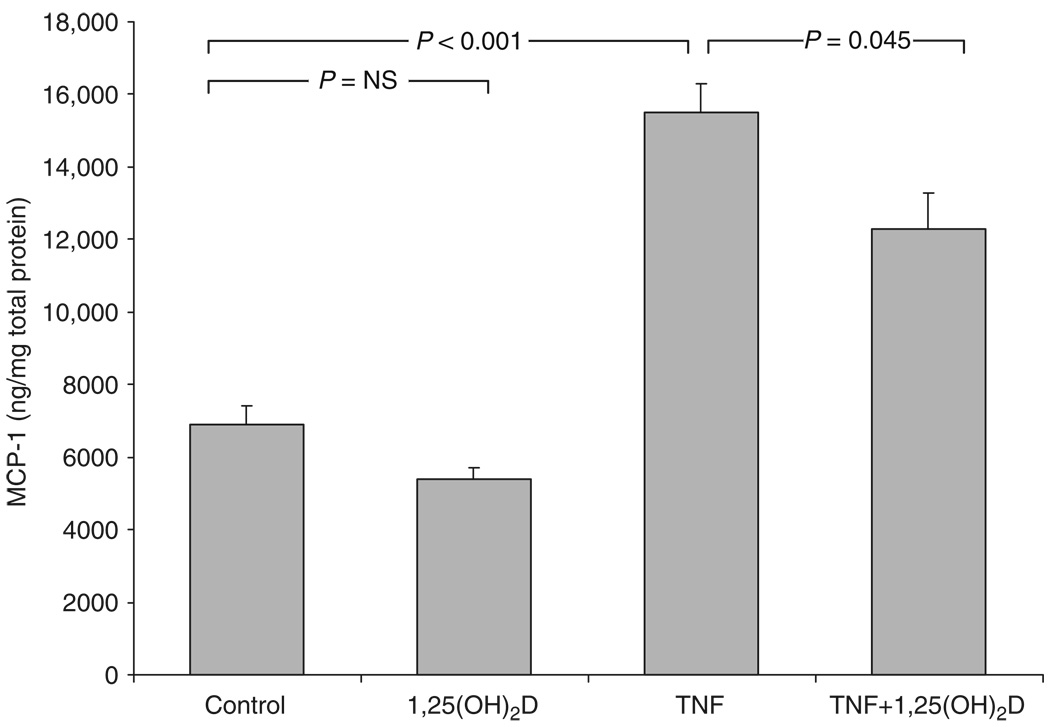
To investigate further a link between the vitamin D hormonal system and renal inflammation, additional studies were carried out in vitro. Results in Figure 6 showed that inflammatory activation of proximal tubular epithelial cells with tumor necrosis factor-α stimulated MCP-1 production by these cells, and this was attenuated following coincubation with 1,25(OH)2D (P=0.045), suggesting a possible role for vitamin D as a modulator of renal inflammation.
Figure 6. 1,25(OH)2D suppresses expression of MCP-1 synthesis in primary cultures of human proximal renal tubular epithelial cells.
Cells (n=4) were treated for 24 h with 1,25-dihydroxyvitamin D (1,25(OH)2D) (10−7 m), TNF-α (1 ng/ml) or TNF-α +1,25(OH)2D (10−7 m) before measuring MCP-1 protein in the supernatant and standardized to total cellular protein levels. Statistical significance between groups is shown as P-values.
DISCUSSION
Consistent with previously published studies, we confirmed that increased renal impairment is associated with decreased circulating levels of 1,25(OH)2D,16–18 although this was not associated with significant changes in CYP27B1 mRNA expression. To the best of our knowledge, this is the first study to describe the expression of vitamin D-related genes in material from patients undergoing a diagnostic kidney biopsy. As such, the only other available CKD model for comparison is a rat model that has utilized three-fourth nephrectomy, respectively, to mimic CKD.30 In these rats, the renal impairment caused by substantial loss of renal mass was associated with a compensatory increase in mRNA for CYP27B1, and decreased expression of CYP24A1 and VDR mRNA in the kidney remnant, with resulting normalized serum 1,25(OH)2D levels. Clearly, it is difficult to draw a direct comparison between the rat model and our data. However, indirect measurement of renal endocrine function in humans, with falling serum 1,25(OH)2D levels already as a consequence of mild CKD seems to support our observations.16–18 One possible explanation for the lack of change in CYP27B1 expression with renal impairment is that the predominant determinant of decreased serum 1,25(OH)2D in CKD patients is indeed loss of functional renal mass. Alternatively, CKD may be associated with altered expression of CYP27B1 and CYP24A1 mRNA splice variants that are not readily detected by standard real-time RT–PCR but are still able to modulate 25OHD metabolism.21,31 A further explanation is that the analysis of mRNA for CYP27B1 mRNA does not adequately reflect changes in the protein for this enzyme. Previous studies have shown that, during normal physiology, there is a strong correlation between expression of mRNA for CYP27B1 and capacity to synthesize 1,25(OH)2D.32,33 However, more recent analysis of the suppression of 1,25(OH)2D production in hypophosphatemic hyp mice concluded that control of CYP27B1 activity in these animals occurred at a post-transcriptional level.34 Owing to the constraints of tissue availability, we were unable to evaluate CYP27B1 protein expression or enzyme activity in renal biopsies. It is therefore possible that the decreased capacity for synthesis of 1,25(OH)2D associated with CKD also involves a decline in protein rather than mRNA for CYP27B1.
Data from this study indicate that another crucial determinant of endocrine and autocrine 1,25(OH)2D in CKD is serum 25OHD. Linear regression showed a strong correlation between the active form of vitamin D and its precursor, which contrasts the lack of direct correlation between serum 25OHD and 1,25(OH)2D concentrations previously reported in normal subjects.35 From this observation and the apparent retention of CYP27B1 expression in situ, we can hypothesize that CKD patients with 25OHD sufficiency will maintain adequate 1,25(OH)2D production. A central question concerning the low levels of circulating 25OHD concentrations in CKD is whether this actively contributes to renal inflammation with progression of renal disease. To address the contribution of circulating 25OHD concentration, renal inflammation and progression of renal disease issue further, analysis of serum vitamin D metabolites was carried out according to CKD disease type. Although there was a trend toward lower levels of 25OHD in more severe forms of CKD, we did not find a statistically significant variation with disease severity. Nevertheless, these data provide support for future studies to assess the impact of vitamin D supplementation on changes in renal impairment associated with CKD.
In CKD, a progressive decline in renal function is associated with worsening tubulointerstitial and glomerular scarring.36 Data presented here show a close association between kidney function and the histological quantification of tubulointerstitial fibrosis. Renal scarring is associated with an immune cell infiltrate consisting primarily of macrophages,37 which increases with deteriorating renal function27 in response to local synthesis of the inflammatory chemokine MCP-1, with accelerated tissue inflammation and injury.27,37,38 This is illustrated by the high levels of macrophage infiltration and MCP-1 in acute renal inflammation, which parallels the significant decline in renal impairment seen in these patients. In the light of accumulating evidence for the importance of the vitamin D hormonal system for the modulation of local inflammation, in particular renal inflammation,8–10,24,29 several of our observations are important, notably the logistic regression analyses (Table 4). Although these data suggest that enhancement of the vitamin D endocrine system is anti-inflammatory, it is also possible that the effect of 25OHD is being mediated at a local, autocrine/paracrine level. Specifically, characterization of the acute inflammation disease group revealed increased expression of CYP27B1 mRNA and protein despite decreased circulating levels of 1,25(OH)2D. Analysis of interstitial infiltration by immune cells and immune staining of consecutive tissue sections for macrophages and CYP27B1 protein suggests that activated macrophages are the likely source of this enhanced CYP27B1 expression,39 although renal epithelial cells26 and endothelial cells40 may also contribute to localized synthesis of 1,25(OH)2D following inflammation. The observed positive trend between CYP27B1 and CYP24 mRNA in acute inflammation also suggest a role for autocrine synthesis of 1,25(OH)2D, as described for bone25 and is in contrast to its known regulation in renal tubular cells.25,26 Indeed, the addition of exogenous 1,25(OH)2D was able to suppress TNF-α-induced MCP-1 expression by renal epithelial cells in vitro, an effect likely caused by 1,25(OH)2D targeting of nuclear factor-κB.29 Renal conversion of 25OHD to 1,25(OH)2D may provide an, as yet unrecognized, anti-inflammatory function for renal CYP27B1 activity.
MATERIALS AND METHODS
Patient recruitment and tissue collection
Following local ethical committee approval (RRK2608), patients underwent renal biopsy in the department of nephrology at the Queen Elizabeth Hospital (Birmingham, UK), for investigation of proteinuria and/or hematuria and/or renal impairment. Patients taking vitamin D metabolites were excluded. Renal biopsy material was divided and one part was snap frozen. The other part was fixed in formalin–saline and glutaraldehyde for routine pathological study. Relevant clinical data are shown in Table 1 and the main pathological diagnoses are shown in Table 2.
Laboratory analysis
Serum and urinary creatinine was measured using the method of Jaffe41 and CrCl calculated from a 24-h urine collection, performed before renal biopsy. Urinary MCP-1 was quantified using a commercially available sandwich ELISA kit (R&D Systems, Minneapolis, MN, USA), and levels corrected for urinary creatinine. Serum 25OHD (intraassay variation 5–6%, interassay variation 7–8%) (specificity for 25OHD2 75% and 25OHD3 100%) and 1,25(OH)2D (intraassay variation 5–8%, interassay variation 9–10%) (specificity for 1,25(OH)2D2 91% and 1,25(OH)2D3 100%) were measured using an IDS Gamma-B radioimmunoassay (IDS, Boldon, UK). Intact parathyroid hormone was measured using an immunoradiometric method, the Nichols intact parathyroid hormone assay (Nichols Institute Diagnostics, San Clemente, CA, USA).
Quantitative (real-time) RT-PCR analysis of CYP27B1, CYP24A1 VDR, and MCP-1 mRNA in renal biopsies
Total RNA was isolated from frozen kidney biopsies using the GenElute RNA extraction system (Sigma, Poole, UK). Aliquots (1.5 µg) of RNA from each biopsy were then reverse transcribed using AMV reverse transcriptase (Promega, Southampton, UK). Gene-specific PCR was then carried out using an ABI 7700 sequence detection system (PE Biosystems, Warring, UK) as described previously,42 using primers and probes outlined below under the following conditions: 50 °C for 2 min; 95 °C for 10 min; followed by 44 cycles of 95 °C for 15 s and 60 °C for 1 min.
Primers and probes for PCR reactions
Primer and probe sequences for CYP27B1, VDR, and CYP24A1 were as follows: CYP27B1, forward primer 5′-TTGGCAAGCGCAGCTGTAT-3′, reverse primer 5′-TGTGTTAGGATCTGGGCCAAA-3′, Taq-Man probe 5′-TTGCAATTCAAGCTCTGCCAGGCG-3′; VDR, forward primer 5′-CTTCAGGCGAAGCATGAAGC-3′, reverse primer 5′-CCTTCATCATGCCGATGTCC-3′, TaqMan probe 5′-AAGGCACTATTCACCTGCCCCTTCAA-3′; CYP24A1, forward primer 5′-CAAACCGTGGAAGGCCTATC-3′, reverse primer 5′-AGTCTTCCCCTTCCAGGATCA-3′, TaqMan probe 5′-ACTACCGCAAAGAAGGCTACGGGCTG-3′. The primers and probe for MCP-1 were commercially available assays on demand (Applied Biosystems, UK).
Measurement of chronic kidney damage
An interactive image analysis system was used to outline and measure the extent of glomerular and interstitial scarring, expressed as percentage of cortical cross-sectional area, in biopsies stained with periodic acid–methenamine silver. This measure, the index of chronic damage, is a strong predictor of renal outcome.36
Analysis of macrophage infiltration and CYP27B1 expression of renal tissue
Immunohistochemical detection of tissue macrophages and interactive image analysis was performed as previously described.27 Coded sections stained for CD68 were visualized at ×200 magnification and the image captured digitally by an Aequitas image database and image archive management system (Dynamic Data Links, Cambridge, UK). Each image was then converted to a two-color scale image by Aequitas image analysis software. By altering the threshold, the image was processed so that positive staining was represented by black pixels and measured as a percentage of the area of total image analyzed. For each patient, the mean measurement of five randomly selected nonconfluent microscopic fields of renal cortex was determined. Glomerular staining was excluded from the analysis by the computer software. Sections where background staining made it impossible to digitally differentiate specific staining were excluded from analysis. Immunohistochemical analysis of CYP27B1 protein on paraffine embedded human kidney biopsies was performed using previously described methods.43
Primary culture of human proximal tubular epithelial cells
Human proximal tubular epithelial cells were grown using described methodologies.44 Cells were used up to three passages for experimental treatments (24 h) included TNF-α (1 ng/ml), 1,25(OH)2D (10−7 m) alone and TNF-α (1 ng/ml) with 1,25(OH)2D (10−7 m). Epithelial MCP-1 production was quantified with the commercially available sandwich ELISA kit according to manufacturer’s (R&D Systems) instructions and levels corrected for total cellular protein.
Statistical analysis
Data were expressed as mean ± s.d. unless otherwise (threshold cycle for amplification of the target gene (CT) minus CT for 18S rRNA) stated. Statistical analysis on real-time PCR was performed on dCT values. Importantly, the quantification of mRNA from the gene of interest provides a larger difference to the house-keeping gene, when fewer target mRNA can be found in tissue. This can lead to the description of a negative association, where a positive association is described and vice versa. Comparison between groups was undertaken with paired and unpaired t-tests, analysis of variance, or the Mann–Whitney rank-sum test where appropriate. Linear regression analyses were performed to determine relationship between data variables, with appropriate transformations of items with non-normal distributions. Stepwise multivariable linear regression analysis was performed to identify statistically significant predictors. Final models were assessed for colinearity and regression diagnostics checked to ensure models were reasonable fit to data. Relationships are presented as standardized β-coefficient with the P-value. Analyses were performed using SPSS for Windows, version 12.0, and the level of significance was set at P<0.05.
Multiple logistic regression models allowed for adjustment of OR for potential confounding effects of age, gender, ethnicity, and index of chronic renal scarring. Logistic models were fitted using the SAS statistical package and their appropriateness assessed using Hosmer–Lemeshow goodness-of-fit tests and colinearity diagnostics.
ACKNOWLEDGMENTS
We thank Guerman Molostvov and Sean James for their help with immunohistochemistry.
Source of support: This work (MH) was supported by a grant from the Foundation for Nephrology and NIH grant R01AI076292.
Footnotes
Part of these studies were presented at the 37th annual meeting of the American Society of Nephrology, 27 October to 1 November 2004; St Louis, Missouri, USA.
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Vanhooke JL, Prahl JM, Kimmel-Jehan C, et al. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1{alpha}-hydroxylase promoter activity in the skin. Proc Natl Acad Sci USA. 2006;103:75–80. doi: 10.1073/pnas.0509734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228:764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 3.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 4.Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol. 2005;233:115–124. doi: 10.1016/j.cellimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 6.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin–angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 8.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Acharya M, Tian J, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Zhou J, Minto AW, et al. Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int. 2006;70:882–891. doi: 10.1038/sj.ki.5001624. [DOI] [PubMed] [Google Scholar]
- 11.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–524. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 13.Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 14.Mawer EB, Taylor CM, Backhouse J, et al. Failure of formation of 1,25-dihydroxycholecalciferol in chronic renal insufficiency. Lancet. 1973;1:626–628. doi: 10.1016/s0140-6736(73)92197-1. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed KY, Varghese Z, Wills MR, et al. Long-term effects of small doses of 1,25-dihydroxycholecalciferol in renal osteodystrophy. Lancet. 1978;1:629–632. doi: 10.1016/s0140-6736(78)91137-6. [DOI] [PubMed] [Google Scholar]
- 16.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 17.LaClair RE, Hellman RN, Karp SL, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–1033. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Ishimura E, Nishizawa Y, Inaba M, et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999;55:1019–1027. doi: 10.1046/j.1523-1755.1999.0550031019.x. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 20.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Ren S, Nguyen L, et al. Splice variants of the CYP27b1 gene and the regulation of 1,25-dihydroxyvitamin D3 production. Endocrinology. 2007;148:3410–3418. doi: 10.1210/en.2006-1388. [DOI] [PubMed] [Google Scholar]
- 22.St-Arnaud R, Arabian A, Travers R, et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 23.Akeno N, Saikatsu S, Kawane T, et al. Mouse vitamin D-24-hydroxylase: molecular cloning, tissue distribution, and transcriptional regulation by 1α,25-dihydroxyvitamin D3. Endocrinology. 1997;138:2233–2240. doi: 10.1210/endo.138.6.5170. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz U, Amann K, Orth SR, et al. Effect of 1,25(OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998;53:1696–1705. doi: 10.1046/j.1523-1755.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 25.Anderson PH, O’Loughlin PD, May BK, et al. Modulation of CYP27B1 and CYP24 mRNA expression in bone is independent of circulating 1,25(OH)2D3 levels. Bone. 2005;36:654–662. doi: 10.1016/j.bone.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Bland R, Zehnder D, Hughes SV, et al. Regulation of vitamin D-1α-hydroxylase in a human cortical collecting duct cell line. Kidney Int. 2001;60:1277–1286. doi: 10.1046/j.1523-1755.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 27.Eardley KS, Zehnder D, Quinkler M, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–1197. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 28.Eardley KS, Kubal C, Zehnder D, et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Yuan W, Sun L, et al. 1,25-Dihydroxyvitamin D3 targeting of NF-κB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- 30.Takemoto F, Shinki T, Yokoyama K, et al. Gene expression of vitamin D hydroxylase and megalin in the remnant kidney of nephrectomized rats. Kidney Int. 2003;64:414–420. doi: 10.1046/j.1523-1755.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 31.Ren S, Nguyen L, Wu S, et al. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem. 2005;280:20604–20611. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 32.Anderson PH, O’Loughlin PD, May BK, et al. Quantification of mRNA for the vitamin D metabolizing enzymes CYP27B1 and CYP24 and vitamin D receptor in kidney using real-time reverse transcriptase-polymerase chain reaction. J Mol Endocrinol. 2003;31:123–132. doi: 10.1677/jme.0.0310123. [DOI] [PubMed] [Google Scholar]
- 33.Anderson PH, O’Loughlin PD, May BK, et al. Determinants of circulating 1,25-dihydroxyvitamin D3 levels: the role of renal synthesis and catabolism of vitamin D. J Steroid Biochem Mol Biol. 2004;89–90:111–113. doi: 10.1016/j.jsbmb.2004.03.089. [DOI] [PubMed] [Google Scholar]
- 34.Yuan B, Xing Y, Horst RL, et al. Evidence for abnormal translational regulation of renal 25-hydroxyvitamin D-1α-hydroxylase activity in the hyp-mouse. Endocrinology. 2004;145:3804–3812. doi: 10.1210/en.2004-0192. [DOI] [PubMed] [Google Scholar]
- 35.Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88:185–191. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 36.Howie AJ, Ferreira MA, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant. 2001;16:1163–1169. doi: 10.1093/ndt/16.6.1163. [DOI] [PubMed] [Google Scholar]
- 37.Sean Eardley K, Cockwell P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005;68:437–455. doi: 10.1111/j.1523-1755.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- 38.Grandaliano G, Gesualdo L, Ranieri E, et al. Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: a pathogenetic role in interstitial monocytes recruitment. J Am Soc Nephrol. 1996;7:906–913. doi: 10.1681/ASN.V76906. [DOI] [PubMed] [Google Scholar]
- 39.Stoffels K, Overbergh L, Giulietti A, et al. Immune regulation of 25-hydroxyvitamin-D3-1α-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 40.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 41.O’Leary N, Pembroke A, Duggan PF. A simplified procedure for eliminating the negative interference of bilirubin in the Jaffe reaction for creatinine. Clin Chem. 1992;38:1749–1751. [PubMed] [Google Scholar]
- 42.Zehnder D, Evans KN, Kilby MD, et al. The ontogeny of 25-hydroxyvitamin D(3)1α-hydroxylase expression in human placenta and decidua. Am J Pathol. 2002;161:105–114. doi: 10.1016/s0002-9440(10)64162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zehnder D, Bland R, Walker EA, et al. Expression of 25-hydroxyvitamin D3-1α-hydroxylase in the human kidney. J Am Soc Nephrol. 1999;10:2465–2473. doi: 10.1681/ASN.V10122465. [DOI] [PubMed] [Google Scholar]
- 44.van Kooten C, Lam S, Daha MR. Isolation, culture, characterization and use of human renal tubular epithelial cells. J Nephrol. 2001;14:204–210. [PubMed] [Google Scholar]