Abstract
Objectives
The purposes of this study were to characterize the direct effect of the C-terminal fragment of fibrinogen γ chain (γC)on microvascular endothelial permeability and to examine its molecular mechanism of action.
Methods and Results
Intravital microscopy was performed to measure albumin extravasation in intact mesenteric microvasculature, followed by quantification of hydraulic conductivity in single perfused microvessels. Transendothelial electric resistance was measured in microvascular endothelial cells in combination with immunoblotting and immunocytochemistry. The results show that γC induced time- and concentration-dependent increases in protein transvascular flux and water permeability and decreases in endothelial barrier function, coupled with Rho GTPase activation, myosin light chain phosphorylation and stress fiber formation. Depletion of RhoA via siRNA knockdown or pharmacological inhibition of RhoA signaling attenuated γC-induced barrier dysfunction. Imaging analyses demonstrated binding of γC to endothelial cells; the interaction was inhibited during blockage of the αvβ3 integrin. Furthermore, in vivo experiments showed that the microvascular leak response to γC was attenuated in integrin β3−/− animals.
Conclusion
Fibrinogen-γ C-terminus directly interacts with the microvascular endothelium causing fluid and protein leak. The endothelial response to γC involves an integrin receptor-mediated, RhoA-dependent signaling pathway that leads to paracellular hyperpermeability.
Keywords: Fibrinogen degradation products, microvascular permeability, signal transduction, Rho-GTPase, thrombosis
Introduction
Fibrinogen is comprised of double polypeptides termed α β and γ chains, which form an elongated structure with two outer globular D domains connected to central E domains through coiled-coil segments (1–3). As essential steps of the coagulation cascade, thrombin cleavage of fibrinogen and conversion to cross-linked fibrin occur in response to activation of intrinsic or extrinsic factors, followed by fibrinolysis that generates fibrin degradation products (FDPs) (Supplement Figure I, please see www.ahajournals.org). Typical FDPs include D-dimer fragments and soluble monomers containing the C-termini of fibrinogen-α, β and γ chains, clinically measured as markers of coagulation disorder (4–7).
The fibrinolysis cascade exerts physiological functions and pathogenic impact well beyond its role in hemostasis. Increased plasma levels of fibrinogen have been correlated with atherosclerosis, stroke, myocardium infarction, and peripheral vascular disease (5,6). Some fibrinogen proteolytic products have been shown to participate in cell proliferation, migration and adhesion (6–8). An effect on endothelial barrier properties has been documented (9–12); however, consensus has not been reached as to whether or not fibrinogen as intact protein increases vascular permeability, and even less is known regarding the receptor mechanism and signal transduction that mediates the endothelial response to specific subunits or degradation products, which are known to act distinctively based on their unique chemical structures and molecular conformations (1,3,8). Investigation is often confounded by the multi-domain structure of fibrinogen and its diverse cellular targets ranging from platelets and leukocytes to endothelial cells (7).
Among various fibrinogen subunits, the C-terminus of γ chain (γC) is of particular importance, as it contains reactive regions that mediate fibrin cross-linking, clot formation, and interactions with other molecules (13–15). While this domain encompasses a major component of the D fragments seen in the circulation of hypercoagulatory subjects (13,14), intravascular coagulation is not the only source of production. During severe trauma, burn and myocardial infarction, low molecular weight FDPs are produced from extravascular matrix or injured tissues, where upregulated or activated metalloproteinases, proteases, and leukocyte-derived elastases promote fibrinogen degradation (8, 16, 17). The abundance of these proteolytic products in the vascular wall and extracellular matrix under various injurious conditions supports their potential involvement in the pathogenesis of vascular inflammation.
In an initial effort to develop therapeutic agents for pathological angiogenesis, we have cloned and purified recombinant human fibrinogen-related proteins and characterized their biochemical properties (18,19). The studies have led to the identification of a novel function of fibrinogen-γ to induce apoptosis and suppress capillary tube formation through interacting with endothelial cells via a sequence exposed in its C-terminus, whereas intact fibrinogen fails to effect (18). The lack of reactivity may result from cryptic endothelial binding sites in the native form, consistent with crystallographic evidence of a dipole structure containing pocketed binding motifs (15). We postulate that the molecular conformations of fibrinogen degradation products are critical in determining their cellular targets and biological functions, and that truncated peptides or fragments with exposed endothelial-reactive regions may be more potent than the intact protein in altering vascular function. The current study extends these original observations to a further characterization of the specific C-terminal product γC151–411, focusing on its endothelial effect and mechanism of action in regulating barrier properties. The data provide complementary in vivo and in vitro evidence for a direct effect of this molecule on microvascular permeability, along with insights into the receptor mechanism and signal transduction that mediates the endothelial response to γC.
Materials and Methods
For details please see www.ahajournals.org. The γC fragment (residue 151–411, 30 kD) was produced by cloning its cDNA into the pET21a vector and overexpressed in E. coli. The recombinant protein was re-natured through urea step gradient followed by dialysis in refolding buffer with a final product resuspended in endotoxin-free PBS. Similar procedures were applied to express and purify other products, including GFP-γC, truncated γC with the C-terminal dodecapeptides deleted, and the C-termini of αE and β chains. Sprague-Dawley rats and C57BL6 mice (wild-type and integrin β3−/−) were used according to the IACUC-approved protocols. Intravital microscopy was performed to examine the mesenteric microcirculation. For measuring protein extravasation, fluorescently labeled albumin was injected and changes in the intraluminal vs. extraluminal fluorescence intensity over time were recorded (20,21). Hydraulic conductivity (Lp) was determined using a modified Landis micro-occlusion technique that measures fluid filtration across unit areas of the capillary wall under controlled perfusion pressures (21). Transcellular electrical resistance (TER) was measured in rat lung microvascular endothelial monolayers using electric cell-substrate impedance sensor (ECIS). Additionally, Rho-GTPase activity was examined using pull-down assays (22), and protein expression and phosphorylation were determined by Western blotting. Statistical analyses were performed using paired t test for self-comparison and ANOVA for inter-group comparison followed by Tukey’s HSD analysis.
Results
γC-induced microvascular leakage
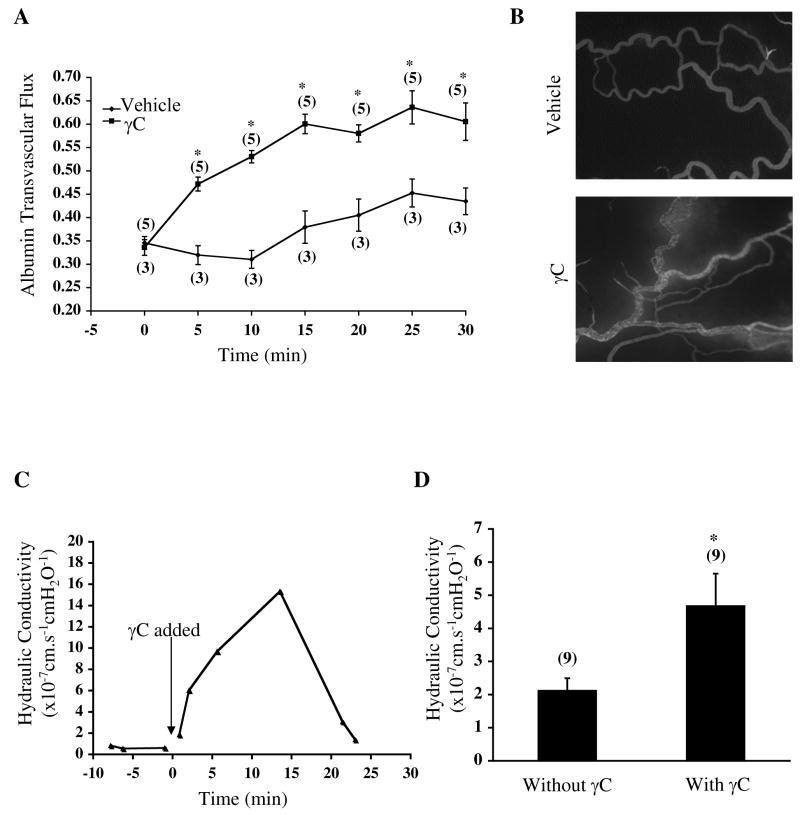
Administration of recombinant γC (10 μg/mL) promoted plasma protein extravasation in mesenteric microvessels (Figure 1A, B). Compared to vehicle, γC induced a higher level of albumin flux. The leak response occurred rapidly, reached maximum at ~15 min and was reversible upon clearance of γC. In cannulated microvessels without the presence of platelets or leukocytes, perfusion with γC elevated hydraulic conductivity (Figure 1C, D), indicating a direct effect on endothelial permeability. The time course of Lp increase corresponded to that of protein leak in intact microvasculature. Control perfusate containing vehicle did not cause any change in Lp. Neither γC nor vehicle altered the microvessel diameter.
Figure 1.
γC induces plasma fluid and protein leakage in microvessels. A, albumin extravasation in rat mesenteric microvessels during treatment with γC (10 μg/mL) or vehicle-Numbers in parentheses represent numbers of animals. *P<0.05 vs. sham. B, representative images of the mesenteric microvasculature 10 min after treatment. C, typical tracing of hydraulic conductivity response to γC (10 μg/mL). D, mean Lp values before and after γC. Perfusion of γC increased Lp from 2.14±0.35×10−7 cm/s/cmH2O to 4.70±0.96×10−7 cm/s/cmH2O within 15 min. Numbers in parentheses represent numbers of vessels. *P<0.05 vs. basal without γ C.
γC-induced endothelial barrier dysfunction
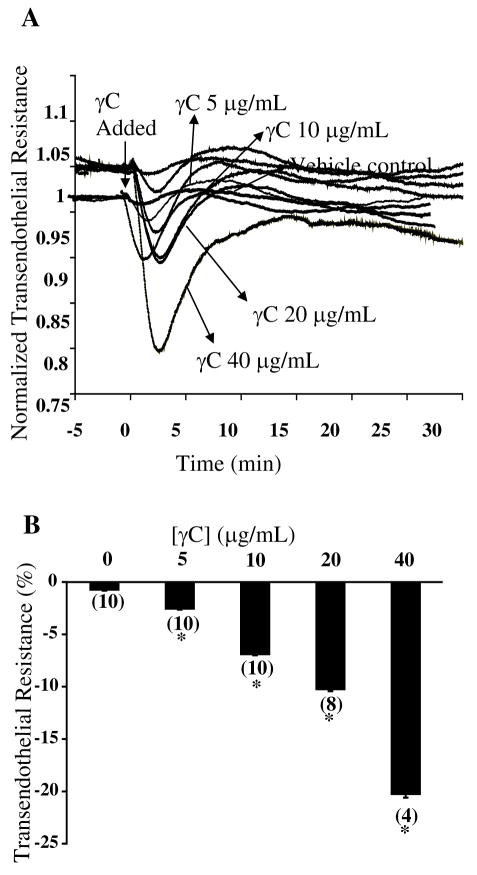
In microvascular endothelial monolayers, γC caused a significant reduction in transcellular electric resistance (Figure 2), indicative of impaired cell-cell adhesive barrier properties. Dose-response studies showed that endothelial TER decreased as a function of γC concentrations within a pathophysiological range (5–40 μg/mL). At lower concentrations, the response was relatively mild and transient which recovered within 10–15 min. A high concentration (40 μg/mL) caused severe hyperpermeability that persisted for several hours.
Figure 2.
γC induces endothelial barrier dysfunction in microvascular endothelial cell monolayers. A, time courses of γC-induced changes in TER. B, dose-responsive effects of γC on TER. Numbers in parentheses represent numbers of culture wells. *P<0.05 vs. basal without γC.
Differential effects of FDPs
We compared the permeability effects of native fibrinogen and its various degradations products, including fragment D (FFD) and the C-termini of α β, and γ chains (αEC, βC, and γC) (Supplement Figure II, please see www.ahajournals.org). Intact fibrinogen at 4 mg/mL (pathophysiological concentration) did not significantly decrease TER or increase microvascular hydraulic conductivity over the same time course as seen with γC (Figure IIA, B). Fragment D induced a significant decline in TER; however, it seemed to be less potent than γC within the same micromolar range (1–2 μmol/L FFD vs. 1.3 μmol/L γC) (Figure IIC). In addition, both αEC and βC slightly reduced barrier function but the effects did not reach a statistically significant level (Figure IID). Furthermore, a truncated mutant of γC lacking the last 12 residues in the C-terminus, γC399tr, exerted the same hyperpermeability potency as the wild-typeγC (Figure IID), indicating that the extreme C-terminal dodecapeptide sequence is not required for the permeability response.
RhoA signaling
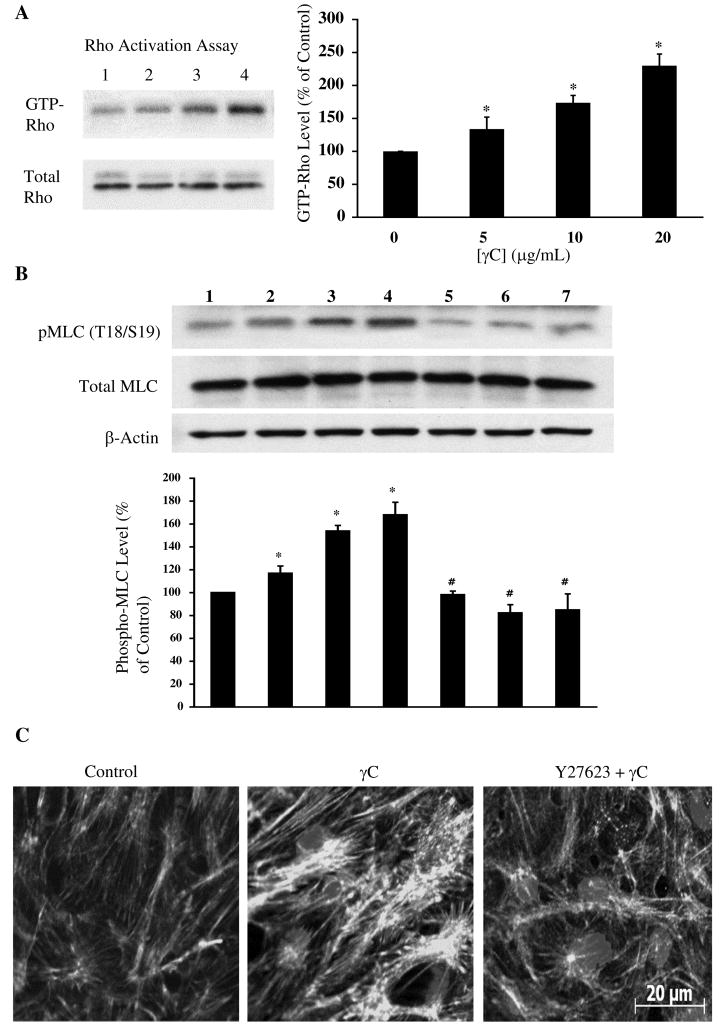
In microvascular endothelial cells, γC induced Rho-GTPase activation and myosin light chain phosphorylation at threonine18/serine19 without changing total protein levels (Figure 3A, B). Concomitantly, actin stress fibers and intercellular gaps were frequently observed. The responses were diminished in the presence of the Rho kinase inhibitor Y27632 (Figure 3C). Further, siRNA knockdown of RhoA expression (Figure 4A) did not significantly alter the basal permeability but prevented the TER response to γC (Figure 4B). Likewise, blockage of RhoA signaling with Y27632 inhibited barrier dysfunction in endothelial monolayers (Figure 4C) and prevented albumin leakage in microvessels caused by γC (Figure 4D).
Figure 3.
γC activates RhoA concomitantly with contractile cytoskeleton response in microvascular endothelial cells. A, pull-down assay shows Rho activation by γC. Lanes 1–4, γC at 0, 5, 10, 20 μg/mL, respectively. B, γC induces myosin light chain phosphorylation at Thr18/Ser19 and the effect is blocked by the RhoA kinase inhibitor Y27632. Lanes 1–4, γC at 0, 5, 10, 20 μg/mL; lanes 5–7, 20 μg/mL γC plus Y27632 at 1, 5, 10 μmol/L, respectively. C, immunofluorescent staining of F-actin in endothelial cells treated with vehicle, γC (20 μg/mL) and γC plus Y27632 (5 μmol/L).
Figure 4.
Inhibition of RhoA attenuates γC-induced endothelial barrier dysfunction and microvascular hyperpermeability. A, siRNA transfection depletes RhoA expression. Lane C, siControl; lanes T1-6, replicates of RhoA siRNA transfection. B, RhoA knockdown attenuates γC-induced TER decrease. *P<0.05 vs. vehicle, #P<0.05 vs. siControl. C, the RhoA kinase inhibitor Y27632 dose-dependently inhibits TER decrease to γC. D, γC-induced albumin leakage in microvasculature is prevented by Y27632 (1 μmol/L, 30 min). *P<0.05 vs. vehicle; #P<0.05 vs. γC only.
γC binding endothelial cells via integrin αvβ3
Figure 5A demonstrates binding of GFP-γC to the endothelial cell membrane, an interaction inhibited by pre-incubation with the αvβ3 integrin-blocking antibody. Further in vivo measurements of mesenteric microcirculation showed that γC (10 μg/mL) caused a significant increase in albumin transvascular flux; the leak response was greatly attenuated in integrin β3-deficient mice (Figure 5B).
Figure 5.
γC binds to the endothelial cell membrane via integrin αvβ3 mediating barrier dysfunction. A, immunofluorescent and corresponding DIC images of microvascular endothelial cells interacting with GFP-γC (left panels); the binding is diminished during antibody blockage of the αvβ3 integrin (right panels). Bar = 10 μm. B, γC (10 μg/mL) induces albumin leakage in mesenteric microvasculature in wild-type mice, whereas the leak response is attenuated in integrin β3−/− mice and not significantly different from control. Albumin transvascular flux was expressed as changes in fluorescence intensity ratios normalized to baseline 15 min after treatment. *P<0.05 vs. vehicle; #P<0.05 vs. wild-type + γC.
Discussion
This study reports a direct effect of γC on endothelial barrier dysfunction and microvascular leakage. We generated a recombinant protein based on the globular C-domain of human fibrinogen-γ chain, a component of fibrin degradation products that we previously showed to modulate angiogenesis (18). Administration of γC at pathophysiological concentrations induced a time- and dose-dependent increase in albumin transvascular flux and microvascular hydraulic conductivity in vivo and a decrease in transendothelial electric resistance across microvascular endothelial monolayers. The hyperpermeability effect was coupled with RhoA activation and endothelial cytoskeletal changes characterized by myosin light chain phosphorylation, actin stress fibers and intercellular gap formation. The cellular responses were greatly attenuated during depletion of RhoA expression or inhibition of RhoA function. In microvessels, blockage of RhoA signaling did not significantly alter the basal permeability but prevented γC-induced albumin leakage. Further imaging analyses demonstrated binding of γC to the endothelial cell membrane through the αvβ3 integrin, and that γC-induced microvascular leak response was greatly attenuated in β3-deficient animals. Taken together, the results suggest that the C-terminal fragment of fibrinogen-γ interacts with the microvascular endothelium causing barrier dysfunction via αvβ3-mediated, RhoA-dependent signaling. Selective blockage of this fragment or targeting its intracellular signaling while maintaining hemostasis may represent a new direction of drug development for the control of vascular inflammation.
The focus of this study is the 30-kDa fragment of fibrinogen-γ chain, rather than the 340-kDa native protein, with a practical view to characterize the molecular biology of this small proteolytic product as a pathogenic factor or therapeutic target for barrier injury. To the best of our knowledge, these data comprise the first line of evidence for a direct impact of γC on microvascular endothelium. In this regard, although elevated circulating fibrinogen has been correlated with increased risk of coronary and peripheral vascular diseases (4–7), it remains controversial as to whether it merely represents an acute phase protein elaborated during inflammation, or it is the cause of inflammation (22). Likewise, while an effect of fibrinogen to promote albumin transendothelial flux has been reported (9), there is evidence that the intact protein fails to cause pulmonary edema (12); rather, it attenuates vascular leakage caused by other inflammatory agents (10). In this study, we observed a minimal permeability response to native fibrinogen. Furthermore, the fragment D containing cross-linked C-termini of α β and γ chains was capable of altering endothelial barrier function; however, its effect seemed to be less potent than γC. This finding is in line with our previous data that native fibrinogen does not interact with endothelial cells in the same fashion as γC and polymerized C-fragments have limited endothelial-binding capability owing to cryptic binding sites (18). Indeed, recent crystallographic studies reveal a structure featuring multiple binding motifs embedded in pockets that are only reactive under certain conformations (1,3,15). Thus, proteolytic products with exposed endothelial-reactive regions may play a more important role than the intact protein in regulating endothelium-dependent vascular functions. This may explain why Ancrod, a fibrinogenolytic agent, is ineffective in treating myocardial ischemia-reperfusion, as it reduces plasma levels of fibrinogen but simultaneously increases its degradation products that may exacerbate vascular injury (6).
Our data provide a comparative analysis of the permeability effects of different fibrinogen derivates that share the C-terminal homology. While the dimerized C-termini of α β, and γ chains were capable of causing barrier dysfunction, individual αEC or βC chain effected to a much less extent than the D fragment or γC, consistent with the hypothesis that the γC domain may play a predominant role in the permeability response by providing critical molecular sites for interacting with endothelial cells. Supporting this is recent literature that emphasizes the structural stability and functional importance of the C-terminal sequences of γ chain relative to other subunits in coagulation, calcium binding, and interactions with multiple molecules and cells (7,13,15, 23). Unlike γC, the crystal structure of αC remains elusive and its function has not been well studied due, in part, to its intrinsic instability (3). Likewise, relatively less information is available regarding the structure-function relationship of βC, and there is limited evidence pointing to a direct physiological impact of these segments on vascular permeability.
Specific sequences in fibrinogen-γ that potentially participate in the interaction with circulating cells and the vascular wall include residues 117–133 that bind ICAM-1 (24), 377–395 that bind CD11b/CD18 (23,25) and 400–411 that bind integrin αIIβ3 (13,26). Recent studies reveal the potential of residues 151–399 within the C-terminus as a binding motif for the endothelial αvβ3 integrin in suppression of angiogenesis and tumor growth (8,18,19). Given the signaling role of αvβ3 in vascular function and our previous data on its effect in regulating microvascular permeability (27), we suspect that this integrin serves as a receptor for γC to target the endothelial barrier. The current study supports a direct binding interaction between γC and αvβ3. The in vivo data that β3 knockout prevents γC-induced microvascular leakage further supports the functional importance of this integrin in mediating the barrier response. The lack of permeability response to γC in β3-deficient microvessels may result from the phenotypic changes and vascular defects in these mice; however, further studies are required to explain the underlying mechanisms. On the other hand, the ability of γC to interact with the endothelial integrin receptor may not depend on its extreme C-terminal dodecapeptide (aa400–411), as deletion of these residues did not reduce the hyperpermeability potency of γC, consistent with our previous finding that truncated γC mutants maintain the ability to recognize αvβ3 (18). Interestingly, a previous study suggests that fragment D increases bovine pulmonary artery endothelial permeability in the absence of the γ-chain extreme C-terminus (11). The study varies from ours in that different preparations of fibrinolytic products were tested with different experimental models. Within this context, although a high level of structural homology exits among the C-termini of fibrinogen-α β and γ chains as well as across species (humans and rodents) (28), heterogeneous responses have been observed in endothelial cells of different species or vascular origins. Furthermore, it is possible that other integrins or non-integrin receptors are involved in the γC-endothelium interaction (29, 30). While a search for detailed molecular structures is underway, we thought to first establish the intracellular signaling pathway that leads to γC-induced hyperpermeability.
We found that activation of RhoA served as a critical step in the transduction of γC action. The central role of this Rho-GTPase in modulating endothelial barrier function during inflammation has been recognized (31–34). However, whether it mediates hyperpermeability to any particular fibrinogen degradation product remains to be determined. A novel aspect of the current study is that it provides direct evidence for RhoA-dependent hyperpermeability to the C-terminal fragment of fibrinogen-γ. The concomitant changes in endothelial adhesive barrier function and myosin light chain phosphorylation and stress fiber formation were prevented by RhoA depletion or inhibition, indicating the RhoA-stimulated cytoskeleton contraction as a potential cellular process underlying paracellular hyperpermeability. Indeed, cascades of intracellular reactions cross-talk with the Rho-pathway (35), where activation of RhoA can be triggered by receptor tyrosine kinases or the β integrins (36–38), and its downstream targets range from signaling molecules, such as protein kinase C and myosin light chain kinase (39,40), to barrier structural components, such as actin, myosin, microtubules, and junctions (31–33,41,42). In addition, the MAPK cascades have been linked to RhoA signaling. A recent study reports the involvement of ERK1/2 in fibrinogen-induced permeability (9). However, based on our observation that inhibition of ERK1/2 activation did not block γC-induced barrier dysfunction (data not shown), it is unlikely that this pathway serves as the major effector in γC-RhoA signaling.
Both the transcellular and paracellular pathways have been proposed to explain macromolecule transport across the microvascular endothelium during inflammation (34,43); their relative contribution to vascular leakage varies depending on experimental conditions, inflammatory stimuli, vessel subtypes, and the presence or absence of blood components and immune cells (44,45). Taking advantage of the availability of multiple permeability models, we performed a comprehensive analysis of endothelial barrier function under conditions where these confounding factors were dissected. The study was initiated with intravital microscopy that measures plasma protein extravasation in the intact microvasculature indicative of the overall exchange status in the presence of circulating cells (20). We then measured hydraulic conductivity in single perfused microvessels without platelets and leukocytes, examining the direct effect of γC on intact endothelium (21). This in situ experiment is mechanistically important because the Lp values are reflective of convective transport mainly through the paracellular pathway (21,41). Following this was a study in endothelial monolayers of microvascular origin for monitoring the dynamics of transcellular electric resistance, an indicator of cell-cell and cell-matrix adhesive barrier properties. Information derived from these experiments does not exclude the participation of circulating components but highlights the direct endothelial effect of γC. Moreover, although we recognize the potential involvement of other cellular pathways such as transcytosis, our data emphasize the importance of the paracellular pathway, supported by evidence that RhoA activation causes myosin light chain phosphorylation, actin polymerization, and endothelial cell contraction; these responses are known to mediate paracellular permeability (31–34,41). The rapid, reversible nature of γC-induced hyperpermeability is consistent with a dynamic regulation of cell-cell adhesions, rather than permanent cell detachment or apoptosis, which may occur in late stages (8–24 hr) or after prolonged (>12 hr) challenge of γC (11,18).
In summary, we report that the C-terminal fragment of fibrinogen γ-chain is capable of disrupting the microvascular endothelial barrier leading to fluid and protein leakage. The hyperpermeability response may result from a direct interaction of γC with the endothelial αvβ3 integrin receptor and is mediated by RhoA-dependent signaling. This finding indicates an intriguing possibility that proteolytic products containing the C-terminal fragments of fibrinogen act as inflammatory mediators causing vascular dysfunction during tissue injury or thrombotic disorder. In addition, γC-induced microvascular hyperpermeability may have implications in the pathophysiological regulation of angiogenesis, tumor development and wound healing. Investigative efforts shall be continued to characterize the biochemical nature and molecular basis of γC-endothelium interactions.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants HL-073324 (MH Wu) and HL-061507 and HL-070752 (SY Yuan).
We acknowledge Dr. Nathalie Gaudreault and Mr. Bert Frederich for their contributions to the imaging analysis and animal surgery. We also thank Mr. Chris Pivetti and Ms. Olesya Litovka for their help with the manuscript preparation.
References
- 1.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 2.Doolittle RF. Determining the crystal structure of fibrinogen. J Thromb Haemost. 2004;2:683–689. doi: 10.1111/j.1538-7933.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- 3.Lord ST. Fibrinogen and fibrin: scaffold proteins in hemostasis. Curr Opin Hematol. 2007;14:236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- 4.Wakai A, Gleeson A, Winter D. Role of fibrin D-dimer testing in emergency medicine. Emerg Med J. 2003;20:319–325. doi: 10.1136/emj.20.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saigo M, Hsue PY, Waters DD. Role of thrombotic and fibrinolytic factors in acute coronary syndromes. Prog Cardiovasc Dis. 2004;46:524–538. doi: 10.1016/j.pcad.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Zacharowski K, Zacharowski P, Reingruber S, Petzelbauer P. Fibrin(ogen) and its fragments in the pathophysiology and treatment of myocardial infarction. J Mol Med. 2006;84:469–477. doi: 10.1007/s00109-006-0051-7. [DOI] [PubMed] [Google Scholar]
- 7.Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem. 2007;14:2925–2936. doi: 10.2174/092986707782360015. [DOI] [PubMed] [Google Scholar]
- 8.Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost. 2006;4:932–939. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 9.Tyagi N, Roberts AM, Dean WL, Tyagi SC, Lominadze D. Fibrinogen induces endothelial cell permeability. Mol Cell Biochem. 2008;307:13–22. doi: 10.1007/s11010-007-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allard MF, Doerschuk CM, Brumwell ML, Belzberg A, Hogg JC. Oleic acid-induced lung injury in rabbits: effect of fibrinogen depletion with Arvin. J Appl Physiol. 1988;64:920–928. doi: 10.1152/jappl.1988.64.3.920. [DOI] [PubMed] [Google Scholar]
- 11.Ge M, Ryan TJ, Lum H, Malik AB. Fibrinogen degradation product fragment D increases endothelial monolayer permeability. Am J Physiol. 1991;261:L283–289. doi: 10.1152/ajplung.1991.261.4.L283. [DOI] [PubMed] [Google Scholar]
- 12.Lo SK, Del Vecchio PJ, Lum H, Malik AB. Fibrin contact increases endothelial permeability to albumin. J Cell Physiol. 1992;151:63–70. doi: 10.1002/jcp.1041510111. [DOI] [PubMed] [Google Scholar]
- 13.Farrell DH. Pathophysiologic roles of the fibrinogen γ chain. Curr Opin Hematol. 2004;11:151–155. doi: 10.1097/01.moh.0000131440.02397.a4. [DOI] [PubMed] [Google Scholar]
- 14.Mosesson MW. Fibrinogen γ chain functions. J Thromb Haemost. 2003;1:231–238. doi: 10.1046/j.1538-7836.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 15.Yee VC, Pratt KP, Côté HC, Trong IL, Chung DW, Davie EW, Stenkamp RE, Teller DC. Crystal structure of a 30 kDa C-terminal fragment from the γ chain of human fibrinogen. Structure. 1997;5:125–138. doi: 10.1016/s0969-2126(97)00171-8. [DOI] [PubMed] [Google Scholar]
- 16.Prager MD, Baxter CR, Hartline B. Proteolytic activity in burn wounds exudates and comparison of fibrin degradation products and protease inhibitors in exudates and sera. J Burn Care Rehabil. 1994;15:130–136. doi: 10.1097/00004630-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Wada H, Nobori T, Nakatani K, Onishi K, Nishikawa M, Shiku H, Kazahaya Y, Sawai T, Koiki K, Matsuda M. Elevated Plasma Levels of Fibrin Degradation Products by Granulocyte-Derived Elastase in Patients with Disseminated Intravascular Coagulation. Clin Appl Thromb Hemost. 2005;11:391–400. doi: 10.1177/107602960501100405. [DOI] [PubMed] [Google Scholar]
- 18.Akakura N, Hoogland C, Takada YK, Saegusa J, Ye X, Liu FT, Cheung AT, Takada Y. The COOH-terminal globular domain of fibrinogen γ chain suppresses angiogenesis and tumor growth. Cancer Res. 2006;66:9691–9697. doi: 10.1158/0008-5472.CAN-06-1686. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama K, Erickson HP, Ikeda Y, Takada Y. Identification of amino acid sequences in fibrinogen γ-chain and tenascin C C-terminal domains critical for binding to integrin αvβ3. J Biol Chem. 2000;275:16891–16898. doi: 10.1074/jbc.M000610200. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Armenante PM, Durán WN. Transient analysis of macromolecular transport across microvascular wall and into interstitium. Am J Physiol. 1993;265:H993–999. doi: 10.1152/ajpheart.1993.265.3.H993. [DOI] [PubMed] [Google Scholar]
- 21.Reynoso R, Perrin RM, Breslin JW, Daines DA, Watson KD, Watterson DM, Wu MH, Yuan S. A role for long chain myosin light chain kinase (MLCK-210) in microvascular hyperpermeability during severe burns. Shock. 2007;28:589–595. doi: 10.1097/SHK.0b013e31804d415f. [DOI] [PubMed] [Google Scholar]
- 22.Breslin JW, Yuan SY. Involvement of RhoA and Rho kinase in neutrophil-stimulated endothelial hyperpermeability. Am J Physiol Heart Circ Physiol. 2004;286:H1057–1062. doi: 10.1152/ajpheart.00841.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kerlin B, Cooley BC, Isermann BH, Hernandez I, Sood R, Zogg M, Hendrickson SB, Mosesson MW, Lord S, Weiler H. Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood. 2004;103:1728–1734. doi: 10.1182/blood-2003-08-2886. [DOI] [PubMed] [Google Scholar]
- 24.Ugarova TP, Lishko VK, Podolnikova NP, Okumura N, Merkulov SM, Yakubenko VP, Yee VC, Lord ST, Haas TA. Sequence γ377–395(P2), but not γ190–202(P1), is the binding site for the αMI-domain of integrin αMβ2 in the γC-domain of fibrinogen. Biochemistry. 2003;42:9365–9373. doi: 10.1021/bi034057k. [DOI] [PubMed] [Google Scholar]
- 25.Sans E, Delachanal E, Duperray A. Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian cell expression model: implication of ICAM-1 cytoplasmic domain and Rho-dependent signaling pathway. J Immunol. 2001;166:544–551. doi: 10.4049/jimmunol.166.1.544. [DOI] [PubMed] [Google Scholar]
- 26.Flick MJ, Du X, Witte DP, Jirousková M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett JS. Platelet-fibrinogen interactions. Ann N Y Acad Sci. 2001;936:340–354. doi: 10.1111/j.1749-6632.2001.tb03521.x. [DOI] [PubMed] [Google Scholar]
- 28.Wu MH. Endothelial focal adhesions and barrier function. J Physiol. 2005;569:359–366. doi: 10.1113/jphysiol.2005.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouma H, III, Fuller GM. Partial Chemical Characterization of rat fibrinogen. J Biol Chem. 1975;250:4678–4683. [PubMed] [Google Scholar]
- 30.Bach TL, Barsigian C, Yaen CH, Martinez J. Endothelial cell VE-cadherin functions as a receptor for the β15–42 sequence of fibrin. J Biol Chem. 1998;273:30719–30728. doi: 10.1074/jbc.273.46.30719. [DOI] [PubMed] [Google Scholar]
- 31.Gorlatov S, Medved L. Interaction of fibrin(ogen) with the endothelial cell receptor VE-cadherin: mapping of the receptor-binding site in the NH2-terminal portions of the fibrin β chains. Biochemistry. 2002;41:4107–4116. doi: 10.1021/bi0160314. [DOI] [PubMed] [Google Scholar]
- 32.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- 33.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 34.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 35.van Nieuw Amerongen GP, van Hinsbergh VW. Endogenous RhoA inhibitor protects endothelial barrier. Circ Res. 2007;101:7–9. doi: 10.1161/CIRCRESAHA.107.156513. [DOI] [PubMed] [Google Scholar]
- 36.Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-β-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol. 2005;288:L294–306. doi: 10.1152/ajplung.00213.2004. [DOI] [PubMed] [Google Scholar]
- 37.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation. 2006;13:237–247. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- 38.Miao H, Li S, Hu YL, Yuan S, Zhao Y, Chen BP, Puzon-McLaughlin W, Tarui T, Shyy JY, Takada Y, Usami S, Chien S. Differential regulation of Rho GTPases by β1 and β3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J Cell Sci. 2002;115:2199–2206. doi: 10.1242/jcs.115.10.2199. [DOI] [PubMed] [Google Scholar]
- 39.Harrington EO, Shannon CJ, Morin N, Rowlett H, Murphy C, Lu Q. PKCδregulates endothelial basal barrier function through modulation of RhoA GTPase activity. Exp Cell Res. 2005;308:407–421. doi: 10.1016/j.yexcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCα and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol. 2005;289:L176–185. doi: 10.1152/ajplung.00003.2005. [DOI] [PubMed] [Google Scholar]
- 41.Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, Drenckhahn D. Rho and Rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol. 2002;539:295–308. doi: 10.1113/jphysiol.2001.013117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol. 2004;201:55–70. doi: 10.1002/jcp.20055. [DOI] [PubMed] [Google Scholar]
- 43.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 44.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 45.Sarelius IH, Kuebel JM, Wang J, Huxley VH. Macromolecule permeability of in situ and excised rodent skeletal muscle arterioles and venules. Am J Physiol Heart Circ Physiol. 2006;290:H474–480. doi: 10.1152/ajpheart.00655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.