Abstract
Fluorescence emission is nearly isotropic in space. With typical optical components the collection efficiency is 1% or less. In this preliminary report, we describe a novel approach to transforming the normally isotropic emission into directional emission with a collection efficiency near 50%. This can be accomplished for fluorophores located near a semi-transparent silver film on a glass substrate. The emission couples with the surface plasmon resonance on the silver surface and enters the transparent substrate at a sharply defined angle, the surface plasmon angle for the emission wavelength. We estimate that 40–70% of the total emission enters the substrate at the plasmon angle and can thus be directed towards a detector. Background emission from fluorophores distant from the silver does not couple with the plasmon and is not detected. Different emission wavelengths couple at different angles allowing spectral discrimination without additional optics. Surface plasmon-coupled emission represents a new technology which can be used for high detection efficiency with microfluidic and/or surface-bound assay formats.
Fluorescence detection is the basis of numerous measurements in the biological sciences, biotechnology, and medical diagnostics. While fluorescence is a highly sensitive method, there is always a need for increased sensitivity to detect smaller and smaller numbers of target molecules. Numerous approaches are used to obtain increased detectability, including amplified assays such as ELISA [1] and PCR [2], probe with multiple fluorophores such as the phycobiliproteins [3,4], long wavelength excitation [5,6], and/or gated detection to decrease the background emission [7,8]. Several fundamental factors limit the sensitivity of fluorescence methods, typically photodestruction of the fluorophores and the extent of background fluorescence. However, sensitivity is also limited by the collection efficiency of the detection optics.
The importance of light collection efficiency can be seen by consideration of the requirements for single molecule detection [9–11]. A typical fluorophore can undergo a finite number of excitation–relaxation cycles prior to photochemical destruction. For photostable molecules such as tetramethylrhodamine photodestruction occurs after about 105 cycles. However, the number of photons detectable from a single fluorophore is typically much smaller, near 103 photons. This difference is due to the isotropic distribution of fluorescence, which makes it difficult to capture more than small fraction of the total emission. According to Keller and co-workers [11], even sensitive and efficient detection systems capture only about 1% of the total emission, and typically less. Some authors report higher efficiencies near 5%. Efficient collection of the emission is a promising approach to increased sensitivity.
In the present paper, we describe a novel method to efficiently collect about 50% of the total emission while simultaneously reducing the contribution of unwanted background signal. Our approach depends on localization of the probe chemistry near a thin silver film on a transparent substrate, typically 10–200 nm from the surface. At these distances the emission couples with the surface plasmon resonance of the silver and enters the transparent substrate at the surface plasmon angle [12,13]. This coupling can be highly efficient to over 90% for molecules with the proper orientation and distance from the surface [13]. Remarkably, directional emission occurs if the fluorophores are excited by using or not using the evanescent field due to plasmon resonance [14,15]. This phenomenon of surface plasmon-coupled emission (SPCE) may be considered to be the reverse of surface plasmon resonance absorption in which the reflectivity is minimum at the angle of incidence for plasmon resonance [16,17]. That is, instead of absorption of the incident light at its plasmon angle, SPCE occurs at the plasmon angle of the emission, and is highly directional.
In this initial report, we studied the angular dependence of the emission of fluorophores in polyvinyl alcohol (PVA) spin-coated on a continuous semi-transparent silver film. We found the emission to be sharply distributed around two angles symmetric from an axis normal to the glass–PVA interface. Additionally, we found that fluorophores that are more distant from the surface did not couple into the metal, allowing background rejection in assay formats with surface-localized chemistry. And finally, we found that fluorophores with different emission maxima emit at different angles, allowing spectral discrimination without additional dispersion optics.
Materials and methods
Sample preparation
Glass microscope slides (plain, Corning) were covered with a continuous 50 nm thick silver film, which was vapor deposited by EMF (Ithaca, NY). The samples were prepared by spin-coating (3000 rpm) of 0.5% PVA (MW 13,000; Aldrich, St. Louis, MO) solution in water containing sulforhodamine 101 (S101; 95%, Aldrich). The thickness of the PVA film was estimated by measurement of the optical densities of S101 in spin-coated film and in 15 μm thick film obtained from the same stock solution by evaporation. The optical density of S101 in 0.5% PVA spin-coated film was 0.0016 and in control 15 μm film the optical density was 1.52. We estimated the thickness of our spin-coated films to be about 15 nm.
Fluorescence measurements
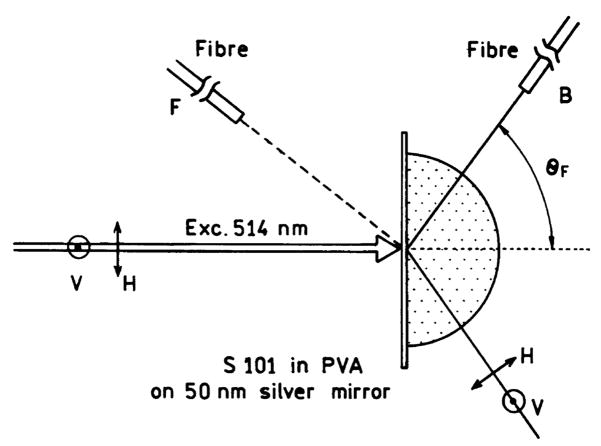
The microscope slide with sample was attached with index-matching fluid to a hemicylindrical prism made of BK7 glass and positioned on a precise rotary stage (Fig. 1). The stage was equipped with an arm of about 15 cm long for fiber optic detection, which allowed observation at any angle relative to the incident angle. The incident light was normal to the glass–PVA interface. The air slit of 200 μm was placed on the fiber input. The output of the fiber was directed to the SLM 8000 spectrofluorometer for emission spectra and steady-sate intensity measurements. The 514 nm excitation was from a pulsed mode-locked argon ion laser (76 MHz repetition rate, 120 ps half-width). Scattered incident light at 514 nm was suppressed on observation by using a holographic supernotch-plus filter (Kaiser Optical System, Ann Arbor, MI). Unless otherwise indicated the incident light was polarized horizontally in the laboratory axis that is within the plane shown in Fig. 1.
Fig. 1.
Experimental set-up for directional emission (reverse Kretschmann configuration).
Results
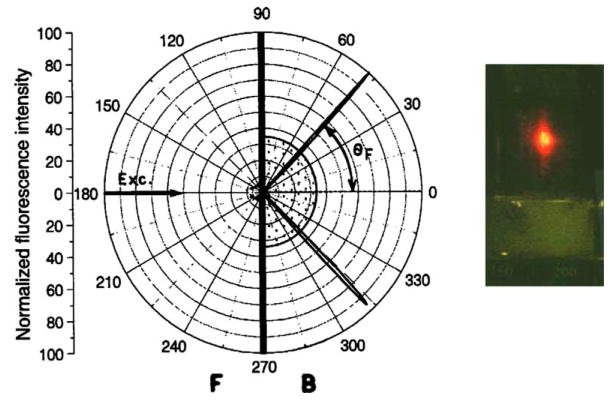
We measured the emission intensity for the complete range of angles (θ) from 0° to 360°. For a typical fluorescent solution, even for a rigid solution if the excitation is vertically polarized, the emission intensity would be the same for all angles of observation (θ in Fig. 1). Remarkably, the emission was found to be strongly directional into the hemicylinder at two angles 47° and 313° (Fig. 2). We believe that these intensity maxima at specific angles are due to SPCE. By SPCE we mean the excited fluorophores couple with electron oscillations in the surface, the surface plasmons. These plasmons in turn emit into the glass hemicylinder.
Fig. 2.
The angular distribution of fluorescence intensity of sulforhodamine 101 (S101) spin-coated with polyvinyl alcohol (PVA) on silvered microscope slide. The silver layer was 50 nm thick (EMF, Ithaca, NY). The spin-coated sample was prepared from 0.5% PVA solution which results in the thickness of near 15 nm. About half of the entire emission is coupled to the cone. The photograph on the right was taken at about 47° from the distance of about 1 m.
Examination of Fig. 2 suggests that a large fraction of the total emission occurs via the plasmons. We integrated the intensities observed from the back (IB, −90° to +90°) and the front (IF) of the sample (90°–270°). This ratio was found to be 0.96, indicating that half of the emission occurred via the surface plasmon. Emission spectra were collected by observation from the front and the back of the sample (Fig. 3). These spectra were found to be the same as expected for S101, showing that the directional intensity peaks seen in Fig. 2 were not due to scattered light. The emission is over 10-fold more intense for a directional peak at 47° than for the free-space emission observed at 149°.
Fig. 3.
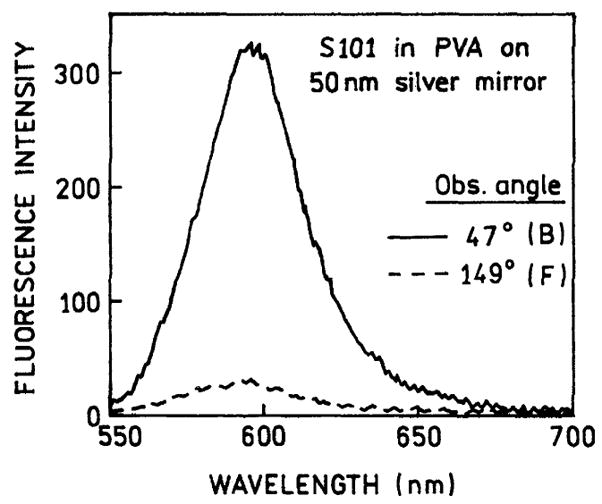
Spectrum of the directional emission of S101 in PVA spin-coated on 50 nm silver mirror observed with the reverse Kretschmann configuration (—). Also shown is a fluorescence spectrum observed on the front of the sample (- - -).
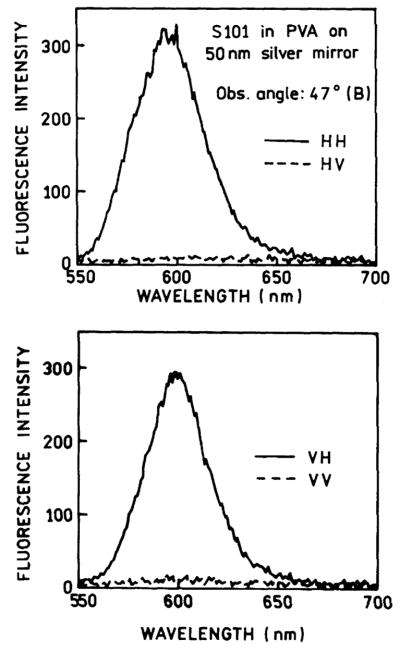
In a recent paper [18], we described the polarization properties of SPCE. Fig. 4 (top) shows the emission spectra when the incident light was horizontally (H) polarized. The emission is much more intense when the observation polarizer is horizontally oriented (HH). Additionally, the polarization (or anisotropy) of the emission is over 0.5 (over 0.4) which cannot occur for one-photon excitation of an isotropic fluorescent solution with free-space emission. It should be recalled that horizontally polarized light is often used to determine the G-factor for anisotropy measurements. With horizontally polarized excitation and right angle observation the emission intensities are expected to be equal [19]. Because of this unusual polarization, we also examined the emission intensities with vertically polarized excitation (Fig. 4 bottom). Once again the horizontally polarized emission was much more intense than the vertically polarized emission. These observations can be understood by recalling that surface plasmon resonance absorption only occurs for light with the electric vector transverse to the plane of incidence. Similarly for SPCE, the coupling only occurs for fluorophores with their transition moment transverse to the plane of observation. We also measured the polarization with observation from the front side. For horizontally polarized excitation the emission was roughly equally intense for horizontal or vertical observations (Fig. 5), as expected for typical free-space emission. These observed polarization properties can be regarded as proof that emission occurs by coupling to the surface plasmons.
Fig. 4.
Fluorescence spectra of S101 in PVA spin-coated on 50 nm silver mirror observed at 47° (defined by θ in Fig. 2). (Top) Excitation horizontally polarized and emission observed through horizontally (—) and vertically (- - -) oriented polarizer. (Bottom) Excitation vertically polarized.
Fig. 5.
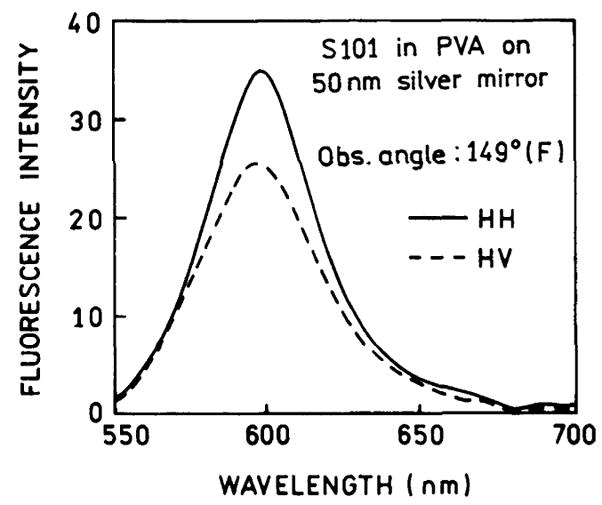
Parallel (HH) and perpendicular (HV) intensities of fluorescence emitted to the front of the sample (F). Emission observed from the front of excitation (angles 90°–270° on Fig. 2) is not highly polarized.
In surface plasmon resonance it is known that the angle of minimum reflectivity depends on the refractive index of the solution extending to about 200 nm into the sample. Hence, we reasoned that SPCE could only occur for fluorophores which were within 200 nm of the silver surface. We tested this idea by placing an ethanolic solution of pyridine 2 (Py2) behind the S101–PVA film. PVA is weakly soluble in alcohol. The film did not dissolve and the S101 did not wash out of the PVA during the measurements. Fig. 6 shows emission spectra from the front and back of the sample containing both S101 in the PVA film and Py2 in the bathing solution. We chose concentrations so that the emission observed from the front is dominated by the Py2 mock-impurity. We anticipate that S101 is close to the metal film and is a much weaker coupling to the Py2 which is more distant from the film. When observed from the back, which is the SPCE, the emission is almost completely due to S101. This result shows that it is possible to selectively observe fluorophores near the silver film and “suppressed” the emission from more distal fluorophores, by observation of the SPCE.
Fig. 6.
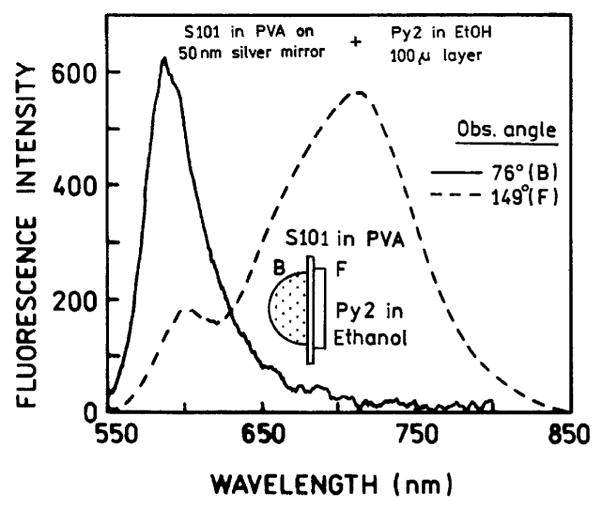
Fluorescence of S101/PVA on silver layer in the presence of Py2 background emission observed in front (- - -) at 149° and along directional fluorescence (—) at 76°. The background was a 100 μm thick ethanol solution of Py2 attached to the front of the slide with a demountable 0.1 mm cuvette. Note, the θF angle increased from 47° to about 76° due to higher refractive index of EtOH.
Conclusion
Fluorophores near a metallic silver surface couple with the surface plasmon. This process is efficient and collects 50% of the total emission with no complex optical elements. The sample configuration is simple and applicable to any surface-bound assay. Background from the distal parts of the sample is not observed due to lower coupling at longer distances. We believe that surface plasmon-coupled emission will have numerous applications to high sensitivity fluorescence detection.
Acknowledgments
This work was supported by the National Center for Research Resources, RR-08119, the National Institute of Biomedical Imaging and Bioengineering, EB-000682 and EB-00980, and the Human Genome Institute HG002655.
References
- 1.Gosling JP. A decade of development in immunoassay methodology. Cell. 1990;36:1408–1427. [PubMed] [Google Scholar]
- 2.Walker NJ. A technique whose time has come. Science. 2002;296:557–559. doi: 10.1126/science.296.5567.557. [DOI] [PubMed] [Google Scholar]
- 3.White JC, Stryer L. Photostability studies of phycobiliprotein fluorescent labels. Anal Biochem. 1987;161:442–452. doi: 10.1016/0003-2697(87)90473-8. [DOI] [PubMed] [Google Scholar]
- 4.Kronick MN. The use of phycobiliproteins as fluorescent labels in immunoassays. J Immunol Methods. 1986;92:1–13. doi: 10.1016/0022-1759(86)90496-5. [DOI] [PubMed] [Google Scholar]
- 5.Daehne S, Resch-Genger U, Wolfbeis OS, editors. Near-Infrared Dyes for High Technology Applications. Kluwer Academic Publishers; New York: 1998. p. 458. [Google Scholar]
- 6.Casay GA, Shealy DB, Patonay G. Near infrared fluorescence probes. In: Lakowicz JR, editor. Topics in Fluorescence Spectroscopy, vol. 4: Probe Design and Chemical Sensing. Plenum Press; New York: 1994. pp. 183–222. [Google Scholar]
- 7.Diamandis EP. Immunoassays with time-resolved fluorescence spectroscopy: principles and applications. Clin Biochem. 1988;21:139–150. doi: 10.1016/0009-9120(88)90001-x. [DOI] [PubMed] [Google Scholar]
- 8.Lövgren T, Pettersson K. Time-resolved fluoroimmunoassay advantages and limitations. In: Van Dyke K, Van Dyke R, editors. Luminescence Immunoassay and Molecular Applications. CRC Press; New York: 1990. pp. 234–250. [Google Scholar]
- 9.Ambrose WP, Goodwin PM, Jett JH, Van Orden A, Wemer JH, Keller RA. Single-molecule fluorescence spectroscopy at ambient temperature. Chem Rev. 1999;99:2929–2956. doi: 10.1021/cr980132z. [DOI] [PubMed] [Google Scholar]
- 10.Soper SA, Nutter HL, Keller RA, Davis LM, Shera EB. The photophysical constants of several fluorescent dyes pertaining to ultrasensitive fluorescence spectroscopy. Photochem Photobiol. 1993;57(6):972–977. [Google Scholar]
- 11.Van Orden A, Machara NP, Goodwin PM, Keller RA. Single-molecule identification in flowing sample streams by fluorescence burst size and intraburst fluorescence decay rate. Anal Chem. 1998;70(7):1444–1451. doi: 10.1021/ac970545k. [DOI] [PubMed] [Google Scholar]
- 12.Benner RE, Dornhaus R, Chang RK. Angular emission profiles of dye molecules excited by surface plasmon waves at a metal surface. Opt Commun. 1979;30(2):145–149. [Google Scholar]
- 13.Weber WH, Eagen CF. Energy transfer from an excited dye molecule to the surface plasmons of an adjacent metal. Opt Lett. 1979;4(8):236–238. doi: 10.1364/ol.4.000236. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko F, Nakano T, Terakado M, Shinbo K, Kato K, Kawakami T, Wakamatsu T. Emission light from prism/silver/rhodamine-B LB film and multiple surface plasmon excitations in the ATR Kretschmann configuration. Mater Sci Eng C. 2002;22:409–412. [Google Scholar]
- 15.Nakano T, Kobayashi H, Shinbo K, Kato K, Kaneko F, Kawakami T, Wakamatsu T. Emission light properties from Ag/rhodamine-B LB films due to surface plasmon excitations in the Kretschmann and reverse configurations. Mater Res Soc Symp Proc. 2001;660:8351–8356. [Google Scholar]
- 16.Haroche S, Kleppner D. Cavity quantum electrodynamics. Phys Today. 1989:24–30. [Google Scholar]
- 17.Haroche S, Raimond JM. Cavity quantum electrodynamics. Sci Am. 1993:54–62. [Google Scholar]
- 18.Lakowicz JR. Radiative decay engineering 3. Surface plasmon-coupled directional emission. Anal Biochem. 2003 doi: 10.1016/j.ab.2003.09.039. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakowicz JR. Principles of Fluorescence Spectroscopy. 2. Kluwer Academic Press/Plenum Publishers; New York: 1999. p. 698. [Google Scholar]