Abstract
Background
Recombinant human leptin (r-metHuLeptin) has demonstrated efficacy in improving hormonal and metabolic parameters in leptin-deficient states, and it has been suggested that leptin replacement may reverse metabolic adaptations during weight loss iterventions. Pharmacokinetics of subcutaneously administered r-metHuLeptin have been recently published, but whether pharmacokinetic parameters are altered by short-term fasting, adiposity, and/or gender has not yet been evaluated. We sought to characterize pharmacokinetic parameters following subcutaneous r-metHuLeptin administration at a range of physiologic to pharmacologic doses in the fed state and during 3-day complete fasting in lean and obese individuals, including men and women.
Methods
We analyzed pharmacokinetic profiles in 5 lean men, 5 obese men, and 5 lean women following subcutaneous administration of physiologic (0.01 mg/kg), supraphysiologic (0.1 mg/kg), and pharmacologic (0.3 mg/kg) doses of r-metHuLeptin given once in the fed state and once daily during 3-day complete caloric deprivation (fasting).
Results
With r-metHuLeptin administration at 0.01 mg/kg, leptin levels ranged up to ~7 ng/ml in lean men, ~20 ng/ml in obese men, and ~30 ng/ml in lean women. There was a significant effect of 3-day fasting to decrease baseline leptin levels, maximal concentration (Cmax), and area under the curve (AUC) (all P<0.0001) and to increase clearance (P<0.001), most prominently in lean men (P<0.0001 across groups). Administration of r-metHuLeptin at 0.1 mg/kg resulted in leptin levels up to ~70 ng/ml in lean men, ~100 ng/ml in obese men, and ~150 ng/ml in lean women. At this dose, there was a similar effect of fasting on the above parameters as well as a decrease in half-life (P=0.02), consistent with increased clearance, but the effect of fasting was overall less pronounced compared with the 0.01 mg/kg dose. With r-metHuLeptin administration at 0.3 mg/kg, leptin levels ranged up to ~150 ng/ml in lean men, ~300 ng/ml in obese men, and ~400 ng/ml in lean women. At this dose, fasting increased clearance to a lesser degree (P=0.046), mainly in lean men, suggesting that the fasting-induced increase in leptin clearance by the kidneys can plateau. Within each group, subjects lost ~3–4 kg of body weight after 3 days of fasting (all P<0.0001), but the amount and time course of weight loss did not differ according to the dose of r-metHuLeptin administered or circulating leptin levels achieved.
Conclusions
Short-term fasting in healthy individuals results in increased clearance of leptin; this contributes to hypoleptinemia, which may serve as a signal to increase energy intake in the setting of caloric restriction. Obese individuals with greater energy stores at baseline have a blunted response to the fasting-induced increase in leptin clearance. Also, women have a differential response to fasting with primarily decreased leptin production rather than increased clearance. These findings and the resulting formulas for calculating doses for r-metHuLeptin administration have important implications for the future therapeutic use of r-metHuLeptin in conjunction with hypocaloric diets for the treatment of obesity.
Keywords: Leptin, pharmacokinetics, subcutaneous, fasting, gender, adiposity
BACKGROUND
The adipocyte-secreted hormone leptin[1] has demonstrated therapeutic efficacy in several disease states associated with leptin deficiency, ranging from absolute congenital leptin deficiency[2] to acquired relative leptin deficiency. The latter includes individuals with congenital and acquired severe lipodystrophy,[3] HIV-associated lipodystrophy,[4] and hypothalamic amenorrhea,[5] who have metabolic and/or neuroendocrine abnormalities associated with low leptin levels. In contrast, recombinant methionyl human leptin (r-metHuLeptin) administration for 24 weeks to obese, leptin-resistant individuals studied in the fed state had modest effects to induce weight loss in a dose-dependent manner (up to 5.8 kg placebo-corrected at the highest dose of 0.3 mg/kg).[6] A different formulation of leptin (pegylated human recombinant leptin) administered for almost 6 weeks to overweight men during a very low calorie diet also resulted in weight loss (2.8 kg placebo-corrected) and reduced appetite compared to placebo.[7] Recently, administration of leptin in combination with pramlintide (a human analog of amylin) for 24 weeks in overweight and obese subjects resulted in greater weight loss (12.7%) compared to pramlintide alone (8.4%) (www.amylin.com).
Compensatory neuroendocrine changes may limit the efficacy of weight loss attempts and contribute to the high rate of recidivism and weight regain in the management of obesity. Since leptin plays a key role in mediating the adaptation to starvation,[8] r-metHuLeptin has been administered to obese individuals after 10% reduced body weight in a recent uncontrolled study of sequential design and found to reverse changes in thyroid hormone levels, energy expenditure, and sympathetic nervous system tone.[9] Whether r-metHuLeptin has greater and/or more sustained effects on weight loss in conjunction with more stringent and/or long-term hypocaloric diets for the treatment of obesity has not yet been evaluated in a controlled fashion, but if leptin ameliorates neuroendocrine and metabolic adaptations associated with weight loss, leptin replacement during caloric restriction may be advantageous in achieving and sustaining weight loss.
Thus, knowledge of how r-metHuLeptin pharmacokinetics may be influenced by energy deficiency is of critical importance for the potential future therapeutic use of r-metHuLeptin. We previously published pharmacokinetic data based on intravenous administration of r-metHuLeptin,[10] and more recently based on subcutaneous (s.c.) administration.[11] We have demonstrated in our prior study involving r-metHuLeptin administration to lean men during short-term starvation[8] that higher doses of r-metHuLeptin were necessary to achieve replacement levels in the fasting condition than that predicted by formulas based on non-fasting conditions. However, the effect of short-term complete caloric restriction on r-metHuLeptin pharmacokinetics has not yet been systematically evaluated in different populations (lean vs. obese subjects, men vs. women) to determine whether dosing needs to be adjusted in the fasting vs. fed condition. Thus, we conducted a pharmacokinetic study using subcutaneous r-metHuLeptin at doses ranging from physiologic to supra-physiologic to pharmacologic in lean and obese individuals, including men and women, who were studied in the fed state and as well as during 72-hour fasting conditions.
METHODS
Human Subjects
Five healthy normal-weight men with body mass index (BMI) < 25 kg/m2, five obese otherwise healthy men with BMI > 30 kg/m2, and five lean healthy women with BMI < 25 kg/m2 were admitted to the General Clinical Research Center (GCRC) at the Beth Israel Deaconess Medical Center (BIDMC) for separate 3-day fasting and 1-day fed studies with administration of r-metHuLeptin at three different doses (0.01 mg/kg, 0.1 mg/kg, 0.3 mg/kg). All subjects participated in 3 studies in the fed condition (Part A) and 3 separate 72-hour fasting studies (Part B). This study was approved by the BIDMC Institutional Review Board. Written informed consent was obtained from subjects. Clinical quality r-metHuLeptin was supplied by Amgen, Inc. (Thousand Oaks, California) and administered under an Investigational New Drug application submitted to the Food and Drug Administration. Subjects were counseled to keep their dietary habits stable over the course of the study and were contacted several days before each admission as a reminder to keep their diet and activity stable during the days preceding the admission. During each study, subjects were not allowed to exercise strenuously but could move freely within their room. The rooms were generally maintained at typical room temperatures but not specifically thermoregulated across all admissions. For lean men and obese men, fed studies were scheduled after all fasting studies had been completed. For lean women, the first day of each study was scheduled during the early follicular phase of their menstrual cycle. Given this constraint, fed admissions for lean women were scheduled either between or after the fasting studies.
Part A: Subcutaneous administration of r-metHuLeptin in the fed state
Subjects were admitted to the GCRC the night before the study day and received an isocaloric diet with breakfast at 7am (20% of daily calories), lunch at 2pm (35% of calories), dinner at 6pm (35% of calories), and a snack at 10pm (10% of calories). r-metHuLeptin was administered s.c. in the abdomen at a dose of 0.01mg/kg the following morning at 8:00am. Serum leptin levels were measured at time 0 before each dose of r-metHuLeptin administered and at times: +30 minutes, +1hr, + 2hr, +3hr, +4hr, +5hr, +6hr, +8hr, +10hr, +12hr, +18hr, and +24hr after the dose. Subjects were admitted again 1 to 12 weeks later for 1-day admissions following a similar protocol, except with r-metHuLeptin administration at a dose of 0.1 or 0.3 mg/kg. Eight subjects received the 0.01 and 0.1 mg/kg dose on consecutive days at 8am as the 0.01 mg/kg dose was not expected to alter baseline leptin levels 24 hours later based on the half-life of r-metHuLeptin. Fasting body weight with subjects dressed in a standard hospital gown was obtained on the morning of each study (prior to blood sampling) using the same scale in the GCRC.
Part B: Subcutaneous administration of r-metHuLeptin in the fasting state
For each fasting study, subjects were admitted to the GCRC the night before the first study day and received a standardized snack (748 kcal) at 10pm, after which they fasted (receiving only calorie-free liquids, NaCl [500 mg], KCl [40 meq], and a standard multivitamin with minerals once per day) until study day 3 at 10pm when they received a standardized snack (225 kcal). For the first fasting study, r-metHuLeptin was administered s.c. at a dose of 0.01 mg/kg once daily at 8am for 3 consecutive days. Serum leptin levels were measured at time 0 before each dose and +30 minutes, +1hr, + 2hr, +3hr, +4hr, +5hr, +6hr, +8hr, +10hr, +12hr, and +18hr after the dose. Subjects were admitted again 2 to 4 weeks later for a second fasting study except with r-metHuLeptin administration at 0.1 mg/kg once daily for 3 consecutive days, and 3 to 10 weeks after that for a third fasting study with r-metHuLeptin administration at 0.3 mg/kg once daily for 3 consecutive days, following a similar protocol to that of the first fasting study, except for the dose of r-metHuLeptin. Fasting body weight with subjects dressed in a standard hospital gown was obtained on the morning of each day using the same scale in the GCRC.
Measurements
Leptin levels (ng/ml) were measured using RIA (Linco Research [St Louis, MO], now Millipore [Billerica, MA]), with a sensitivity of 0.5 ng/ml, in-house intra-assay and inter-assay coefficients of variation (CVs) of 7% and 18–20%, respectively. This assay measures both endogenous leptin and exogenous r-metHuLeptin. Samples were run in dilution as needed so that results fell within the linear part of the standard curve. Samples for the same subject were run within the same assay (in duplicate) to decrease inter-assay variability.
Noncompartmental Pharmacokinetic Analysis
Pharmacokinetic analyses were performed on baseline-subtracted leptin concentrations, using leptin values obtained immediately prior to dosing as baseline. After baseline-subtraction, peak serum concentration (Cmax), Tmax (defined as the time that Cmax was reached), terminal-phase elimination half-life (t1/2), and area under the serum concentration versus time curve from zero to infinity (AUC0-∞) were calculated (WinNonlin Version 5.0.1, Pharsight, Mountain View, CA) as previously described.[10] The apparent clearance (CL/F) of r-metHuLeptin is the ratio of actual dose administered to AUC0-∞, where F (an unknown factor herein) is the bioavailability factor after s.c. administration and represents the fraction of drug absorbed into the systemic circulation relative to that available after direct systemic administration, since other administration routes are associated with a percentage lost through factors such as degradation, hepatic metabolism, etc.
Baseline Level of Leptin
In general, the endogenous leptin levels were assumed to be at steady state before fasting or r-metHuLeptin administration, and their levels can be described by the following differential equations:
| Equation 1 |
In Equation 1, Rsyn, [L], and (L) represent the endogenous production rate, amount of leptin in the body, and serum concentration of leptin, respectively, and CL defines the clearance for leptin. At time 0 (steady state), before fasting or r-metHuLeptin administration, there was no change in leptin concentration, thus d[L]/dt = 0, and Rsyn = CL* (L), therefore,
| Equation 2 |
where (L) equals to the endogenous leptin concentration before any experimental conditions were implemented. The intravenous infusion rate of exogenous r-metHuLeptin required to raise serum leptin levels can be calculated as Rsyn=Rinf =CL*(L). Since only s.c. r-metHuLeptin was administered in the current study, the equation is further modified as Rinf,s.c =(CL/F)*(L). Since CL/F was estimated for each treatment day, the estimation for Rinf,s.c was performed without any assumption on “F”.
Statistical Analysis
Data are presented as mean ± SD. Statistical analyses on the relationships between pharmacokinetic parameters from each administered dose versus each subject group and day of fasting (assigning Day 0 for fed condition, Day 1 as first fasting day, Day 2 as second fasting day, and Day 3 as the third fasting day) were explored with an analysis of variance (ANOVA) model using JMP software (Version 5.1, SAS Institute, Cary, NC). An exploratory ANOVA model using administered dose, fasting day, and subjects as covariates to specifically investigate the effects on fasting was also performed. An unadjusted significance level of 0.05 was used for all comparisons.
RESULTS
Five normal-weight men (mean age=22.2±0.9, mean BMI=22.0±0.5 kg/m2), five obese otherwise healthy men (mean age=23.4±1.5, mean BMI=32.0±1.0 kg/m2), and five lean healthy women (mean age=20.4±0.7, mean BMI=21.9±0.7 kg/m2) participated in 3 fed and 3 fasting studies (one fed and one 72-hour fasting condition at each of the 3 different doses of r-metHuLeptin). r-metHuLeptin was generally well tolerated with no systemic side effects occurring in any subjects. Two subjects (1 lean woman and 1 obese man) had mild injection site reactions at the 0.3 mg/kg dose with development of well-demarcated, mildly pruritic, but non-painful erythematous patches on the abdomen several hours after the injection, which resolved without intervention over the course of a few days.
During each of the 72-hour fasting conditions, subjects lost a significant amount of body weight of ~3–4 kg (with obese men losing slightly more weight than lean men and lean women) (Table I). Importantly, within each subject group, body weight at the beginning of each fasting study was very similar (within 0.5 to 1 kg), indicating full recovery of body weight back to baseline between each of the fasting studies. The amount of weight lost and the time course of weight loss were remarkably similar across the three doses of r-metHuLeptin administered for each subject group (Table I).
Table I.
Body weight (kg) of lean men (n=5), obese men (n=5), and lean women (n=5) during 72-hour complete fasting with administration of r-metHuLeptin at 3 different doses, mean ± SD. Overall P-value across days 1 to 4 of fasting by repeated measures ANOVA.
| Fed | Fasting | ||||||
|---|---|---|---|---|---|---|---|
| Subject Group |
r-metHuLeptin Dose |
Day 1 | Day 2 | Day 3 | Day 4 | P-value | |
| Lean | 0.01 mg/kg | 67.1 ± 6.7 | 65.8 ± 5.0 | 64.7 ± 5.2 | 63.7 ± 5.4 | 62.6 ± 5.2 | <0.001 |
| Men | 0.1 mg/kg | 66.9 ± 6.6 | 66.1 ± 5.4 | 64.5 ± 5.2 | 63.5 ± 5.2 | 62.4 ± 5.3 | <0.001 |
| 0.3 mg/kg | 67.2 ± 6.7 | 66.2 ± 5.8 | 64.5 ± 5.8 | 63.5 ± 5.9 | 62.8 ± 5.6 | <0.001 | |
| Obese | 0.01 mg/kg | 105.4 ± 12.1 | 105.3 ± 10.9 | 103.6 ± 10.6 | 102.5 ± 10.4 | 101.6 ± 10.2 | <0.001 |
| Men | 0.1 mg/kg | 105.2 ± 12.4 | 105.1 ± 10.5 | 102.8 ± 10.1 | 101.4 ± 10.0 | 100.5 ± 9.8 | <0.001 |
| 0.3 mg/kg | 107.1 ± 13.6 | 104.9 ± 11.7 | 103.1 ± 11.5 | 101.8 ± 11.5 | 101.0 ± 11.2 | <0.001 | |
| Lean | 0.01 mg/kg | 59.7 ± 7.1 | 59.2 ± 7.3 | 57.8 ± 7.2 | 56.9 ± 7.0 | 56.2 ± 7.0 | <0.001 |
| Women | 0.1 mg/kg | 58.9 ± 6.5 | 59.4 ± 7.7 | 58.0 ± 7.7 | 56.9 ± 7.6 | 56.2 ± 7.5 | <0.001 |
| 0.3 mg/kg | 58.8 ± 6.8 | 59.1 ± 7.5 | 57.6 ± 7.2 | 56.8 ± 7.3 | 56.0 ± 6.8 | <0.001 | |
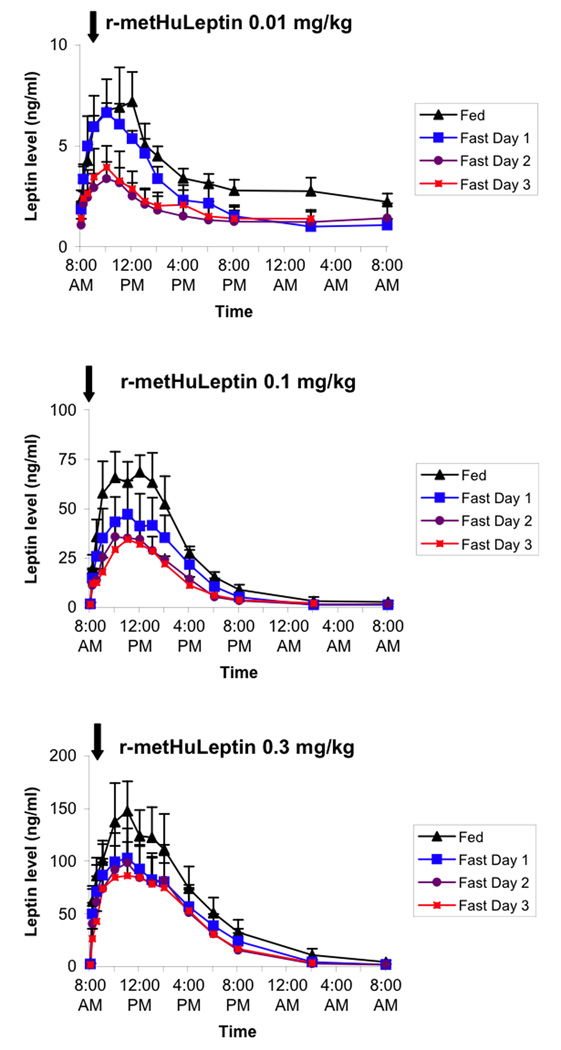
Figure 1 shows pharmacokinetic profiles of leptin levels following administration of 0.01, 0.1, and 0.3 mg/kg doses of r-metHuLeptin in lean men during the fed condition as well as with 1 to 3 days of complete fasting. In the fed state, serum leptin levels increased from a baseline level of ~2 ng/ml to physiologic ranges (~6–7 ng/ml) with the 0.01 mg/kg dose, to supraphysiologic levels (~50–70 ng/ml) with the 0.1 mg/kg dose, and to pharmacologic levels (~100–150 ng/ml) with the 0.3 mg/kg dose. At the 0.01 mg/kg dose, fasting for 1 day had minimal effect on leptin levels but levels decreased by ~50% after 2 days of fasting with minimal additional effect of the third day of fasting. With higher doses of r-metHuLeptin, the effect of fasting to decrease leptin levels was less marked but still present.
Figure 1.
Pharmacokinetic profiles of leptin following administration of 0.01, 0.1, and 0.3 mg/kg of r-metHuLeptin once in the fed condition and once daily during 3 days of complete fasting in healthy lean men (n=5). Arrow indicates dose of r-metHuLeptin administered just after measurement of baseline leptin levels at 8:00am. Leptin levels are actual (not baseline-corrected).
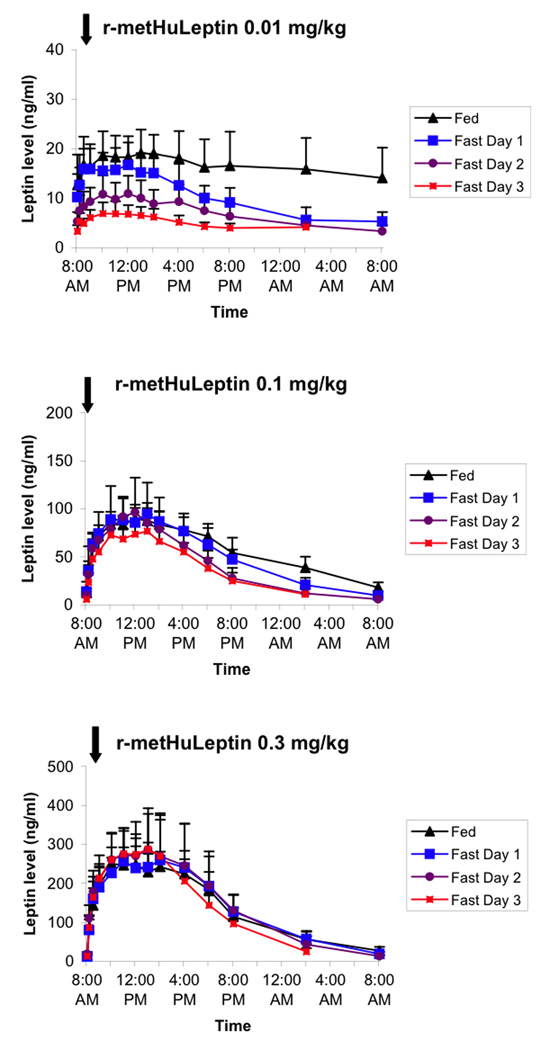
In obese men, administration of r-metHuLeptin in the fed condition resulted in higher leptin levels than lean men, in the range of 15–20 ng/ml with the 0.01 mg/kg dose, 75–100 ng/ml with the 0.1 mg/kg dose, and 250–300 ng/ml with the 0.3 mg/kg dose (Figure 2). At the 0.01 mg/kg dose, fasting resulted in a progressive decrease in leptin levels by ~2/3, but there was minimal effect of fasting at 0.1 and 0.3 mg/kg doses.
Figure 2.
Pharmacokinetic profiles of leptin following administration of 0.01, 0.1, and 0.3 mg/kg of r-metHuLeptin once in the fed condition and once daily during 3 days of complete fasting in healthy obese men (n=5). Arrow indicates dose of r-metHuLeptin administered just after measurement of baseline leptin levels at 8:00am. Leptin levels are actual (not baseline-corrected).
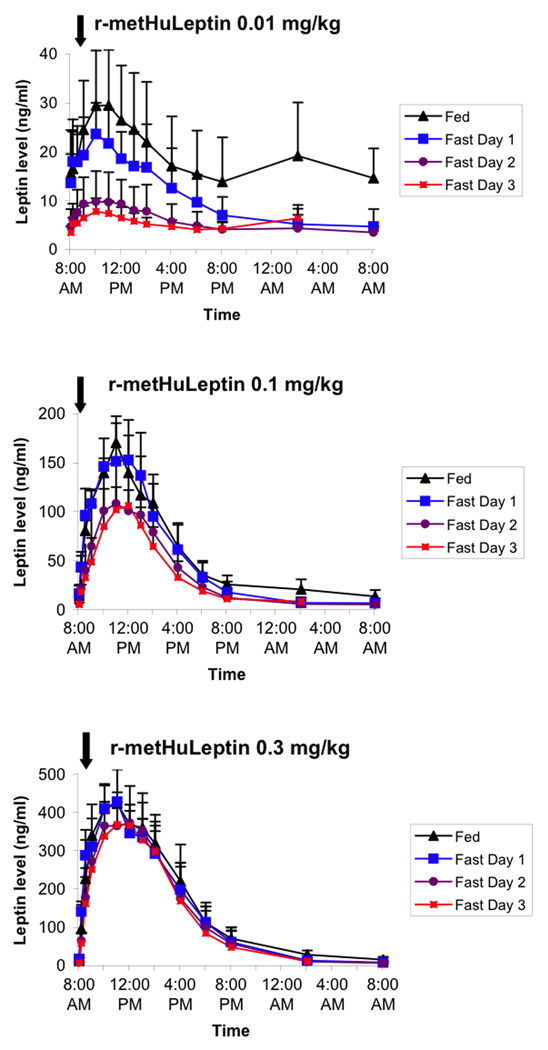
In lean women, administration of r-metHuLeptin in the fed condition resulted in even higher peak leptin levels than lean or obese men, ranging from 20–30 ng/ml with the 0.01 mg/kg dose, 100–150 ng/ml with the 0.1 mg/kg dose, and 350–450 ng/ml with the 0.3 mg/kg dose (Figure 3). A clear effect of fasting on serum leptin levels was again seen in response to the 0.01 mg/kg dose, with a decrease in leptin levels by ~2/3 on the second and third days of fasting. The effect of fasting was still present with the 0.1 mg/kg dose (again mainly during days 2 and 3 of fasting), but nearly absent with the 0.3 mg/kg dose.
Figure 3.
Pharmacokinetic profiles of leptin following administration of 0.01, 0.1, and 0.3 mg/kg of r-metHuLeptin once in the fed condition and once daily during 3 days of complete fasting in healthy lean women (n=5). Arrow indicates dose of r-metHuLeptin administered just after measurement of baseline leptin levels at 8:00am. Leptin levels are actual (not baseline-corrected).
We then calculated pharmacokinetic parameters (baseline leptin level [Lo], Tmax, Cmax, t1/2, area under the curve [AUC], and CL/F) for each group of subjects during the fed condition and during each day of fasting at the 3 different doses of r-metHuLeptin. Table II presents pharmacokinetic parameters with the physiologic 0.01 mg/kg dose. As expected, there was a significant difference between groups in the baseline leptin level as well as a progressive decrease in baseline leptin levels with complete fasting. The percent decrease was more pronounced in lean women and obese men (~25% of baseline) although absolute leptin levels achieved with fasting were lower in lean men. The half-life of r-metHuLeptin administered s.c. was ~2–3 hours, with no effect of fasting and no difference between subject groups. Tmax was generally around 2–3 hours (except for obese men in the fed condition). Cmax was significantly different between groups and decreased with fasting, as suggested by the pharmacokinetic profiles. Although obese men had higher absolute leptin levels than lean men, baseline correction resulted in Cmax levels that were similar to but somewhat higher than in lean men, although women had higher Cmax than lean men even after baseline correction. The difference in AUC was ~3-fold higher in obese men and lean women compared to lean men in the fed condition. AUC decreased significantly with fasting in each group. CL/F demonstrated an inverse relationship with AUC. In lean men, clearance increased by ~2-fold increase on the second day of fasting compared to the fed state and first day, and remained at a similar level on the third day. Fasting also increased clearance in lean women and obese men by a similar degree, although the absolute CL/F was higher in lean men. Because of the increased clearance with fasting, the dose required to raise serum leptin levels by 1 ng/ml was ~2-fold higher in all groups after 2 to 3 days of fasting.
Table II.
Leptin pharmacokinetic parameters (mean ± SD) and projected dose for r-metHuLeptin in lean men (n=5), obese men (n=5), and lean women (n=5) after r-metHuLeptin administration at a dose of 0.01 mg/kg in the fed and 72-hour fasting conditions.
| Parameter | Subject | Fed | 72-hour Fasting | P-valuea | |||
|---|---|---|---|---|---|---|---|
| Group | Day 1 | Day 2 | Day 3 | Fasting | Group | ||
| Lo | <0.0001 | <0.0001 | |||||
| (ng/ml) | Lean Men | 2.32 ± 0.48 | 1.85 ± 0.39 | 1.07 ± 0.31 | 1.42 ± 0.72 | ||
| Obese Men | 12.67 ± 6.15 | 11.92 ± 2.90 | 5.27 ± 1.95 | 3.32 ± 1.21 | |||
| Lean Women | 15.54 ± 8.90 | 13.65 ± 5.90 | 4.72 ± 3.57 | 3.53 ± 1.26 | |||
| t1/2 | 0.34 | 0.64 | |||||
| (hr) | Lean Men | 2.84 ± 0.98 | 1.44 ± 0.46 | 2.84 ± 2.50 | 1.93 ± 0.28 | ||
| Obese Men | 4.71 ± 2.16 | 1.69 ± 0.60 | 1.64 ± 0.31 | 2.75 ± 1.49 | |||
| Lean Women | 3.08 ± 1.24 | 1.32 ± 0.87 | 3.34 ± 2.86 | 2.99 ± 1.01 | |||
| Tmax | 0.02 | 0.003 | |||||
| (hr) | Lean Men | 3.00 ± 1.00 | 2.20 ± 1.10 | 2.40 ± 0.55 | 2.00 ± 0.71 | ||
| Obese Men | 6.20 ± 3.35 | 3.40 ± 0.89 | 3.00 ± 1.00 | 3.00 ± 0.71 | |||
| Lean Women | 2.80 ± 0.84 | 2.20 ± 1.10 | 2.60 ± 1.34 | 2.80 ± 1.30 | |||
| Cmax | <0.0001 | <0.0001 | |||||
| (ng/ml) | Lean Men | 5.97 ± 1.65 | 5.26 ± 1.03 | 2.43 ± 0.75 | 2.60 ± 0.59 | ||
| Obese Men | 7.91 ± 2.37 | 5.19 ± 2.22 | 5.88 ± 2.13 | 4.21 ± 1.17 | |||
| Lean Women | 14.51 ± 2.96 | 11.15 ± 3.49 | 5.49 ± 2.80 | 4.62 ± 1.60 | |||
| AUC0→∞ | <0.0001 | <0.0001 | |||||
| (ng*h/mL) | Lean Men | 33.18 ± 8.68 | 25.01 ± 5.72 | 18.23 ± 14.62 | 12.27 ± 2.95 | ||
| Obese Men | 113.9 ± 56.6 | 29.93 ± 17.07 | 47.03 ± 19.90 | 35.43 ± 13.81 | |||
| Lean Women | 110.5 ± 45.0 | 45.91±31.73 | 37.40±13.90 | 47.81±23.90 | |||
| CL/F | 0.0008 | <0.0001 | |||||
| (mL/h)/kg | Lean Men | 316.3 ± 72.3 | 415.1 ± 84.5 | 858.2 ± 565.7 | 848.3 ± 176.4 | ||
| Obese Men | 129.0 ± 113.0 | 417.1 ± 196.9 | 238.9 ± 81.1 | 313.7 ± 105.8 | |||
| Lean Women | 107.4 ± 54.8 | 316.7 ± 220.5 | 306.5 ± 139.6 | 243.8 ± 92.1 | |||
| Dose | 0.0008 | <0.0001 | |||||
| (µg/kg)/d | Lean Men | 7.59 ± 1.73 | 9.96 ± 2.03 | 20.60 ± 13.58 | 20.36 ± 4.23 | ||
| Obese Men | 3.10 ± 2.71 | 10.01 ± 4.73 | 5.73 ± 1.95 | 7.53 ± 2.54 | |||
| Lean Women | 2.58 ± 1.32 | 7.60 ± 5.29 | 7.36 ± 3.35 | 5.85 ± 2.21 | |||
P-value for effect of fasting by ANOVA adjusting for group and subject factors. P-value for effect of group by ANOVA adjusting for fasting and subject factors.
L0 (ng/ml) represents the baseline level of endogenous level measured before administration of r-metHuLeptin
Tmax (hr) represents the time to maximal concentration
Cmax (ng/mL) represents the maximal concentration of serum leptin, corrected for the baseline leptin level
t1/2 (hrs) represents terminal-phase elimination half-life of leptin
AUC0→∞(ng*h/mL) represents the area under the serum leptin vs. time curve from time zero to infinity
CL/F [(mL/hour)/kg] represents the clearance divided by the bioavailability factor (F) Dose [(µg/kg)/day] represents the daily dose of r-metHuLeptin required to raise serum leptin levels by 1 ng/ml
At the supraphysiologic 0.1 mg/kg dose of r-metHuLeptin, baseline leptin levels were again significantly different between groups and decreased in response to fasting, following a similar pattern as with the 0.01 mg/kg dose, although baseline leptin levels on the 3rd day of fasting were higher with the 0.1 vs. 0.01 mg/kg dose in lean women and obese men (Table III). The half-life was around 2–3 hours, with a significant difference between groups (longer in obese men). Cmax was different between groups, but as suggested by the pharmacokinetic profiles, the effect of fasting was no longer significant, mainly due to the lack of change in lean women and obese men, whereas Cmax still decreased in lean men with fasting. However, AUC was significantly decreased by fasting in all groups with a clear difference between groups. Clearance and the dose to raise serum leptin levels by 1 ng/ml increased with fasting, especially in lean men.
Table III.
Leptin pharmacokinetic parameters (mean ± SD) and projected dose for r-metHuLeptin in lean men (n=5), obese men (n=5), and lean women (n=5) after r-metHuLeptin administration at a dose of 0.1 mg/kg in the fed and 72-hour fasting conditions.
| Parameter | Subject | Fed | 72-hour Fasting | P-valuea | |||
|---|---|---|---|---|---|---|---|
| Group | Day 1 | Day 2 | Day 3 | Fasting | Group | ||
| Lo | 0.0003 | <0.0001 | |||||
| (ng/ml) | Lean Men | 2.11 ± 0.50 | 1.74 ± 0.60 | 1.33 ± 0.53 | 1.69 ± 0.89 | ||
| Obese Men | 15.49 ± 8.64 | 13.79 ± 3.10 | 9.76 ± 4.20 | 5.92 ± 1.93 | |||
| Lean Women | 14.36 ± 11.12 | 16.40 ± 8.06 | 6.75 ± 4.93 | 5.36 ± 1.89 | |||
| t1/2 | 0.02 | <0.0001 | |||||
| (hr) | Lean Men | 2.89 ± 0.24 | 1.40 ± 0.48 | 2.31 ± 0.87 | 1.82 ± 0.58 | ||
| Obese Men | 4.72 ± 1.51 | 2.72 ± 0.89 | 3.18 ± 1.08 | 3.10 ± 1.20 | |||
| Lean Women | 2.08 ± 1.01 | 1.47 ± 0.27 | 1.73 ± 0.57 | 1.73 ± 0.57 | |||
| Tmax | 0.37 | 0.003 | |||||
| (hr) | Lean Men | 3.00 ± 1.58 | 3.20 ± 1.30 | 3.00 ± 1.22 | 3.20 ± 1.10 | ||
| Obese Men | 4.60 ± 0.89 | 4.60 ± 1.14 | 3.40 ± 0.55 | 4.00 ± 1.58 | |||
| Lean Women | 3.20 ± 0.84 | 3.20 ± 0.84 | 3.00 ± 1.00 | 3.00 ± 1.00 | |||
| Cmax | 0.08 | <0.0001 | |||||
| (ng/ml) | Lean Men | 72.56 ± 12.19 | 50.05 ± 16.37 | 36.20 ± 5.90 | 33.88 ± 9.38 | ||
| Obese Men | 77.26 ± 10.10 | 83.42 ± 30.86 | 92.40 ± 32.99 | 78.43 ± 14.71 | |||
| Lean Women | 107.4 ± 19.4 | 144.1 ± 31.9 | 106.4 ± 36.7 | 106.4 ± 36.7 | |||
| AUC0→∞ | 0.002 | <0.0001 | |||||
| (ng*h/mL) | Lean Men | 507.4 ± 44.1 | 326.3 ± 102.2 | 236.7 ± 39.4 | 206.9 ± 56.9 | ||
| Obese Men | 1051 ± 202.9 | 1168 ± 290.6 | 915.7 ± 300.0 | 778.2 ± 121.5 | |||
| Lean Women | 620.2 ± 157.1 | 887.7 ± 231.4 | 676.5 ± 267.4 | 676.5 ± 267.4 | |||
| CL/F | <0.0001 | <0.0001 | |||||
| (mL/h)/kg | Lean Men | 198.4 ± 18.6 | 330.4 ± 98.5 | 431.5 ± 68.3 | 507.0 ± 108.0 | ||
| Obese Men | 97.90 ± 18.15 | 122.5 ± 39.33 | 131.6 ± 20.65 | 146.7 ± 25.1 | |||
| Lean Women | 170.2 ± 44.8 | 119.0 ± 31.0 | 171.6 ± 79.1 | 171.6 ± 79.1 | |||
| Dose | <0.0001 | <0.0001 | |||||
| (µg/kg)/d | Lean Men | 4.76 ± 0.45 | 7.93 ± 2.36 | 10.36 ± 1.64 | 12.17 ± 2.59 | ||
| Obese Men | 2.35 ± 0.44 | 2.94 ± 0.94 | 3.16 ± 0.50 | 3.52 ± 0.60 | |||
| Lean Women | 4.08 ± 1.08 | 2.86 ± 0.74 | 4.12 ± 1.90 | 4.12 ± 1.90 | |||
P-value for effect of fasting by ANOVA adjusting for group and subject factors. P-value for effect of group by ANOVA adjusting for fasting and subject factors.
L0 (ng/ml) represents the baseline level of endogenous level measured before administration of r-metHuLeptin
Tmax (hr) represents the time to maximal concentration
Cmax (ng/mL) represents the maximal concentration of serum leptin, corrected for the baseline leptin level
t1/2 (hrs) represents terminal-phase elimination half-life of leptin
AUC0→∞ (ng*h/mL) represents the area under the serum leptin vs. time curve from time zero to infinity
CL/F [(mL/hour)/kg] represents the clearance divided by the bioavailability factor (F)
Dose [(µg/kg)/day] represents the daily dose of r-metHuLeptin required to raise serum leptin levels by 1 ng/ml
Finally, at the 0.3 mg/kg dose of r-metHuLeptin, the effect of fasting on baseline leptin levels was less pronounced (P=0.049), especially in obese men, whereas baseline levels still decreased modestly in lean men by ~30% and in lean women by 60% (Table IV). Half-life remained around 2–3 hours, again slightly longer in men. Cmax was significantly different across groups, with much higher levels in lean women and obese men than lean men, but no significant effect of fasting. Similarly, AUC differed across groups with an only borderline significant effect of fasting. Clearance was still significantly increased by fasting, but the effect was less pronounced compared to lower r-metHuLeptin doses due to an increased clearance in lean men but not obese men or lean women. Correspondingly, the dose to raise serum leptin levels by 1 ng/ml was ~4-fold higher in lean men than lean women and obese men.
Table IV.
Leptin pharmacokinetic parameters (mean ± SD) and projected dose for r-metHuLeptin in lean men (n=5), obese men (n=5), and lean women (n=5) after r-metHuLeptin administration at a dose of 0.3 mg/kg in the fed and 72-hour fasting conditions.
| Parameter | Subject | Fed | 72-hour Fasting | P-valuea | |||
|---|---|---|---|---|---|---|---|
| Group | Day 1 | Day 2 | Day 3 | Fasting | Group | ||
| Lo | 0.049 | <0.0001 | |||||
| (ng/ml) | Lean Men | 2.97 ± 0.68 | 2.22 ± 0.84 | 1.73 ± 0.73 | 1.62 ± 0.64 | ||
| Obese Men | 16.24 ± 5.00 | 12.75 ± 4.57 | 19.63 ± 4.99 | 13.72 ± 2.97 | |||
| Lean Women | 12.60 ± 7.78 | 16.99 ± 5.45 | 8.07 ± 3.16 | 6.95 ± 1.05 | |||
| t1/2 | 0.0002 | <0.0001 | |||||
| (hr) | Lean Men | 2.94 ± 0.95 | 1.91 ± 0.50 | 1.76 ± 0.38 | 1.98 ± 0.28 | ||
| Obese Men | 3.69 ± 1.13 | 3.44 ± 1.34 | 2.87 ± 0.48 | 2.40 ± 0.54 | |||
| Lean Women | 2.91 ± 0.90 | 1.52 ± 0.35 | 1.45 ± 0.40 | 1.92 ± 0.45 | |||
| Tmax | 0.52 | 0.03 | |||||
| (hr) | Lean Men | 3.60 ± 0.89 | 2.20 ± 0.84 | 3.00 ± 1.87 | 3.80 ± 1.30 | ||
| Obese Men | 3.40 ± 1.67 | 6.00 ± 2.12 | 4.80 ± 2.05 | 3.40 ± 1.67 | |||
| Lean Women | 2.80 ± 0.84 | 3.00 ± 1.22 | 3.40 ± 1.52 | 3.80 ± 1.48 | |||
| Cmax | 0.25 | <0.0001 | |||||
| (ng/ml) | Lean Men | 168.7 ± 61.7 | 106.8 ± 40.1 | 101.3 ± 34.4 | 88.39 ± 32.13 | ||
| Obese Men | 254.3 ± 36.7 | 288.8 ± 95.8 | 296.1 ± 98.0 | 301.6 ± 86.8 | |||
| Lean Women | 440.7 ± 85.2 | 437.8 ± 77.4 | 386.7 ± 38.2 | 399.6 ± 76.8 | |||
| AUC0→∞ | 0.056 | <0.0001 | |||||
| (ng*h/mL) | Lean Men | 1243 ± 304.2 | 871.5 ± 252.9 | 773.6 ± 300.4 | 747.3 ± 300.8 | ||
| Obese Men | 2948 ± 521.9 | 3241 ± 899.4 | 3065 ±1076 | 2717 ± 647.1 | |||
| Lean Women | 3300 ± 871 | 2945 ± 552 | 2874 ± 583 | 2738 ± 624 | |||
| CL/F | 0.046 | <0.0001 | |||||
| (mL/h)/kg | Lean Men | 252.7 ± 58.7 | 374.3 ± 133.2 | 479.5 ± 308.6 | 482.8 ± 269.4 | ||
| Obese Men | 104.7 ± 20.13 | 104.6 ± 34.66 | 108.9 ± 37.57 | 116.0 ± 29.90 | |||
| Lean Women | 95.4 ± 21.6 | 105.0 ± 21.2 | 107.7 ± 20.4 | 114.1 ± 25.0 | |||
| Dose | 0.046 | <0.0001 | |||||
| (µg/kg)/d | Lean Men | 6.06 ± 1.41 | 8.98 ± 3.20 | 11.51 ± 7.41 | 11.59 ± 6.47 | ||
| Obese Men | 2.51 ± 0.48 | 2.51 ± 0.83 | 2.61 ± 0.90 | 2.78 ± 0.72 | |||
| Lean Women | 2.29 ± 0.52 | 2.52 ± 0.51 | 2.58 ± 0.49 | 2.74 ± 0.60 | |||
P-value for effect of fasting by ANOVA adjusting for group and subject factors. P-value for effect of group by ANOVA adjusting for fasting and subject factors.
L0 (ng/ml) represents the baseline level of endogenous level measured before administration of r-metHuLeptin
Tmax (hr) represents the time to maximal concentration
Cmax (ng/mL) represents the maximal concentration of serum leptin, corrected for the baseline leptin level
t1/2 (hrs) represents terminal-phase elimination half-life of leptin
AUC0→∞ (ng*h/mL) represents the area under the serum leptin vs. time curve from time zero to infinity
CL/F [(mL/hour)/kg] represents the clearance divided by the bioavailability factor (F)
Dose [(µg/kg)/day] represents the daily dose of r-metHuLeptin required to raise serum leptin levels by 1 n
DISCUSSION
In this dose-ranging pharmacokinetic study of subcutaneous r-metHuLeptin administration, we present pharmacokinetic data on lean and obese individuals (men and women) in response to varying doses of r-metHuLeptin ranging from physiologic (0.01 mg/kg) to supraphysiologic (0.1 mg/kg) to pharmacologic (0.3 mg/kg) during fed and fasting conditions. Our study design allowed us to determine the effect on leptin pharmacokinetics of progressive days of complete fasting (up to 3 days) as well as the effect of progressively higher levels of leptin. We report novel findings of increased clearance of leptin with fasting in healthy subjects, as well as important differences in obese vs. lean individuals and men vs. women with respect to the effect of fasting and/or increasing r-metHuLeptin doses on pharmacokinetic parameters.
In rodent models[12,13] as well as in humans[14,15], the kidney is the main site of leptin clearance, and renal leptin excretion has been calculated to account for ~80% of all leptin removed from plasma.[14] Leptin levels are only transiently increased in rats undergoing unilateral nephrectomy with no change in leptin production, indicating that renal tissue can rapidly adapt to restore normal leptin elimination.[16] In humans, information on leptin kinetics is relatively limited. Using simultaneous measurement of leptin levels in a radial artery and an abdominal vein draining subcutaneous adipose tissue, leptin production from abdominal adipose tissue and, by extrapolation, whole body leptin production in men have been found to correlate directly with percent body fat.[17] Although endogenous leptin clearance and half-life were reportedly unrelated to adiposity, only two obese individuals were included in that study.[17] In contrast, our prior pharmacokinetic study in a larger number of subjects (men and women) based on intravenous r-metHuLeptin administration and direct measurement of whole body leptin kinetics found decreased leptin clearance as well as increased leptin production with obesity.[10] Although leptin production could not be directly measured herein using subcutaneous r-metHuLeptin because of the unknown bioavailability factor, we report similar findings of decreased leptin clearance in obese men in the fed state (~one-third to one-half that of lean men).
A major finding of this study was that fasting has a significant effect to decrease baseline leptin levels, Cmax, and AUC, while increasing clearance. In lean men, Cmax decreased by half, and clearance doubled by the second day of complete fasting, with minimal effect of the third day of fasting. This resulted in a doubling of the dose of r-metHuLeptin required to raise serum leptin levels by 1 ng/ml. Although leptin production rates could not be directly calculated, the approximate doubling of leptin clearance in the setting of a ~50% decline in baseline leptin levels with fasting suggests that leptin production remained stable. The effect of fasting to increase leptin clearance persisted across all three doses of administered r-metHuLeptin, but was less pronounced with increasing r-metHuLeptin dose. This suggests that sufficiently high levels of leptin can cause the increase in leptin clearance to reach a plateau. From a teleologic perspective, increasing leptin clearance with fasting (which thereby decreases leptin levels even further) may send a more intense signal to increase caloric ingestion in a setting of perceived starvation.
The finding of decreased t1/2 with fasting is also consistent with the alterations in clearance. At the lowest dose of r-metHuLeptin, the effect of fasting on t1/2 was not statistically significant. However, pharmacokinetic profiles after low-dose r-metHuLeptin administration may be more sensitive to baseline correction and fluctuations of endogenous leptin levels, whereas supraphysiologic and pharmacologic doses provide more robust pharmacokinetic profiles that are less affected by baseline correction, which permits more rigorous assessment of t1/2. Thus, at the 0.1 and 0.3 mg/kg doses, an effect of fasting to decrease t1/2 by ~1/3 in lean men is apparent and consistent with the increased clearance.
As expected, obese men had higher baseline leptin levels at all time points compared to lean men. Although the absolute difference in baseline leptin levels between lean and obese men was only ~10 ng/ml, the same dose of r-metHuLeptin administered in obese men resulted in ~3 to 4-fold higher AUC than in lean men at each of the doses administered. The higher AUC even after baseline correction reflects a decrease in clearance in obese vs. lean men. These findings are consistent with our prior intravenous pharmacokinetic study demonstrating that obese individuals have decreased leptin clearance as well as increased production.[10] At the physiologic 0.01 mg/kg dose, fasting had an effect to reduce Cmax by ~50% and AUC by ~2/3 in obese men. This effect could still be seen with the supraphysiologic r-metHuLeptin dose but to a lesser degree; however, it was essentially lost with pharmacologic r-metHuLeptin. Thus, obesity has an effect to blunt the response to fasting, but mainly at pharmacologic leptin levels. From a teleologic perspective, this response may be considered appropriately adaptive since a decrease in efficiency of leptin clearance may create less of a drive to eat during starvation in obese individuals who have greater energy stores at baseline (and thus less risk of malnutrition). This is also consistent with the notion that leaner individuals are more sensitive to the effects of energy deprivation to perturb neuroendocrine function than their heavier counterparts.[18] Although alterations in leptin pharmacokinetics may play a role in the regulation of energy homeostasis in obesity, the factors that influence an individual’s drive to eat are clearly complex and multifactorial with contributions of both internal physiological changes (e.g. decreased glucose and insulin) as well as external environmental cues.
In this study, we also report leptin pharmacokinetic parameters based on subcutaneous r-metHuLeptin administration in women for the first time. As expected, leptin levels at baseline were ~5 to 6-fold higher in lean women compared to men, despite similar BMI. Again, despite an absolute difference in baseline leptin levels of only ~10–15 ng/ml between lean men and women, there was a striking difference in Cmax and AUC (~2 to 3-fold higher in women) after the same dose of r-metHuLeptin administered. In fact, lean women had even higher leptin levels at each dose of r-metHuLeptin compared to obese men. Otherwise, Tmax and t1/2 were similar in lean women as in lean men. Fasting results in an increase in clearance of ~2 to 3-fold in lean women at the 0.01 mg/kg dose. These findings are in contrast to a prior study using the technique of arteriovenous balance to estimate whole body leptin kinetics, which found a decrease in leptin production after 22 hours of fasting but no change in leptin clearance in lean and obese women.[19] Again, this discrepancy may relate to the different methodologies used (direct measurement of whole body leptin kinetics herein vs. indirect estimates in the prior study) and/or the much shorter duration of fasting in the prior study.[19]
At higher r-metHuLeptin doses (0.1 and 0.3 mg/kg), there was no effect of fasting on clearance in lean women, but baseline leptin levels still decreased by ~40–60% with fasting. This suggests that women may have a different pharmacokinetic response to fasting than men such that the fasting-induced decrease in baseline leptin levels in women relates more to decreased leptin production rather than increased leptin clearance. This issue cannot be fully resolved unless a similar study was performed using intravenous r-metHuLeptin. However, a lack of increase in leptin clearance during acute fasting in women may prevent further decreases in leptin levels and thus protect against perturbations in neuroendocrine function during acute energy deprivation, since disruption of reproductive function may have more significant implications on procreation in women than in men. This is consistent with our prior studies on the role of leptin in the neuroendocrine response to fasting demonstrating that whereas 72-hour fasting decreases testosterone and LH pulsatility in lean men (which are fully restored with leptin replacement),[8] the same duration of fasting has only minimal effects on the reproductive axis in lean women.[20] However, it is important to note that the lack of change in clearance was noted mainly at supraphysiologic and pharmacologic leptin levels, whereas an effect of fasting on clearance was present at the 0.01 mg/kg dose of r-metHuLeptin.
It is well-established that women have higher baseline leptin levels than men even after adjusting for differences in fat mass, which may relate to differences in body fat distribution and/or effect of sex steroids (i.e. effect of estrogen to increase leptin production and/or effect of testosterone to decrease leptin levels).[21–23] If fasting in women primarily causes a decrease in leptin production vs. an increase in clearance, it is possible that changes in sex steroids (not measured herein) could contribute, but this would require further evaluation.
Strengths of this study include: the novel elucidation of the effect of fasting on leptin pharmacokinetics; the evaluation of the same subjects in both the fed and fasting conditions using the same dosing protocol in each subject to minimize inter-subject variability, which helps to compensate for the relatively small n; the study of a range of r-metHuLeptin doses from physiologic to supraphysiologic to pharmacologic; the insights provided by evaluating body weight during short-term complete fasting with r-metHuLeptin administration at a range of doses; and the inclusion of both lean and obese individuals and both men and women. Although obese women were not directly studied, the comparison of obese men to lean men and of lean women to lean men permits the effects of gender and adiposity to be elucidated. Although no other commercially available assays were used herein, we have previously demonstrated that DSL correlates reasonably well with Linco,[11] and thus concentration-independent pharmacokinetic parameters should be similar if the DSL assay were used.
CONCLUSIONS
Short-term fasting in healthy individuals results in increased clearance of leptin, which may serve as a signal to increase energy intake in the setting of caloric restriction. Although the paradigm of our study was complete fasting rather than the more typical hypocaloric diets used to induce weight loss, these findings indicate that higher doses of r-metHuLeptin may be required for the same effect if r-metHuLeptin is used with caloric restriction (especially very low calorie diets) for the treatment of obesity. Obese individuals have a blunted response to fasting, which may reflect their greater energy stores at baseline, and thus may need less of an adjustment in r-metHuLeptin doses with caloric restriction. Women have a differential response to fasting with decreased leptin production rather than increased clearance, but because of their higher baseline leptin levels and greater increase in leptin levels in response to a given dose of r-metHuLeptin, their dosing requirements are more similar to that of obese men than lean men. These findings have important implications for the future therapeutic use of r-metHuLeptin in conjunction with hypocaloric diets for the treatment of obesity.
ACKNOWLEDGEMENTS
This study was supported by NIH Grant MO1-RR01032; NIH Grant R01-58785, Amgen grant, and BIDMC discretionary grant to CSM; and NIH Grant K23 RR018860 to JLC. CSM has received honorarium for one lecture and grant support through BIDMC-Harvard from Amylin, Inc. JLC and SLW have no conflicts of interest to report.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 01;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002 Oct;110(8):1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002 Feb 21;346(8):570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006 Jul;91(7):2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 5.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004 Sep 02;351(10):987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 6.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999 Oct 27;282(16):1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 7.Hukshorn CJ, Westerterp-Plantenga MS, Saris WH. Pegylated human recombinant leptin (PEG-OB) causes additional weight loss in severely energy-restricted, overweight men. Am J Clin Nutr. 2003 Apr;77(4):771–776. doi: 10.1093/ajcn/77.4.771. [DOI] [PubMed] [Google Scholar]
- 8.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003 May;111(9):1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005 Dec;115(12):3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab. 2004 Jun;89(6):2672–2677. doi: 10.1210/jc.2003-031931. [DOI] [PubMed] [Google Scholar]
- 11.Chan JL, Wong SL, Orlova C, Raciti P, Mantzoros CS. Pharmacokinetics of recombinant methionyl human leptin after subcutaneous administration: variation of concentration-dependent parameters according to assay. J Clin Endocrinol Metab. 2007 Jun;92(6):2307–2311. doi: 10.1210/jc.2006-2864. [DOI] [PubMed] [Google Scholar]
- 12.Cumin F, Baum HP, Levens N. Leptin is cleared from the circulation primarily by the kidney. Int J Obes Relat Metab Disord. 1996 Dec;20(12):1120–1126. [PubMed] [Google Scholar]
- 13.Zeng J, Patterson BW, Klein S, Martin DR, gogo-Jack S, Kohrt WM, et al. Whole body leptin kinetics and renal metabolism in vivo. Am J Physiol. 1997 Dec;273(6 Pt 1):E1102–E1106. doi: 10.1152/ajpendo.1997.273.6.E1102. [DOI] [PubMed] [Google Scholar]
- 14.Meyer C, Robson D, Rackovsky N, Nadkarni V, Gerich J. Role of the kidney in human leptin metabolism. Am J Physiol. 1997 Nov;273(5 Pt 1):E903–E907. doi: 10.1152/ajpendo.1997.273.5.E903. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MD, Moller N, Nair KS, Eisenberg P, Landt M, Klein S. Regional leptin kinetics in humans. Am J Clin Nutr. 1999 Jan;69(1):18–21. doi: 10.1093/ajcn/69.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Landt M, Martin DR, Zeng J, Miller SB, Kohrt WM, Patterson BW. Plasma leptin concentrations are only transiently increased in nephrectomized rats. Am J Physiol. 1998 Sep;275(3 Pt 1):E495–E499. doi: 10.1152/ajpendo.1998.275.3.E495. [DOI] [PubMed] [Google Scholar]
- 17.Klein S, Coppack SW, Mohamed-Ali V, Landt M. Adipose tissue leptin production and plasma leptin kinetics in humans. Diabetes. 1996 Jul;45(7):984–987. doi: 10.2337/diab.45.7.984. [DOI] [PubMed] [Google Scholar]
- 18.Alvero R, Kimzey L, Sebring N, Reynolds J, Loughran M, Nieman L, et al. Effects of fasting on neuroendocrine function and follicle development in lean women. J Clin Endocrinol Metab. 1998 Jan;83(1):76–80. doi: 10.1210/jcem.83.1.4512. [DOI] [PubMed] [Google Scholar]
- 19.Klein S, Horowitz JF, Landt M, Goodrick SJ, Mohamed-Ali V, Coppack SW. Leptin production during early starvation in lean and obese women. Am J Physiol Endocrinol Metab. 2000 Feb;278(2):E280–E284. doi: 10.1152/ajpendo.2000.278.2.E280. [DOI] [PubMed] [Google Scholar]
- 20.Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996 Sep;81(9):3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 22.Elbers JM, Asscheman H, Seidell JC, Frolich M, Meinders AE, Gooren LJ. Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. J Clin Endocrinol Metab. 1997 Oct;82(10):3267–3270. doi: 10.1210/jcem.82.10.4284. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, et al. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol. 1997 Aug;154(2):285–292. doi: 10.1677/joe.0.1540285. [DOI] [PubMed] [Google Scholar]