Abstract
In this letter, we report the observation of metal-enhanced chemiluminescence. Silver Island films, in close proximity to chemiluminescence species, can significantly enhance luminescence intensities; a 20-fold increase in chemiluminescence intensity was observed as compared to an identical control sample containing no silver. This suggests the use of silver nanostructures in the chemiluminescence-based immunoassays used in the biosciences today, to improve signal and therefore analyte detectability. In addition, this finding suggests that surface plasmons can be directly excited by chemically induced electronically excited luminophores, a significant finding toward our understanding of fluorophore-metal interactions and the generation of surface plasmons.
The use and utility of chemiluminescence reactions and materials as an analytical tool needs little introduction.1–4 As compared to fluorescence-based detection, chemiluminescence offers practical simplicity, significantly reduced background interference as the entire sample is not externally excited, and the fact that no optical filters are required. Chemiluminescent detection is however currently limited by the choice of probes available and to some degree the toxicity and the need for particular reagents to create chemically induced electronic excited states.1–4 In contrast, fluorescence does suffer from the need for relatively more complex and expensive detection instrumentation, the need for emission filters, unwanted biological autofluorescence, and generally poor fluorophore photostability.5 Fluorescence does however offer a considerably larger choice of probes.5 For both detection technologies, there is an urgent unequivocal need for an increased luminescence yield, as this would benefit the overall detectability and therefore in the context of bioassays, the sensitivity toward a particular analyte.1–5
In this regard, our laboratories have recently developed a technology which we have shown can increase the system quantum yield,6–8 enhance the photostability of the fluorophore6–8 and by using spatially localized multiphoton excitation9 can readily alleviate unwanted background autofluorescence. In all of these examples of metal-enhanced fluorescence (MEF),10 also called radiative decay engineering,11 and surface-enhanced fluorescence,8 we have employed nanosecond decay time fluorophores in close proximity (<10 nm)6–11 to a variety of different shaped12,13 and sized14 metallic nanostructures. Our current thinking of the MEF phenomenon with fluorophores is one whereby optically excited fluorophores induce surface plasmons (mirror dipoles),14,15 Fig. 1—middle, which in turn radiate the photophysical properties of the excited state. This was subtly different to our early reports7,10 were we believed that the fluorophore itself radiated, Fig. 1—top, its photophysical properties thought to be modified by a resonance interaction with the surface plasmons.7,10
FIG. 1.
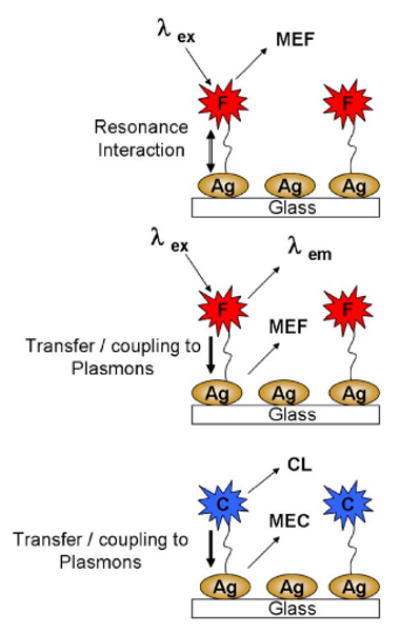
(Color online) Graphical representations of MEF (top and middle) and for MEC (Bottom). F—Fluorophore, C—Chemiluminescence species/probe and CL—Chemiluminescence.
However, recent complementary work from our laboratories with continuous silver films and fluorophores has clearly shown that plasmons can radiate coupled fluorescence under various optical conditions,16 with the coupled emission being completely p-polarized irrespective of the mode of excitation.16,17 Subsequently, we have developed the radiating plasmon model14,15 and have also demonstrated its plausibility experimentally.14 In these experiments, larger silver nanostructures have been shown to be more efficient at coupling and therefore radiating fluorescence than smaller ones.14 In this regard, it is known that the extinction properties (CE) of metal particles can be expressed as both a combination of both absorption (CA) and scattering (CS) factors, when the particles are spherical and have sizes comparable to the incident wavelength of light, i.e., in the Mie limit18,19
| (1) |
where k1 = 2πn1/λ0 is the wavevector of the incident light in medium I and α is the polarizability of a sphere with radius r, n1 is the refractive index, and λ0 is the incident wavelength. The term |α|2 is square of the modulus of α18,19
| (2) |
where ε1 and εm are the dielectric and the complex dielectric constants of the metal, respectively. The first term in Eq. (1) represents the cross section due to absorption, CA, and the second term, the cross section due to scattering, CS. Current interpretation of metal-enhanced fluorescence14 is one underpinned by the scattering component of the metal extinction, i.e., the ability of fluorophore-coupled plasmons to radiate (plasmon scatter).20 Intuitively, larger particles have wavelength distinctive scattering spectra (CS) as compared to their absorption spectra (CA),18,19 facilitating plasmon coupled emission from the larger nanoparticles.
Subsequently, in this paper, we show that chemically induced electronic excited states (chemiluminescence species) can also couple to surface plasmons, Fig. 1—bottom, producing emission intensities >20-fold, as compared to a control sample containing no surface silver nanostructures. This finding not only further facilitates our understanding of plasmon-fluorophore (luminophore) interactions, but suggests that this approach may be of significance for optically amplifying chemiluminescence-based clinical assays, potentially increasing analyte/biospecies detectability.
To demonstrate the broad potential utility of this approach, we have used several standard chemiluminescence kits (Omnioglow, West Springfield, MA) as the source of chemiluminescence. These chemiluminescence reactions are well known,1–4 light being generated by the random depopulation of a chemically induced electronic excited state of a luminophore, pumped by the initial oxidation via a peroxide solution. In this study, both green and blue kits were employed. For each sample, ≈70 μl of reaction mixture was sandwiched between partially silvered APS-coated glass plates, Fig. 2—top. The Silver Island Films (SiFs) are readily produced by the reduction of silver nitrate by glucose, as reported by our laboratories many times.7 This procedure readily deposits island-shaped silver noncontinuous deposits, approximately 200 nm in diameter, 40 nm high and with a ≈40% mass surface coverage on cleaned glass substrates, as shown by atomic force microscopy analysis.7
FIG. 2.
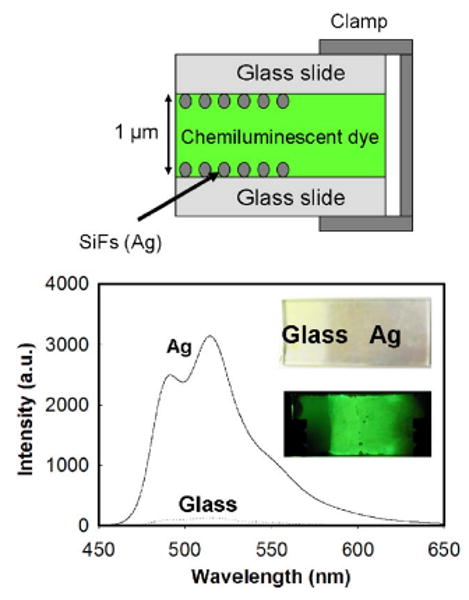
(Color online) Experimental sample setup (Top), and chemiluminescence emission intensity from both the glass and the silvered surface (Ag) (Bottom). Inset—photographs of the silvered and glass surfaces, with (inset - top) and without (inset - bottom) chemiluminescence material in the sandwich. The enhancement factor was >20, i.e., intensity on Ag/intensity on glass.
The bottom of Fig. 2 shows the green luminescence emission from between the silvered plates (Ag) and from glass, a control sample by which to calculate the enhancement ratio, i.e., intensity on silver/intensity on glass. Interestingly, the enhanced luminescence intensity was >20-fold brighter from the silver, as compared to glass, where both spectra are identical when normalized (not shown). The bottom inset of Fig. 2 shows both the silvered plates as well as with the chemiluminescence material sandwiched in between. The emission intensity is clearly visible from between the plates, yet only just visible from the glass control portion of the slide. Previous studies of the MEF phenomenon have reported the coupling to surface plasmons to be effective up to about 10 nm from the surface.7,10,11 This suggests that with an approximate 1 micron solution thickness, the true enhancement is much higher than ≈20, and in the range of 100–1000 fold, given that only 2% of the solution is actually in contact with the silver. This finding is of major significance for chemiluminescence-based detection, and suggests the amplified optical detection of either analytes or biospecies in close proximity to silver nanostructures.
Finally, we undertook several detailed control experiments to ascertain whether silver could catalyze the chemiluminescence reaction and account for the enhanced optical signatures observed, as compared to an interpretation in terms of a chemiluminescence-based radiating plasmon model. The top of Fig. 3 shows the luminescence intensity as a function of time. Clearly, the enhanced luminescence from the SiFs is visible, with the initial intensity on silver ≈3100 a.u. (at t=0) as compared to <150 on glass. We subsequently compared the rates of loss of luminescence after the curves were normalized (Fig. 3—top inset). The rate of loss of luminescence, which is due to the depletion of solution reactants and therefore depletion over time of excited states, was found to follow first-order decay kinetics and could simply be modeled to an exponential function of the form:
FIG. 3.
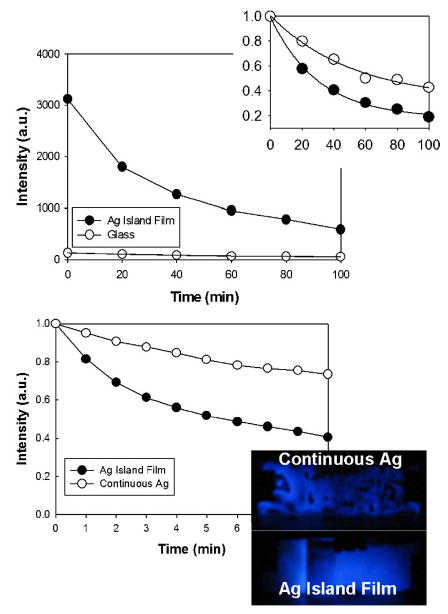
(Color online) Chemiluminescence intensity measured on both SiFs and glass as a function of time (Top) and the data normalized (Top—inset). Normalized chemiluminescence intensity on both SiFs and a continuous silver film (Bottom). Photograph of the emission from both the continuous silver film and the SiFs (Bottom—inset). Ag—Silver. SiFs—Silver Island Film.
| (3) |
where C is the intensity at time t=∞, B is a pre-exponential factor, and k is the rate of luminescence depletion, units S−1. The rate of depletion on silver was found to be 1.7 times faster than on glass, 0.034 versus 0.019 s−1, respectively. Two explanations could initially describe this observation: First, silver catalysis of the chemiluminescence reaction, or second, the high rate of transfer/coupling of the chemiluminescence to surface plasmons, rapidly reducing the excited state lifetime of the chemiluminescence species. To eliminate silver-based catalysis of the chemiluminescence reaction as an explanation for the enhanced signals, we additionally measured the luminescence rates on both SiFs and a continuous silver strip. Interestingly, the rate of loss of luminescence was still found to be greater on the SiFs as compared to the continuous silver strip (Fig. 3—bottom). In addition, the emission intensity was very low indeed from the continuous strip of silver (Fig. 3—bottom inset). Given that the continuous strip is indeed darker and that the rate is slower than on SiFs, then silver-based catalysis can be eliminated as a possible explanation of the observation of increased signal intensities on the SiFs. It is known that continuous metallic surfaces traditionally quench close proximity luminescence (<5 nm),7,10,11,15 accounting for the weaker intensity observed. Its is also known that the generation of surface plasmons in continuous metallic strips occurs only under certain unique conditions,15 as compared to the simplicity of creating surface plasmons in subwavelength sized particles.15,18,19 Subsequently, these observations suggest that chemically induced electronic excited states (chemiluminescence species) can readily induce/couple to surface plasmons, facilitating metal-enhanced chemiluminescence. Further, the reduced rate and increased emission intensity observed here, is very consistent with our reported findings for nanosecond decay time fluorophores sandwiched between identical silver nano-structures, similarly suggesting that the radiating plasmon model14,15 is most likely also applicable to chemically induced electronic excited states.
In conclusion, we have described the observation of Metal-Enhanced Chemiluminescence (MEC), which shows very similar characteristics to those observed with fluorophores and silver nanostructures, i.e., MEF. Greater than 20-fold enhancements in luminescence intensity have been readily observed by this approach, suggesting the approaches' utility in chemiluminescence-based assays to improve analyte detectability.
Acknowledgments
This work was supported by the NIH (Grant No. GM070929) and the National Center for Research Resources (Grant No. RR008119). Partial salary support to two of others (C.D.G. and J.R.L.) from UMBI is also acknowledged.
Contributor Information
Mustafa H. Chowdhury, Center for Fluorescence Spectroscopy, Medical Biotechnology Center, University of Maryland School of Medicine, 725 West Lombard Street, Baltimore, Maryland 21201
Kadir Aslan, Institute of Fluorescence, Laboratory for Advanced Medical Plasmonics, Medical Biotechnology Center, University of Maryland Biotechnology Institute, 725 West Lombard Street, Baltimore, Maryland 21201.
Stuart N. Malyn, Institute of Fluorescence, Laboratory for Advanced Medical Plasmonics, Medical Biotechnology Center, University of Maryland Biotechnology Institute, 725 West Lombard Street, Baltimore, Maryland 21201
Joseph R. Lakowicz, Center for Fluorescence Spectroscopy, Medical Biotechnology Center, University of Maryland School of Medicine, 725 West Lombard Street, Baltimore, Maryland 21201
Chris D. Geddes, Institute of Fluorescence, Laboratory for Advanced Medical Plasmonics, Medical Biotechnology Center, University of Maryland Biotechnology Institute, 725 West Lombard Street, Baltimore, Maryland 21201 Center for Fluorescence Spectroscopy, Medical Biotechnology Center, University of Maryland School of Medicine, 725 West Lombard Street, Baltimore, Maryland 21201.
References
- 1.Garcia-Campana AM, Baeyens WR. Anal Chem. Marcel Dekker; New York: 2001. [Google Scholar]
- 2.Wampler JE. In: Chemi- and Bioluminescence. Burr JG, editor. Marcel Dekker; New York: 1985. pp. 1–44. [Google Scholar]
- 3.Berthold F. In: Luminescence Immunoassays and Molecular Applications. Van Dyke K, Van Dyke R, editors. CRC Press; Boca Raton, FL: 1990. pp. 11–25. [Google Scholar]
- 4.Nieman T. Chemiluminescence: Theory and Instrumentation, Overview, in Encyclopedia of Analytical Science. Academic; Orlando, FL: 1995. pp. 608–613. [Google Scholar]
- 5.Lakowicz JR. Principles of Fluorescence Spectroscopy. Kluwer/Academic/Plenum; New York: 1997. [Google Scholar]
- 6.Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CD. Curr Opin Biotechnol. 2005;16:55. doi: 10.1016/j.copbio.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geddes CD, Aslan K, Gryczynski I, Malicka J, Lakowicz JR. In: Topics in Fluorescence Spectroscopy. Geddes CD, Lakowicz JR, editors. Kluwer/Academic/Plenum; New York: 2005. pp. 405–448. [Google Scholar]
- 8.J. R. Lakowicz, C. D. Geddes, I. Gryczynski, J. Malicka, Z. Gryczynski, K. Aslan, J. Lukomska, E. Matveeva, J. Zhang, R. Badugu, and J. Huang, J. Fluoresc. 14, 425 (2004).
- 9.J. R. Lakowicz, I. Gryczynski, J. Malicka, Z. Gryczynski, and C. D. Geddes, J. Fluoresc. 12, 299 (2002).
- 10.C. D. Geddes and J. R. Lakowicz, J. Fluoresc. 12, 121 (2002).
- 11.Lakowicz JR. Anal Biochem. 2001;298:1. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslan K, Leonenko Z, Lakowicz JR, Geddes CD. J Phys Chem B. 2005;109:3157. doi: 10.1021/jp045186t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslan K, Lakowicz JR, Geddes CD. J Phys Chem B. 2005;109:6247. doi: 10.1021/jp044235z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aslan K, Leonenko Z, Lakowicz JR, Geddes CD, Fluoresc J. 2005;15:643. doi: 10.1007/s10895-005-2970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakowicz JR. Anal Biochem. 2005;337:171. doi: 10.1016/j.ab.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakowicz JR. Anal Biochem. 2004;324:153. doi: 10.1016/j.ab.2003.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.C. D. Geddes, I. Gryczynski, J. Malicka, Z. Gryczynski, and J. R. Lakowicz, J. Fluoresc. 14, 119 (2004).
- 18.Yguerabide J, Yguerabide E. Anal Biochem. 1998;262:137. doi: 10.1006/abio.1998.2759. [DOI] [PubMed] [Google Scholar]
- 19.Yguerabide J, Yguerabide E. Anal Biochem. 1998;262:157. doi: 10.1006/abio.1998.2760. [DOI] [PubMed] [Google Scholar]
- 20.Aslan K, Lakowicz JR, Geddes CD. Curr Opin Chem Biol. 2005;9:538. doi: 10.1016/j.cbpa.2005.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


