Abstract
Background
The consistent association between obesity and colorectal cancer is thought to be explained by metabolic disturbances common, but not exclusive, to the obese.
Methods
We assessed the relation between metachronous neoplasia and the components of metabolic syndrome (MetS) as defined by the National Cholesterol Education Program’s Adult Treatment Panel III in 2,392 participants of two previously conducted chemoprevention trials. Waist circumference, fasting plasma glucose, trigylcerides, high-density lipoprotein, and systolic and diastolic blood pressure were measured at baseline.
Results
MetS classification was associated with increased odds of metachronous neoplasia among women [odds ratio (OR), 1.37; 95% confidence interval (95% CI), 1.01–1.85] but not among men (OR, 0.99; 95% CI, 0.81–1.21). High waist circumference in men (OR, 1.41; 95% CI, 1.15–1.72) and women (OR, 1.41; 95% CI, 1.05–1.90) and elevated fasting glucose in women (OR, 1.46; 95% CI, 1.09–1.96), as defined by Adult Treatment Panel III cutpoints, were associated with increased odds, whereas none of the other criteria were independently associated with metachronous neoplasia. When each trait was evaluated using quartiles, elevated glucose among women and large waist circumference among men were significantly associated with metachronous lesions. Exploratory analysis of waist circumference and fasting glucose suggested an interaction, where only the combination of large waist circumference and elevated glucose conferred significant increased odds of metachronous neoplasia among both men (OR, 1.36; 95% CI, 1.04–1.78; Pinteraction = 0.08) and women (OR, 1.83; 95% CI, 1.26–2.67; Pinteraction = 0.12).
Conclusions
These results suggest that, of the specific components of MetS, those that capture impaired glucose uptake increased the odds of metachronous neoplasia.
Introduction
Colorectal cancer is thought to arise as a consequence of a limited set of molecular events that largely originate with relatively benign adenomatous and possibly serrated lesions for which a small fraction, if left in situ, will progress to cancer (1, 2). Depending on the study population, the proportion of males and females, and extent of family history, 20% to 35% of persons over 50 years old harbor one or more types of colorectal neoplasm (3, 4). In postpolypectomy populations, metachronous lesions occur at a rate of 20% to 30% per year (5, 6). The propensity to develop colorectal adenomas identifies a sizable subgroup of the population at enhanced risk for subsequent adenoma formation and for colorectal cancer. This represents a group of people for whom adequate surveillance and risk-lowering behavior interventions is essential for cancer risk reduction.
Obesity, defined as body mass index (BMI) ≥ 30 kg/m2, has consistently been identified as a risk factor for colorectal cancer, particularly for men (7–10). This same association is present for colorectal preneoplasia (11–21) and the propensity for metachronous lesions (22), supporting an effect of obesity across the natural history of the cancer process. The underlying physiologic reasons for this association remain unclear. It has been postulated that the elevated risk for colorectal neoplasia associated with obesity is mediated through a disturbed intermediate metabolic state similar to that identified as a set of risk factors for cardiovascular disease and diabetes recognized as metabolic syndrome (MetS) (23). Along with advancing age and physical inactivity, obesity, particularly visceral or abdominal obesity, increases individual risk for several metabolic disturbances (e.g., dyslipidemia, hypertension, elevated plasma glucose, and chronic inflammation) (24). The underlying mechanisms for carcinogenesis with MetS are unclear, although inflammation and associated oxidative stress as well as chronic exposure to tumor-promoting effects of a hyperinsulimic state have been suggested (23).
Several studies have used the diagnostic criteria for MetS or specific traits of the syndrome to assess whether the MetS classification or feature(s) thereof identifies a group of individuals at elevated risk for colorectal neoplasia. Results from these studies have been largely consistent. Risk for incident colorectal neoplasia (25–29), metachronous colorectal neoplasia (30), colorectal cancer (31–34), and death from colorectal cancer (35) is increased in persons who meet the diagnostic criteria of MetS, have evidence of impaired glucose control, or have large waist circumferences or visceral adiposity. Results of these studies, along with those of animal studies of adiposity and metabolic disturbance and colon carcinogenesis (23), support MetS and features of MetS as an exposure or set of exposures that act across the continuum of colorectal carcinogenesis. Thus, investigation of these factors may better capture the magnitude of risk conferred by metabolic changes and prove useful in identifying individuals who might benefit from interventions targeted at the underlying metabolic changes associated with aging, sedentary behavior, and visceral obesity.
Given available pharmacologic agents (e.g., insulin sensitizers and agents for lowering glucose, blood pressure, and lipids) and the benefits of behavioral changes for the prevention of the principal disturbances of MetS, understanding the component contributions to the risk of metachronous lesions may help prioritize risk reduction approaches for adenoma formers and design of future polyp prevention trials. Thus, the present study aimed to examine whether metabolic disturbance, as captured by MetS status, or specific metabolic traits provide insight beyond measures of obesity (e.g., BMI and waist circumference) among a cohort of adenoma formers (36).
Materials and Methods
Study Population
Analyses were conducted on a subset of participants from the Wheat Bran Fiber trial and the Ursodeoxycholic Acid (UDCA) trial, the details of which have been reported elsewhere (37, 38). A brief description of each study is provided below. The University of Arizona Human Subjects Committee and the Institutional Review Board approved both studies. Written informed consent was obtained before study enrollment.
The Wheat Bran Fiber trial was a randomized double-blind controlled trial conducted to compare the effect of a high-fiber versus low-fiber cereal supplement on metachronous adenomas among individuals who had undergone colonoscopy and had one or more adenomas removed. Participants were recruited between 1990 and 1995, and 1,429 participants were randomized to either a high-fiber or a low-fiber treatment group. A total of 1,310 (91.7%) participants completed one or more follow-up colonoscopies ≥6 months after the initial qualifying exam and were followed for an average of 43.4 months.
The UDCA trial was a randomized double-blind controlled trial conducted to compare the effect of UDCA versus placebo on metachronous adenomas among individuals who had undergone colonoscopy and had one or more adenomas removed. Between 1996 and 2000, 1,285 participants were randomized to either the treatment or the placebo group. A total of 1,192 (92.8%) participants completed the trial by undergoing one or more follow-up colonoscopies ≥6 months after randomization and were followed for an average of 31.0 months.
In both studies, individuals with a personal history of inflammatory bowel disease or hereditary colon cancer syndromes were excluded. Neither intervention had a significant effect on the development of metachronous neoplasia (37, 38).
We restricted our analysis to participants with available data on all five components of MetS, as defined by the National Cholesterol Education Program’s Adult Treatment Panel III (ATP III) report, which includes waist circumference, triglycerides, high-density lipoprotein (HDL) cholesterol, blood pressure, and fasting glucose (39). Measurements for the five components were available for 1,305 of the 1,310 Wheat Bran Fiber trial participants and 1,087 of the 1,192 UDCA trial participants, whereas waist circumference or blood pressure data were not available for the remaining 110 participants. The combined study population available for the current analysis is 2,392.
Assessment of MetS Components
Participants were instructed to measure their waist circumference at the smallest circumference of their natural waist, which is usually just above the belly button, and report this measurement to the nearest 1/16th of an inch. Blood pressure was measured by a clinic nurse during the registration process. Blood samples were drawn from fasting participants, placed in tubes containing heparin, and sent for standard clinical analyses of blood chemistry, measuring fasting glucose and blood lipid levels, including triglycerides, total cholesterol, and HDL.
Colorectal Neoplasia Ascertainment
The findings of follow-up colonoscopies, including adenoma characteristics such as size, location, histology, and number of adenomas, were abstracted from the medical record and pathology report(s) for each participant. The primary endpoint of our analysis, metachronous neoplasia, is defined as detection of one or more colorectal adenoma or a colorectal cancer occurrence ≥6 months after baseline. Neoplasia were classified as advanced if ≥1 cm in size and/or with tubulovillous or villous histology or adenocarcinoma. Occurrence of more than one neoplasm during the follow-up period was classified as multiple. Distal neoplasia was defined as anatomic location in the distal colorectum that extends from the rectum through the splenic flexure, whereas proximal location was defined as that proximal to the splenic flexure.
Assessment of Other Variables
Self-administered questionnaires were used to obtain data on sociodemographic variables, family history of colorectal cancer in a first-degree relative, history of polyps before the qualifying colonoscopy, diabetes, aspirin use, and cigarette smoking. Dietary intake was assessed using the Arizona Food Frequency Questionnaire, a self-administered, semiquantitative food frequency questionnaire that asks about usual dietary intake in the previous year. Details regarding the development of the Arizona Food Frequency Questionnaire and assessment of validity and reliability have been reported (40, 41).
Analysis
We assessed the main effects of MetS components as well as the composite MetS derived variable on metachronous neoplasia detection using unconditional logistic regression. When evaluating MetS components, we initially used the risk factor cutpoints prescribed in the ATP III definition of MetS: waist circumference >40 inches for men and >35 inches for women, triglycerides ≥150 mg/dL, HDL cholesterol <40 mg/dL for men and <50 mg/dL for women, blood pressure ≥130/≥85 mm Hg, and fasting glucose ≥100 mg/dL. MetS is defined as the presence of three or more of the five risk factors. We then categorized the component exposures into quartiles based on sex-specific distributions for evaluation of dose-response patterns. Multinomial logistic regression was used to model advanced and nonadvanced metachronous neoplasia outcomes and multiple and single metachronous neoplasia outcomes. We also evaluated the effects of MetS components on distal and proximal metachronous neoplasia.
All analyses were conducted separately for men and women. All models were adjusted for age and study. We considered potential confounders, including smoking status, family history of colorectal cancer, history of colorectal polyps, aspirin use, energy intake, fiber, calcium, red meat, and alcohol consumption. None of the additional covariates generated a substantial change in the odds ratios (OR) for the main effects.
To evaluate whether diabetics in our study population influenced our results, we excluded 213 self-reported diabetics as well as 35 participants with missing diabetic status and ran all models again. No substantial differences were seen in the ORs (data not shown); thus, all participants were retained in the final models. We evaluated whether the treatment assignment from the trials affected our results by running all models for only the placebo arm of the UDCA trial and the low-fiber arm of the Wheat Bran Fiber trial. Although we saw some fluctuation in the effect estimates, the interpretations remained the same (data not shown). Study heterogeneity was evaluated by comparing study-specific ORs for each exposure and any metachronous neoplasia outcome. The magnitude of effect was generally similar or moderately stronger for the UDCA sample compared with the Wheat Bran Fiber sample. We also created a study–by–exposure interaction variable and tested for heterogeneity using the likelihood ratio test. None of the effects were significantly modified by study (data not shown).
The components of MetS are often described as a set of clustered or correlated risk factors with potential joint effects as a justification for the MetS diagnosis. We evaluated the correlation between each of the components of MetS and explored the possibility of an interaction between waist circumference and glucose using the dichotomous cutpoints from the ATP III definition with the likelihood ratio test. All statistical analyses were conducted using SAS software version 9.1 (SAS Institute).
Results
Of the 1,609 men and 783 women available for analysis, 672 (41.8%) men and 277 (35.4%) women met at least three criteria for MetS classification. During follow-up, metachronous neoplasia was found in 800 (49.7%) men and 305 (39.0%) women, which included 14 cancers in men and 1 cancer in women.
Baseline characteristics of the study population by the composite MetS classification are presented in Table 1 separately for men and women. No differences between the groups were seen for age, race, or education. Men and women classified with MetS reported more energy consumption and other dietary nutrients than participants not classified with MetS. Alcohol consumption was slightly lower among participants with MetS. No differences in smoking status among men were observed, although women with MetS were more often former smokers and less often current smokers. As expected, participants with MetS presented with a higher mean BMI and a higher mean waist-to-hip ratio and were more likely to report diabetes. For men, there was more use of aspirin in the presence of MetS, although no difference in aspirin use was observed for women. Differences in self-reported history of colorectal polyps and family history of colorectal cancer were small. Because all participants qualified for the trials by presenting with at least one adenoma, we also show baseline adenoma characteristics in Table 1 as a cross-sectional evaluation of the association between MetS and clinical adenoma features. No differences in adenoma size or number of adenomas were found at the start of the trials. However, more proximal adenomas were observed for both men and women with MetS, with more villous histology apparent among women with MetS.
Table 1.
Baseline characteristics of study population by MetS classification
| Characteristics* | MetS |
|||
|---|---|---|---|---|
| Men (n = 1,609) |
Women (n = 783) |
|||
| No (n= 937) | Yes (n = 672) | No (n = 506) | Yes (n = 277) | |
| Demographics | ||||
| Age, y | 66.1 ± 8.9 | 65.8 ± 8.2 | 65.1 ± 9.4 | 66.7 ± 7.8 |
| White (ethnicity/race)† | 894 (95.8) | 624 (94.4) | 482 (95.8) | 261 (94.6) |
| Education, y† | 14.1 ± 2.5 | 13.9 ± 2.3 | 13.4 ± 2.2 | 13.0 ± 2.1 |
| Dietary intake | ||||
| Energy, kcal/d | 2,095.6 ± 749.4 | 2,209.9 ± 773.2 | 1,552.6 ± 577.0 | 1,593.2 ± 614.7 |
| Protein, g/d | 76.9 ± 28.2 | 82.6 ± 29.2 | 58.4 ± 21.0 | 62.4 ± 27.6 |
| Carbohydrates, g/d | 281.9 ± 111.5 | 300.1 ± 119.0 | 222.7 ± 91.8 | 223.2 ± 94.1 |
| Total dietary fiber, g/d | 22.9 ± 10.1 | 24.1 ± 10.8 | 19.2 ± 8.9 | 19.7 ± 10.5 |
| Total fat, g/d | 71.2 ± 31.4 | 74.8 ± 31.9 | 50.2 ± 24.2 | 53.3 ± 25.8 |
| Calcium, mg/day | 968.8 ± 443.3 | 1,038.0 ± 446.7 | 834.7 ± 399.5 | 876.5 ± 461.2 |
| Red meat, g/d | 61.8 ± 41.7 | 67.4 ± 44.5 | 33.6 ± 24.3 | 42.6 ± 34.7 |
| Alcohol, g/d | 10.6 ± 18.0 | 9.1 ± 16.2 | 3.6 ± 6.3 | 2.8 ± 5.5 |
| Nondietary factors | ||||
| Smoking status† | ||||
| Never | 237 (25.5) | 171 (25.8) | 240 (47.7) | 110 (47.4) |
| Former | 578 (62.3) | 415 (62.5) | 175 (34.8) | 109 (39.9) |
| Current | 113 (12.2) | 78 (11.7) | 88 (17.5) | 34 (12.5) |
| BMI† | 26.5 ± 3.4 | 30.3 ± 4.2 | 25.2 ± 4.3 | 30.6 ± 5.4 |
| Waist-to-hip ratio | 0.93 ± 0.05 | 0.97 ± 0.05 | 0.79 ± 0.08 | 0.86 ± 0.07 |
| Diabetes (self-reported)† | 50 (5.4) | 107 (16.3) | 10 (2.0) | 46 (16.7) |
| Aspirin use‡ | 283 (30.2) | 230 (34.2) | 102 (20.2) | 53 (19.1) |
| Previous polyps†§ | 372 (43.0) | 281 (45.5) | 178 (39.3) | 101 (40.2) |
| Family history of colorectal cancer†|| | 186 (20.7) | 138 (21.2) | 122 (25.3) | 70 (26.2) |
| Adenoma characteristics | ||||
| Size of largest adenoma, mm† | 8.5 ± 5.7 | 8.3 ± 5.3 | 8.7 ± 6.4 | 9.1 ± 5.8 |
| No. adenomas† | 1.8 ± 1.3 | 1.8 ± 1.6 | 1.5 ± 1.1 | 1.7 ± 1.0 |
| Proximal adenomas† | 457 (50.6) | 379 (57.8) | 213 (44.1) | 135 (50.2) |
| Tubulovillous/villous† | 186 (19.9) | 141 (21.0) | 107 (21.2) | 71 (25.6) |
Count and percentage for categorical variables; mean ± SD for continuous variables.
Participants with missing data: education 21, BMI 18, size of largest adenoma 1, number of adenomas 5, White 19, smoking status 24, diabetes 35, previous polyps 205, family history of colorectal cancer 93, proximal adenomas 81, and tubulovillous/villous histology 2.
Regular use of aspirin in the previous month.
History of previous polyps before qualifying colonoscopy.
History of colorectal cancer in parent or sibling.
Using the cutpoints prescribed by the ATP III definition and detailed in Materials and Methods, the estimates of the main effects of the components of MetS and the composite MetS classification for any metachronous adenoma and associated outcomes are presented in Table 2. The odds of any metachronous neoplasia were 1.37 [95% confidence interval (CI 1.01–1.85)] times higher in women classified with MetS compared with women not classified with MetS. Similar estimates were found for nonadvanced neoplasia and multiple neoplasia outcomes among women. In contrast, there was no association observed among men between MetS and any metachronous neoplasia (OR, 0.99; 95% CI, 0.81–1.21) or other outcome measures.
Table 2.
Association between ATP III clinical identification of the MetS and metachronous adenomas
| Any metachronous neoplasia(men n = 800 and women n = 305) |
Nonadvanced neoplasia(men n = 563 and women n = 207) |
Advanced neoplasia(men n = 236 and women n = 97) |
Single metachronous neoplasia(men n = 382 and women n = 163) |
Multiple metachronous neoplasia(men n = 418 and women n = 142) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | OR* (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | |
| Men (n = 1,609) | ||||||||||
| Waist circumference | ||||||||||
| Low‡ | 46.7 | 1.00 | 32.9 | 1.00 | 13.8 | 1.00 | 23.2 | 1.00 | 23.5 | 1.00 |
| High | 54.2 | 1.41 (1.15–1.72) | 38.1 | 1.41 (1.13–1.76) | 16.1 | 1.41 (1.05–1.90) | 24.5 | 1.25 (0.97–1.60) | 29.6 | 1.58 (1.23–2.02) |
| Triglycerides (mg/dL) | ||||||||||
| <150 | 50.6 | 1.00 | 35.0 | 1.00 | 15.6 | 1.00 | 24.1 | 1.00 | 26.5 | 1.00 |
| ≥150 | 48.5 | 0.94 (0.77–1.15) | 35.0 | 0.97 (0.78–1.20) | 13.4 | 0.87 (0.64–1.17) | 23.2 | 0.93 (0.73–1.19) | 25.3 | 0.95 (0.75–1.21) |
| HDL | ||||||||||
| High§ | 50.7 | 1.00 | 35.4 | 1.00 | 15.2 | 1.00 | 24.1 | 1.00 | 26.6 | 1.00 |
| Low | 47.5 | 0.92 (0.74–1.14) | 34.0 | 0.94 (0.74–1.19) | 13.4 | 0.87 (0.63–1.21) | 22.9 | 0.91 (0.69–1.19) | 24.6 | 0.93 (0.71–1.21) |
| Blood pressure (mm Hg) | ||||||||||
| <130/<85 | 49.6 | 1.00 | 33.9 | 1.00 | 15.7 | 1.00 | 24.5 | 1.00 | 25.1 | 1.00 |
| ≥130/≥ 85 | 49.8 | 0.95 (0.77–1.16) | 35.8 | 1.01 (0.81–1.27) | 13.9 | 0.79 (0.59–1.07) | 23.2 | 0.92 (0.72–1.18) | 26.6 | 0.98 (0.76–1.25) |
| Fasting glucose (mg/dL) | ||||||||||
| <100 | 49.6 | 1.00 | 34.7 | 1.00 | 14.9 | 1.00 | 25.1 | 1.00 | 24.5 | 1.00 |
| ≥100 | 49.8 | 1.01 (0.82–1.23) | 35.2 | 1.03 (0.82–1.28) | 14.5 | 0.96 (0.71–1.29) | 22.8 | 0.91 (0.71–1.16) | 27.0 | 1.12 (0.87–1.43) |
| MetS|| | ||||||||||
| 0–2 criteria | 50.9 | 1.00 | 35.0 | 1.00 | 15.2 | 1.00 | 24.6 | 1.00 | 25.6 | 1.00 |
| 3–5 criteria | 49.1 | 0.99 (0.81–1.21) | 35.0 | 1.01 (0.81–1.26) | 14.0 | 0.93 (0.69–1.25) | 22.6 | 0.91 (0.71–1.17) | 26.5 | 1.07 (0.84–1.37) |
| Women (n = 783) | ||||||||||
| Waist circumference | ||||||||||
| Low‡ | 36.0 | 1.00 | 22.5 | 1.00 | 13.5 | 1.00 | 20.5 | 1.00 | 15.5 | 1.00 |
| High | 44.1 | 1.41 (1.05–1.90) | 33.3 | 1.72 (1.23–2.41) | 10.5 | 0.89 (0.55–1.42) | 21.3 | 1.20 (0.83–1.74) | 22.7 | 1.72 (1.17–2.53) |
| Triglycerides (mg/dL) | ||||||||||
| <150 | 37.3 | 1.00 | 23.9 | 1.00 | 13.3 | 1.00 | 20.8 | 1.00 | 16.5 | 1.00 |
| ≥150 | 41.2 | 1.14 (0.85–1.53) | 29.8 | 1.28 (0.92–1.78) | 11.2 | 0.88 (0.56–1.38) | 20.9 | 1.05 (0.73–1.51) | 20.3 | 1.27 (0.87–1.86) |
| HDL | ||||||||||
| High§ | 38.1 | 1.00 | 24.9 | 1.00 | 13.1 | 1.00 | 20.8 | 1.00 | 17.4 | 1.00 |
| Low | 41.1 | 1.13 (0.82–1.55) | 30.4 | 1.28 (0.89–1.82) | 10.7 | 0.86 (0.52–1.42) | 21.0 | 1.06 (0.71–1.57) | 20.1 | 1.21 (0.80–1.83) |
| Blood pressure (mm Hg) | ||||||||||
| <130/<85 | 36.1 | 1.00 | 23.7 | 1.00 | 12.4 | 1.00 | 20.9 | 1.00 | 15.2 | 1.00 |
| ≥130/≥ 85 | 41.4 | 1.13 (0.83–1.52) | 28.9 | 1.16 (0.82–1.63) | 12.4 | 1.04 (0.66–1.63) | 20.7 | 1.02 (0.71–1.48) | 20.7 | 1.27 (0.86–1.89) |
| Fasting glucose (mg/dL) | ||||||||||
| <100 | 35.0 | 1.00 | 23.4 | 1.00 | 11.5 | 1.00 | 18.9 | 1.00 | 16.1 | 1.00 |
| ≥100 | 45.0 | 1.46 (1.09–1.96) | 31.2 | 1.51 (1.08–2.11) | 13.8 | 1.39 (0.89–2.17) | 23.8 | 1.49 (1.04–2.14) | 21.2 | 1.44 (0.98–2.11) |
| MetS|| | ||||||||||
| 0–2 criteria | 36.0 | 1.00 | 23.3 | 1.00 | 12.7 | 1.00 | 20.0 | 1.00 | 16.0 | 1.00 |
| 3–5 criteria | 44.4 | 1.37 (1.01–1.85) | 32.3 | 1.52 (1.08–2.13) | 12.0 | 1.06 (0.67–1.69) | 22.4 | 1.27 (0.88–1.84) | 22.0 | 1.50 (1.02–2.21) |
OR from logistic regression adjusted for age and study.
OR from multinomial logistic regression adjusted for age and study.
Waist circumference classification for men: low V40 inches, high ≤40 inches; women: low ≤35 inches, high >35 inches.
HDL cholesterol classification for men: high HDL ≥40 mg/dL, low HDL <40 mg/dL; women: high HDL ≥50 mg/dL, low HDL <50 mg/dL.
ATP III clinical identification of the MetS.
Men with a waist circumference >40 inches had an increased odds of metachronous neoplasia (OR, 1.41; 95% CI, 1.15–1.72) as did women with a waist circumference >35 inches (OR, 1.41; 95% CI, 1.05–1.90). These estimates were similar or modestly stronger for nonadvanced neoplasia and multiple neoplasia outcomes for men and women and similar for men with an advanced neoplasia outcome. Women with a fasting glucose ≥ 100 mg/dL exhibited an increased odds of metachronous neoplasia (OR, 1.46; 95% CI, 1.09–1.96), with similar estimates for nonadvanced, advanced, single, and multiple neoplasia. No association for elevated glucose was found for men (OR, 1.01; 95% CI, 0.82–1.23) with metachronous neoplasia or any other outcome. No evidence was found for an association between triglycerides, HDL cholesterol, or blood pressure with metachronous neoplasia, in either men or women, when these continuous traits were dichotomized using the ATP III cutpoints.
Further exploration of the component traits of MetS are presented for men (Table 3) and women (Table 4), with sex-specific quartile categorizations. The odds of metachronous neoplasia increased monotonically across the quartiles of waist circumference for men; compared with the first quartile of waist circumference, the ORs (95% CI) were 1.13 (0.85–1.50), 1.36 (1.03–1.80), and 1.61 (1.21–2.14) for the second, third, and fourth quartiles, respectively (Table 3). Similarly suggestive patterns were seen for nonadvanced, advanced, single, and multiple neoplasia outcomes in men. Weak, nonsignificant increased odds in the fourth quartile of waist circumference for metachronous neoplasia and multiple neoplasia were observed among women (Table 4). Women in the fourth quartile of waist circumference showed a 1.64 (95% CI, 1.03–2.61) increase in odds for nonadvanced neoplasia compared with women in the first quartile.
Table 3.
Association between MetS components in quartiles and metachronous neoplasia in men (n = 1,609)
| Any metachronous neoplasia (n = 800) |
Nonadvanced neoplasia (n = 563) |
Advanced neoplasia (n = 236) |
Single metachronous neoplasia (n = 382) |
Multiple metachronous neoplasia (n = 418) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | OR* (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | |
| Waist circumference (inches) | ||||||||||
| 34.6 ± 1.6 | 44.7 | 1.00 | 31.1 | 1.00 | 13.6 | 1.00 | 20.5 | 1.00 | 24.2 | 1.00 |
| 37.8 ± 0.7 | 47.4 | 1.13 (0.85–1.50) | 34.8 | 1.19 (0.88–1.62) | 12.5 | 0.97 (0.63–1.49) | 24.9 | 1.28 (0.91–1.81) | 22.4 | 1.00 (0.70–1.42) |
| 40.5 ± 0.9 | 52.4 | 1.36 (1.03–1.80) | 35.1 | 1.33 (0.98–1.81) | 17.2 | 1.44 (0.96–2.15) | 24.5 | 1.38 (0.98–1.96) | 27.8 | 1.35 (0.97–1.89) |
| 45.4 ± 2.9 | 54.6 | 1.61 (1.21–2.14) | 39.3 | 1.67 (1.22–2.28) | 15.3 | 1.48 (0.97–2.26) | 25.1 | 1.53 (1.07–2.19) | 29.6 | 1.69 (1.20–2.39) |
| Triglycerides (mg/dL) | ||||||||||
| 75.1 ± 14.8 | 48.2 | 1.00 | 32.4 | 1.00 | 15.7 | 1.00 | 21.4 | 1.00 | 26.8 | 1.00 |
| 114.8 ± 11.3 | 51.3 | 1.12 (0.84–1.48) | 36.9 | 1.19 (0.87–1.62) | 14.3 | 0.96 (0.64–1.45) | 26.1 | 1.29 (0.91–1.83) | 25.1 | 0.97 (0.69–1.36) |
| 162.8 ± 17.8 | 49.9 | 1.06 (0.80–1.40) | 34.7 | 1.08 (0.79–1.47) | 15.1 | 1.01 (0.67–1.51) | 24.9 | 1.20 (0.85–1.70) | 24.9 | 0.95 (0.67–1.33) |
| 296.6 ± 108.1 | 49.6 | 1.08 (0.82–1.43) | 36.1 | 1.15 (0.85–1.57) | 13.5 | 0.94 (0.62–1.42) | 22.6 | 1.10 (0.77–1.56) | 27.1 | 1.08 (0.77–1.51) |
| HDL (mg/dL) | ||||||||||
| 33.6 ± 3.9 | 47.8 | 1.00 | 34.7 | 1.00 | 13.1 | 1.00 | 23.7 | 1.00 | 24.1 | 1.00 |
| 41.9 ± 1.9 | 49.1 | 1.06 (0.80–1.40) | 33.9 | 1.01 (0.74–1.37) | 15.0 | 1.18 (0.77–1.79) | 24.7 | 1.07 (0.76–1.50) | 24.4 | 1.05 (0.74–1.48) |
| 49.1 ± 2.3 | 51.3 | 1.12 (0.85–1.47) | 37.0 | 1.11 (0.82–1.50) | 14.3 | 1.13 (0.74–1.72) | 23.2 | 1.04 (0.73–1.46) | 28.1 | 1.20 (0.85–1.68) |
| 64.9 ± 11.6 | 50.9 | 1.06 (0.81–1.40) | 34.5 | 1.00 (0.74–1.35) | 16.4 | 1.25 (0.83–1.87) | 23.5 | 1.03 (0.73–1.44) | 27.4 | 1.10 (0.79–1.54) |
| Systolic blood pressure (mm Hg) | ||||||||||
| 109.7 ± 6.2 | 47.5 | 1.00 | 32.6 | 1.00 | 14.8 | 1.00 | 25.5 | 1.00 | 22.0 | 1.00 |
| 122.9 ± 2.9 | 49.7 | 1.06 (0.79–1.41) | 34.3 | 1.07 (0.77–1.47) | 15.4 | 1.03 (0.67–1.57) | 23.0 | 0.93 (0.65–1.32) | 26.8 | 1.22 (0.85–1.75) |
| 134.9 ± 4.2 | 49.4 | 1.00 (0.75–1.31) | 34.7 | 1.04 (0.77–1.41) | 14.6 | 0.88 (0.59–1.33) | 20.9 | 0.82 (0.58–1.16) | 28.4 | 1.20 (0.86–1.69) |
| 154.7 ± 10.4 | 52.3 | 1.06 (0.79–1.42) | 38.3 | 1.17 (0.85–1.62) | 14.0 | 0.82 (0.53–1.26) | 26.1 | 1.06 (0.75–1.51) | 26.1 | 1.07 (0.75–1.54) |
| Diastolic blood pressure (mm Hg) | ||||||||||
| 65.9 ± 5.1 | 47.7 | 1.00 | 33.2 | 1.00 | 14.6 | 1.00 | 23.5 | 1.00 | 24.3 | 1.00 |
| 75.5 ± 2.2 | 52.0 | 1.30 (0.96–1.78) | 38.1 | 1.37 (0.98–1.92) | 13.9 | 1.16 (0.73–1.84) | 23.5 | 1.14 (0.77–1.67) | 28.5 | 1.48 (1.02–2.16) |
| 81.2 ± 1.6 | 50.7 | 1.26 (0.96–1.65) | 35.4 | 1.24 (0.92–1.68) | 15.2 | 1.28 (0.86–1.91) | 24.4 | 1.16 (0.83–1.61) | 26.3 | 1.37 (0.99–1.91) |
| 91.8 ± 5.9 | 48.5 | 1.19 (0.89–1.60) | 33.8 | 1.18 (0.85–1.62) | 14.7 | 1.25 (0.81–1.92) | 23.2 | 1.07 (0.75–1.53) | 25.3 | 1.33 (0.93–1.91) |
| Fasting glucose (mg/dL) | ||||||||||
| 89.8 ± 5.8 | 47.6 | 1.00 | 32.7 | 1.00 | 14.9 | 1.00 | 23.2 | 1.00 | 24.4 | 1.00 |
| 98.9 ± 1.9 | 49.4 | 1.11 (0.85–1.46) | 34.9 | 1.15 (0.85–1.55) | 14.5 | 1.03 (0.69–1.55) | 24.1 | 1.09 (0.78–1.52) | 25.3 | 1.13 (0.81–1.58) |
| 106.6 ± 2.7 | 48.3 | 1.04 (0.78–1.37) | 34.2 | 1.08 (0.79–1.48) | 14.0 | 0.94 (0.62–1.43) | 22.6 | 0.99 (0.70–1.41) | 25.6 | 1.08 (0.76–1.53) |
| 140.0 ± 37.9 | 53.7 | 1.28 (0.97–1.69) | 38.4 | 1.34 (0.99–1.83) | 15.2 | 1.12 (0.74–1.69) | 24.9 | 1.21 (0.86–1.71) | 28.8 | 1.35 (0.96–1.89) |
OR from logistic regression adjusted for age and study.
OR from multinomial logistic regression adjusted for age and study.
Table 4.
Association between MetS components in quartiles and metachronous neoplasia in women (n = 783)
| Any metachronous neoplasia (n = 305) |
Nonadvanced neoplasia (n = 207) |
Advanced neoplasia (n = 97) |
Single metachronous neoplasia (n = 163) |
Multiple metachronous neoplasia (n = 142) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | OR* (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | % | OR† (95% CI) | |
| Waist circumference (inches) | ||||||||||
| 27.8 ± 1.5 | 37.9 | 1.00 | 23.2 | 1.00 | 14.7 | 1.00 | 20.2 | 1.00 | 17.7 | 1.00 |
| 31.7 ± 0.9 | 35.1 | 0.87 (0.58–1.32) | 22.7 | 0.91 (0.56–1.49) | 12.4 | 0.81 (0.45–1.47) | 20.1 | 0.93 (0.56–1.54) | 14.9 | 0.81 (0.46–1.42) |
| 35.0 ± 1.2 | 38.8 | 1.02 (0.68–1.54) | 26.5 | 1.14 (0.71–1.83) | 12.2 | 0.84 (0.46–1.53) | 21.4 | 1.07 (0.64–1.76) | 17.4 | 0.98 (0.57–1.68) |
| 40.9 ± 3.4 | 44.1 | 1.32 (0.88–1.98) | 33.5 | 1.64 (1.03–2.61) | 10.3 | 0.79 (0.42–1.47) | 21.5 | 1.18 (0.71–1.96) | 22.6 | 1.51 (0.89–2.54) |
| Triglycerides (mg/dL) | ||||||||||
| 78.5 ± 14.9 | 38.0 | 1.00 | 24.0 | 1.00 | 14.0 | 1.00 | 20.0 | 1.00 | 18.0 | 1.00 |
| 118.8 ± 10.4 | 34.4 | 0.83 (0.55–1.26) | 24.1 | 0.92 (0.57–1.47) | 10.3 | 0.68 (0.37–1.28) | 20.0 | 0.92 (0.56–1.53) | 14.5 | 0.72 (0.41–1.27) |
| 164.6 ± 17.2 | 45.4 | 1.30 (0.87–1.95) | 30.1 | 1.34 (0.84–2.14) | 15.0 | 1.19 (0.67–2.14) | 23.7 | 1.30 (0.79–2.15) | 21.7 | 1.32 (0.78–2.22) |
| 283.2 ± 97.2 | 38.1 | 0.95 (0.63–1.43) | 27.8 | 1.09 (0.68–1.74) | 10.3 | 0.72 (0.38–1.35) | 19.6 | 0.95 (0.57–1.59) | 18.6 | 0.96 (0.56–1.63) |
| HDL (mg/dL) | ||||||||||
| 41.9 ± 5.6 | 41.4 | 1.00 | 30.5 | 1.00 | 10.9 | 1.00 | 20.9 | 1.00 | 20.5 | 1.00 |
| 54.0 ± 3.1 | 37.4 | 0.88 (0.59–1.32) | 23.0 | 0.74 (0.46–1.18) | 14.4 | 1.27 (0.69–2.34) | 19.3 | 0.87 (0.52–1.44) | 18.2 | 0.90 (0.53–1.52) |
| 65.1 ± 3.5 | 36.7 | 0.83 (0.56–1.25) | 23.1 | 0.71 (0.45–1.12) | 13.3 | 1.13 (0.61–2.09) | 19.9 | 0.88 (0.53–1.45) | 16.8 | 0.78 (0.46–1.32) |
| 83.9 ± 11.3 | 40.0 | 0.91 (0.61–1.36) | 28.9 | 0.89 (0.57–1.39) | 11.1 | 0.96 (0.51–1.84) | 23.2 | 1.07 (0.66–1.75) | 16.8 | 0.75 (0.44–1.27) |
| Systolic blood pressure (mm Hg) | ||||||||||
| 107.9 ± 7.0 | 33.8 | 1.00 | 20.8 | 1.00 | 13.0 | 1.00 | 18.4 | 1.00 | 15.5 | 1.00 |
| 123.0 ± 2.9 | 39.3 | 1.14 (0.74–1.76) | 27.4 | 1.27 (0.77–2.09) | 11.9 | 0.94 (0.50–1.80) | 25.0 | 1.42 (0.85–2.39) | 14.3 | 0.85 (0.47–1.56) |
| 135.2 ± 4.5 | 38.1 | 1.06 (0.70–1.60) | 25.2 | 1.10 (0.68–1.77) | 12.6 | 0.97 (0.53–1.76) | 19.7 | 1.08 (0.65–1.81) | 18.4 | 1.04 (0.61–1.78) |
| 156.2 ± 12.4 | 45.4 | 1.37 (0.89–2.10) | 33.5 | 1.58 (0.97–2.57) | 11.9 | 1.01 (0.53–1.94) | 21.1 | 1.30 (0.75–2.23) | 24.3 | 1.44 (0.83–2.50) |
| Diastolic blood pressure (mm Hg) | ||||||||||
| 65.8 ± 4.7 | 36.0 | 1.00 | 23.0 | 1.00 | 13.0 | 1.00 | 20.1 | 1.00 | 15.9 | 1.00 |
| 75.2 ± 2.3 | 42.5 | 1.31 (0.87–1.97) | 25.8 | 1.25 (0.77–2.01) | 16.8 | 1.43 (0.81–2.54) | 22.8 | 1.26 (0.77–2.07) | 19.8 | 1.39 (0.81–2.39) |
| 80.3 ± 0.7 | 39.4 | 1.21 (0.80–1.83) | 29.1 | 1.42 (0.89–2.28) | 10.3 | 0.86 (0.45–1.63) | 21.8 | 1.17 (0.71–1.93) | 17.6 | 1.29 (0.74–2.24) |
| 90.1 ± 6.1 | 39.2 | 1.16 (0.79–1.70) | 28.9 | 1.35 (0.87–2.09) | 10.0 | 0.80 (0.44–1.46) | 19.3 | 1.02 (0.63–1.65) | 19.8 | 1.35 (0.81–2.24) |
| Fasting glucose (mg/dL) | ||||||||||
| 85.7 ± 4.6 | 29.9 | 1.00 | 19.0 | 1.00 | 10.9 | 1.00 | 16.7 | 1.00 | 13.1 | 1.00 |
| 94.0 ± 2.1 | 37.4 | 1.33 (0.88–2.00) | 26.7 | 1.46 (0.91–2.35) | 10.4 | 1.04 (0.55–1.97) | 21.2 | 1.39 (0.84–2.30) | 16.3 | 1.27 (0.73–2.22) |
| 100.8 ± 1.9 | 47.3 | 1.97 (1.30–3.01) | 33.1 | 2.14 (1.32–3.47) | 14.2 | 1.68 (0.90–3.15) | 23.7 | 1.87 (1.11–3.14) | 23.7 | 2.13 (1.22–3.70) |
| 129.5 ± 36.0 | 43.7 | 1.70 (1.13–2.56) | 29.0 | 1.74 (1.08–2.81) | 14.7 | 1.63 (0.89–2.99) | 22.6 | 1.65 (0.99–2.75) | 21.1 | 1.78 (1.03–3.07) |
OR from logistic regression adjusted for age and study.
OR from multinomial logistic regression adjusted for age and study.
Among men, a weak increase in odds of metachronous neoplasia was observed in the fourth quartile of fasting glucose, and across the various outcomes, although none are statistically significant, whereas for women the odds of metachronous neoplasia increased significantly with higher fasting glucose levels. Compared with the first quartile of fasting glucose, the ORs (95% CI) were 1.33 (0.88–2.00), 1.97 (1.30–3.01), and 1.70 (1.13–2.56) for the second, third, and fourth quartiles, respectively. Similar evidence was seen for nonadvanced, advanced, single, and multiple neoplasia outcomes in women.
No significant associations were observed across the quartiles of systolic blood pressure or diastolic blood pressure in men or women; further, no evidence of an association was found across the quartiles of triglycerides or HDL cholesterol with metachronous neoplasia in either group.
We considered whether the association between components of MetS might vary by proximal versus distal metachronous neoplasia. All exposure variables were evaluated for an association with any proximal neoplasia and then for an association with any distal neoplasia. In general, the results were either similar to the results for any metachronous neoplasia and not appreciably different by location or subtly stronger for proximal neoplasia (data not shown). The most notable finding was a monotonic dose-response for fasting glucose and proximal metachronous neoplasia in women. Compared with the first quartile of glucose, the ORs (95% CI) for proximal lesions were 1.61 (1.02–2.55), 1.84 (1.15–2.95), and 2.03 (1.28–3.19) for the second, third, and fourth quartiles, respectively. The association between fasting glucose and distal metachronous neoplasia in women was weaker and not significant. Compared with the first quartile of glucose, the ORs (95% CI) for distal lesions were 0.88 (0.52–1.48), 1.60 (0.97–2.64), and 1.40 (0.86–2.29) for the second, third, and fourth quartiles, respectively. The location-specific results for fasting glucose in men mirrored the results for any metachronous neoplasia seen in Table 3 and did not differ appreciably between proximal and distal outcomes.
Table 5 shows Pearson correlation coefficients for the component traits of MetS, separately for men and women, to evaluate how these traits cluster and to explore potential reasons for differences by sex in the associations with colorectal neoplasia. We found that fasting glucose levels were more strongly correlated with other components of MetS among women than among men. Most notably, waist circumference and glucose had a correlation coefficient of 0.16 among men, whereas the correlation was 0.32 among women.
Table 5.
Correlation of MetS components
| Waist circumference | Triglycerides | HDL cholesterol | Systolic blood pressure | Diastolic blood pressure | Glucose | MetS | |
|---|---|---|---|---|---|---|---|
| Men (n = 1,609) | |||||||
| Waist circumference | 1.00 | ||||||
| Triglycerides | 0.20 | 1.00 | |||||
| HDL cholesterol | −0.28 | −0.43 | 1.00 | ||||
| Systolic blood pressure | 0.15 | 0.08 | −0.03 | 1.00 | |||
| Diastolic blood pressure | 0.22 | 0.11 | −0.05 | 0.53 | 1.00 | ||
| Glucose | 0.16 | 0.15 | −0.16 | 0.11 | −0.05 | 1.00 | |
| MetS | 0.47 | 0.46 | −0.45 | 0.33 | 0.26 | 0.29 | 1.00 |
| Women (n = 783) | |||||||
| Waist circumference | 1.00 | ||||||
| Triglycerides | 0.36 | 1.00 | |||||
| HDL cholesterol | −0.33 | −0.44 | 1.00 | ||||
| Systolic blood pressure | 0.20 | 0.16 | −0.06 | 1.00 | |||
| Diastolic blood pressure | 0.24 | 0.15 | −0.11 | 0.55 | 1.00 | ||
| Glucose | 0.32 | 0.23 | −0.23 | 0.16 | 0.09 | 1.00 | |
| MetS | 0.57 | 0.50 | −0.45 | 0.34 | 0.26 | 0.41 | 1.00 |
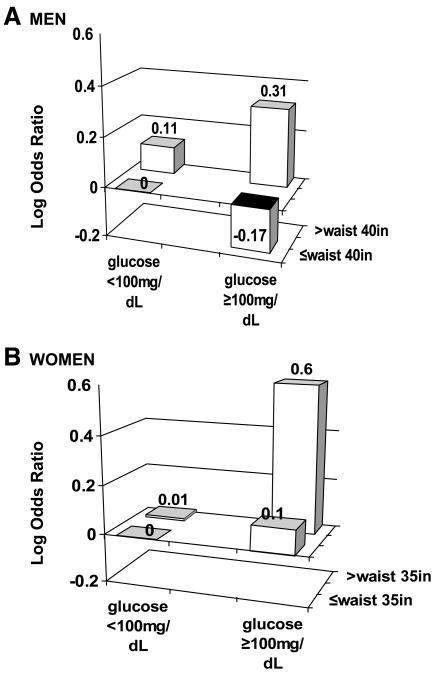
To better understand the potential joint effect between waist circumference and glucose, we conducted an exploratory analysis to evaluate the possibility of an interaction between waist circumference and fasting glucose levels using the dichotomous cutpoints from the ATP III definition (Fig. 1). When compared with men with a small waist circumference and low fasting glucose, men with a large waist circumference and low fasting glucose did not experience a substantial increase in odds of metachronous neoplasia (OR, 1.12; 95% CI, 0.81–1.56) nor did men with a small waist circumference and high fasting glucose (OR, 0.84; 95% CI, 0.65–1.08). In contrast, men with a large waist circumference and high fasting glucose experienced a modest but significant increase in odds of metachronous neoplasia (OR, 1.36; 95% CI, 1.04–1.78); the likelihood ratio test for interaction between waist circumference and fasting glucose among men produced P = 0.08. Similarly, compared with women with a small waist circumference and low fasting glucose, women with a large waist circumference and low fasting glucose experienced no increase in odds of metachronous neoplasia (OR, 1.01; 95% CI, 0.65–1.57), nor was there an increase in odds for women with a small waist circumference and high fasting glucose (OR, 1.10; 95% CI, 0.73–1.65). Women with a large waist circumference and high fasting glucose experienced a significant increase in odds of metachronous neoplasia (OR, 1.83; 95% CI, 1.26–2.67); the likelihood ratio test for interaction between waist circumference and fasting glucose among women produced P = 0.12. We further explored this interaction between waist circumference and fasting glucose with other outcomes and found the effect modification was almost entirely consistent across the nonadvanced/advanced, single/multiple, and proximal versus distal outcomes, with the combined effect strongest among women (data not shown).
Figure 1.
A. Waist circumference and fasting glucose interaction and metachronous colorectal neoplasia among men. B. Waist circumference and fasting glucose interaction and metachronous colorectal neoplasia among women.
Discussion
The results from this prospective study showed that women who met the ATP III diagnostic criteria for MetS had significantly higher odds of developing metachronous colorectal neoplasia than women who did not meet the criteria. Among men, MetS was not associated with the development of metachronous neoplasia. For the individual component traits, elevated blood pressure, triglyceride levels, and HDL levels were not significantly associated with metachronous neoplasia in men or women. For women, elevated glucose and large waist circumference were independently positively associated with increased odds for metachronous neoplasia. For men, waist circumference was associated with increased odds of metachronous neoplasia, whereas elevated glucose was not. Further analyses of the individual components by sex-specific quartiles support an association between waist size and metachronous neoplasia in men and between glucose levels and metachronous neoplasia in women.
The differences between men and women may reflect the stronger correlation between waist circumference and glucose in women compared with men. Furthermore, Kim et al. reported that fasting plasma glucose is more strongly correlated with insulin-mediated glucose uptake (a more direct measure of insulin sensitivity and metabolic disturbance) among the obese than in normal-weight individuals (42); thus, fasting glucose may better capture insulin resistance among individuals with a large waist circumference. When we evaluated the interaction between waist circumference and fasting glucose levels, only the combination of large waist circumference and high fasting glucose was associated with a significant increase in odds of metachronous neoplasia in both men and women.
Although largely equivocal and somewhat limited, a differential effect of MetS and key component traits on the anatomic location of colorectal neoplasia, most principally a stronger association for proximal neoplasia, has been suggested (43, 44). The general patterns of association with metachronous lesions were similar for proximal and distal locations in our data. We did observe a stronger association between glucose levels and proximal lesions compared with distal lesions for women. For men, we observed no difference by anatomic location of metachronous adenoma and MetS or its components.
We observed no clear differences in the association between MetS or the components and features of adenomatous lesions recognized as more clinically significant (size, histology, and multiplicity). MetS in women and glucose and waist circumference as single and interacting traits in men and women were, in general, similarly associated across lesion type and multiplicity, with no evidence favoring a strong differential effect on the type or number of lesions.
Our results are consistent with the majority of studies conducted on the relation between MetS and any feature of MetS and incident colorectal neoplasia and support the evidence that adiposity as some measure (e.g., waist circumference or BMI) and dysregulation in glucose, possibly in combination with adiposity, act as risk enhancers for colorectal neoplasia. As an example, our results are similar to those of Stocks et al., who reported more than a doubling of odds for colorectal cancers with the presence of at least two of the following: elevated BMI, elevated blood pressure, and elevated fasting glucose, although they did not evaluate other component traits of MetS (45). In addition, our results are consistent with those reported by Flood et al., where insulin and glucose levels were associated with increased odds for metachronous adenoma among a nested subsample of individuals participating in the Polyp Prevention Trial (30).
Our sex-stratified analysis suggests that the combined effect of elevated glucose and large waist circumference is likely similar for men and women, although it may differ in magnitude, with the effect more pronounced in women. These results are distinct from studies that used BMI as the sole measure of body size, where the association appears to be stronger among men, as found in a pooled analysis of seven prospective studies of metachronous colorectal neoplasia (22).
The consistent association between colorectal neoplasia risk and biomarkers of glucose and insulin, as opposed to lipid metabolism, supports metabolic disturbances more aligned with insulin resistance, although the exact biologic mechanism that explains the association is unknown (23, 46). A direct role for insulin is supported by early animal studies of Tran et al., where direct insulin exposure, and not other metabolic disturbances, was associated with higher tumor burden in azoxymethane-treated rats (47). More recently, Ealey et al. (48) found that the tumor-promoting activity of insulin in mice exposed to azoxymethane was absent in animals deficient for insulin-like growth factor-I (IGF-I), results that suggest that the tumor-promoting effect of insulin may depend on the availability of IGF-I. IGF-I is a potent promitogen in the colonocyte for which substantial evidence supports a tumor-promoting role in human colorectal cancers (49). Paradoxically, in a recent study published by our group, IGF-I levels in a substudy of 299 men were found to be inversely related to odds of metachronous adenoma (50), results that were corroborated by Flood et al. (51). Additional work is needed to clarify the joint effects of insulin and IGF-I and their relation to degree and type of adiposity in the risk for metachronous adenoma.
Our data do not support a simple relationship wherein measures of body size act as simple surrogates for elevated glucose; the correlation between waist circumference and fasting glucose was not strong, and differed by sex, with a stronger correlation among women. Elevated glucose without large waist circumference was not associated with increased odds for metachronous neoplasia. It is only in those individuals, men and women, with both excess central adiposity and elevated glucose that we observed the enhanced odds of metachronous neoplasia. Our results likely reflect the inadequacy of the measures to capture impaired glucose uptake and the dependence of fasting plasma glucose on obesity for capturing impaired glucose uptake in an individual (42). The inability to directly assess impaired glucose uptake as a measure of insulin sensitivity is a limitation of this and other large population studies where oral glucose challenge testing is prohibitive. Inclusion of insulin and derivation of surrogates of insulin sensitivity such as the Homeostasis Model Assessment of Insulin Resistance might prove superior in estimating the underlying insulin resistance and should be considered.
Additional limitations of our study derive from the limited criteria that are used to define MetS, and the measured state of MetS as defined by the ATP III definition, as some portion of subjects may represent normal metabolic status as a result of pharmacologic control. Physical activity is a recognized protective factor in colorectal carcinogenesis (52) whose absence as a major determinant of glucose homeostasis in the definition of MetS may result in unmeasured confounding and possibly explain differences between men and women (53), although our population is largely sedentary in nature. Large waist and high adiposity are also marked by several systemic changes other than hyper-insulinemia that are not captured by the component traits of MetS, most notably altered patterns of adipocyte-derived cytokines and adipokines (54). Although the in vivo consequence of disturbances in the fat mass is unclear, protumorigenic roles for adipocyte-derived factors such as leptin in cell culture models of colon tumors are hypothesized as direct acting factors in tumorigenesis via interleukin-6 production and transinterleukin-6 signaling (55), a proinflammatory cytokine response pathway (54). These noninsulin effects may act alone or in combination with the tumor-promoting effects of insulin and IGF-I as determinants of colorectal adenoma development. Additional studies are needed to determine the joint or independent role of such factors in the biology of human colorectal neoplasia development.
Strengths of our study include the prospective nature of the data on metachronous adenoma, high follow-up rate, the large sample size for sex-specific analyses, the use of colonoscopy in all subjects, and clinical measures of the MetS components with the exception of waist circumference (56, 57), which was self-measured following instruction. The generalizability of our results is limited due to the specific nature of our population as postpolypectomy patients.
Our results offer additional evidence that fasting plasma glucose and large waist circumference increase odds of metachronous colorectal neoplasia, where the combination of these two measures may better capture insulin resistance. The strength of evidence accumulated to date warrants classification of metabolic factors related to glucose as risk factors for colorectal carcinogenesis. As such, future colorectal cancer prevention efforts might be rationally designed around the reduction of risk associated with impaired glucose uptake including use of pharmacologic agents adopted in the cardiovascular and diabetes prevention settings. With the high prevalence of obesity, sedentary behavior, and the aging of the U.S. population, insulin resistance identifies a common disease prevention target for behavioral and pharmacologic interventions to effectively increase the overall public health benefit.
Acknowledgments
Grant support: USPHS grants CA-41108, CA-23074, and CA-77145 and Specialized Program of Research Excellence in Gastrointestinal Cancer grant CA95060; National Cancer Institute Career Development Award 1K07CA10629-01A1 (E.T. Jacobs).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 2.Jass JR. Colorectal cancer: a multipathway disease. Crit Rev Oncog. 2006;12:273–87. doi: 10.1615/critrevoncog.v12.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 3.Strul H, Kariv R, Leshno M, et al. The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average-risk individuals aged 40–80 years. Am J Gastroenterol. 2006;101:255–62. doi: 10.1111/j.1572-0241.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med. 1993;328:901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 6.Yamaji Y, Mitsushima T, Ikuma H, et al. Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese. Gut. 2004;53:568–72. doi: 10.1136/gut.2003.026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 8.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2007;13:4199–206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 11.Neugut AI, Lee WC, Garbowski GC, et al. Obesity and colorectal adenomatous polyps. J Natl Cancer Inst. 1991;83:359–61. doi: 10.1093/jnci/83.5.359. [DOI] [PubMed] [Google Scholar]
- 12.Bayerdorffer E, Mannes GA, Ochsenkuhn T, Kopcke W, Wiebecke B, Paumgartner G. Increased risk of ‘high-risk’ colorectal adenomas in overweight men. Gastroenterology. 1993;104:137–44. doi: 10.1016/0016-5085(93)90845-4. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7:253–63. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 15.Bird CL, Frankl HD, Lee ER, Haile RW. Obesity, weight gain, large weight changes, and adenomatous polyps of the left colon and rectum. Am J Epidemiol. 1998;147:670–80. doi: 10.1093/oxfordjournals.aje.a009508. [DOI] [PubMed] [Google Scholar]
- 16.Kono S, Handa K, Hayabuchi H, et al. Obesity, weight gain and risk of colon adenomas in Japanese men. Jpn J Cancer Res. 1999;90:805–11. doi: 10.1111/j.1349-7006.1999.tb00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutron-Ruault MC, Senesse P, Meance S, Belghiti C, Faivre J. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr Cancer. 2001;39:50–7. doi: 10.1207/S15327914nc391_7. [DOI] [PubMed] [Google Scholar]
- 18.Erhardt JG, Kreichgauer HP, Meisner C, Bode JC, Bode C. Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps—a case control study. Eur J Nutr. 2002;41:35–43. doi: 10.1007/s003940200004. [DOI] [PubMed] [Google Scholar]
- 19.Terry MB, Neugut AI, Bostick RM, et al. Risk factors for advanced colorectal adenomas: a pooled analysis. Cancer Epidemiol Bio-markers Prev. 2002;11:622–9. [PubMed] [Google Scholar]
- 20.Larsen IK, Grotmol T, Almendingen K, Hoff G. Lifestyle as a predictor for colonic neoplasia in asymptomatic individuals. BMC Gastroenterol. 2006;6:5. doi: 10.1186/1471-230X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf LA, Terry PD, Potter JD, Bostick RM. Do factors related to endogenous and exogenous estrogens modify the relationship between obesity and risk of colorectal adenomas in women? Cancer Epidemiol Biomarkers Prev. 2007;16:676–83. doi: 10.1158/1055-9965.EPI-06-0883. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs ET, Ahnen DJ, Ashbeck EL, et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: a pooling study. Am J Epidemiol. 2009 doi: 10.1093/aje/kwn401. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Lim YJ, Kim YH, et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev. 2007;16:1543–6. doi: 10.1158/1055-9965.EPI-07-0199. [DOI] [PubMed] [Google Scholar]
- 26.Lee GE, Park HS, Yun KE, et al. Association between BMI and metabolic syndrome and adenomatous colonic polyps in Korean men. Obesity (Silver Spring) 2008;16:1434–9. doi: 10.1038/oby.2008.216. [DOI] [PubMed] [Google Scholar]
- 27.Wang YY, Lin SY, Lai WA, Liu PH, Sheu WH. Association between adenomas of rectosigmoid colon and metabolic syndrome features in a Chinese population. J Gastroenterol Hepatol. 2005;20:1410–5. doi: 10.1111/j.1440-1746.2005.03971.x. [DOI] [PubMed] [Google Scholar]
- 28.Chan AO, Jim MH, Lam KF, et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA. 2007;298:1412–9. doi: 10.1001/jama.298.12.1412. [DOI] [PubMed] [Google Scholar]
- 29.Oh TH, Byeon JS, Myung SJ, et al. Visceral obesity as a risk factor for colorectal neoplasm. J Gastroenterol Hepatol. 2008;23:411–7. doi: 10.1111/j.1440-1746.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- 30.Flood A, Mai V, Pfeiffer R, et al. Elevated serum concentrations of insulin and glucose increase risk of recurrent colorectal adenomas. Gastroenterology. 2007;133:1423–9. doi: 10.1053/j.gastro.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006;107:28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 32.Sturmer T, Buring JE, Lee IM, Gaziano JM, Glynn RJ. Metabolic abnormalities and risk for colorectal cancer in the Physicians’ Health Study. Cancer Epidemiol Biomarkers Prev. 2006;15:2391–7. doi: 10.1158/1055-9965.EPI-06-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol. 2006;164:652–64. doi: 10.1093/aje/kwj253. [DOI] [PubMed] [Google Scholar]
- 34.Stocks T, Lukanova A, Johansson M, et al. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes. 2008;32:304–14. doi: 10.1038/sj.ijo.0803713. [DOI] [PubMed] [Google Scholar]
- 35.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–91. [PubMed] [Google Scholar]
- 36.Jacobs ET, Martinez ME, Alberts DS, et al. Association between body size and colorectal adenoma recurrence. Clin Gastroenterol Hepatol. 2007;5:982–90. doi: 10.1016/j.cgh.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberts DS, Martinez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342:1156–62. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 38.Alberts DS, Martinez ME, Hess LM, et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst. 2005;97:846–53. doi: 10.1093/jnci/dji144. [DOI] [PubMed] [Google Scholar]
- 39.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 40.Martinez ME, Marshall JR, Graver E, et al. Reliability and validity of a self-administered food frequency questionnaire in a chemoprevention trial of adenoma recurrence. Cancer Epidemiol Biomarkers Prev. 1999;8:941–6. [PubMed] [Google Scholar]
- 41.Ritenbaugh C, Aickin M, Taren D, et al. Use of a food frequency questionnaire to screen for dietary eligibility in a randomized cancer prevention phase III trial. Cancer Epidemiol Biomarkers Prev. 1997;6:347–54. [PubMed] [Google Scholar]
- 42.Kim SH, Abbasi F, Reaven GM. Impact of degree of obesity on surrogate estimates of insulin resistance. Diabetes Care. 2004;27:1998–2002. doi: 10.2337/diacare.27.8.1998. [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Lim YJ, Kim Y-H, et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev. 2007;16:1543–6. doi: 10.1158/1055-9965.EPI-07-0199. [DOI] [PubMed] [Google Scholar]
- 44.Morita T, Tabata S, Mineshita M, Mizoue T, Moore MA, Kono S. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces Health Study. Asian Pac J Cancer Prev. 2005;6:485–9. [PubMed] [Google Scholar]
- 45.Stocks T, Lukanova A, Johansson M, et al. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes. 2007;32:304–14. doi: 10.1038/sj.ijo.0803713. [DOI] [PubMed] [Google Scholar]
- 46.Kim S, Keku TO, Martin C, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323–8. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev. 1996;5:1013–5. [PubMed] [Google Scholar]
- 48.Ealey KN, Xuan W, Lu S, Archer MC. Colon carcinogenesis in liver-specific IGF-I-deficient (LID) mice. Int J Cancer. 2008;122:472–6. doi: 10.1002/ijc.23102. [DOI] [PubMed] [Google Scholar]
- 49.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs ET, Martinez ME, Alberts DS, et al. Plasma insulin-like growth factor I is inversely associated with colorectal adenoma recurrence: a novel hypothesis. Cancer Epidemiol Biomarkers Prev. 2008;17:300–5. doi: 10.1158/1055-9965.EPI-07-0764. [DOI] [PubMed] [Google Scholar]
- 51.Flood A, Mai V, Pfeiffer R, et al. Serum concentrations of insulin-like growth factor and insulin-like growth factor binding protein 3 and recurrent colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2008;17:1493–8. doi: 10.1158/1055-9965.EPI-08-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colditz GA, Cannuscio CC, Frazier AL. Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Causes Control. 1997;8:649–67. doi: 10.1023/a:1018458700185. [DOI] [PubMed] [Google Scholar]
- 53.Blaak E. Sex differences in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2008;11:500–4. doi: 10.1097/MCO.0b013e32830467d3. [DOI] [PubMed] [Google Scholar]
- 54.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–78. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 55.Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507–15. doi: 10.1093/carcin/bgl018. [DOI] [PubMed] [Google Scholar]
- 56.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165–77. doi: 10.1017/s0007114599001348. [DOI] [PubMed] [Google Scholar]
- 57.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]