Abstract
Background
Clinical trials in children with moderate to severe persistent asthma are limited.
Objective
To determine if azithromycin or montelukast are inhaled corticosteroid-sparing.
Methods
The budesonide dose [with salmeterol (50 mcg) twice daily] necessary to achieve control was determined in children 6–17 years of age with moderate to severe persistent asthma. After a budesonide-stable period of 6 weeks, children were randomized in a double-masked, parallel, multi-center study to receive once nightly azithromycin, montelukast, or matching placebos, plus the established controlling dose of budesonide (minimum 400 mcg BID) and salmeterol twice daily. Primary outcome was time from randomization to inadequate asthma control following sequential budesonide dose reduction.
Results
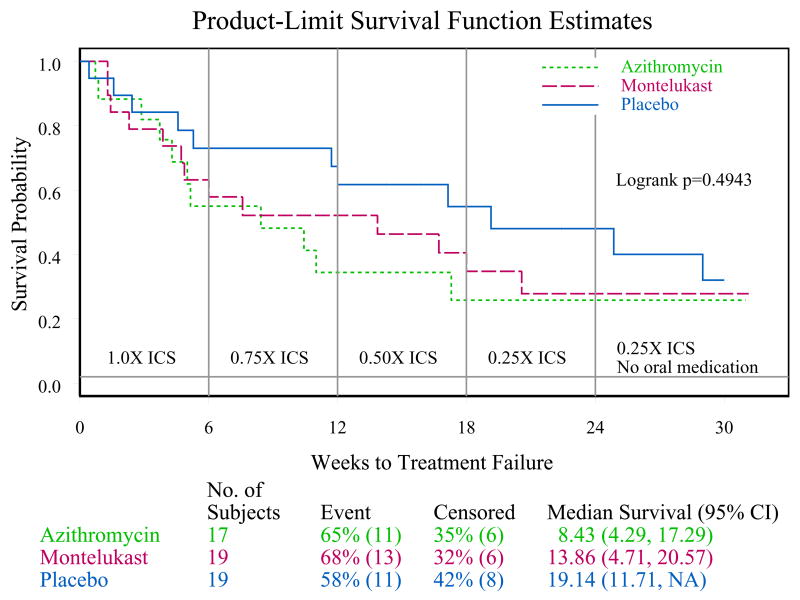
Of 292 children screened, only 55 were randomized. Inadequate adherence to study medication (n=80) and improved asthma control under close medical supervision (n=49) were the major reasons for randomization failure. A futility analysis was requested by the Data Safety Monitoring Board. In data available for analyses, no differences were noted for either treatment compared to placebo in time to inadequate control status (median, weeks (95% CL) azithromycin: 8.4 (4.3, 17.3), montelukast 13.9 (4.7, 20.6), placebo 19.1 (11.7, infinity)), with no difference between the groups (logrank test, p = 0.49). The futility analysis indicated that even if the planned sample size was reached, results of this negative study were unlikely to be different and the trial was prematurely terminated.
Conclusion
Based upon these results, neither azithromycin nor montelukast is likely to be an effective ICS-sparing alternative in children with moderate to severe persistent asthma.
Keywords: Asthma, Moderate to severe, Children, Macrolide, Leukotriene receptor antagonist, Clinical trial
INTRODUCTION
Children with moderate to severe persistent asthma comprise a small percentage of all children with asthma, but account for most of its morbidity (1). The few studies of medications to control severe asthma (2, 3) were conducted in the early 1990s before availability of newer medications that have improved clinical control while limiting side effects. Therapy with medium to high doses of ICS supplemented with additional controller medication(s) is generally effective in achieving control of bothersome symptoms (4, 5). Concern over systemic side effects (linear growth, bone parameters, adrenal cortical function) from long-term use of medium to high dose ICS medications (e.g., (6)) has prompted physicians to seek non-steroid medications that would allow ICS doses to be decreased. The Childhood Asthma Research and Education (CARE) Network initiated the Montelukast or Azithromycin for Reduction of Inhaled Corticosteroids in Childhood Asthma (MARS) protocol in moderate to severe persistent asthma in children to explore non-corticosteroid medications that might allow reduction of daily doses of ICS so as to lower the incidence of corticosteroid side effects.. Two medication classes were chosen as candidates, a macrolide and a leukotriene receptor antagonist. A macrolide was chosen based on studies showing improvement in clinical and pulmonary function outcomes in adults with asthma on a wide range of ICS doses (e.g., (7, 8)). Macrolides been shown to have anti-inflammatory effects in both animal and human studies (e.g., (9), and suggested for the effect in pandiffuse bronchiolitis (e.g., (10) and cystic fibrosis (11). Leukotriene receptor antagonists are an adjunctive therapy in persistent asthma (5, 12). Montelukast, a leukotriene receptor antagonist, has been shown to allow for reduction of ICS used chronically in adults while maintaining clinical stability of participants (e.g., (13). There is only one study demonstrating the ability of montelukast to improve symptom control in children with symptoms on low-dose ICS (14).
We report the findings of MARS, a trial targeting children 6–17 years of age with moderate to severe persistent asthma who achieved control on medium to high doses of ICS administered with salmeterol, to determine the potential ICS-sparing effects of a leukotriene receptor antagonist, montelukast, and a macrolide, azithromycin.
METHODS
Study Participants
Participants were recruited at five CARE Network centers from August 2006 to March 2007 (see Online Repository). Each center’s Institutional Review Board approved the study, and parents/guardians provided informed consent, with verbal assent given by children younger than 7 years and written assent from older children.
Inclusion criteria were physician-diagnosed asthma, age 6 to less than 18 years, and demonstration of moderate to severe persistent asthma by history of symptoms and current controller medication use over the previous month, i.e., inadequately controlled while receiving medium or high dose ICS alone that could be stepped-up by addition of salmeterol, or controlled with high dose ICS in combination with a long acting bronchodilator agent or other controller medication where the dose of ICS could be reduced (5). Pre-bronchodilator values of FEV1 had to be ≥ 80% predicted for consideration of step-down at enrollment, or ≥ 50% predicted if inadequately controlled and step-up planned. All children demonstrated ability to perform reproducible spirometry, and had airway lability demonstrated either by an improvement in FEV1 of ≥ 12% after 4 puffs of albuterol or airway hyperresponsiveness reflected by a 20% fall in FEV1 after a methacholine dose of ≤ 12.5 mg/ml. Exclusion criteria included asthma too severe as indicated by more than 3 hospitalizations in the preceding 12 months, history of intubation or mechanical ventilation within the last year, or any history of hypoxic seizure due to asthma; history of severe sinusitis requiring sinus surgery within the past 12 months; use of maintenance oral or systemic antibiotics for treatment of an ongoing condition; contraindication for use of azithromycin or montelukast; presence of lung disease other than asthma; use of digoxin, ergotamine or dihydroergotamine, triazolam, carbamazepine, cyclosporine, hexobarbital, and phenytoin, and other macrolides.
Protocol
The overall study design is depicted in Figure 1 in Online Repository. Participants had their historically reported ICS dosing converted to an equivalent budesonide BID regimen. All participants also received salmeterol dry powder (Serevent Diskus®) 50 mcg BID throughout the run-in and post randomization periods. During the run-in, participants demonstrated evidence of inadequate control on ICS plus salmeterol, with subsequent documentation that step-up to a higher dose of ICS (to a maximum of 1600 mcg daily, again with salmeterol) established control. Criteria to define inadequately controlled and controlled status were modeled after those used by the Asthma Clinical Research Network (15) and are presented in Table 1. The process used to make these determinations utilized an algorithm (Figure 2 in Online Repository). Documentation of normal liver enzymes and QTc interval on EKG was required prior to initiation of double-blind therapy that may include a macrolide. The participants were monitored via clinic visits every 2–4 weeks and interim phone calls, emphasizing adherence to all study medications and use of a daily diary to document symptoms and doses of albuterol required. The daily dose of budesonide at randomization was a minimum of 800 mcg to allow for a maximum of 4-fold reduction of dose, and a maximum of 1600 mcg to allow for patient safety considering side effects of high dose ICS. Participants were excluded if they were unable to use the study drug delivery systems or to adhere to ≥80% of days with use of salmeterol Diskus® and oral capsules and of diary card completion during the run-in (pre-randomization) period.
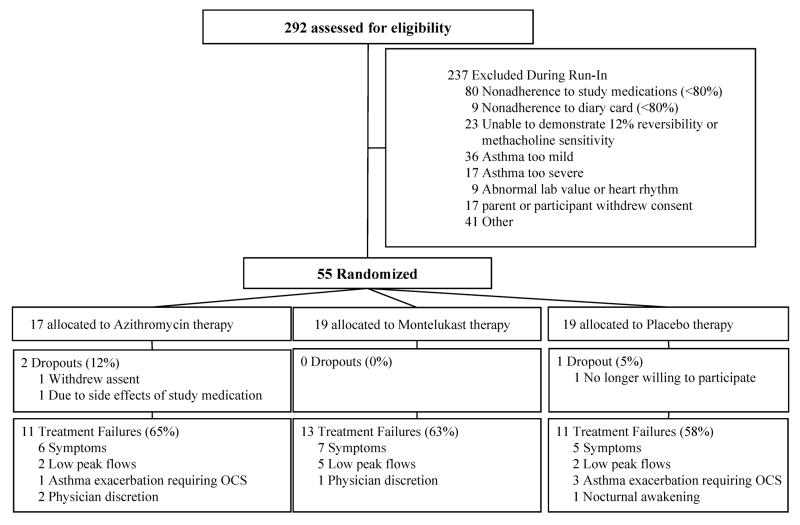
Figure 1.
Cascade of enrollment.
Table 1.
Criteria for Assigning Status of Control and Inadequate Control of Asthma during both run-in and the double-blind treatment period
Control:
|
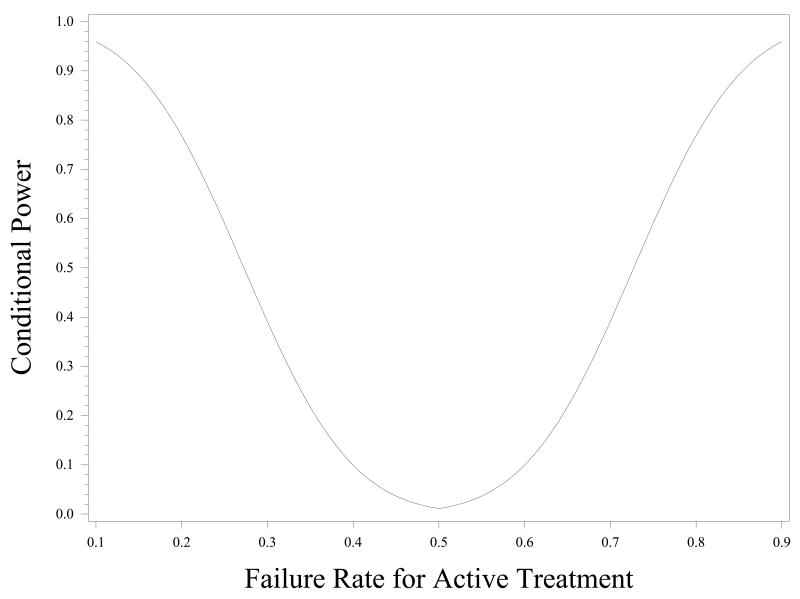
Figure 2.
The Conditional power in MARS, calculated as the probability of rejecting the null hypothesis of no active treatment effect if the trial had reached the target enrollment (210 randomized children), given that the active treatment and placebo groups displayed approximately a 0.5 failure rates at the interim stage (55 randomized children).
When asthma control criteria were met by the increased dose of ICS, a patient was randomized to one of the three treatment arms, (1) placebo (one placebo montelukast tablet and one or two placebo azithromycin capsules) once daily, (2) azithromycin 250 mg (for those 25–40 kg) or 500 mg (for those >40 kg) once daily (11) (one placebo montelukast tablet and one or two capsules each containing 250 mg azithromycin) (azithromycin was chosen as the macrolide because it was well tolerated during long-term use in children with cystic fibrosis (11) and did not interfere with the P450 liver enzymes, thus avoiding interference with prednisolone elimination known to occur with clarithromycin (16)), or (3) montelukast 5 mg or 10 mg once daily (based on age as indicated in the package insert). The three treatment arms were stratified according to clinical center and dose of budesonide (800 mcg/day vs. 1600 mcg/day) that achieved asthma control during run-in.
Following randomization, participants received the dose of ICS that achieved control (“1X”) + salmeterol BID for an additional six weeks. They then proceeded through three 6-week periods of ICS reduction, first to 75% of the control dose (“0.75X”), then 50% of the control dose (“0.5X”) and then 25% of the control dose (“0.25X”), each using salmeterol 50 mcg BID as concomitant medication. After the lowest dose was achieved and control maintained for an additional 6 week, the active study medication was changed to placebo (blinded to participant) and follow-up was continued for an additional 6 weeks. The ICS dosing and salmeterol were open label. Budesonide was purchased from AstraZeneca and administered as Pulmicort® Turbuhaler® 200 mcg/inhalation. Serevent® Diskus® 50 mcg/inhalation delivery systems were donated by GlaxoSmithKline (GSK). Singulair® and matching placebo were donated by Merck. Azithromycin (Zithromax®) 250 mg tablets were purchased from Pfizer. Proclinical Pharmaceutical Services over-encapsulated the azithromycin tablets in accordance with United States Pharmacopeia standards (17), in order to make identical active drug and placebo opaque capsules.
Electronic peak expiratory flow (PEF) measurements (AM1, Jaeger-Toenies GmbH, Hoechburg, Germany), asthma symptom scores, and albuterol use were recorded in diaries twice daily. Adherence to inhaled medication was estimated from the Diskus® dose indicator, and oral dose medication adherence was assessed on the basis of capsule count and an Electronic Drug Exposure Monitor (eDEM; AARDEX Ltd, Zug, Switzerland).
Participants were provided with albuterol MDI (Ventolin®, GSK), prednisone (10 mg tablets), and a written asthma action plan. Prednisone was initiated for an asthma exacerbation by the study physician if any one of the following occurred: > 12 puffs rescue albuterol in 24 hours, a diary card indicating severe symptoms that lead to inability to sleep or perform daily activities, or PEF < 70% personal best before albuterol; symptom code of 3 (severe symptoms leading to inability to sleep or perform daily activities) for ≥ 48 hours; PEF drop to < 50% personal best despite albuterol. Prednisone dosage was 2 mg/kg/day (maximum of 60 mg) for 2 days, then 1 mg/kg/day (maximum of 30 mg) for 2 days, and may have been extended at physician discretion.
Polymerase Chain Reaction (PCR) Assays for detection of Mycoplasma pneumoniae and Chalmydia pneumoniae
Nasal washes were obtained at randomization, at week 18, and end of trail (either after the last planned visit or at time of treatment failure). The assays developed for use in MARS and their sensitivities are presented in the Online Repository.
Outcome variables
The primary outcome was time to inadequate asthma control, with criteria for inadequate asthma control identical to that used during run-in (Table 1). Participants were terminated from the study for a significant adverse event related to study medication, including presence of significant abdominal pain or diarrhea, elevation of liver enzymes, or development of an abnormal QTc interval or evidence of a cardiac rhythm abnormality.
Sample size and statistical analysis
The primary outcome variable was time until inadequate control of asthma by comparing each active treatment regimen to placebo. It was assumed that the failure rate in the placebo group would be 50%, and a clinically relevant effect size for each of the azithromycin and montelukast groups would be a failure rate of only 20%. Therefore, a two-sided, 0.025 significance level test with 90% statistical power for detecting an effect size of a 50% failure rate versus a 20% failure rate, and allowing for a 10% drop-out rate, would require 70 randomized children per treatment regimen (for a total of 210 randomized children, 42 per clinical center). The significance level was set at 0.025 to account for the two primary comparisons of each active treatment to placebo.
Because the primary outcome variable is expressed as a time-to-event variable, Kaplan-Meier survival curves were constructed for graphical displays. The logrank test was applied to assess statistical significance. Stratification for clinical center and ICS dose at randomization (800 mcg or 1600 mcg) was planned.
The initial study timeline indicated that participants were to be enrolled over 15 months. One of every four children enrolled was expected to be randomized. Thus, with a total of 210 randomized children needed, we expected that 56 children would be enrolled each month or about 11 children per clinical center per month.
RESULTS
Study Progress
Flow of participants into study enrollment was slower than expected, with a rate of 22/month compared to the expected 56/month. The 292 enrolled participants had historical characteristics consistent with moderate to severe persistent asthma, with 82% taking medium to high dose ICS (800 mcg budesonide or equivalent or higher): almost one-half of these were inadequately controlled prior to study entry by symptom report. Eighteen percent of enrolled participants had inadequately controlled asthma while taking a lower dose of ICS, 400 mcg budesonide equivalent; these participants were given a higher dose of ICS + salmeterol during the run-in study phase to determine if they would qualify. Of the 292 total enrolled, 55 (19%) were randomized. Length of run-in was median 9 weeks (range 6–21 weeks). Reasons for not achieving randomization are shown in Figure 1. The two most common reasons were: 1) controlled asthma during the run-in phase (no symptoms or no airway lability demonstrated) (n=59, 25%) on low dose ICS (400 mcg budesonide BID) + salmeterol, and 2) adherence below the standard of 80% (n=89, 37%) (e.g., adherence with Diskus® in these children was a median of 70%, range 17–77%). There were no differences in ages of the children who did not reach randomization criteria (mean ages in years, too severe 12.3, too mild 11.9, non-adherent 11.8) compared to those who were successfully randomized (11.4 years). Elevated liver enzymes (n=6) and a prolonged QTc interval (n =3) were infrequent reasons for ineligibility.
The three treatment groups were well balanced, with characteristics at the time of randomization consistent with guideline definitions of moderate to severe persistent asthma controlled with use of moderate to high dose ICS and salmeterol (Table 2). After randomization, 100% of expected visits and 89% of expected phone contacts were completed, along with 90% adherence to diary entries. Adherence to medications estimated from salmeterol Diskus® counter and from Electronic Drug Exposure Monitor records for use of study capsules were 85 and 89%, respectively. There was no difference in adherence to use of study procedures or medications between treatment groups. Analysis of adherence within study groups for capsule (azithromycin or placebo) and tablet (montelukast or placebo) use monitored by eDEM found differences only by minority (Caucasian vs. Minority), with Caucasians doing better than minorities for both capsules and tablets (capsules: 92.2% in Caucasians compared to 81.5% in minorities, with a standard error of the difference = 4.4%, p=0.02; tablets: 91.6% in Caucasians compared to 80.7%, with a standard error of the difference = 4.7%, p=0.02). There was insufficient power to detect a difference when analyzed by treatment group. Rates of adherence for use of Diskus did not differ by gender, ethnicity, SES, or age group overall. There were differences in rates of adherence of use of Diskus among age groups in the placebo and montelukast arms: within the placebo group, adherence was 93.7% for ages 10–14 years compared to 80.7% for those 15–18 years, with a standard error of the difference = 4.7, p=0.0495; for the Montelukast group, adherence was 96.0% for ages 6–9 years compared to 83.4% for those 15–18 years, with a standard error of the difference = 5.6, p=0.0417). For those who had treatment failure, adherence was < 80% for use of Diskus for only 1 patient who was in the placebo group and only at the treatment failure visit. Adherence for oral medications in those achieving treatment failure criteria was < 80% for 1 in the azithromycin group, 1 in the montelukast group, and 3 in the placebo group. Three subjects who completed the full study had adherence < 80 % throughout the treatment phase, 2 in azithromycin and 1 in montelukast.
Table 2.
Characteristics at time of randomization
| MARS Cohort (N=55) | |
|---|---|
| Demographics/Asthma History | |
| Age (years) | 11.2 ± 2.6 |
| Male (%)* | 58.2 |
| Minority (%) | 45.5 |
| Atopic characteristics | |
| Positive aeroallergen ST (%) | 64.7 |
| Positive aeroallergen ST (#) | 2.2 ± 2.1 |
| Total serum IgE (kU/L) [median (Q1,Q3)] | 246 (62,483) |
| Peripheral blood eosinophils (%)[median (Q1,Q3)] | 3.5 (2.4,7.0) |
| Pulmonary Function | |
| Pre-bronchodilator FEV1 (% predicted) | 101.9 ± 13.7 |
| Pre-bronchodilator FEV1/FVC (%) | 80.5 ± 8.6 |
| Exhaled nitric oxide (ppb) [median (Q1,Q3)] | 9.3 (6.1, 16.2) |
| Methacholine PC20 (mg/ml) [median (Q1,Q3)] | 1.1 (0.4, 3.8) |
| Other characteristics | |
| ACQ score [median (Q1,Q3)] | 0.33 (0,0.83) |
| Overall Quality of Life (PAQLQ(S)) [median (Q1,Q3)] | 6.6 (6.1,6.9) |
| Overall Caregiver Quality of Life (PACQLQ) [median (Q1,Q3)] | 6.8 (6.2,7.0) |
| Dose of ICS (budesonide) at randomization = 800 mcg/day (%) | 60% |
Early Discontinuation of the Study
The a priori study monitoring plan prescribed that the Data Safety Monitoring Board (DSMB) examine study progress at 6 and 13 months after initiation of enrollment. At the second planned review, enrollment and efficiency of randomization were lower than expected, yielding a much lower than projected number of study participants. As such, the DSMB requested a futility analysis.
The observed data indicated that a reasonable assumption for the true failure rate (that is the proportion of participants who become uncontrolled) in the placebo group was approximately 0.5. Conditional power was calculated to determine what would be the probability of rejecting the null hypothesis of no active treatment effect at the end of the trial (210 randomized children) given that all the groups, active treatments and placebo, displayed approximately a 0.5 failure rate at the interim stage (55 randomized children). Figure 2 illustrates the conditional power curve for a range of true failure rates for either of the active treatments. On the right of the figure are power calculations for higher failure rates in the treatment groups than in the placebo, and on the left, power calculations for higher failure rates in the placebo group than in the treatment groups. Figure 2 shows that there was only 50% power to detect a true failure rate of 0.25 or 0.75 in the treatment groups as compared with a failure rate of 0.50 in the placebo group. Using the lower 95% confidence limit of the failure rates observed among the first 36 randomized children to the active treatments (e.g, 0.28) as the best-case scenario, the maximum conditional power was 40% to detect a significant active treatment effect if the study had continued to completion using the originally planned sample size (210 randomized children). For these reasons, the DSMB judged it to be futile for the MARS trial to continue, and recommended to the NHLBI that the trial be terminated.
Outcomes of those randomized
Of the 55 randomized children, 10 (18.2%) remained controlled throughout ICS reduction until the last planned study visit at 30 weeks after randomization. Seven (12.7%) were still controlled when the study was stopped by the DSMB at a median of 15.9 weeks (range 2.4–23.4 weeks) after randomization. Thirty-five (63.6%) reached inadequately controlled status following randomization at a median 5.1 weeks (range 0.4–29.0) after randomization. There were no differences between those who remained controlled compared to those who reached inadequately controlled status by age (p=0.10), gender (p=0.13), minority status (p=0.19), race (p=0.13), ethnicity (p=0.96), or income (p=0.73). Three subjects were withdrawn due to reasons other than inadequate control: abdominal pain within 24 hours of drug administration (azithromycin, n=1) and withdrawal of assent (azithromycin and placebo, n=1 each).
Of the 35 who were discontinued for attaining inadequately controlled status reasons were an exacerbation requiring oral corticosteroid in 4 participants, symptoms or albuterol use >6 days/week on average over 2 weeks in 16 (2 of these also had nocturnal awakenings >=2 days/week), nocturnal awakening with minimal accompanying daytime symptoms in 3, PEF readings on the diary card <80% expected >6 days/week on average over 2 weeks in 9, and physician discretion in 3. Reasons for reaching inadequately controlled status before the first reduction of ICS at 6 weeks after randomization (N=21, 60% of those reaching inadequately controlled status) were symptoms in 10 (48%), need for OCS in 3 (13%), PEF values <80% of the pre-bronchodilator value at the randomization visit without accompanying symptoms in 6 (29%), and physician discretion 2 (10%).
Among the 55 randomized children, time to inadequate asthma control after randomization is shown in Figure 3. Table 3 summarizes the proportion of children within each of the three randomized groups who met the criteria for inadequate asthma control. There is a great deal of overlap among the three confidence intervals, which is mostly attributable to the small sample size within each randomized group. The logrank test was applied to compare the three randomized groups with respect to the time until inadequate asthma control (failure) and it yielded a non-significant result (p = 0.49).
Figure 3.
Product-limit survival function estimates for time to return of inadequate control for children assigned to azithromycin (n=17), montelukast (n=19), and placebo (n=19).
Table 3.
Proportion of participants reaching inadequately controlled status after randomization
| Study group | Inadequately Controlled Subjects N (%) | 95% Confidence Interval (%) |
|---|---|---|
| Azithromycin (n=17) | 11 (64.7 %) | 38.3, 85.8 |
| Montelukast (n=19) | 13 (68.4%) | 43.5, 87.4 |
| Placebo (n=19) | 11 (57.9%) | 33.5, 79.8 |
PCR assays to detect Mycoplasma pneumoniae and Clamydia pneumoniae were done on the 55 participants as designated in the protocol (Figure 1 in Online Repository), at randomization, visit 5 and completion of the study at visit 7, or at a treatment failure visit. None of the 140 samples assayed had evidence of DNA from Mycoplasma pneumoniae and Clamydia pneumoniae.
DISCUSSION
Moderate to severe persistent asthma in children accounts for a significant proportion of both morbidity and mortality from asthma, but interventions to improve these outcomes have not been thoroughly or systematically studied. The MARS trial was an attempt to study this group of children, using therapeutic interventions considered to be relevant to their disease course given that significant side effects can occur from the high doses of inhaled corticosteroids required to control symptoms and prevent morbidity. While there are high risks of sampling error and bias when a trial is discontinued at an early stage, the futility analysis predicted that it was highly unlikely that either azithromycin or montelukast would demonstrate an ICS-sparing effect on the established outcome measure of inadequate control even if considerably more than the targeted 210 participants had been randomized and studied.
Major findings of the MARS study were the low rate of identification of moderate to severe persistent asthma as defined in the NAEPP Asthma Guidelines (4, 5) was lower than expected, failure to randomize due to remaining controlled on low dose ICS + long acting bronchodilator agent in the context of close monitoring despite historically not achieving control with comparable ICS dosing, and lack of adherence to protocol-required levels even with substantial education and encouragement. These findings suggest that there are not as many children with moderate to severe persistent asthma as thought and that many children who appear to have moderate to severe asthma may not have underlying “severe” disease, but rather may have poor adherence to prescribed medication. These findings are consistent with observations of Jones et al. (20) that poor adherence to prescribed therapy was significantly associated with difficult-to-control asthma. The clinical implications of these observations are striking.
Atypical organisms have been noted as a cause of exacerbations of asthma in both children and adults (e.g., (21)). These organisms colonize airways of adults with asthma (22), but are found infrequently in children with stable asthma (23). Our finding of negative PCR from nasal wash samples for both C. pneumoniae and M. pneumoniae during periods of both stable and uncontrolled asthma is consistent with previous findings in children and do not provide evidence for use of macrolides for their antibiotic activity in patients without evidence of respiratory tract infection.
The study design, including a lengthy run-in phase, may have impeded randomization, but it was necessary to assure that the study population was appropriate for the research question and to protect patient safety. Time to return of inadequate control of asthma as ICS doses were reduced was chosen as the primary outcome as a method to quantify effectiveness of azithromycin and montelukast in adding to control of children with moderate to severe persistent asthma. More than fifty percent of the randomized study participants became inadequately controlled before the first reduction of ICS, as the median time to inadequately controlled status was 5.1 weeks with the first ICS reduction scheduled at 6 weeks. Presence of symptoms or albuterol use >6 days in a 14-day interval, need for OCS, or physician concern over patient safety were the predominant reasons for attainment of inadequately controlled status overall (77%). However, nine subjects (26%) achieved inadequately controlled status based on low PEF measurements without sufficient symptoms reported, 6 of whom had symptoms 3 days or less during the 14-day intervals when peak flow criteria were met. The PEF criteria identified inadequate control twice as often before the first reduction of ICS than later in the trial. Perhaps the occurrence of low PEF reading without accompanying symptoms is too sensitive an indicator of inadequate control. Careful monitoring in the context of a clinical trial might well be sufficient to allow symptoms alone, perhaps along with a higher level of symptoms and a lower level of pulmonary function then that set in the current study, to take the predominant role in defining inadequate control of asthma, both during run-in to improve efficiency of randomization and to prolong time in the trial. This suggestion is supported by the findings of McCoy et al. (24) that symptoms on diary cards were a much stronger predictor of exacerbations than a low peak flow, although both were less predictive than overall risk assessment based on previous history of severe asthma (p=0.007).
There were many lessons learned about the study of children with moderate to severe persistent asthma. The study of children with moderate to severe persistent asthma presented many unexpected barriers to completion of the MARS protocol. The number of children with historically moderate to severe asthma willing to participate was well below the expected enrollment projections. The outcome chosen for study, reduction of inhaled corticosteroid dose, required a complex run-in period. A large subpopulation of participants were not able to meet the adherence requirements during this run-in, limiting the randomization efficiency below any other study done in the CARE Network It is possible that the pattern of poor adherence during the run-in is a reflection of how children with more severe disease use therapy chronically. It is also possible to speculate that their historical pattern of severe disease may be due in part to ongoing poor adherence to appropriate therapy rather than underlying disease severity. The rapid time to inadequate control emphasizes the inherent variability of moderate to severe asthma, which may weaken further study of the disease using lack of control as an endpoint. Moderate to severe asthma may be better studied using a longer study interval with a goal of prevention of exacerbations in addition to symptom control, perhaps followed by a reduction in ICS dose. While analyses of adherence were with small sample sizes and are exploratory only, there were suggestions that those with minority status had less adherence as compared to Caucasians with use of oral medications and that older children had less adherence with use of Diskus as compared to younger children. These findings may be useful in future studies of children with moderate to severe asthma.
In summary, this is the first study in children to evaluate whether leukotriene receptor antagonists or macrolides are ICS-sparing in those children who achieved asthma control on medium to high doses of ICS administered with salmeterol. Given the results of the futility analysis in the MARS trial, it is not likely that either azithromycin or montelukast is effective in reducing ICS doses used chronically for children with moderate to severe asthma.
Supplementary Material
Acknowledgments
The authors acknowledge the excellent technical assistance of Magda Dwidar in development of the real-time PCR assays and performing the Mycoplasma pneumoniae and Chalmydia pneumoniae testing.
Abbreviations
- CARE Network
Childhood Asthma Research and Education Network
- DSMB
Data Safety Monitoring Board
- ICS
Inhaled Corticosteroids
- MARS
Montelukast or Azithromycin for Reduction of Inhaled Corticosteroids in Childhood Asthma
- PCR
Polymerase Chain Reaction assays
- PEF
peak expiratory flow
Footnotes
Clinical implications: Experience during the run-in of this clinical trial suggests that many children who appear with “severe asthma” may really have poor medication adherence that demands aggressive attention rather than step-up of medication. (32 words of limit of 35)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore W, Peters S. Severe asthma: An overview. J Allergy Clin Immunol. 2006;117:487–494. doi: 10.1016/j.jaci.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Ball B, Hill M, Brenner M, Sanks R, Szefler S. Effect of low-dose troleandomycin on glucocorticoid kinetics and airway hyperresponsiveness in severely asthmatic children. Annals of Allergy. 1990;65:37–45. [PubMed] [Google Scholar]
- 3.Kamada A, Hill M, Iklé D, Brenner A, Szefler S. Efficacy and safety of troleandomycin therapy in severe, steroid-requiring asthmatic children. J Allergy Clin Immunol. 1993;91:873–882. doi: 10.1016/0091-6749(93)90345-g. [DOI] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma--Update on selected topics 2002. National Institutes of Health. National Heart, Lung, and Blood Institute; Bethesda, MD.: 2002. National Asthma Education and Prevention Program Expert Panel Report. [Google Scholar]
- 5.National Asthma Education and Prevention Program Expert Panel Report. Expert Panel Report 3:Guidelines for the Diagnosis and Management of Asthma. Department of Health and Human Services; Bethesda, MD: 2007. [Google Scholar]
- 6.Visser M, van der Veer E, Postma D, Arends L, de Vries T, Brand P, Duiverman E. Side-effects of fluticasone in asthmatic children: no effects after dose reduction. Eur Respir J. 2004;24:420–425. doi: 10.1183/09031936.04.00023904. [DOI] [PubMed] [Google Scholar]
- 7.Kostadima E, Tsiodras S, Alexopoulos E, Kaditis A, Mavrou I, Georgatou N, Papamichalopoulos A. Clarithromycin reduces the severity of bronchial hyperresponsiveness in patients with asthma. Eur Respir J. 2004;23:714–717. doi: 10.1183/09031936.04.00118404. [DOI] [PubMed] [Google Scholar]
- 8.Black P, Blasi F, Jenkins C, Scicchitano R, Mills G, Rubinfeld A, Ruffin R, Mullins P, Dangain J, Cooper B, David D, Allegra L. Trial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniae. Am J Respir Crit Care Med. 2001;164:536–541. doi: 10.1164/ajrccm.164.4.2011040. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe A, Bush A. Anti-inflammatory effects of macrolides in lung disease. Pediatr Pulmonol. 2001;31:464–73. doi: 10.1002/ppul.1076. [DOI] [PubMed] [Google Scholar]
- 10.Oda H, Kadota J, Kohno S, Hara K. Erthromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchilits. Chest. 1994;49:1116–1123. doi: 10.1378/chest.106.4.1116. [DOI] [PubMed] [Google Scholar]
- 11.Saiman L, Marshall B, Mayer-Hamblett N, Burns J, Quittner A, Cibene D, Coquillette S, Fieberg A, Accurso F, Campbell P f t M S Group. Azithromycin in patients with cystic fibrosis chronically infected with pseudomonas aeruginosa: A randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 12.Johnston N, Mandhane P, Dai J, Duncan J, Greene J, Lambert K, Sears M. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007;120:e702–e712. doi: 10.1542/peds.2006-3317. [DOI] [PubMed] [Google Scholar]
- 13.Lofdahl C, Reiss T, Leff J, Israel E, Noonan M, Finn A, Seidenberg B, Capizzi T, Kundu S, Godard P. Randomised, placebo controlled trial of effect of a leukotiene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patients. BMJ. 1999;319:87–90. doi: 10.1136/bmj.319.7202.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons FE, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, Laessig W, Schuster A, Perez-Frias J, Sekerel BE, Menten J, Leff JA. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr. 2001;138(5):694–8. doi: 10.1067/mpd.2001.112899. [DOI] [PubMed] [Google Scholar]
- 15.Lemanske RJ, Sorkness C, Mauger E, Lazarus S, Boushey H, Fahy J, Drazen J, Chinchilli V, Craig T, Fish J, Ford J, Israel E, Kraft M, Martin R, Nachman S, Peters S, Spahn J, Szefler S. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–2603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 16.Fost D, Leung D, Martin R, Brown E, Szefler S, Spahn J. Inhibition of methylprednisolone elimination in the presence of clarithromycin therapy. J Allergy Clin Immunol. 1999;103:1031–1035. doi: 10.1016/s0091-6749(99)70175-2. [DOI] [PubMed] [Google Scholar]
- 17.United States Pharmacopeial Convention, I. The United States Pharmacopeia/the National Formulary (USP 28/NF 23) Rockville, MD: 2005. Dissolution; pp. 2412–2415. [Google Scholar]
- 18.Szefler S, Phillips B, Martinez F, Chinchilli V, Lemanske R, Strunk R, Zeiger R, Larsen G, Spahn J, Bacharier L, Bloomberg G, Guilbert T, Heldt G, Morgan W, Moss M, Sorkness C, Taussig L. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Sorkness C, Lemanske R, Mauger D, Strunk R, Szefler S, Zeiger R, Bacharier L, Bloomberg G, Covar R, Guilbert T, Heldt G, Larsen G, Mellon M, Morgan W, Moss M, Spahn J, Taussig L. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 20.Jones C, Clement L, Morphew T, Kwong K, Hanley-Lopez J, Lifson F, Ops L, Guterman J. Achieving and maintaining asthma control in an urban pediatric disease management program: The Breathmobile Program. J Allergy Clin Immunol. 2007;119:1445–1453. doi: 10.1016/j.jaci.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Biscione G, Corne J, Chauhan A, Johnston S. Increased frequency of detection of Chlamydophila pneumoniae in asthma. Eur Respir J. 2004;24:745–749. doi: 10.1183/09031936.04.00049004. [DOI] [PubMed] [Google Scholar]
- 22.Gencay M, Rudiger J, Tamm M, Soler M, Prruchoud A, Roth M. Increased frequency of Chlamydia pneumoniae antibodies in patients with asthma. Am J Respir Crit Care Med. 2001;163:1097–1100. doi: 10.1164/ajrccm.163.5.2003162. [DOI] [PubMed] [Google Scholar]
- 23.Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, Heilbronner C, Iniguez JL, Chaussain M, Nicand E, Raymond J, Gendrel D. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–6. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 24.McCoy K, Shade D, Irvin C, Mastronarde J, Hanania N, Castro M, Anthonisen N. Predicting episodes of poor asthma control in treated patients wtih asthma. J Allergy Clin Immunol. 2006;2006:1226–1233. doi: 10.1016/j.jaci.2006.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.