Abstract
Steady-state haemoglobin (Hb) desaturation is a common finding in sickle cell anaemia (Hb SS) that could predispose to stroke by limiting oxygen delivery to the brain. To determine its association with the risk of overt stroke, we examined the relationship between daytime Hb saturation measured by pulse oximetry (SpO2) and cerebral artery blood flow velocity measured by transcranial Doppler ultrasonography (TCD), an established risk factor for overt stroke in Hb SS. We studied 181 children using multivariable models to control for known determinants of TCD velocity, including age, haematocrit, and a measure of stenosis. We found that SpO2 correlated significantly and inversely with TCD velocity in both the right and left middle cerebral arteries. Hb desaturation was associated with increased cerebral artery blood flow velocities and increased odds of abnormal TCD velocities, hence increased risk of stroke. About 5% of the variation in TCD velocity could be ascribed to Hb saturation while controlling for other determinants of TCD velocity. In conclusion, Hb saturation is a determinant of TCD velocity and a risk factor for stroke in children with Hb SS.
Keywords: sickle cell disease, stroke, risk factor, pulse oximetry, transcranial Doppler ultrasonography
INTRODUCTION
Steady-state haemoglobin (Hb) desaturation is common in patients with sickle cell anaemia (Hb SS) (Quinn and Ahmad 2005). It has several causes, including a rightward shift of the oxy-haemoglobin dissociation curve due to chronic anaemia (Needleman, et al 1999) and the properties of sickle Hb in solution (Seakins, et al 1973, Ueda, et al 1979). Hb desaturation was once thought to be relatively benign, but it has now been associated with several complications of Hb SS. Nocturnal Hb desaturation appears to increase the frequency of acute painful episodes (Hargrave, et al 2003) and central nervous system events, such as seizures, transient ischaemic attacks and strokes (Kirkham, et al 2001). Recently, we showed that daytime steady-state Hb desaturation is also a risk factor for clinically overt stroke in children with Hb SS (Quinn and Sargent 2008).
The mechanisms by which Hb desaturation could contribute to the risk of stroke are not entirely clear. Setty et al. showed that nocturnal oxygen desaturation in patients with sickle cell disease was associated with endothelial, leukocyte, and erythrocyte activation and adhesion as well the release of pro-adhesive and pro-inflammatory mediators (Setty, et al 2003). These pathophysiologic events could lead to vaso-occlusion and stroke. Hb desaturation also decreases arterial oxygen content and, potentially, oxygen delivery to the brain (Gupta, et al 1997, Jones, et al 1981). This could also predispose to stroke in patients with Hb SS, especially in regions of the brain distal to stenotic cerebral vasculopathy.
We wished to better understand the relationship between Hb desaturation and stroke in Hb SS. Increased cerebral artery blood flow velocity as measured by transcranial Doppler ultrasonography (TCD) is an established risk factor for overt stroke in children with Hb SS (Adams, et al 1992). Higher TCD velocities are associated with higher risk of stroke. Therefore, we investigated the association between daytime Hb saturation and TCD velocity. We hypothesised that daytime Hb saturation correlated inversely with TCD velocity, because Hb desaturation should cause a compensatory increase in cerebral blood flow velocity to maintain oxygen delivery to the brain (Gupta, et al 1997).
METHODS
Overview and Study Subjects
We performed a cross-sectional study of the Dallas Newborn Cohort (DNC), a newborn inception cohort of children with sickle cell disease (Quinn, et al 2004), to determine whether daytime Hb saturation correlated with TCD measurements of cerebral artery blood flow velocity. We studied all cohort patients with Hb SS or sickle-β0-thalassemia (Hb Sβ0) who had screening TCD studies for clinical care. We analysed patients with Hb SS and Hb Sβ0 together because the diseases are clinically indistinguishable and Hb Sβ0 is uncommon.
Definitions and Measurements
We collected the time-averaged maximum mean velocities (TAMMV) in the right and left middle cerebral arteries (RMCA, LMCA), the right and left anterior cerebral arteries (RACA, LACA), the right and left distal internal carotid arteries (RdICA, LdICA), and the vertebrobasilar system (VB). The highest TAMMV in each artery was used for the analysis, consistent with clinical care. We also used the single highest TAMMV in the entire VB, consistent with clinical care, so we could not study the individual contributions of the three arteries in the VB. Only the first TCD study of any patient was used, so each patient was represented only once in each analysis. TCD examinations were performed according to the STOP study protocol (Adams, et al 1998) by a STOP-certified technician using a non-imaging system (2-Mhz pulsed Doppler ultrasonograph, model TC8080, Nicolet Viasys Healthcare, Madison, Wisconsin).
We measured Hb saturation in room air (SpO2) during “well” clinic visits by trained clinical technicians using the Nellcor N-395 pulse oximeter (Nellcor Puritan Bennett Inc., Pleasanton, CA, USA). We recorded the daytime SpO2 that was nearest to the date of TCD study.
Known determinants of TCD velocity (TAMMV) include age, haematocrit (Hct), metabolic demands, and degree of stenosis (Taormina and Nichols 1996). To control for these confounders in our analysis of the effect of SpO2 on TAMMV we recorded the patient’s age at the time of the TCD study and his or her most recent Hct (relative to the time of the TCD study). All patients were supine, still, and quietly awake during TCD measurements, consistent with clinical care, to ensure similar metabolic demands.
We did not have a direct measurement of degree of stenosis, because this is not provided by TCD. Further there is no “gold standard” for the measurement of degree of stenosis besides conventional radiocontrast angiography, which our patients did not undergo because of its increased risk in SCD patients and the lack of a medical indication for the study. There is also no validated measure of degree of stenosis by magnetic resonance angiography. Therefore, we developed a novel, proxy measurement of stenosis by considering the ratio of the TAMMV in the artery of interest (e.g., RMCA or LMCA) to the TAMMV in the VB in the same patient. Sickle cerebral vasculopathy is rare in the vertebrobasilar system, so VB blood flow velocity will be dependent on physiologic factors, such as age, Hct, metabolic demand, and SpO2, rather than stenosis. As such, this ratio (e.g., RMCA:VB) should provide a reasonable approximation of the proportion of the TAMMV in the artery of interest (e.g., RMCA) that might be due to stenosis and not age, Hct, metabolic demands, and SpO2. We tested the characteristics of this proxy measure before including it in statistical models.
Statistical Analysis
Summary statistics were calculated for all variables of interest. We used Spearman correlation to test the two primary hypotheses that SpO2 was inversely correlated with TAMMV in the RMCA and LMCA. We pre-specified that these two vessels would be used in the primary hypothesis tests. We chose these two vessels because they are interrogated by TCD for the determination of risk of stroke and we knew that dICA measurements were not always obtained. Correlations with other vessels and between other variables of interest were performed as exploratory analyses. We compared the characteristics of MCA:VB as a proxy measure of degree of stenosis to the expected performance of such a measure. Group medians were compared using the Kruskal-Wallis test.
If we found SpO2 to be significantly correlated with TAMMV, we then planned to perform multiple linear regression to estimate the effect of SpO2 on TAMMV in the right and left MCA while simultaneously controlling for age, Hct, and stenosis. We tested for multicollinearity and the required assumptions of multiple linear regression. We also planned to model the odds of having an abnormal TCD velocity (≥ 200 cm/s) given SpO2 using multiple logistic regression to simultaneously control for age, Hct, and stenosis. Receiver-operating characteristic (ROC) curves would be generated, if appropriate, using the predicted probabilities from the logistic regression model as the test variable and abnormal TCD velocity as the dichotomous state variable. These logistic regression models would not be useful clinically as predictive tools, because a measure of stenosis is a covariate; rather, these models define the relative physiologic contributions of age, Hct, SpO2, and stenosis to TAMMV.
Data were analysed using SPSS version 17 statistical software for Mac (SPSS, Inc., Chicago IL). Subjects with missing measurements were excluded from each analysis; no data were imputed. Because we had two primary hypothesis tests, we nominally controlled for multiple statistical comparisons study-wide by considering only P values less than 0.025 to be statistically significant.
RESULTS
Characteristics of Patients
We identified 181 patients who had screening TCD studies; 53.8% were male and 97.8% had Hb SS. Of these, 167 (92%) and 181 (100%) had measurements of SpO2 and Hct for analysis. Characteristics of the patients are shown in Table 1. The median interval between the TCD study and the most recent Hct and SpO2 was 28 and 49 days, respectively. Fourteen patients had received a transfusion in the 3 months preceding the TCD study. None of the patients was transfused or prescribed hydroxyurea in the interval between the TCD study and the measurements of Hct and SpO2. Some arteries could not be insonated in a minority of patients due to technical difficulties, patient non-compliance, or severe stenosis (Table 1).
Table 1.
Characteristics of Patients.
| Variable | Mean | Median | 10th – 90th Percentiles |
Na |
|---|---|---|---|---|
| Age (years) | 8.0 | 7.4 | 3.2 – 13.5 | 181 |
| Hct (%) | 22.8 | 22.2 | 17.8 – 28.1 | 181 |
| SpO2 (%) | 96.6 | 97.0 | 92 – 100 | 167 |
| TCD Velocities (cm/s) | ||||
| RMCA | 143 | 141 | 104 – 183 | 178 |
| LMCA | 150 | 148 | 103 – 189 | 175 |
| RACA | 121 | 122 | 80 – 162 | 164 |
| LACA | 118 | 115 | 82 – 154 | 157 |
| RdICA | 122 | 122 | 79 – 126 | 82 |
| LdICA | 124 | 121 | 85 – 166 | 82 |
| VB | 108 | 106 | 79 – 135 | 168 |
| Proxy for Stenosis | ||||
| RMCA:VB | 1.37 | 1.33 | 1.03 – 1.74 | 166 |
| LMCA:VB | 1.43 | 1.37 | 1.02 – 1.92 | 165 |
Number of subjects who had data for analysis of each variable
Correlation of SpO2 with TAMMV
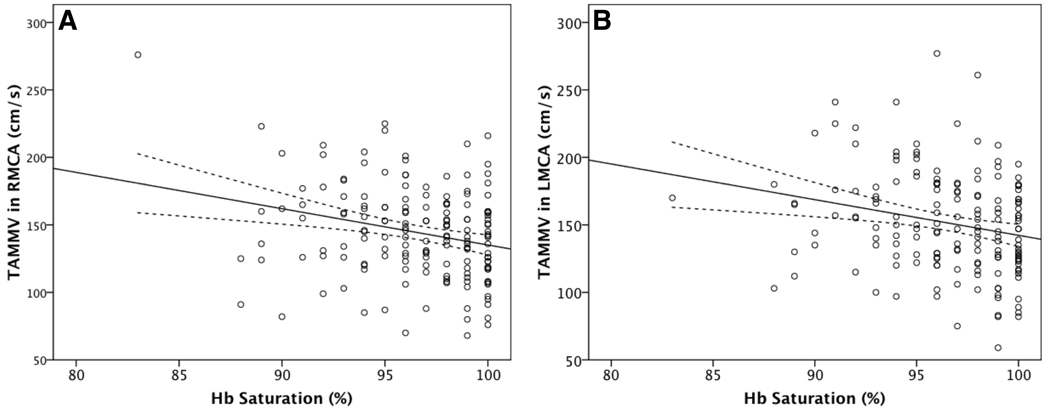
We found that daytime SpO2 correlated significantly with TAMMV in the right and left MCA in the direction hypothesised (Figure 1; Table 2). Lower Hb saturations were associated with higher TAMMVs. The same relationship was demonstrated in the anterior and posterior circulations (Table 2). Age and Hct were also inversely correlated with SpO2 (data not shown), a previously described relationship (Quinn and Ahmad 2005).
Figure 1. Correlation between Hb saturation and cerebral artery blood flow velocity (TAMMV).
Panels A and B show the correlations in the right and left middle cerebral arteries, respectively. Also shown are the regression line and its 95% confidence interval (dotted lines).
Table 2.
Correlations between SpO2 and TCD velocities.
| Artery | Spearman ρ | P |
|---|---|---|
| RMCA | −0.216 | 0.006 |
| LMCA | −0.244 | 0.002 |
| RACA | −0.269 | 0.001 |
| LACA | −0.192 | 0.020 |
| RdICA | −0.240 | 0.028 |
| LdICA | −0.278 | 0.012 |
| VB | −0.238 | 0.003 |
Characteristics of the proxy measure of stenosis
We evaluated the characteristics of the MCA:VB ratio as proxy measure for degree of stenosis. As would be theoretically expected, MCA:VB was not significantly correlated with SpO2, Hct, or age, but it did correlate with the TAMMV in the MCA on the same side (Table 3). Further, MCA:VB was higher in patients who had elevated TAMMVs (≥170 cm/s) in the MCA on the same side compared to patients whose TAMMVs were not elevated. The median MCA:VB was 1.3, 1.5, and 1.6 for patients with normal (<170 cm/s), conditional (170 – 199 cm/s), and abnormal (≥ 200 cm/s) TAMMVs, respectively (P = 0.001). Because we planned to use MCA:VB as a predictor (covariate) of TAMMV in the MCA in multivariable models, the expected correlation between MCA and MCA:VB might have limited its usefulness. However, RMCA explained only 16% of the variation in RMCA:VB, and LMCA explained only 22% of the variation in LMCA:VB, indicating that the majority of the variation in MCA:VB is unrelated to simply the TAMMV in the MCA. All these characteristics supported the use of MCA:VB as a proxy measure for degree of stenosis in the multivariable models.
Table 3.
Correlations between the proxy measure of stenosis and age, Hct, SpO2, and TAMMV in the right and left MCA.
| RMCA:VB | LMCA:VB | |||
|---|---|---|---|---|
| Spearman ρ | P | Spearman ρ | P | |
| Age | −0.157 | 0.044 | −0.035 | 0.652 |
| Hct | 0.104 | 0.183 | 0.052 | 0.510 |
| SpO2 | 0.54 | 0.511 | −0.25 | 0.763 |
| TAMMV | 0.437a | <0.001 | 0.517b | <0.001 |
Correlation with TAMMV in the RMCA
Correlation with TAMMV in the LMCA
Multivariable models
We then performed multiple linear regression with stepwise selection using the TAMMV in the right or left MCA as the dependent variable and age, Hct, SpO2, and the proxy measure of stenosis (MCA:VB) as the independent variables. The distribution of SpO2 was right-skewed, but transformation of SpO2 to a normal distribution did not change the performance of the models. Therefore, we did not transform SpO2 in the final models. The residual analyses suggested that the distributions were reasonably close to normal, linear, and homoscedastic. We found a significant linear relationship between SpO2 and the TAMMV in both the right and left MCA while controlling for age, Hct, and the proxy measure of stenosis (Table 4). As expected, lower SpO2 was associated with higher TAMMVs. Age and Hct were also inversely correlated with TAMMV. The proxy measure of stenosis (MCA:VB) was directly associated with TAMMV. Overall, the models explained about half the variation in TAMMV, and SpO2 explained about 5% of the variation while controlling for covariates.
Table 4.
Predictors of TAMMV in the right and left MCA.
| TAMMV in RMCA | TAMMV in LMCA | |||||
|---|---|---|---|---|---|---|
| β | partial r2 | P | β | partial r2 | P | |
| Intercept | 367.26 | – | – | 310.52 | – | – |
| Age | −3.03 | 0.18 | <0.001 | −2.12 | 0.10 | <0.001 |
| Hct | −3.20 | 0.19 | <0.001 | −3.25 | 0.20 | <0.001 |
| SpO2 | −1.98 | 0.06 | 0.002 | −1.57 | 0.04 | 0.017 |
| MCA:VB | 46.79 | 0.28 | <0.001 | 56.67 | 0.42 | <0.001 |
| model r2 = 0.50 | model r2 = 0.52 | |||||
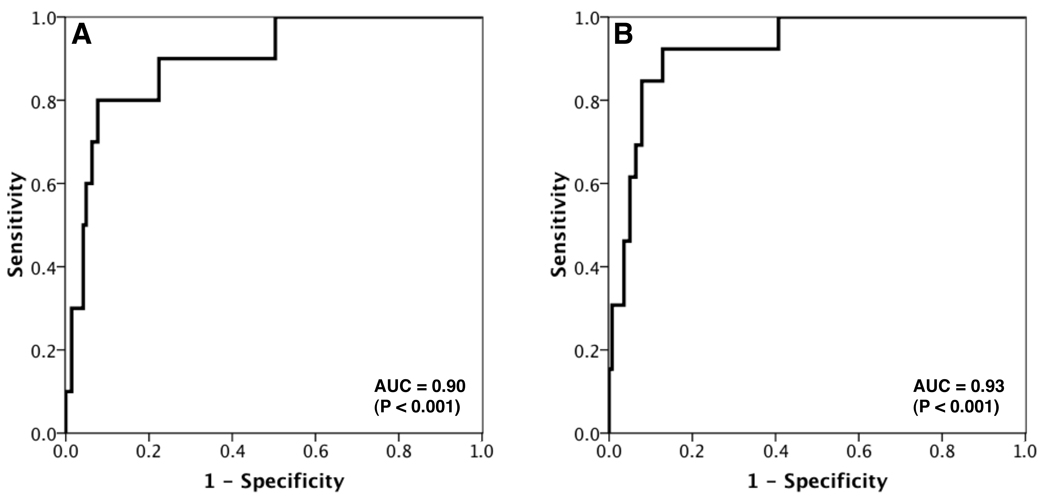
We then modeled the odds of having an abnormal TCD velocity (TAMMV ≥ 200 cm/s) in either the right or the left MCA. While simultaneously controlling for age, Hct, and the proxy measure of stenosis (MCA:VB), we found that each unit (1% absolute) decrease in SpO2 gave an odds ratio for an abnormal TCD velocity of 1.3 in the RMCA (P = 0.021) and 1.4 in the LMCA (P = 0.010). Figure 2 shows the ROC curves for prediction of abnormal TCD velocity by the models. The areas under the ROC curves were 0.90 (P < 0.001) and 0.93 (P < 0.001) for the RMCA and LMCA, indicating excellent prediction of abnormal TCD velocities by SpO2.
Figure 2. Receiver-operating characteristic (ROC) curves for the prediction of abnormal TCD velocities by Hb saturation.
Panels A and B show the ROC curves for the prediction of abnormal TCD velocities (≥ 200 cm/s) in the right and left middle cerebral arteries, respectively, using a combination of Hb saturation, haematocrit, age, and a proxy measure of degree of stenosis as predictors. The x-axis indicates the false positive rate (1 – specificity). The y-axis indicates sensitivity (the proportion of patients who were correctly classified).
DISCUSSION
We show that daytime Hb saturation is a determinant of TCD velocity in children with Hb SS. Desaturation is associated with increased cerebral artery blood flow velocities and increased odds of abnormal TCD velocities, thus increased risk of stroke. A similar inverse relationship between Hb saturation and TCD velocity was shown in a small (N=30), uncontrolled study of determinants of intellectual function in children with Hb SS (Hogan, et al 2006). Here we control for confounders of TCD velocity in a much larger sample and show that about 5% of the variation in TCD velocity can be ascribed to Hb saturation alone. Although stenosis itself is the dominant risk factor, our study adds to growing body of evidence for an additional role of Hb desaturation in the pathophysiology of stroke in Hb SS (Hogan, et al 2006, Kirkham and Datta 2006, Kirkham, et al 2001, Quinn and Sargent 2008, Setty, et al 2003). We propose that desaturation, which decreases arterial oxygen content, limits oxygen delivery to the brain and predisposes to stroke, especially in regions distal to stenotic cerebral vasculopathy where compensatory increases in blood flow may be blunted. Moreover, the compensatory increase in cerebral blood flow due to desaturation could increase the turbulence of blood flow at the site of stenosis and further predispose to vessel injury and stroke.
Our findings do not yet directly affect clinical practice, but they add to our knowledge about the genesis of stroke in Hb SS and have at least two additional implications. First, the measurement of cerebral artery blood flow velocity by TCD is more complex than a simple measure of the degree of stenosis in a blood vessel. Although stenosis is a dominant factor, TCD is actually a composite measure that integrates a number of related factors that could increase the risk of stroke in children with Hb SS, including the degree of anaemia and Hb saturation. Second, Hb saturation is a potentially modifiable risk factor. Supplemental oxygen would be cumbersome to use, but treatment with hydroxyurea (Singh, et al 2008) or red blood cell transfusions (Kress, et al 1999) can alleviate desaturation in Hb SS, which might be part of therapeutic benefit of both agents.
Our study has a number of limitations. First, we cannot ascribe a causal relationship between Hb desaturation and TCD velocity because this is a cross-sectional study. Even so, our findings are consistent with controlled physiologic experiments in animal models (Jones, et al 1981) and humans without Hb SS (Gupta, et al 1997). Second, we used a novel proxy measure of degree of stenosis (MCA:VB). We used this proxy because there is no appropriate “gold standard” for children with Hb SS. Nevertheless, this proxy is supported by biologic rationale, and it performed as expected for a measure of degree of stenosis. Additionally, the significant inverse relationship between SpO2 and TCD velocity remains if we remove this proxy measure from the models entirely (data not shown). That is, our conclusions are the same with or without the use of MCA:VB as a covariate, but the inclusion of MCA:VB may improve the accuracy of our models. Third, we retrospectively assembled data that were obtained and documented for the purposes of routine clinical practice, so there were missing data and the possibility of different types of bias, such as ascertainment bias. However, we analysed every patient in our cohort who had a screening TCD study, and 92 and 100% of patients had measurements of SpO2 and Hct. Fourth, the measurements of SpO2 and Hct were not simultaneous with the TCD study, because this is not our clinical practice, but the median interval between the TCD and these measurements was short (1 – 1.5 months). Finally, we did not ascertain patients’ oxy-haemoglobin dissociation curves, so we do not know whether desaturated patients are hypoxaemic and cannot quantify oxygen extraction. Nevertheless, Hb concentration and saturation are the dominant determinants of arterial oxygen content, so anemia and Hb desaturation will greatly limit the bulk delivery of oxygen to the brain irrespective of dissolved oxygen content.
In conclusion, Hb saturation is a determinant of TCD velocity in children with Hb SS. Desaturation is associated with increased cerebral artery blood flow velocities and increased odds of abnormal TCD velocities, thus increased risk of stroke. Hb saturation is an easily measured and potentially modifiable risk factor. It could be studied as a potential therapeutic target to prevent stroke.
Acknowledgment
We thank Drs. George Buchanan and Naveed Ahmad for their review of this manuscript. This work was supported in part by grants from the National Institutes of Health (CTQ and MMD: U54-HL70588, KL2-RR024983) and First American Real Estate Services, Inc. (MMD).
This work was supported in part by grants from the National Institutes of Health (CTQ and MMD: U54-HL70588, KL2-RR024983) and First American Real Estate Services, Inc. (MMD).
REFERENCES
- Adams R, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- Adams RJ, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- Gupta AK, et al. Thresholds for hypoxic cerebral vasodilation in volunteers. Anesth Analg. 1997;85:817–820. doi: 10.1097/00000539-199710000-00018. [DOI] [PubMed] [Google Scholar]
- Hargrave DR, et al. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- Hogan AM, et al. Physiological correlates of intellectual function in children with sickle cell disease: hypoxaemia, hyperaemia and brain infarction. Dev Sci. 2006;9:379–387. doi: 10.1111/j.1467-7687.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- Jones MD, Jr, et al. Effects of changes in arterial O2 content on cerebral blood flow in the lamb. Am J Physiol. 1981;240:H209–H215. doi: 10.1152/ajpheart.1981.240.2.H209. [DOI] [PubMed] [Google Scholar]
- Kirkham FJ, Datta AK. Hypoxic adaptation during development: relation to pattern of neurological presentation and cognitive disability. Dev Sci. 2006;9:411–427. doi: 10.1111/j.1467-7687.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham FJ, et al. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357:1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- Kress JP, et al. Determination of hemoglobin saturation in patients with acute sickle chest syndrome: a comparison of arterial blood gases and pulse oximetry. Chest. 1999;115:1316–1320. doi: 10.1378/chest.115.5.1316. [DOI] [PubMed] [Google Scholar]
- Needleman JP, et al. Measurement of hemoglobin saturation by oxygen in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:423–428. doi: 10.1002/(sici)1099-0496(199912)28:6<423::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol. 2005;131:129–134. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CT, et al. Survival of children with sickle cell disease. Blood. 2004;103:4023–4027. doi: 10.1182/blood-2003-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CT, Sargent JW. Daytime steady-state haemoglobin desaturation is a risk factor for overt stroke in children with sickle cell anaemia. Br J Haematol. 2008;140:336–339. doi: 10.1111/j.1365-2141.2007.06927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seakins M, et al. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1973;52:422–432. doi: 10.1172/JCI107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty BN, et al. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–1455. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- Singh SA, et al. Resolution of chronic hypoxemia in pediatric sickle cell patients after treatment with hydroxyurea. Pediatr Blood Cancer. 2008;50:1258–1260. doi: 10.1002/pbc.21480. [DOI] [PubMed] [Google Scholar]
- Taormina MA, Nichols FT., 3rd Use of transcranial Doppler sonography to evaluate patients with cerebrovascular disease. Neurosurg Clin N Am. 1996;7:589–603. [PubMed] [Google Scholar]
- Ueda Y, et al. An increased Bohr effect in sickle cell anemia. Blood. 1979;53:472–480. [PubMed] [Google Scholar]