Abstract
This article addresses a long-standing clinical and theoretical debate regarding the potential relationship between speech and nonspeech behaviors in the developing system. The review is motivated by the high popularity of nonspeech oral motor exercises (NSOMEs), including alimentary behaviors such as chewing, in the treatment of speech disorders in young children. The similarities and differences in the behavioral characteristics, sensory requirements, and task goals for speech and nonspeech oromotor behaviors are compared. Integrated theoretical paradigms and empirical data on the development of early oromotor behaviors are discussed. Although the efficacy of NSOMEs remains empirically untested at this time, studies of typical developmental speech physiology fail to support a theoretical framework promoting the use of NSOMEs. Well-designed empirical studies are necessary, however, to establish the efficacy of NSOMEs for specific clinical population and treatment targets.
Keywords: Speech, oral-motor, nonspeech oral motor exercises (NSOMEs), speech physiology, task specificity, motor development
This issue is devoted to an ongoing research and clinical discussion that has elicited a wide range of opinion concerning the influence of nonspeech oral motor behaviors on developing speech.1 On one end of the continuum is the representation of nonspeech and speech oromotor activities as distinct classes of behaviors with differences in sensorimotor systems and in behavioral, neuromotor, and sensory goals;2-4 on the other end of the continuum is the assertion that speech patterns are modifications of extant nonspeech oromotor patterns.5,6 Empirical support for either extreme (or intermediate) positions will have significant consequences for theoretical models of speech and for the guiding framework for speech treatments across both child and adult clinical populations.
Fundamental to this debate are empirical findings characterizing the acquisition of motor skills for speech and nonspeech behaviors such as sucking and chewing. The discovery of a common developmental origin (or, from an empirical standpoint, a common functional organization) for these divergent motor behaviors would provide evidence of shared neurological control and reveal clear behavioral precursors to later emerging speech skills. Recent evidence in early oromotor development can inform the hypothesized mechanisms underlying these behaviors.
During the first year of life, children display a wide variety of speech and nonspeech oral motor behaviors, including sucking, chewing, silent spontaneous orofacial movements, vocalizations (i.e., cooing), babbling (e.g., variegated and reduplicative), and speaking (i.e., first words). Only a few investigations have examined the development of motor control for these behaviors, leaving many questions regarding the relationship between speech and nonspeech oromotor behaviors unanswered.
THE EARLY DEVELOPMENT OF NONSPEECH AND SPEECH BEHAVIORS
Orofacial movements are abundant in the developing fetus. Using ultrasound, de Vries and colleagues7 documented jaw movements and sucking/swallowing motions in fetuses as young as 10 to 12 and 12 to 14 weeks, respectively. Perinatally, infants exhibit a growing repertoire of orofacial movements that are reflexive (e.g., sucking) or arise from spontaneous neural activity of the developing motor system.8 Other facial movements are exploratory;9 earliest appearing of these include, but are not limited to, non-nutritive sucking, mouthing of objects (e.g., hands and toys), vocalizing, babbling, and imitating facial movements and speech sounds.10,11 From these exploratory behaviors, infants become perceptually attentive to themselves and their environment9,12 and, presumably, goaldirected behaviors begin to emerge (i.e., sucking, chewing, babbling, and speech)13 (see also Green and Wilson8).
Some of the most robust theories of motor control entail task specificity in the neural control of a motor behavior, suggesting the coordinative organization for a given behavior is task specific or goal dependent14-16(also see recent reviews by Bunton17 and Weismer3). The development of motor control for a given behavior arises through the sensorimotor experiences with the effectors (i.e., muscles and structures) as they interact with the environment in the context of a particular task (e.g., as implemented in Guenther's Directions into Velocities of Articulators, or DIVA, model of speech production, detailed later). This perspective on task specificity in motor development shifts the emphasis to the unique requirements of a task and the immature, developing neuromotor system's adaptation to each task's emergent demands. From this perspective, these are important questions: (1) How distinct or redundant are the goals of different oromotor behaviors? (2) What are the task-specific demands for each effector involved, and are the functional synergies among these effectors unique to each task? and (3) What innate and environmental factors uniquely influence the development of motor control of each target behavior?
Table 1 summarizes the behavioral demands and effectors used for the early-appearing oromotor behaviors. This overview highlights the disparate sensorimotor demands required of sucking, chewing, and babbling.
Table 1.
Description of Early Oromotor Characteristics, Goals, and Frequencies
| Behavior | Movement Characteristics | Behavioral Goal | Predominant Jaw Movement Frequency |
|---|---|---|---|
| Sucking | Vertical lingual motion (coupled with mandibular motion) generates negative pressure in the oral cavity. | Draw liquid bolus into oral cavity prior to swallowing. | Non-nutritive: 1-2 Hz Nutritive: 1 Hz |
| Mastication | Lingual motion positions the bolus; mandibular motion generates occlusal force. | Break down a solid bolus prior to swallowing. | 0.88-2.11 Hz |
| Speech | Rapid and more independent motions of the lips, tongue, jaw, soft palate, and pharynx modulate vocal tract resonance and aeroacoustic valving. | Aerodynamic expiratory energy, transformed to acoustic energy at the larynx; the spectral shape of this acoustic energy is shaped by the relative positions of the articulators. | 3-4 Hz |
Sucking
Sucking occurs in utero by 12 to 14 weeks of gestation in humans.7 Full-term infants suck reflexively, and effectively, immediately following birth. As with later-appearing vocalizations, the primary articulators involved in nutritive sucking are the lips, jaw, tongue, and hard and soft palate. Unlike emergent vocalizations, synchronous motion of the tongue and lower jaw serve to express liquid, which is further facilitated by the pressure seal created by the lips; these coordinated actions generate the strong negative intraoral pressures that characterize sucking. More specifically, the lateral edges of the tongue turn upward while the tongue body moves in a characteristic peristaltic wave to direct the fluid into the pharynx. The hard palate provides an opposing support against which tongue movements compress the nipple. The soft palate elevates to seal the superior border of the oral cavity to prevent nasal regurgitation. Productive nutritive sucking requires significant force generated by the jaw and tongue, and cycles at ~1 Hz.18,19 In contrast, non-nutritive sucking (i.e., sucking without nutritive benefit) cycles at ~1 to 2 Hz.18,19 Although sucking is a reflexive behavior at birth, it becomes more regular with age.20
Chewing
The functional objective of chewing is bolus breakdown, which is accomplished by the shearing/grinding forces of the mandible and teeth. The tongue forms the food into a cohesive bolus in preparation for the swallow. Once an adequate labial seal is achieved, peristaltic motions of the tongue move the bolus back into the pharynx21 and the soft palate closes to prevent nasal regurgitation. The muscular forces generated for chewing are much greater than those generated for speech.22,23 Chewing rate ranges from 0.88 to 2.11 Hz during its earliest emergence.24 Munching, the earliest chewing behavior, begins with the introduction of semisolid foods (i.e., baby cereal and pureed food) between 4 and 6 months of age. Mature chewing, in sharp contrast to mature speech, is characterized by the rotary motion of the jaw.
The rotary jaw movement pattern has been observed in infants as young as 18 months of age25 and is reportedly established by 24 to 30 months of age;26 however, recent work by Wilson and Green (unpublished data, 2008) suggests that the development of rotary jaw motion for chewing is completely established sometime after 30 months of age.
Early Speech
The singular goal of articulatory movements during speech is to generate a narrowly specified acoustic output by modifying the shape of the vocal tract. Speech movements of the jaw, lips, tongue, and other speech systems subserve this goal exclusively, even to the extent that incompatible homeostatic behaviors (e.g., breathing for life purposes) can be briefly suppressed. Movements for speech must be rapid and accurate, and they are produced with much less muscular force than they are for chewing. In adults, speech requires only ~20% of the maximum force seen in chewing;22,23 however, the relative force generation by children to produce speech is unknown.
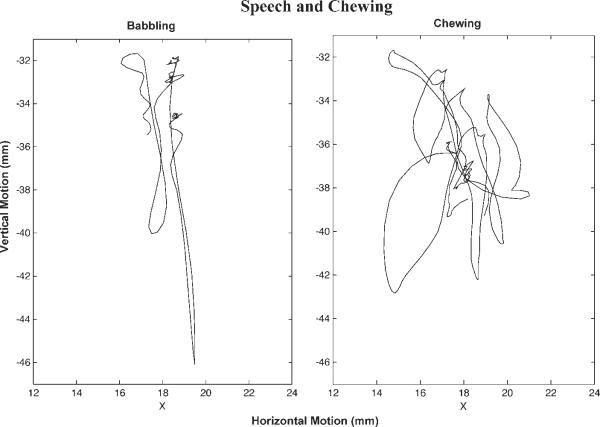
Similar to sucking and chewing, jaw movement plays a principal role in the production of developing speech; the movements of the tongue and lower lip must be coordinated with those of the jaw. During early speech, the movement of the jaw is characterized as a succession of vertical oscillations (i.e., reciprocal depression and elevation);27 these motions work to shape the sound composition of early utterances. These movements, which are confined primarily to the midsagittal plane, are in sharp contrast to the highly variable vertical, lateral, and rotary movements observed during early chewing (Wilson and Green, unpublished data, 2008; and Wilson28) (Fig. 1). The oscillatory motions of the jaw for speech are ~3 to 4 Hz, which is much faster than those during sucking or chewing (Table 1).
Figure 1.
Jaw motion epochs (in the frontal plane) for babbling and chewing in a young child. Note the relatively constrained motion of the jaw for babbling in contrast to the variable motion observed for early chewing.
Early speech is produced with relatively unstable lip and jaw coordination.29 As children age, the lower and upper lip become increasingly independent of each other, allowing for the generation of a greater repertoire of vocal tract configurations.29 Also involved in the goal of speech production and the generation of a variety of vowels and consonant sounds are the tongue, velum, and larynx. In a recent investigation of developmental changes in the spatiotemporal dynamics of the jaw and tongue, Cheng and colleagues30 determined that coordination (i.e., temporal coupling and spatiotemporal stability) between the tongue and jaw generally increased with age. However, data describing tongue movement during the early stages of speech development are unavailable.
DIFFERENCES BETWEEN NONSPEECH AND SPEECH BEHAVIORS IN THEIR USE OF SENSORY INFORMATION
A particularly crucial distinction among oral motor behaviors is their incorporation and dependence on sensory information (Table 2). Models of speech development uniformly demonstrate the critical importance of sensory feedback (e.g., auditory, cutaneous, proprioceptive) for refining motor control during the early stages of speech development. The integration of sensory information into ongoing movements differs significantly among oral behaviors; recognizing these differences is central to understanding the marked differences between the sensorimotor demands and the goals of speech and nonspeech behaviors. Incorporation of sensory information can occur by several very potent mechanisms, including error correction based on feedback, feedforward or efferent copy, or overt compensation.31-34
Table 2.
Description of Sensory Information for Early Oromotor Behaviors
| Behavior | Feedback |
|---|---|
| Sucking | Information from oral cavity regarding bolus volume, consistency, taste, and flow. |
| Bolus delivery method (i.e., nursing versus bottle) | |
| Appetite satiation | |
| Chewing | Information from oral cavity and periodontal receptors including bolus volume, consistency, and taste. |
| Bolus breakdown requirements | |
| Rate of bolus breakdown | |
| Rate of feeding | |
| Bolus delivery method (i.e., spoon feeding versus self-feeding) | |
| Appetite Satiation | |
| Speech | Auditory feedback |
| Proprioceptive feedback from position articulators | |
| Communicative partner(s) | |
| Communicative demands (i.e., environmental noise). | |
| Language and cognitive processing |
Note: The differences in sensory feedback between the two nonspeech oromotor behaviors (i.e., sucking and chewing) are in bold. The differences in sensory feedback between speech and the other two nonspeech oromotor behaviors are italicized.
Children learning to suck must accommodate changes in bolus volume, flow, and consistency, as well as variations in nipple size and shape (i.e., nursing versus bottle use). These varying characteristics are continuously detected by oral sensory receptors and integrated with very short latency (i.e., < 35 milliseconds) into ongoing sucking activity. The specific neural substrate for human sucking is largely unknown, although animal models are relatively well understood. Finan and Barlow35 suggest “that the sucking motor pattern of the human fetus and infant reflects the output of similar pattern generating circuitry.”
Children learning to chew encounter unique sensorimotor challenges. Children must learn to accommodate a wide range of food consistencies and, relative to the protective functions of the oral motor system and the airway, must shape and manage the bolus continuously. Bolus characteristics are registered by periodontal and oral cavity mucosa receptors, as well as mandibular proprioceptors, which project to trigeminal nuclei and the mandibular region of the cortex.36-38 The ongoing modulation of masticatory force incorporates these inputs in achieving efficient bolus breakdown. Oral sensory information might be expected to change with the emergence of dentition, although these changes are yet to be described in humans. Consequently, little is known about how dental emergence affects the coordinative organization of chewing (Wilson and Green, unpublished data, 2008).
Speech development must be similarly influenced by sensorimotor feedback from the oral cavity. Current models of speech development primarily emphasize the essential role of auditory feedback for the development of speech motor control.39-42 Guenther's39 DIVA model of speech production, for example, characterizes speech motor acquisition from its inception in babbling. During the babbling process, auditory feedback is used to shape articulatory objectives, and presumably, the motor control parameters underlying them. By babbling, children learn the acoustic consequences of their articulatory movements.
In addition to learning to map from articulatory movement to acoustic output, the model proposed by Westerman and Miranda42 incorporates the importance of establishing a visual speech map for early vowel learning. The visual speech map likely plays a role in speech development as infants appear to learn visual cues associated with speech sounds as early as 4.5 months of age.43,44 In contrast, there has been no indication that visual (or auditory) input contributes to the development of motor control for sucking or chewing.
The previous descriptions (and those in Tables 1 and 2) highlight the differences in behavioral, neuromotor, and sensory goals among early-appearing oromotor behaviors. Despite these numerous fundamental differences, clinicians and researchers commonly consider these behaviors to be functionally related, overlaid, or developmentally dependent, which may perhaps arise from the obvious sharing of primary anatomical structures and dependencies.
WHY EARLY OROMOTOR BEHAVIORS MAY BE PERCEIVED AS RELATED
The notion that early oromotor behaviors are related and interdependent has great intuitive appeal for a variety of reasons.45 The first and most obvious one is that many of the same effectors are active across each of these behaviors.46 In fact, the shared primary structures for both chewing and speech have prompted some investigators to assign a precursory role for chewing in the development of speech.5 Current hypotheses regarding the role of chewing in speech development are founded on the assumption that oromotor skills for chewing are established early in ontogeny relative to those used for speech.5
Frame/Content Theory
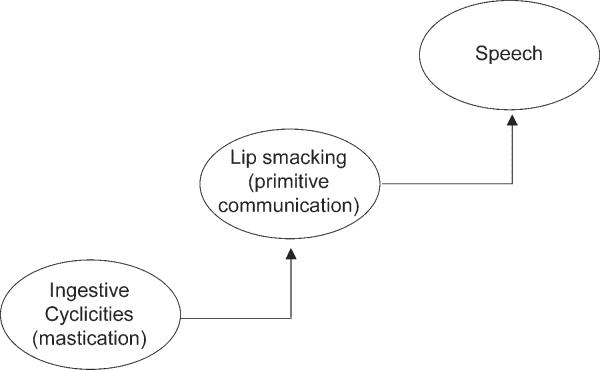
MacNeilage5 proposed that chewing is a precursor to speech in his frame/content theory of the evolution of speech production. He subscribes to Jacob's47 notion of “evolutionary tinkering,” which suggests that new motor behaviors are simply modified extensions of preexisting behaviors. According to MacNeilage, early in phylogeny, lip smacking (which is posited to be a primitive form of communication) emerged from the cyclic oscillation of the mandible during mastication. Presumably, the jaw movements acquired for lip smacking evolved into those used for speech (Fig. 2). MacNeilage proposes that human development parallels this evolutionary sequence, where the rhythmic oscillation of the mandible evident during mastication provides the framework for the syllable, the primary unit of speech production.
Figure 2.
Theoretical framework for the frame/content theory.
Central Pattern Generator
Along with the shared peripheral structures, MacNeilage and others have proposed that early oromotor behaviors have a common neurodevelopmental origin, such as a brainstem central pattern generator. Studies on nonhuman animals have identified a brainstem central pattern generator (CPG) that provides the central coordination of rhythmic oscillatory behaviors such as sucking and chewing. Currently, there is indirect evidence of the existence of a homologous CPG in humans, and several investigators have postulated that it may coordinate the cyclic jaw movements observed in babble and early speech. Grillner48 suggests that, “In analogy with the control of limb movements, the innate programs to control different sounds sequences in animals may be generated through a control of the activity in the respiratory brainstem generator and fractionations of the central pattern generators for mastication and swallowing (p. 227).” Lund and Kolta49 concur, and they suggest it is highly plausible that the speech system stems from the masticatory CPG rather than a distinct control center specific to speech production. However, the notion that a CPG provides a common developmental origin for all rhythmic oromotor behaviors, although appealing, remains speculative.20
WHY EARLY OROMOTOR BEHAVIORS MAY NOT BE RELATED
If distinct oral motor behaviors such as chewing, sucking, and speech share a common developmental origin, they might be expected to exhibit similarities in their coordinative organization in their earliest phases of development. However, data by Steeve and colleagues20 have recently demonstrated that distinct patterns of muscle activation are evident across chewing, sucking, and speech as early as 9 months of age. In general, findings by Moore and colleagues (Table 3) provided clear evidence of very broad differences in the coordinative organization between speech and non-speech behaviors, and they noted that these differences are evident from infancy2,20,24,50- through adulthood.51,52 For example, studies of mandibular muscle activity patterns have shown that immature and mature speech is characterized by the co-contraction of the antagonist muscle groups; whereas immature and mature chewing is characterized by the reciprocal activation of the antagonist muscle groups (Table 3).
Table 3.
Description of Empirical Studies on the Development of Early Oromotor Behaviors
| Author | Ages | Task(s) |
|---|---|---|
| Moore et al51 | Adults (n=7) | Chewing, continuous jaw oscillations, speech and nonspeech jaw motion, and continuous speech |
| Moore52 | Adults (n=6) | Chewing, jaw oscillations, and speech |
| Moore and Ruark2 | 15-month-olds (n=7) | Sucking, chewing, and early speech |
| Green et al24 | 12-48 months longitudinal (n=4) | Chewing |
| Ruark and Moore50 | 24-29-month-olds (n=7) | Chewing, lip protrusion, syllable repetition, and speech |
| Steeve et al20 | 9-month-olds (n=15) | Sucking, chewing, and babbling |
In addition, if “alimentary behaviors” such as sucking and chewing were necessary or appropriate precursors to speech, motor control might be expected to develop earlier for these behaviors than for speech. A comparison of findings across studies suggests, however, that jaw movement patterns for speech become adult-like earlier than for chewing (Wilson and Green, unpublished data, 2008; Green et al53). Specifically, jaw movements of 1-year olds during a simple speech utterance were statistically indistinguishable from those of adults.51 In contrast, Wilson and Green (unpublished data, 2008) observed that the adult-like rotary pattern of mandibular movement was not apparent even at 30 months of age. Moore and colleagues have concluded that protracted development of jaw control for chewing does not support the suggestion that early alimentary behaviors form the basis for later-appearing speech.
Current Clinical Trends for Early Intervention
How can this research on task-specific physiological development of early oromotor behaviors translate into clinical theory and practice? Is there compelling evidence that speech development exploits any of the coordinative organization afforded by other oromotor behaviors? Can proficiency in speech, regardless of its physiological infrastructure, be enhanced by practiced proficiency in nonspeech tasks?
One of the prevailing trends in clinical management of motor speech disorders in children is the use of nonspeech oromotor exercises (NSOMEs), some of which can include alimentary behaviors such as sucking and chewing.54 NSOMEs, in a variety of forms, have been used in the treatment of speech disorders for many years.55 The use of NSOMEs are guided by the tacit, although untested, hypothesis that NSOMEs (including alimentary behaviors) provide a foundation from which speech can develop, and that this foundation can be exploited broadly in the treatment of speech delay (e.g., childhood apraxia of speech, late speech development, complex and severe motor impairments). Others contend that the complexity of speech precludes its decomposition into basic elements (i.e., nonspeech behaviors) in remediation of delayed or disordered speech.
In any case, the efficacy of NSOMEs remains empirically untested, neither proved nor disproved. In lieu of direct evaluation of treatment efficacy of targeted patient populations using well-defined methods, our best estimations of NSOMEs' presumed effects can be informed indirectly by these few investigations of the physiological development of speech and nonspeech oromotor behaviors in typically developing children. The quantitative physiological work by Moore and colleagues consistently reveals differing coordinative organization across these earliest appearing oral motor behaviors (Table 3). Further, as detailed in Tables 1 and 2, the task demands for each of these behaviors are easily seen to be distinct on many levels, with strikingly different internal and external influences. Thus these studies of typical developmental speech physiology fail to support a theoretical framework promoting the use of NSOMEs, and they might reasonably be taken to support the notion of speech as a unique behavior, emerging independently among other oral motor behaviors.
Footnotes
Learning Outcomes: As a result of this activity, the reader will be able to (1) summarize some of the major developmental milestones in the development of speech and nonspeech behaviors (e.g., sucking and chewing), (2) describe differences in the use of sensory information during speech and nonspeech activities in infants and children, (3) summarize at least one model that presumes interdependence between nonspeech and speech behaviors in development, (4) provide some arguments that suggest that chewing, sucking, and speech do not share a common developmental origin, and (5) evaluate the current status of treatment efficacy research in the use of nonspeech oral motor exercises (NSOMEs).
REFERENCES
- 1.Maas E, Robin DA, Austermann Hula SN, et al. Principles of motor learning in treatment of motor speech disorders. Am J Speech Lang Pathol. 2008;17(3):277–298. doi: 10.1044/1058-0360(2008/025). [DOI] [PubMed] [Google Scholar]
- 2.Moore CA, Ruark JL. Does speech emerge from earlier appearing oral motor behaviors? J Speech Hear Res. 1996;39(5):1034–1047. doi: 10.1044/jshr.3905.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weismer G. Philosophy of research in motor speech disorders. Clin Linguist Phon. 2006;20(5):315–349. doi: 10.1080/02699200400024806. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler W. Speech motor control is task-specific: evidence from dysarthria and apraxia of speech. Aphasiology. 2003;17:3–36. [Google Scholar]
- 5.MacNeilage PF. The frame/content theory of evolution of speech production. Behav Brain Sci. 1998;21(4):499–546. doi: 10.1017/s0140525x98001265. [DOI] [PubMed] [Google Scholar]
- 6.Ballard KJ, Robin DA, Folkins JW. An integrative model of speech motor control: a response to Ziegler. Aphasiology. 2003;17:37–48. [Google Scholar]
- 7.de Vries JL, Visser GH, Prechtl HF. The emergence of fetal behaviour. I. Qualitative aspects. Early Hum Dev. 2008;7(4):301–322. doi: 10.1016/0378-3782(82)90033-0. [DOI] [PubMed] [Google Scholar]
- 8.Green JR, Wilson EM. Spontaneous facial motility in infancy: a 3D kinematic analysis. Dev Psychobiol. 2006;48(1):16–28. doi: 10.1002/dev.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson EJ. Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annu Rev Psychol. 1988;39:1–41. [Google Scholar]
- 10.Chen X, Striano T, Rakoczy H. Auditory-oral matching behavior in newborns. Dev Sci. 2004;7(1):42–47. doi: 10.1111/j.1467-7687.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 11.Meltzoff AN, Moore MK. Newborn infants imitate adult facial gestures. Child Dev. 1983;54:702–709. [PubMed] [Google Scholar]
- 12.Rochat P, Morgan R. The function and determinants of early self-exploration. In: Rochat P, editor. The Self in Infancy: Theory and Research. Elsevier Science B.V.; 1995. [Google Scholar]
- 13.Thelen E. Developmental origins of motor coordination: Leg movements in human infants. Dev Psychobiol. 1985;18:1–22. doi: 10.1002/dev.420180102. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein NA. The Co-ordination and Regulation of Movements. Pergamon; Oxford, United Kingdom: 1967. [Google Scholar]
- 15.Turvey MT. Coordination. Am Psychol. 1990;45:938–953. doi: 10.1037//0003-066x.45.8.938. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay S, Houle G, Ostry DJ. Specificity of speech motor learning. J Neurosci. 2008;28(10):2426–2434. doi: 10.1523/JNEUROSCI.4196-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunton K. Speech versus nonspeech: different tasks, different neural organization. Semin Speech Lang. 2008;29 doi: 10.1055/s-0028-1103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finan DS, Barlow SM. The actifier: a device for neurophysiological studies of orofacial control in human infants. J Speech Hear Res. 1996;39(4):833–838. [PubMed] [Google Scholar]
- 19.Woolbridge MW. The `anatomy' of infant sucking. Midwifery. 1986;2:164–171. doi: 10.1016/s0266-6138(86)80041-9. [DOI] [PubMed] [Google Scholar]
- 20.Steeve R, Moore C, Green J, Reilly K, McMurtrey J. Babbling, chewing and sucking: oromandibular coordination at 9 months. J Speech Hear Res. 2008 doi: 10.1044/1092-4388(2008/07-0046). Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson EM, Green JR. Coordinative organization of lingual propulsion during the normal adult swallow. Dysphagia. 2006;21(4):226–236. doi: 10.1007/s00455-006-9053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller EM, Milenkovic PH, MacLeod GE. Perioral tissue mechanics during speech production. In: Eisenfeld J, Delisi C, editors. Mathematics and Computers in Biomedical Applications. North-Holland/Elsevier Science; Amsterdam, The Netherlands: 1984. [Google Scholar]
- 23.Langmore SE, Lehman ME. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. J Speech Hear Res. 1994;37(1):28–37. doi: 10.1044/jshr.3701.28. [DOI] [PubMed] [Google Scholar]
- 24.Green JR, Moore CA, Ruark JL, Rodda PR, Morvee WT, VanWitzenburg MJ. Development of chewing in children from 12 to 48 months: longitudinal study of EMG patterns. J Neurophysiol. 1997;77(5):2704–2716. doi: 10.1152/jn.1997.77.5.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arvedson JC, Lefton-Greif MA. Anatomy, physiology, and development of feeding. Semin Speech Lang. 1996;17(4):261–268. doi: 10.1055/s-2008-1064103. [DOI] [PubMed] [Google Scholar]
- 26.Pinder GL, Faherty AS. Issues in pediatric feeding and swallowing. In: Caruso AJ, Strand EA, editors. Clinical Management of Motor Speech Disorders in Children. Thieme; New York, NY: 1999. pp. 281–318. [Google Scholar]
- 27.Meier RP, McGarvin L, Zakia RA, Willerman R. Silent mandibular oscillations in vocal babbling. Phonetica. 1997;54(34):153–171. doi: 10.1159/000262219. [DOI] [PubMed] [Google Scholar]
- 28.Wilson EM. Madison. Wisconsin; University of Wisconsin-Madison: 2005. Kinematic Description of Chewing Development [dissertation] [Google Scholar]
- 29.Green JR, Moore CA, Higashikawa M, Steeve RW. The physiologic development of speech motor control: lip and jaw coordination. J Speech Lang Hear Res. 2000;43:239–255. doi: 10.1044/jslhr.4301.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng HY, Murdoch BE, Goosee JV, Scott D. Physiologic development of tongue-jaw coordination from childhood to adulthood. J Speech Lang Hear Res. 2007;50:352–360. doi: 10.1044/1092-4388(2007/025). [DOI] [PubMed] [Google Scholar]
- 31.Abbs JH, Gracco VL, Cole KJ. Control of multimovement coordination: sensorimotor mechanisms in speech motor programming. J Mot Behav. 1984;16:195–231. doi: 10.1080/00222895.1984.10735318. [DOI] [PubMed] [Google Scholar]
- 32.Fairbanks G. Systematic research in experimental phonetics: a theory of the speech mechanism as a servosystem. J Speech Dis. 1954;19:133–139. doi: 10.1044/jshd.1902.133. [DOI] [PubMed] [Google Scholar]
- 33.Guenther FH. Cortical interactions underlying the production of speech. J Commun Disord. 2006;39:350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Nasir SM, Ostry D. Somatosensory precision in speech production. Curr Biol. 2006;16:1918–1923. doi: 10.1016/j.cub.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 35.Finan DS, Barlow SM. Intrinsic dynamics and mechanosensory modulation of non-nutritive sucking in human infants. Early Hum Dev. 1998;52(2):181–197. doi: 10.1016/s0378-3782(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 36.Dellow PG, Lund JP. Evidence for central timing of rhythmical mastication. J Physiol. 1971;215:1–13. doi: 10.1113/jphysiol.1971.sp009454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lund JP. Mastication and its control by the brain stem. Crit Rev Oral Biol Med. 1991;2(1):33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- 38.Thexton AJ. Mastication and swallowing: an overview. Br Dent J. 1992;173:197–206. doi: 10.1038/sj.bdj.4808002. [DOI] [PubMed] [Google Scholar]
- 39.Guenther FH. Speech sound acquisition, coarticulation, and rate effects in a neural network model of speech production. Psychol Rev. 1995;102(3):594–621. doi: 10.1037/0033-295x.102.3.594. [DOI] [PubMed] [Google Scholar]
- 40.Ménard L, Schwartz J-L, Boë L-J. The role of vocal tract morphology in speech development: perceptual targets and sensori-motor maps for French synthesized vowels from birth to adulthood. J Speech Lang Hear Res. 2004;47:1059–1080. doi: 10.1044/1092-4388(2004/079). [DOI] [PubMed] [Google Scholar]
- 41.Vihman MM. Variable paths to early word production. J Phonetics. 1993;21:61–82. [Google Scholar]
- 42.Westerman G, Miranda ER. A new model of sensorimotor coupling in the development of speech. Brain Lang. 2004;89:393–400. doi: 10.1016/S0093-934X(03)00345-6. [DOI] [PubMed] [Google Scholar]
- 43.Kuhl PK, Meltzoff AN. The bimodal perception of speech in infancy. Science. 1982;218(4577):1138–1141. doi: 10.1126/science.7146899. [DOI] [PubMed] [Google Scholar]
- 44.Kuhl PK, Meltzoff AN. The intermodal representation of speech in infants. Infant Behav Dev. 1984;7:361–381. [Google Scholar]
- 45.Darley FL, Aronson AE, Brown JR. Motor Speech Disorders. Philadelphia, PA: WB Saunders: 1975. [Google Scholar]
- 46.Lund JP, Appenteng K, Seguin JJ. Analogies and common features in the speech and masticatory control systems. In: Grillner S, Lindblom P, Lubker J, Persson A, editors. Speech Motor Control. Pergamon; New York, NY: 1982. pp. 231–245. [Google Scholar]
- 47.Jacob F. Evolution and tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 48.Grillner S. Possible analogies in the control of innate motor acts and the production of sound in speech. In: Grillner S, Lindblom P, Lubker J, Persson A, editors. Speech Motor Control. Pergamon; New York, NY: 1981. pp. 217–230. [Google Scholar]
- 49.Lund JP, Kolta A. Brainstem circuits that control mastication: do they have anything to say during speech? J Commun Disord. 2006;39(5):381–390. doi: 10.1016/j.jcomdis.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Ruark JL, Moore CA. Coordination of lip muscle activity by 2-year-old children during speech and nonspeech tasks. J Speech Lang Hear Res. 1997;40(6):1373–1385. doi: 10.1044/jslhr.4006.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore CA, Smith A, Ringel RL. Task-specific organization of activity in human jaw muscles. J Speech Hear Res. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- 52.Moore CA. Symmetry of mandibular muscle activity as an index of coordinative strategy. J Speech Hear Res. 1993;36(6):1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green JR, Moore CA, Reilly KJ, et al. Methodological issues in studies of early articulatory development: a response to Dworkin. J Speech Lang Hear Res. 2003;46:1020–1021. [Google Scholar]
- 54.Lof GL, Watson MM. A nationwide survey of nonspeech oral motor exercise use: implications for evidence-based practice. Lang Speech Hear Serv Sch. 2008;39:392–407. doi: 10.1044/0161-1461(2008/037). [DOI] [PubMed] [Google Scholar]
- 55.Fawcus B. Oropharyngeal function in relation to speech. Dev Med Child Neurol. 1969;11(5):556–560. doi: 10.1111/j.1469-8749.1969.tb01484.x. [DOI] [PubMed] [Google Scholar]