Abstract
Objective
To determine whether the cellular inflammatory marker of activated macrophages and monocytes, neopterin (NEO) and the acute phase inflammatory markers sialic acid (SA) and C-reactive protein (CRP) are elevated in pregnancy and further elevated in the pregnancy syndrome preeclampsia.
Methods
Maternal plasma concentrations of NEO, SA and CRP were measured by high sensitivity ELISA or HPLC in 20 non-pregnant women, 40 women with uncomplicated pregnancies, 50 women with transient hypertension of pregnancy alone, 49 women with small for gestational age (SGA) infants without preeclampsia, and 47 women with preeclampsia.
Results
The mean concentration of plasma NEO, SA and CRP were all significantly elevated in all groups of pregnant women compared to non-pregnant women (p<0.001 for all). In addition, maternal plasma NEO concentrations were further elevated in women with preeclampsia compared to the other groups of pregnant women (p<0.01). As expected, the acute phase inflammatory markers CRP and SA correlated positively with each other. However, CRP was also correlated with the activated macrophage and monocyte marker NEO in women with transient hypertension of pregnancy and women with preeclampsia (p<0.05).
Conclusion
The inflammatory markers NEO, SA and CRP are all elevated during pregnancy. However, only NEO, a marker of macrophage and monocyte activation, was further elevated in women with preeclampsia. These data suggest that there is a striking increase in inflammation during pregnancy, and cellular immune activation is further elevated during preeclampsia.
Keywords: pregnancy, preeclampsia, inflammation, neopterin
INTRODUCTION
The pregnancy syndrome preeclampsia complicates 3–7% of all pregnancies and continues to be a major contributor to maternal and neonatal morbidity and mortality. 1, 2 Inflammation is thought to contribute significantly to the pathophysiology of preeclampsia including endothelial and placental dysfunction. 3 The focus of this study was to measure maternal plasma concentrations of three inflammatory markers (CRP, SA and NEO) in pregnant compared to non-pregnant women, and in subjects with the pregnancy complication preeclampsia compared to uncomplicated pregnant subjects as well as subjects with transient hypertension of pregnancy alone and small for gestational age infants alone without preeclampsia.
C-reactive protein (CRP) is an important component of the innate immune system and is primarily produced by the liver as an acute phase reactive protein in response to inflammatory stimuli. 4 Elevated serum CRP provides a sensitive biomarker of chronic systemic inflammation, an independent predictor of future cardiovascular events 5, and elevations in CRP are associated with as well as precede preeclampsia, however this has not been a consistent finding. 6–8
Sialic acid (SA) plays a central role in the biomedical functioning of humans. 9 Similar to CRP, SA concentrations are elevated during inflammatory processes, likely resulting from elevated levels of richly sialylated acute-phase glycoproteins. Elevated levels of SA have recently been associated with an elevated risk of stroke and cardiovascular mortality. 10
Neopterin (NEO) is a specific and unique marker of innate inflammation because it is synthesized and released solely by activated macrophages and monocytes. 11, 12 Measurements of plasma NEO concentrations have been used to evaluate progression of viral infections, renal transplant rejection, severe systemic inflammatory diseases, nephritic syndrome and several autoimmune diseases. 11
Previous data have shown that inflammatory cytokines and cell surface markers are higher in women with preeclampsia compared to women with uncomplicated pregnancies. We hypothesize that plasma concentrations of NEO, SA and CRP will be elevated in pregnant women and further elevated in women with preeclampsia. Moreover plasma concentrations of NEO, SA and CRP will be positively associated with each other, particularly in women with preeclampsia.
METHODS
Study population
This was a nest case-control study of 206 women enrolled between 1997 and 2002 in an ongoing investigation of preeclampsia at Magee-Womens Hospital (Pittsburgh, PA). The study was approved by the Institutional Review Board and informed consent was obtained from all subjects. All pregnant subjects were nulliparous healthy women without known pre-existing medical complications. Exclusion criteria included multiple gestation, prior preeclampsia, illicit drug use and preexisting medical conditions such as diabetes, chronic hypertension, and renal disease.
Twenty women were not pregnant, 40 women had an uncomplicated pregnancy, 47 women had preeclampsia, 50 women had transient hypertension of pregnancy without preeclampsia, and 49 women had SGA infants without preeclampsia. Preeclampsia was diagnosed by the presence of gestational hypertension, proteinuria, and hyperuricemia beginning after the 20th week of pregnancy with resolution of gestational hypertension and proteinuria postpartum. We include hyperuricemia in our classification of preeclampsia as it identifies a more homogeneous group of gestational hypertensive women with a greater frequency of adverse fetal outcomes including preterm birth and small for gestational age infants. 13 Gestational hypertension was defined as a new onset increase in blood pressure including an absolute blood pressure ≥140mmHg systolic and/or ≥90mmHg diastolic after 20 weeks of gestation. Proteinuria was defined as ≥ 300 mg per 24-hour urine collection, ≥ 2+ protein on voided urine sample, ≥ 1+ protein on catheterized urine specimen, or a protein-creatinine ratio of ≥ 0.3. Hyperuricemia was defined as plasma uric acid concentration ≥ 1 SD above reference values at the gestational age the sample was obtained (e.g. term, > 5.5 mg/dL). 14 Diagnosis of preeclampsia was determined retrospectively based on medical chart review by a jury of research and clinical investigators.
Transient hypertension of pregnancy was defined as the presence of gestational hypertension without proteinuria or hyperuricemia. Small for gestational age (SGA) infants were defined by infant birthweight ≤ 10th centile for gestational age, after adjusting for race and gender, in an otherwise uncomplicated pregnancy. SGA infants with clinical or pathological evidence of chronic intrauterine infection or chromosomal abnormalities were excluded from the study.
Blood samples
Maternal venous EDTA plasma samples were collected pre-delivery upon admission to labor and delivery, and EDTA plasma samples from non-pregnant women were collected during the luteal phase of the menstrual cycle. Plasma samples were aliquoted and stored at −70°C for later analysis. Gestational age was determined by best obstetric estimate (first trimester ultrasound when available).
Quantification of Neopterin
Plasma neopterin was measured in duplicate by high performance liquid chromatography (HPLC) with fluorescent detection (Waters 717 plus). The mobile phase was ammonium bicarbonate (10mM, pH 6.8) at a flow rate of 1ml/min. 100µl of plasma was mixed with 100µl of 0.5M perchloric acid, vortexed and centrifuged at 14,000 × g for 5 min. 100µl of collected supernatant was mixed with 40µl of 100mM ammonium bicarbonate buffer at 0.45mM. 20µl of the prepared samples were injected into the equilibrated HPLC system and fluorometric detection performed at an excitation wavelength of 353nm and emission at 438nm. The standard curve was linear from 1.25 to 40nmol/L. A quality control plasma sample was included in each analysis, and the inter-assay variability was 6.5%.
Quantification of C-reactive protein
CRP was measured in duplicate by a high-sensitivity enzyme-linked immunoassay (ELISA) as described previously. 15 The sensitivity of the assay was 0.2 ng/ml, and spike and recovery tests indicated 91 to 103% recovery. The intra-assay variability was 3.9% and inter-assay variability was 7.4%.
Quantification of Sialic acid
Sialic acid was determined in duplicate using a diagnostic kit (Roche). The standard curve was linear from 10 to 125 mg/dl, and the sensitivity of the assay was 5 mg/dl. The inter-assay variability was 9%.
Quantification of plasma creatinine
Creatinine was quantified as an indicator of renal function in the subject groups. Plasma creatinine was measured in duplicate using a modified HPLC method based on that of Kock et al. 16 The inter-assay variability was <4%.
Cellular fibronection (cFN), triglycerides, and uric acid
Cellular fibronectin and triglycerides were quantified as indicators of endothelial dysfunction and dyslipidemia. Plasma cellular fibronectin (cFN) was measured in duplicate for each sample by an ELISA specific for the ED-A domain of human cFN. This ELISA technique is as described by Powers et al. 17 The sensitivity of the ELISA was 12.5ng/ml and the inter-assay variability was 7%.
Plasma triglycerides were measured in duplicate using a diagnostic kit (Pointe Scientific). The coefficient of variation between runs was 7%.
Plasma uric acid was measured in duplicate using a diagnostic kit supplied from Pointe Scientific (Lincoln Park, MI). The assay procedure for uric acid was followed as described by the manufacturer and the inter-assay variability was <6%.
Statistical Analysis
Data are presented as mean ± standard deviation or median and inter-quartile ranges where appropriate. The plasma concentration of CRP was not normally distributed, therefore values were transformed by square root, which normalized the data distribution, and were used for the statistical analyses. The statistical package JMP 5.0.1a software (SAS Institute, Cary, NC) was used to analyze the data. Analysis of variance was performed with Fisher’s protected least significant differences post hoc testing to adjust for multiple comparisons. Correlations were by standard regression analysis with Pearson’s product moment (r) reported. Statistical significance was accepted at p<0.05. Based on previous published differences in CRP between uncomplicated pregnant women compared to women with preeclampsia, a sample size of 19 subjects per group would have 80% power to detect a 2 fold difference between these two groups with an alpha of 0.05. 18 The presence of labor at the time of sample collection (82 in labor and 104 not in labor at sample collection) among the pregnant subjects did not significantly influence any of the markers of inflammation in this study, NEO (p= 0.33), CRP (p=0.76) and SA (p=0.43).
RESULTS
The demographic and clinical characteristics of the study subjects are presented in Table 1. Maternal age was modestly but significantly higher in the women with preeclampsia compared to non-pregnant women, and there was no difference in age between the remaining groups of pregnant women. Maternal pre-pregnancy body-mass index was also greater in women with preeclampsia and the women with transient hypertension of pregnancy compared to non-pregnant women. The average blood pressure before 20 weeks gestation was similar between all groups of pregnant women. As is typical, the average gestational age at delivery was significantly earlier in the women with preeclampsia compared to the other study groups, and infant birth weight and birth weight centile were lower in the preeclampsia and SGA groups compared to the infants of uncomplicated pregnant women. As expected, women with preeclampsia had higher plasma uric acid concentrations compared to all other groups. As reported previously, the median concentrations of cFN and triglycerides, markers of endothelial dysfunction and dyslipidemia respectively, were also significantly elevated in all pregnant women compared to non-pregnant women (Table 1), and the concentration of both were further elevated in women with preeclampsia compared to the other pregnancy groups. In addition, plasma creatinine was elevated in non-pregnant women and women with preeclampsia compared to the remaining groups of pregnant women, and this difference is consistent with the known difference in glomerular filtration rate between preeclamptic women compared to other pregnant women.
Table 1.
Patient demographics, pregnancy information and markers of inflammation.
| Variable | Non-pregnant (n=20) | Uncomplicated pregnancy (n=40) | Transient hypertension of pregnancy (n=50) | Small for gestational age without preeclampsia (n=49) | Preeclampsia (n=47) |
|---|---|---|---|---|---|
| Age (years) a | 22.2±4.0 | 23.8±5.8 | 24.7±7.1 | 23.2±5.0 | 27.1±5.5# |
| Body mass index (kg/m2) a | 22.0±2.8 | 26.1±5.3 | 27.5±6.4# | 24.4±5.4 | 26.6±6.5# |
| Race(% African American) a | 15% | 35% | 38% | 41% | 13% |
| Gestational age at delivery (weeks) a | NA | 38.9±2.6 | 39.3±1.1 | 39.4±1.3 | 36.2±2.8* |
| Average blood pressure before 20 weeks gestation (mmHg) a | NA | 112.3±8.6/ 68.1±5.8 |
114.8±8.1/ 69.5±5.9 |
110.3±8.1/ 66.4±5.3 |
115.2±8.8/ 70.7±7.6 |
| Average blood pressure at delivery (mmHg) a | NA | 119.0±8.5/ 70.8±5.6 |
150.3±10.8*/ 89.9±7.5* |
117.3±8.6/ 69.2±5.8 |
162.5±13.0*/ 94.3±10.5* |
| Infant birth weight (grams) a | NA | 3267±601 | 3251±509 | 2452±261* | 2446±822* |
| Birth weight percentile (%) a | NA | 55.2±26.3 | 46.6±31.3 | 4.1±2.9* | 31.6±31.9* |
| Uric acid(mg/dl) a | 4.7±1.1 | 5.0±1.1 | 5.3±0.9 | 5.5±1.2 | 7.4±1.4* # |
| Creatinine(mg/dl) a | 0.84±0.1* | 0.58±0.1 | 0.57±0.1 | 0.56±0.1 | 0.71±0.2* |
| Triglycerides (mg/dl) b | 62.9 *
(46.1–91.5) |
139.8 #
(190.1–115.7) |
164.3 #
(241.4–127.1) |
168.4 #
(118.3–195.0) |
215.1 *
#
(163.0–304.3) |
| cFN (µg/ml) b | 6.1 *
(3.5–9.1) |
17.0 #
(5.7–25.7) |
22.4 #
(13.5–30.9) |
23.9 #
(13.2–33.3) |
35.9 *
#
(20.1–58.4) |
| Neopterin (nmol/L) b | 10.9 *
(7.6–12.1) |
16.7 #
(11.9–20.1) |
17.2 #
(13.9–20.1) |
16.4 #
(12.7–19.8) |
20.2 *
#
(15.7–23.8) |
| Sialic acid (mg/dl) b | 72.0 *
(62.7–78.8) |
104.4 #
(91.2–106.9) |
102.2 #
(96.7–108.9) |
105.3 #
(91.8–113.6) |
103.9 #
(91.3–111.0) |
| CRP (mg/dl) b | 0.02 *
(0.01–0.25) |
0.74 #
(0.22–1.94) |
0.77 #
(0.29–1.65) |
0.57 #
(0.19–1.49) |
0.74 #
(0.33–1.73) |
Data are mean ± SD
or median (interquartile range)
p<0.05 vs. non-pregnant
p<0.05 vs. uncomplicated pregnant
NA= not applicable
Inflammatory Markers are Elevated in Pregnancy and Preeclampsia
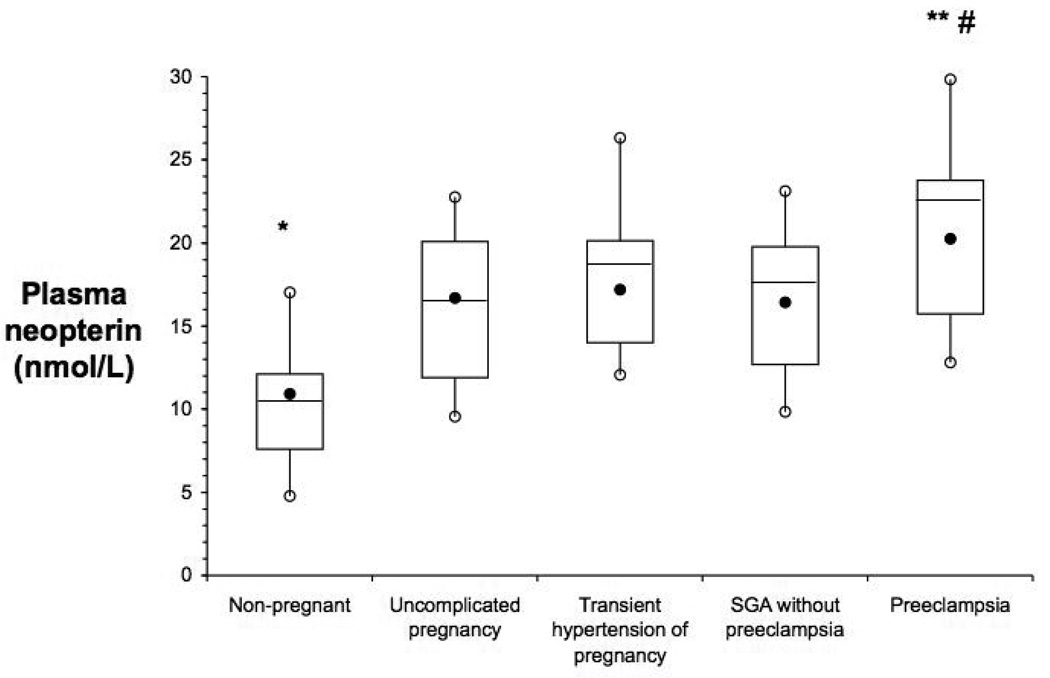
The maternal plasma concentration of the three inflammatory markers, NEO, SA and CRP, were significantly elevated in all groups of pregnant women compared to non-pregnant women (Table 1). In addition, NEO, the specific cellular inflammatory marker of activated monocytes and macrophages, was further elevated among women with preeclampsia (p<0.01) compared to other three pregnancy groups (Figure).
Figure.
Maternal plasma neopterin is elevated in pregnancy and further elevated in preeclampsia. Box and whisker plots of maternal plasma neopterin; the filled black circles are the median, the horizontal line within the box is the mean, the open circles are the 90th and 10th percentiles, and the top and bottom lines of the box are the 75th and 25th percentiles of the data for each group. (#: p<0.005 vs. non-pregnant; **: p<0.005 vs. uncomplicated pregnancy, and *: p<0.01 vs. uncomplicated pregnancy)
We examined the relationship between the inflammatory markers NEO, SA and CRP. As expected, there was a significant positive association between the acute phase inflammatory markers CRP and SA in all groups investigated in this study except the women with transient hypertension of pregnancy (Table 2). There was a significant positive association between the cellular inflammatory marker NEO and the acute phase inflammatory marker CRP in women with transient hypertension of pregnancy and the women with preeclampsia (Table 2). In contrast, there was no association between the cellular inflammatory marker NEO and SA among any of the individual groups investigated.
Table 2.
Correlations between Inflammatory Markers
| Group | CRP vs. Sialic acid | CRP vs. NEO | Sialic acid vs. NEO |
|---|---|---|---|
| All | r=0.47, p<0.0001 | r=0.33, p<0.0001 | r=0.25, p<0.001 |
| Non-pregnant women | r=0.65, p=0.002 | r=0.29, p=0.21 | r=0.27, p=0.24 |
| Uncomplicated Pregnancy | r=0.47, p=0.02 | r=0.22, p=0.18 | r=0.07, p=0.75 |
| Transient Hypertension of Pregnancy | r=0.03, p=0.84 | r=0.29, p=0.041 | r=0.05, p=0.72 |
| SGA infants without Preeclampsia | r=0.50, p<0.001 | r=0.16, p=0.29 | r=0.28, p=0.053 |
| Preeclampsia | r=0.40, p<0.02 | r=0.56, p<0.001 | r=0.16, p=0.28 |
Since NEO was the only inflammatory marker that was both elevated in pregnancy and further elevated in preeclampsia, we further investigated the relationship between NEO and other biological markers. Despite following a similar pattern of being elevated in pregnancy and further elevated in preeclampsia, there was no relationship between NEO and cFN (r=0.04, p=0.77), triglycerides (r=0.2, p=0.18), or uric acid (r=0.0006, p=0.96) among the women with preeclampsia. Similarly, there was no association between NEO and maternal age (r=0.02, p=0.86), BMI (r=0.02, p=0.85), blood pressure (r=0.09, p=0.54), gestational age (r=0.14, p=0.36), infant birth weight (r=0.10, p=0.47), or birth weight centile (r=0.02, p=0.88) among the women with preeclampsia. Interestingly, there was a significant association between NEO and plasma creatinine (r=0.45, p=0.0015) among the women with preeclampsia, and this relationship was also present among the other groups of pregnant women (r=0.17, p=0.05); however, there was no relationship between NEO and plasma creatinine among the non-pregnant women (r=0.09, p=0.69).
DISCUSSION
The objective of the present study was to investigate differences in three inflammatory markers in pregnant compared to non-pregnant women, and to further investigate differences in these markers in the pregnancy complication including preeclampsia. Our primary results from this study were that the cellular inflammatory marker of macrophage and monocyte activation, NEO, as well as the acute phase inflammatory markers CRP and SA, are all significantly elevated during pregnancy compared to non-pregnant women. In addition, NEO was the only inflammatory marker that was further elevated in women with preeclampsia compared to the other pregnancy groups.
Healthy human pregnancy requires adaptation of the maternal immune system in order to preserve maternal immune competence while also maintaining tolerance of the semiallogeneic fetus. Shifts in both the adaptive and innate immune systems contribute to this process. Alterations in innate immunity include both cellular and soluble components, reflected by elevated acute phase proteins, increased numbers of monocytes and granulocytes 19, and a primary role for uterine natural killer cells in both the local placental environment and in the broader maternal system. 20 Preeclampsia has long been hypothesized to have an immunological basis, with excessive immune activation contributing to the endothelial cell dysfunction evident in preeclampsia. 19
Numerous studies have reported finding elevated CRP among women with preeclampsia as well as prior to evident clinical symptoms and as much as 30 years postpartum. 6, 8, 15, 21 In many studies the association between elevated CRP and preeclampsia was significantly attenuated or lost after adjusting for body mass index. 18 On the other hand, studies that have matched for body mass index have been inconsistent with regard to CRP and the risk of preeclampsia. 6 Importantly, elevated CRP (≥4.9 mg/L) in the first trimester has been associated with a 2.5-fold increased risk of preeclampsia among lean women, and this relationship was absent among overweight and obese women. 22 As evidenced in our own data, there is a profound and significant increase in the circulating concentration of CRP among all pregnant women compared to non-pregnant women. Furthermore, it is possible that this pregnancy-mediated elevation in CRP may mask potential differences in CRP concentration between different pregnancy outcomes. In addition, we did not observe a relationship between CRP and body mass index as has been reported in other studies. However, this lack of an association may be the result of the timing of sample collection (pre-delivery third trimester) compared to other studies (first or second trimester).
Similar to CRP, SA is an acute phase inflammatory marker, and has been shown to be a strong predictor of coronary heart disease and cardiovascular mortality. 23 Similar to CRP, we have observed that SA is significantly elevated during pregnancy, and that SA is significantly correlated with CRP. However, no differences in SA concentrations were observed between preeclamptic patients compared to women with uncomplicated pregnancies, transient hypertension or women with SGA infants. Reports regarding SA concentrations during pregnancy have been inconsistent. Sydow et al. reported that serum SA was not significantly elevated in pregnancy 24, whereas Alvi and co-workers did report a significant elevation during pregnancy that was in keeping with data from Goni and colleagues. 25 Reasons for these discrepancies may be varying populations of women studied and assay differences. In addition, heterogeneity among study populations in developed and developing countries and differences in levels of chronic subclinical infection may also contribute to differences in inflammatory markers between studies. 6, 8, 26
Similar to CRP and SA, NEO is also a marker of inflammation. However, NEO is a unique inflammatory marker because it is synthesized solely by activated macrophages and monocytes. 27 There has been limited investigation of NEO in pregnancy and preeclampsia. Bichler et al. were the first to report elevated urinary NEO concentrations in human pregnancy, and Fuith et al. were the first to report elevated serum NEO concentrations in pregnancy. 28, 29 Similar to our own study, Haeger et al. and Kaleli et al. reported that plasma NEO concentrations were elevated in women with preeclampsia compared to normal pregnant women. 30 31 In two different studies, Schrocksnadel et al. reported that NEO levels are elevated in hypertensive pregnant women. 32, 33 More recent data showed that there were no significant differences in NEO concentrations between normal pregnant women and women with preeclampsia between 18 and 38 weeks of pregnancy. 34 However, most of the preeclamptic patients in this study were diagnosed with mild preeclampsia, and smoking, which may increase NEO levels 35, was not an exclusion criterion in the study. The results of our study confirm the findings of elevated NEO levels during pregnancy and the further elevation in preeclampsia, and that the elevation in NEO in preeclampsia is likely not a consequence of gestational hypertension or SGA since NEO was not further elevated in these pregnancy groups. Interestingly, NEO was significantly correlated with CRP in patients with transient hypertension of pregnancy and preeclampsia. This finding suggests a possible convergence of soluble and cell-mediated inflammatory factors in these complications near the end of pregnancy. In contrast, despite a similar pattern of being elevated in pregnancy and further elevated in preeclampsia there was no correlation between NEO and uric acid, the endothelial marker cFN or plasma triglycerides suggesting no direct relationship between NEO and endothelial dysfunction or dyslipidemia observed in preeclampsia. Conversely, NEO was significantly correlated with plasma creatinine concentrations in all pregnant subjects including patients with preeclampsia. Renal clearance is a significant mechanism by which NEO is eliminated. The mean renal clearance of neopterin is 216 ml/min or 1.8 times greater than the glomerular filtration rate of 120ml/min in non-pregnant subjects. 36 Therefore, it is reasonable that NEO should be correlated with plasma creatinine. However, we did not observe a correlation between NEO and creatinine in non-pregnant subjects. One possible explanation for these findings may be that NEO is elevated in pregnancy and further preeclampsia as a result of innate immune system activation, and that differences in renal function may further accentuate these differences.
Limitations of this study include the analysis of inflammatory and biological markers in late pregnancy at the time of clinically recognizable preeclampsia and the differences in gestational age. However, there are several strengths of this study including: the inclusion of a non-pregnant comparison group, the inclusion of other pregnancy complication groups including transient hypertension of pregnancy, a relatively large sample size compared to previous studies, and the quantification of several inflammatory markers as well as other relevant biomarkers.
In conclusion, we have observed that the inflammatory markers NEO, SA and CRP are significantly elevated during pregnancy. In addition, NEO, a marker of activated monocytes and macrophages, is further elevated among women with preeclampsia. These data strengthen the hypothesis that pregnancy is marked by a significant activation of the innate immune system and that this activation is further exaggerated in the pregnancy complication preeclampsia.
ACKNOWLEDGMENT
We gratefully acknowledge the technical assistance of Michael Frank with the analysis of neopterin and Kim Wadas with the determination of CRP. This project was supported by the National Institutes of Health grant numbers P01-HD30367.
Footnotes
DISCLOSURE
The author’s have no conflicts of interest to disclose.
REFERENCES
- 1.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists number 33, January 2002. Diagnosis and Management of Preeclampsia and Eclampsia. Obstet Gynecol. 2002;99:159–161. [Google Scholar]
- 2.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response--a review. Placenta. 2003;24:S21–S27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 3.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 4.Black S, Kushner I, Samols D. C-reactive Protein. Journal of Biological Chemistry. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. Journal of the American College of Cardiology. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 6.Garcia RG, Celedon J, Sierra-Laguado J, et al. Raised C-reactive protein and impaired flow-mediated vasodilation precede the development of preeclampsia. American Journal of Hypertension. 2007;20:98–103. doi: 10.1016/j.amjhyper.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Paternoster DM, Fantinato S, Stella A, et al. C-reactive protein in hypertensive disorders in pregnancy. Clinical & Applied Thrombosis/Hemostasis. 2006;12:330–337. doi: 10.1177/1076029606291382. [DOI] [PubMed] [Google Scholar]
- 8.Teran E, Escudero C, Calle A. C-reactive protein during normal pregnancy and preeclampsia. International Journal of Gynaecology & Obstetrics. 2005;89:299–300. doi: 10.1016/j.ijgo.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Sillanaukee P, Ponnio M, Jaaskelainen IP. Occurrence of sialic acids in healthy humans and different disorders. European Journal of Clinical Investigation. 1999;29:413–425. doi: 10.1046/j.1365-2362.1999.00485.x. [DOI] [PubMed] [Google Scholar]
- 10.Gopaul KP, Crook MA. Sialic acid: a novel marker of cardiovascular disease? Clinical Biochemistry. 2006;39:667–681. doi: 10.1016/j.clinbiochem.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs D, Weiss G, Wachter H. Neopterin, biochemistry and clinical use as a marker for cellular immune reactions. International Archives of Allergy & Immunology. 1993;101:1–6. doi: 10.1159/000236491. [Review] [53 refs]. [DOI] [PubMed] [Google Scholar]
- 12.Hamerlinck FF. Neopterin: a review. Experimental Dermatology. 1999;8:167–176. doi: 10.1111/j.1600-0625.1999.tb00367.x. [Review] [98 refs]./8. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JM, Bodnar LM, Lain KY, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46:1263–1269. doi: 10.1161/01.HYP.0000188703.27002.14. [see comment] [DOI] [PubMed] [Google Scholar]
- 14.Hill LM, Furness C, Dunlop W. Diurnal variation of serum urate in pregnancy. British Medical Journal. 1977;2:1520. doi: 10.1136/bmj.2.6101.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubel CA, Powers RW, Snaedal S, et al. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension. 2008;51:1499–1505. doi: 10.1161/HYPERTENSIONAHA.108.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kock R, Seitz S, Delvoux B, Greiling H. A method for the simultaneous determination of creatinine and uric acid in serum by high-performance-liquid-chromatography evaluated versus reference methods. European Journal of Clinical Chemistry & Clinical Biochemistry. 1995;33:23–29. doi: 10.1515/cclm.1995.33.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Powers RW, Evans RW, Ness RB, Crombleholme WR, Roberts JM. Homocysteine and cellular fibronectin are increased in preeclampsia, not transient hypertension of pregnancy. Hypertension in Pregnancy. 2001;20:69–77. doi: 10.1081/PRG-100104173. [DOI] [PubMed] [Google Scholar]
- 18.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstetrics & Gynecology. 2001;98:757–762. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 19.Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunology Today. 1999;20:114–118. doi: 10.1016/s0167-5699(98)01393-0. [see comment] [DOI] [PubMed] [Google Scholar]
- 20.Moffett-King A. Natural killer cells and pregnancy. Nature Reviews Immunology. 2002;2:656–663. doi: 10.1038/nri886. [erratum appears in Nat Rev Immunol 2002 Dec;2(12):975] [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Qian C, Leshane ES, et al. Two-stage elevation of cell-free fetal DNA in maternal sera before onset of preeclampsia. American Journal of Obstetrics & Gynecology. 2004;190:707–713. doi: 10.1016/j.ajog.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Qiu C, Luthy DA, Zhang C, Walsh SW, Leisenring WM, Williams MA. A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. American Journal of Hypertension. 2004;17:154–160. doi: 10.1016/j.amjhyper.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Lindberg G, Rastam L, Gullberg B, et al. Serum sialic acid concentration predicts both coronary heart disease and stroke mortality: multivariate analysis including 54,385 men and women during 20.5 years follow-up. Int J Epidemiol. 1992;21:253–257. doi: 10.1093/ije/21.2.253. [DOI] [PubMed] [Google Scholar]
- 24.Sydow G, Morack G, Jung U, Semmler K, Christ S. Serum sialic acid levels in cancer, pregnancy and upper respiratory infections. Arch Geschwulstforsch. 1986;56:413–417. [PubMed] [Google Scholar]
- 25.Goni M, Sayeed M, Shah GM, Hussain T. Serum sialic acid levels in normal pregnant and non-pregnant women. Indian J Physiol Pharmacol. 1981;25:356–360. [PubMed] [Google Scholar]
- 26.Lopez-Jaramillo P, Garcia RG, Lopez M. Preventing pregnancy-induced hypertension: are there regional differences for this global problem? J Hypertens. 2005;23:1121–1129. doi: 10.1097/01.hjh.0000170371.49010.4a. [DOI] [PubMed] [Google Scholar]
- 27.Muller MM, Curtius HC, Herold M, Huber CH. Neopterin in clinical practice. Clin Chim Acta. 1991;201:1–16. doi: 10.1016/0009-8981(91)90019-9. [DOI] [PubMed] [Google Scholar]
- 28.Bichler A, Fuchs D, Hausen A, Hetzel H, Reibnegger G, Wachter H. Measurement of urinary neopterin in normal pregnant and non-pregnant women and in women with benign and malignant genital tract neoplasms. Archives of Gynecology. 1983;233:121–130. doi: 10.1007/BF02114788. [DOI] [PubMed] [Google Scholar]
- 29.Fuith LC, Fuchs D, Hausen A, et al. Neopterin, a marker of cell-mediated immune activation in human pregnancy. International Journal of Fertility. 1991;36:372–375. [PubMed] [Google Scholar]
- 30.Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstetrics & Gynecology. 1992;79:19–26. [PubMed] [Google Scholar]
- 31.Kaleli I, Kaleli B, Demir M, Yildirim B, Cevahir N, Demir S. Serum levels of neopterin and interleukin-2 receptor in women with severe preeclampsia. Journal of Clinical Laboratory Analysis. 2005;19:36–39. doi: 10.1002/jcla.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrocksnadel H, Fuchs D, Herold M, Wachter H, Dapunt O. Activated macrophages in the pathologic mechanism of pregnancy-induced hypertension. Zentralblatt fur Gynakologie. 1994;116:274–275. [PubMed] [Google Scholar]
- 33.Schrocksnadel H, Herold M, Steckel-Berger G, Fuchs D, Wachter H, Dapunt O. Cellular simmunity in pregnancy-induced hypertensive diseases. Geburtshilfe Und Frauenheilkunde. 1992;52:592–595. doi: 10.1055/s-2007-1023190. [DOI] [PubMed] [Google Scholar]
- 34.Kronborg CS, Knudsen UB, Moestrup SK, Allen J, Vittinghus E, Moller HJ. Serum markers of macrophage activation in pre-eclampsia: no predictive value of soluble CD163 and neopterin. Acta Obstetricia et Gynecologica Scandinavica. 2007;86:1041–1046. doi: 10.1080/00016340701415236. [DOI] [PubMed] [Google Scholar]
- 35.Schennach H, Murr C, Gachter E, Mayersbach P, Schonitzer D, Fuchs D. Factors influencing serum neopterin concentrations in a population of blood donors. Clin Chem. 2002;48:643–645. [PubMed] [Google Scholar]
- 36.Werner ER, Bichler A, Daxenbichler G, et al. Determination of neopterin in serum and urine. Clinical Chemistry. 1987;33:62–66. [PubMed] [Google Scholar]