Summary
Tuberculosis (TB) is an occupational disease of healthcare workers (HCWs). Administrative and engineering interventions simultaneously implemented in hospitals of developed countries have reduced the risk of nosocomial transmission of M. tuberculosis. We have studied the impact of administrative infection control measures on the risk for latent TB infection (LTBI) among HCWs in a resource-limited, high-burden country. An intervention study was undertaken in a university-affiliated, inner-city hospital in Rio de Janeiro, where routine serial tuberculin skin test (TST) is offered to all HCWs. From October 1998 to February 2001, the following administrative infection control measures were progressively implemented: isolation of TB suspects and confirmed TB inpatients, quick turnaround for acid-fast bacilli sputum tests and HCW education in use of protective respirators. Among 1336 initially tested HCWs, 599 were retested. The number of TST conversions per 1000 person-months during and after the implementation of these measures was reduced from 5.8/1000 to 3.7/1000 person-months (P = 0.006). The most significant reductions were observed in the intensive care unit (from 20.2 to 4.5, P < 0.001) and clinical wards (from 10.3 to 6.0, P < 0.001). Physicians and nurses had the highest reductions (from 7.6 to 0, P < 0.001; from 9.9 to 5.8, P = 0.001, respectively). We conclude that isolated administrative measures for infection control can significantly reduce LTBI among HCWs in high-burden countries and should be implemented even when resources are not available for engineering infection control measures.
Keywords: Infection control, Health personnel, Nosocomial infection, Occupational exposure, Tuberculin test, Tuberculosis
Introduction
Nosocomial transmission of tuberculosis (TB) to healthcare workers (HCWs) was of historic interest until the 1990s, when nosocomial outbreaks of multidrug-resistant TB (MDR-TB) in the USA indicated that it remained an important occupational hazard.1,2 Since then, the Centers for Disease Control and Prevention and the World Health Organization have recommended infection control measures such as respiratory isolation rooms with negative pressure for patients with productive cough and use of personal respiratory protective equipment, i.e. respirators.3,4 Different infection control measures, usually administrative and engineering simultaneously, have been implemented in industrialised countries and resulted in reduced nosocomial tuberculosis transmission.3,5–7 However, the role of individualised infection control measures is difficult to establish. The implementation of all these measures may be neither feasible nor cost-effective in resource-limited nations.2,8 In particular, engineering measures such as negative pressure isolation rooms with high efficiency particulate air (HEPA) filters may be unaffordable. The objective of this study was to evaluate the impact of administrative measures for control of TB transmission in a teaching hospital in Rio de Janeiro, a city with a TB incidence rate 100 per 100 000. Since the rate of tuberculin skin test (TST) conversion among hospital employees is the cornerstone for estimation of the adequacy of tuberculosis control methods, we evaluated the TST conversion rate among HCWs and other hospital employees before and after the implementation of these administrative measures.
Methods
Setting
Clementino Fraga Hospital is located in the north of Rio de Janeiro City, in an area with a 140 per 100 000 inhabitants TB rate. The hospital has 560 clinical and surgical beds and employs ~3400 persons. There is a high turnover of HCWs, with a mean of 60 new employees hired every year. There are no paediatric or obstetric–gynaecological wards. The HCWs’ TST conversion rate before the study was 8.7% per year from 1994 to 1997, or 5.9/1000 person-months. This was considered a high risk when compared to the general population (0.83/1000 person-months in Rio de Janeiro), and so a hospital TB control programme (TCP) was created in 1998. From 1999 to 2001, 197 pulmonary TB cases were diagnosed in the hospital, and 272 in 2002–2003.
Infection control measures
Starting in October 1998, consecutive administrative infection control measures were implemented. A mycobacteriological laboratory was created. The hospital TCP ensured that all patients with sputum sent to the laboratory for acid-fast bacilli (AFB) smear and/or mycobacterial culture were in isolation rooms. This policy required the conversion of two-bed rooms into isolation single rooms. Due to scarce resources, isolation rooms were built without negative pressure. Patients with productive cough were placed in isolation until at least sputum smears were AFB negative. Patients with known human immunodeficiency virus (HIV) infection and abnormal chest radiograms were also isolated. Appropriate isolation procedures were adopted. In particular, all individuals entering the room were required to wear an N-95 respirator. Patients leaving the room for diagnostic tests were required to wear a protective surgical mask, and were educated on cough etiquette and respiratory hygiene by the ward nurse.
In addition, an educational programme on TB was offered to HCWs, especially targeting nurses, residents, attending physicians, visitors and cleaning staff. Personal protective N-95 particulate respirators were offered and their use recommended during the educational programme, which included specific instructions on respirator wearing, maintenance and re-use. No fit-testing of masks or respirators was performed.
The hospital TCP established a ‘one-stop service’ at the TB outpatient clinic, offering registration, pharmacy supplies, accounting and chest radiograms in the same place. All close contacts were investigated and latent TB infection (LTBI) treated when appropriate (adult contact tracing is not routine in Brazil except for high risk individuals). Finally, in 2004 an isolation room with negative pressure was built in the area where the highest risk had previously been detected, the intensive care unit.
Review of medical records and evaluation of isolation rooms
We reviewed patients’ medical records when any respiratory specimen was AFB or M. tuberculosis culture-positive during the study period. Time elapsed from admission to first request of AFB sputum examination, to AFB results, and to date of first treatment prescription was observed.
Tuberculin skin testing and data collection
HCWs were submitted to annual serial TSTs, which were performed by trained professionals using the Mantoux technique. Training included an inter-observer concordance with a standardised trainer of ≥80% out of 100 blinded readings. Whenever possible (compliance of employee), a two-step TST was performed. A positive TST was defined as an induration of ≥10 mm in the initial one- or two-step TST. TST conversion was defined as an increase in the induration of ≥10 mm over the preceding TST for HCWs with an initial two-step TST and an increase of ≥15 mm among HCWs with an initial one-step TST.9 A self-reported questionnaire was also answered by HCWs. Data on age, sex, history of bacillus Calmette–Guérin (BCG) vaccination, tuberculosis exposure, occupation, duration of employment, and work location at time of TST conversion were collected. The presence of BCG scars was assessed by the same trained professional. HCWs with a positive baseline TST or with TST conversion were tested for active TB. When appropriate, LTBI treatment was recommended.
Data analysis
We arbitrarily considered that a minimum of three years would be necessary for the control measures to be effective. Thus, we compared the rates of TST conversion of our first cohort, carried out from February 1999 to December 2001 (first TST from January 1998 to May 1999), to the rates of our second cohort, carried out from September 2002 to December 2003 (first TST from February 2001 to February 2003).
Rates of TST conversion were analysed by work sector and occupation of the HCW. The ‘laboratory’ group included employees from the laboratory, blood bank, radiology and pathology. The ‘physician’ group included staff, assistants and medical residents. The ‘nurse’ group included nurses and nurse aids. The ‘social service’ group included employees from pastoral care, occupational and physical therapy and social services. The ‘housekeeping’ group included personnel from housekeeping, security, engineering and maintenance, laundry, nutrition, and transportation sectors. The ‘administrative clerk’ group included workers from finance, data processing, accounting, medical records, human resources, mailroom, telecommunications, pharmacy, purchasing, and central supply sectors. This latter group was considered the non-exposed group.
Person-days of follow-up were calculated for each individual. TST conversion rates were expressed as the number of TST conversions per 1000 person-months of observation, assuming a uniform distribution of the probability for TST conversion between the last negative TST performed and a documented TST conversion. Differences in TST conversion rates between the two study periods (1999–2001 and 2002–2003) were compared using the exact mid-P probabilities.10 Hazard ratios (crude and adjusted) were also calculated by univariate and multivariate analyses. Finally, the probability of remaining TST negative during both periods was calculated using the Cox regression model.
Results
A total of 406 employees in the first period (1999–2001) and 193 in the second period (2002–2003) were tested, corresponding to 17% of all employees. Among them, 40 converted during the 8351 person-month follow-up. The overall TST conversion rate decreased from 5.8/1000 person-months in the first period to 3.7/1000 person-months in the second period (P = 0.006). The remaining 737 HCWs were lost to follow-up.
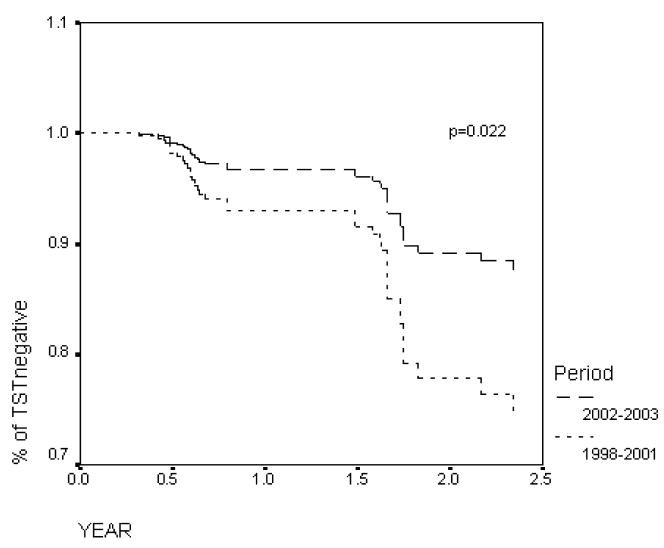
Table I displays the characteristics of the HCWs followed during both study periods. During the first period, 25 TST conversions were observed, 24 after a two-step first TST and one after a one-step TST (15 mm increase over the first test). During the second period, 15 conversions were observed, 11 of them after a two-step TST. Figure 1 displays the probability of remaining TST negative over time during both periods.
Table I.
Characteristics of 599 healthcare workers tested during and after the implementation of administrative M. tuberculosis infection control measures
| Characteristics | 1999–2001 | 2002–2003 | P value |
|---|---|---|---|
| Age (years) (mean ± SD) | 37.3 ± 10.1 | 36.4 ± 11.7 | 0.334 |
| Sex | 0.024 | ||
| Male | 142 (35.0%) | 49 (25.4%) | |
| Female | 264 (65.0%) | 144 (74.6%) | |
| Employment duration (years) (mean ± SD) | 12.5 ± 6.3 | 10.3 ± 8.4 | <0.001 |
| Occupation | |||
| Administrative clerk | 139 (34.2%) | 50 (25.9%) | 0.040 |
| Nurse | 101 (24.9%) | 75 (38.9%) | <0.001 |
| Physician | 67 (16.5%) | 18 (9.3%) | 0.187 |
| Housekeeping | 41 (10.1%) | 26 (13.5%) | 0.221 |
| Social worker | 32 (7.9%) | 9 (4.7%) | 0.145 |
| Laboratory/radiology technician | 26 (6.4%) | 15 (7.8%) | 0.535 |
| Work location | |||
| Administration | 171 (42.1%) | 53 (27.5%) | <0.001 |
| Clinical ward | 94 (23.2%) | 59 (30.6%) | 0.052 |
| Surgery ward | 65 (16.0%) | 25 (13.0%) | 0.329 |
| Outpatient clinics | 33 (8.1%) | 5 (2.6%) | 0.009 |
| Radiology/laboratory/pharmacy | 26 (6.4%) | 15 (7.7%) | 0.535 |
| Intensive care unit | 17 (4.2%) | 18 (9.3%) | 0.012 |
Figure 1.
Observed probability of no tuberculin skin test (TST) conversion in hospital employees by study period (dashed line: 1998–2001; solid line: 2002–2003). Difference between periods: P = 0.022.
Table II displays the reduction in TST conversion rate according to HCWs’ characteristics. A significant reduction of the TST conversion was observed in the intensive care unit and in clinical wards. Significant reductions were observed among physicians and nurses. Finally, absence of BCG scars and of history of exposure to pulmonary TB in the hospital were associated with a decrease in TST conversion. Table III shows crude and adjusted hazard ratios (HR) for TST conversion after the implementation of control measures. On multivariate analysis, the HR remained higher among nurses, but HCWs exposed to pulmonary TB patients were protected from conversion.
Table II.
Number of recent tuberculin skin test (TST) conversions per 1000 person-months during (1999–2001) and after (2002–2003) the implementation of administrative M. tuberculosis infection control measures
| Characteristics |
N/months (conversions/1000 person-months; 95% CI) |
Mid-P value | |
|---|---|---|---|
| 1999–2001 | 2002–2003 | ||
| Overall | 25/4307 (5.8; 4.9–6.7) | 15/3858 (3.7; 2.8–4.6) | 0.006 |
| Sex | |||
| Male | 5/1721 (2.9; 1.1–4.7) | 9/976 (3.0; 1.9–4.1) | 0.928 |
| Female | 20/2586 (7.7; 6.6–8.8) | 6/2881 (5.6; 3.9–7.3) | 0.132 |
| Occupation | |||
| Physician | 5/662 (7.6; 6.3–8.9) | 0/305 (0.0; 0.0–3.5) | <0.001 |
| Nurse | 10/1013 (9.9; 8.1–11.7) | 9/1585 (5.8; 4.3–7.3) | 0.001 |
| Social worker | 1/301 (3.3; 0.4–6.2) | 0/188 (0.0; 0.0–4.1) | 0.279 |
| Laboratory/radiology technician | 2/281 (7.1; 3.4–10.8) | 1/279 (3.6; 1.4–5.8) | 0.218 |
| Housekeeper | 3/485 (6.2; 2.7–9.7) | 2/518 (3.5; 1.8–5.2) | 0.250 |
| Administrative clerk | 4/1566 (2.6; 0.8–4.4) | 3/1018 (2.6; 1.1–3.7) | 0.995 |
| Work location | |||
| Outpatient clinic | 2/304 (6.3; 3.5–9.1) | 0/118 (0.0; 0.0–7.1) | 0.116 |
| Clinical ward | 8/780 (10.3; 9.2–11.4) | 6/1015 (6.0; 4.4–7.6) | <0.001 |
| Intensive care unit | 4/198 (20.2; 15.1–25.3) | 2/424 (4.5; 2.2–6.8) | <0.001 |
| Surgery ward | 2/692 (2.9; 0.5–5.3) | 1/561 (1.7; 0.0–4.1) | 0.409 |
| Radiology/laboratory/pharmacy | 1/250 (4.0; 0.8–7.2) | 3/314 (9.6; 8.5–10.7) | <0.001 |
| Others | 8/2071 (3.9; 2.2–5.6) | 3/1089 (2.4; 1.1–3.7) | 0.340 |
| Ward with respiratory isolation room | |||
| No | 20/3839 (5.2; 4.1–6.3) | 12/2672 (4.3; 3.3–5.3) | 0.336 |
| Yes | 5/419 (11.9; 9.7–14.1) | 3/764 (3.8; 1.7–5.9) | <0.001 |
| BCG scara | |||
| No | 6/665 (9.0; 6.9–11.1) | 3/1083 (2.6; 1.3–3.9) | <0.001 |
| Yes | 18/3542 (5.1; 4.0–6.2) | 8/1969 (3.9; 2.6–5.2) | 0.266 |
| Exposure to pulmonary TB case in hospitalb | |||
| No | 6/461 (13.0; 11.6–14.4) | 6/974 (5.6; 3.9–7.3) | <0.001 |
| Yes | 11/1661 (6.6; 5.1–8.1) | 8/1997 (4.0; 2.7–5.3) | 0.018 |
BCG, bacillus Calmette–Guérin; TB, tuberculosis.
Missing data: one in period 1999–2001 and four in period 2002–2003.
Missing data: eight in period 1999–2001 and one in period 2002–2003.
Table III.
Crude and adjusted hazard risks for recent tuberculin skin test conversion after the implementation of administrative M. tuberculosis infection control measures (2002–2003)
| Characteristics | Crude risk (95% CI) | Adjusted riska (95% CI) |
|---|---|---|
| Overall | ||
| 1998–2001 | 1 | 1 |
| 2002–2003 | 0.46 (0.23–0.89) | 0.24 (0.10–0.54) |
| Sex | ||
| Male | 1 | 1 |
| Female | 1.26 (0.63–2.53) | 1.17 (0.53–2.56) |
| Occupation | ||
| Administrative clerk | 1 | 1 |
| Physician | 1.90 (0.60–6.04) | 1.85 (0.49–6.99) |
| Nurse | 2.52 (1.05–6.00) | 3.97 (0.95–10.61) |
| Social worker | 0.72 (0.88–5.86) | 0.76 (0.09–6.48) |
| Laboratory/radiology technician | 1.91 (0.94–7.40) | 1.63 (0.36–7.33) |
| Housekeeper | 1.77 (0.56–5.59) | 1.79 (0.55–5.83) |
| Work location | ||
| Others | 1 | 1 |
| Outpatient clinic | 1.36 (0.30–6.14) | 0.54 (0.10–2.87) |
| Clinical ward | 2.40 (1.08–5.34) | 2.41 (0.79–7.37) |
| Intensive care unit | 2.52 (0.31–6.83) | 2.01 (0.54–7.41) |
| Surgery ward | 0.65 (0.18–2.34) | 0.37 (0.09–1.57) |
| Radiology/laboratory/pharmacy | 1.81 (0.57–5.68) | 1.51 (0.41–5.62) |
| Ward with respiratory isolation room | ||
| No | 1 | 1 |
| Yes | 1.32 (0.61–2.87) | 0.65 (0.26–1.62) |
| BCG scar | ||
| No | 1 | 1 |
| Yes | 1.00 (0.47–1.15) | 0.78 (0.35–1.77) |
| Exposure to pulmonary TB case in hospital | ||
| No | 1 | 1 |
| Yes | 0.59 (0.28–1.28) | 0.31 (0.13–0.73) |
CI, confidence interval; BCG, bacillus Calmette–Guérin; TB, tuberculosis.
Adjusted for exposure to pulmonary TB case and professional category.
Time elapsed between bacilloscopy request and result received by attending physicians decreased from 6.5 days before 1998 to 8 h by the end of 1999. Out of 1573 suspected cases isolated during both study periods, 469 (35.4%) had pulmonary tuberculosis subsequently diagnosed: 197 in the 1999–2001 period and 272 in the 2002–2003 period, corresponding to a 38.1% increase.
Active TB developed in 32 HCWs (914 per 100 000 incidence rate), of whom 22 had pulmonary TB, four had pleural TB and six had extrapulmonary TB. Five of these 32 HCWs had been previously tuberculin-tested. Among them, three had a positive TST with an induration of >15 mm at baseline, one was diagnosed with TB during investigation of a TST conversion and one had negative serial TSTs.
Discussion
Because prevention of TB nosocomial transmission to HCWs requires financial investment, few studies in resource-limited countries have evaluated the role of different strategies for infection control.11–13 According to a recently published systematic review of TB in HCWs in low- and middle-income countries, Joshi et al. reported that the effectiveness of administrative and engineering measures for TB transmission control varied widely.14 No consistent data on development of TB are available; but the rate of LTBI dropped after implementation of such measures. In Malawi, with a varying degree of compliance with multiple administrative measures in 40 hospitals, there was a non-significant decrease in TB cases among HCWs after one year.12 In a hospital in Thailand, although LTBI incidence rate decreased from 9.3% to 2.2% one to two years after implementation of administrative, personal self-protective and engineering infection-control measures, the incidence of active disease did not and the duration of follow-up was too limited to draw conclusions.11 LTBI rates also dropped in four hospitals in Brazil after the implementation of administrative and engineering measures.13 The use of personal protective equipment in such settings is poor and failure to use respirators is associated with the risk of LTBI.14,15 Joshi et al. concluded that there is limited available evidence suggesting that a reduction in the risk of TB infection is possible with simple administrative control measures, but this would need to be evaluated in larger, better-controlled studies.14
In our study, multiple consecutive administrative and personal infection-control measures were implemented and reduced significantly the annual risk of LTBI. Because of the short time between the initiation of these measures and the long time needed to evaluate its effectiveness on TB nosocomial transmission, the role of each of these measures could not be clearly separated. Nevertheless, no engineering measures were implemented until 2004, when a negative pressure room was built in the intensive care unit. Thus, simple personal and administrative measures were likely to be responsible for the observed drop in the annual risk for LTBI. The protection against TST conversion among HCWs exposed to pulmonary TB suggests that educational measures can be particularly useful. To our knowledge, this is the first study to demonstrate the reduction in nosocomial transmission of LTBI with isolated administrative and personal self-protective measures.
Our results strongly suggest that simple administrative and personal self-protective measures, which are inexpensive and easy to implement in resource-limited countries, can be efficient in reducing occupational acquired LTBI. The reduction was greater in highly exposed professionals: those with closer contact with patients and those who work in places with a high TB incidence. Nevertheless, despite the significant reduction in risk, nurses remained at risk.
Unlike previous studies, which found a high rate of conversion among this category, housekeepers had an intermediate risk for LTBI, before as well as after implementation of preventive measures, an unexpected finding.5,16 This category has the lowest socio-economic status among all analysed categories. Thus, outside hospital LTBI was anticipated to account for a higher rate of LTBI. A high prevalence of cumulative (previous) LTBI at first TST (47.5 at 1998 and 41.7% at 2002) could explain the lack of increment. Louther et al. found that the place of residence was not a confounding factor for professional category exposure. Unfortunately, we do not have this information available for our HCWs.5
We adopted a wide range of isolation criteria, and compliance with this policy was attested by the relatively high percentage of isolation of patients finally diagnosed with active pulmonary TB and lower active TB cases diagnosed in the wards. This percentage is higher than those described in resource-limited countries, and demonstrates that compliance is feasible even in high-burden country hospitals.17,18
One strength of the present study was the careful training of TST readers and the stringent criteria for TST conversion, necessary to avoid misinterpretation (false conversion) of serial TST in BCG-vaccinated populations, in whom a high rate of the booster phenomenon has been documented.9 On the other hand, the study was limited by the participation of <20% of the hospital personnel, which may have resulted in underestimation of the TST conversion rate. Since infection control is not mandatory according to the Brazilian national TB guidelines, only voluntary HCWs were included.19 Exhaustion of susceptible personnel as reported by Fella et al. is unlikely, because of the small number of participants.7 Another potential limitation to this study was the unknown HIV status of participants. Because of the increasing rates of skin test anergy expected as immunity wanes due to progressive HIV infection, both the prevalence of baseline tuberculin reactivity and the rate of TST conversion may have been underestimated.
Another limitation was the absence of a parallel control hospital, with no implementation of these or any measures. However, it is unlikely that the observed reduction in TST conversion was due to reduced TB exposure: the incidence rate of TB in the city has not significantly decreased in the study period, and in-hospital exposure increased by 38%. Although the populations in the two study periods differed significantly in some respects, the differences should have increased, not decreased, the rate of ILTB, since more nurses and more HCWs from highly exposed workplaces were included in the second period.
Finally, studies that rely on TST as indicator of LTBI have known limitations. The association between BCG/history of TB with absence of conversion reduction suggests that part of these conversions may represent boosting, not actual recently acquired LTBI. Nevertheless, TST is still the gold standard for LTBI diagnosis. Future studies using interferon-γ release assays may overcome problems in the identification of the most cost-effective measures for TB infection control in hospitals.20
In conclusion, simple administrative and personal self-protective measures such as isolation of patients and educational programmes can significantly reduce the risk for LTBI among HCWs, although other measures may contribute to attain optimal control. These interventions cannot be sustained without the full support of local and national health authorities. Therefore, there is an urgent need for the implementation of specific laws, which could bring a conceptual legal framework for the systematic application of effective public health policies in resource-limited nations regarding the protection of HCWs against TB and other nosocomial transmitted diseases.
Acknowledgments
We thank R. Raggio Luiz for assistance with statistical analysis.
Funding sources
The study was supported by NIH (ICOHRTA # 5 U2R TW006883–02), by Conselho Nacional de Ensino e Pesquisa-CNPq- Process: 524523/96–7; and by the Academic Tuberculosis Program at Federal University of Rio de Janeiro.
Footnotes
Conflict of interest statement
None declared.
Author query
Tables, P values: please clarify whether any data values should be indicated as significant by using footnote symbols * (P < 0.05) or ** (P < 0.001). (Unexplained bold highlighting has been removed.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pearson ML, Jereb JA, Frieden TR, et al. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis. A risk to patients and health care workers. Ann Intern Med. 1992;117:191–196. doi: 10.7326/0003-4819-117-3-191. [DOI] [PubMed] [Google Scholar]
- 2.Griffith DE, Hardeman JL, Zhang Y, Wallace RJ, Mazurek GH. Tuberculosis outbreak among healthcare workers in a community hospital. Am J Respir Crit Care Med. 1995;152:808–811. doi: 10.1164/ajrccm.152.2.7633747. [DOI] [PubMed] [Google Scholar]
- 3.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 4.Granich R, Binkin NJ, Jarvis WR, et al. Guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings. Geneva: World Health Organization; 1999. p. 51. WHO/CDS/TB/99.269. [Google Scholar]
- 5.Louther J, Rivera P, Feldman J, Villa N, Dehovitz J. Risk of tuberculosis conversion according to occupation among health care workers at a New York city hospital. Am J Resp Crit Care Med. 1997;156:201–205. doi: 10.1164/ajrccm.156.1.9611091. [DOI] [PubMed] [Google Scholar]
- 6.Fennelly KP, Iseman MD. Health care workers and tuberculosis: the battle of a century. Int J Tuberc Lung Dis. 1999;3:363–364. [PubMed] [Google Scholar]
- 7.Fella P, Rivera P, Hale M, Squires K, Sepkowitz K. Dramatic decrease in tuberculin skin test conversion rate among employees at a hospital in New York City. Am J Infect Control. 1995;23:352–356. doi: 10.1016/0196-6553(95)90265-1. [DOI] [PubMed] [Google Scholar]
- 8.Harries AD, Maher D, Nunn P. Practical and affordable measures for the protection of health care workers from tuberculosis in low-income countries. Bull WHO. 1997;75:477–789. [PMC free article] [PubMed] [Google Scholar]
- 9.Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15–21. doi: 10.1164/ajrccm.159.1.9801120. [DOI] [PubMed] [Google Scholar]
- 10.Barnard GA. On alleged gains in power from lower p-values. Stat Med. 1989;8:1469–1477. doi: 10.1002/sim.4780081206. [DOI] [PubMed] [Google Scholar]
- 11.Yanai H, Limpakarnjanarat K, Uthaivoravit W, et al. Risk of Mycobacterium tuberculosis infection and disease among health care workers, Chiang Rai, Thailand. Int J Tuberc Lung Dis. 2003;7:36–45. [PubMed] [Google Scholar]
- 12.Harries AD, Hargreaves NJ, Gausi F, Kwanjana JH, Salaniponi FM. Preventing tuberculosis among health workers in Malawi. Bull WHO. 2002;80:526–531. [PMC free article] [PubMed] [Google Scholar]
- 13.Roth VR, Garrett DO, Laserson KF, et al. A multicenter evaluation of tuberculin skin test positivity and conversion among health care workers in Brazilian hospitals. Int J Tuberc Lung Dis. 2005;9:1335–1342. [PubMed] [Google Scholar]
- 14.Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med. 2006;3:e494. doi: 10.1371/journal.pmed.0030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harries AD, Nyirenda TE, Banerjee A, Boeree MJ, Salaniponi FM. Tuberculosis in health care workers in Malawi. Trans R Soc Trop Med Hyg. 1999;93:32–35. doi: 10.1016/s0035-9203(99)90170-0. [DOI] [PubMed] [Google Scholar]
- 16.Lowenthal G, Keys T. Tuberculosis surveillance in hospital employees: are we doing too much? Infect Control. 1986;7:209–211. doi: 10.1017/s0195941700083958. [DOI] [PubMed] [Google Scholar]
- 17.Wisnivesky JP, Serebrisky D, Moore C, Sacks HS, Iannuzzi MC, McGinn T. Validity of clinical prediction rules for isolating inpatients with suspected tuberculosis. A systematic review. J Gen Intern Med. 2005;20:947–952. doi: 10.1111/j.1525-1497.2005.0185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumberg HM, Watkins DL, Berschling JD, et al. Preventing the nosocomial transmission of tuberculosis. Ann Intern Med. 1995;122:658–663. doi: 10.7326/0003-4819-122-9-199505010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Castelo Filho A, Kritski AL, Barreto AW, et al. II Consenso Brasileiro de Tuberculose: Diretrizes Brasileiras para Tuberculose 2004. J Bras Pneumol. 2004;30(Suppl 1):57–86. [Google Scholar]
- 20.Pai M, Joshi R, Dogra S, et al. Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report. Occup Med Toxicol. 2006;1:7. doi: 10.1186/1745-6673-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]