Abstract
In the human genome the APOBEC3 gene has expanded into a tandem array of genes termed APOBEC3A-H. Several members of this family have potent anti-HIV-1 activity. Here we demonstrate that APOBEC-3B/3C/3F and -3G are expressed in all major cellular components of the CNS. Moreover, we show that both IFN-α and IFN-γ significantly enhance the expression of APOBEC-3G/3F and drastically inhibit HIV-1 replication in primary human brain microvascular endothelial cells (MVECS), the major component of blood brain barrier (BBB). As the viral inhibition can be neutralized by APOBEC3G-specific siRNA, APOBEC3G plays a key role to mediate the anti-HIV-1 activity of IFN-α and/or IFN-γ. Our findings suggest that, in addition to the restriction at viral entry level, the restriction from APOBEC3 family could account for the low-level replication of HIV-1 in MVECS. The manipulation of IFN-APOBEC3 signaling pathway could be a potent therapeutic strategy to prevent HIV invasion to central nervous system.
Introduction
Cellular APOBEC3G (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G) belongs to a family of proteins possessing cytidine deaminase activity. Although for most of them, including APOBEC3A, -3G, -3F, -3B, –3C, –3H, as well as the newly described APOBEC3DE, the normal functions in host cells are still unknown, they have recently been identified to potently inhibit the replication of various retroviruses including human immunodeficiency virus type 1 (HIV-1), simian immunodeficiency virus (SIV), equine infectious anemia virus (EIAV), murine leukemia virus (MLV), foamy virus, as well as hepatitis B virus (HBV) (Dang et al., 2006; Delebecque et al., 2006; Harris et al., 2003; Jarmuz et al., 2002; Kobayashi et al., 2004; Mangeat et al., 2003; Mariani et al., 2003; Navarro et al., 2005; OhAinle et al., 2006; Rosler et al., 2005; Russell et al., 2005; Sasada et al., 2005; Seppen, 2004; Sheehy et al., 2002; Suspene et al., 2005; Zhang et al., 2003). APOBEC3G, which is a powerful antiretroviral host-restriction factor, can either enzymatically edit the newly-synthesized viral DNA or exert an inhibitory effect through a possible non-enzymatic activity at other site(s) of viral life cycle (Chiu et al., 2005; Harris et al., 2003; Luo et al., 2007; Mangeat et al., 2003; Mbisa et al., 2007; Newman et al., 2005; Zhang et al., 2003). For surviving, retroviruses encode various gene products to counteract the inhibition of cytidine deaminases. In the case of HIV-1 and many other lentiviruses, virion infectivity factor (Vif) is encoded to effectively neutralize the anti-viral effect of APOBEC3G, APOBEC3F and others by facilitating the degradation of these cytidine deaminases (Marin et al., 2003; Sheehy et al., 2002; Sheehy, Gaddis, and Malim, 2003; Yu et al., 2003; Zheng et al., 2004). Recent studies, including reports from our laboratories, have demonstrated that APOBEC3G can restrict the replication of incoming viruses in the resting CD4+ T-cells and myeloid dendritic cells (MDCs) (Chen et al., 2006; Chiu et al., 2005; Pion et al., 2006; Stopak et al., 2007). As there is only a trivial amount of virion-associated Vif at the early phase of viral life cycle, this anti-viral activity of APOBEC3G is most likely Vif-unrelated. APOBEC3G exists in two different forms in various cell systems. A low molecular mass (LMM) form that is associated with HIV-1 restriction and a high molecular mass (HMM) complex that lacks enzymatic activity as well as anti-HIV-1 activity (Chen et al., 2006; Chiu et al., 2005; Stopak et al., 2007).
We have recently demonstrated that through a regular IFN-α/β signal transduction pathway, IFN-α can significantly enhance the expression of APOBEC3G in human primary resting but not activated CD4+ T cells and the amounts of APOBEC3G associated with a low molecular mass (LMM) (Chen et al., 2006), albeit different opinions exist (Sarkis et al., 2006; Stopak et al., 2007). Treatment of newly-infected resting CD4+ T cells with IFN-α resulted in significant inactivation of HIV-1 infection, and this inhibitory effect can be counteracted by APOBEC3G-specific short interfering RNA (siRNA), indicating that IFN-α-induced APOBEC3G plays a key role in mediating this anti-HIV-1 process (Chen et al., 2006). Moreover, our most-recent findings show that APOBEC3G and its family members can be upregulated by IFN-α, either exogenously added or endogenously secreted by plasmacytoid dendritic cells (pDCs), and that IFN-α exerts a potent anti-HIV-1 activity in pDCs. Likewise, this inhibitory effect can be neutralized by APOBEC3G-specific siRNA (our unpublished data). Recent studies demonstrate that APOBEC3G and APOBEC3F can be upregulated by a variety of cytokines, including IFNs, in various primary cell systems following distinct patterns of cytokine regulation (Bonvin et al., 2006; Chen et al., 2006; Peng et al., 2006; Sarkis et al., 2006; Stopak et al., 2007; Tanaka et al., 2006; Ying et al., 2007). IFN-α has been shown to exert its antiviral activity through multiple pathways, including PKR (dsRNA dependent protein kinase)/eukaryotic initiation factor-2 (eIF-2), oligoadenylate synthetase (OAS), RNase L, adenosine Deaminase(ADAR1), and protein GTPase Mx/nitric oxide synthease (NOS2) (Samuel, 2001). In addition to these anti-viral pathways, IFN-α exerts its anti-HIV-1 activity through a newly discovered host-defense mechanism involving APOBEC3G in viral target cells such as resting CD4+ T cells, macrophages, myeloid dendritic cells, and pDCs (Chen et al., 2006; Peng et al., 2006; Tanaka et al., 2006).
The central nervous system (CNS) represents another important target for viral invasion. HIV-1 enters the CNS early after infection and can cause serious impairment of motor and cognitive skills known as AIDS dementia complex (Navia, Jordan, and Price, 1986; Spencer and Price, 1992; Tardieu and Boutet, 2002). HIV-1 is known to productively replicate in macrophages and microglia of the CNS, but it also infects, via a CD4-independent pathway, astrocytes and most-importantly brain microvascular endothelial cells (BMVECs), which represent the major component of the blood brain barrier (BBB) (Argyris et al., 2003; Bagasra et al., 1996; Bissel and Wiley, 2004; Bobardt et al., 2004; Liu et al., 2002; Ludwig et al., 1999; Tornatore et al., 1994). Recent studies have indicated that the human mannose receptor is essential for HIV-1 entry into astrocytes, while we and other independent investigators have demonstrated that cell associated heparan and/or chondroitin sulfate proteoglycans are involved in viral entry into BMVECs (Argyris et al., 2003; Bobardt et al., 2004; Liu et al., 2002; Liu et al., 2004). Although, it is generally accepted that neurons do not become infected by HIV-1, recent reports have shown that neuronal progenitor brain cells can be infected by various viral strains (Lawrence et al., 2004). Altogether, these findings strongly suggest the importance of the CNS and its specific cellular components as targets of persistent infection and a viral reservoir.
Until recently, no study had examined the expression and distribution of the important host-defense factor, APOBEC3G, as well as other APOBEC3 family members, including their regulation in the brain, with the exception of a recent single report by Hill et al. demonstrating that expression of APOBEC3G is restricted to neurons in the brains of pigtailed macaques (Hill et al., 2006). In our current study, for the first time we show that a variety of APOBEC3 family genes are widely expressed in human CNS, namely in primary human BMVECS, astrocytes and differentiated post-mitotic mature neurons. Moreover, both IFN-α and IFN-γ can significantly induce the expression of APOBEC-3G/3F and potently inhibit HIV-1 replication in primary human BMVECS. Viral inhibition can be neutralized by APOBEC3G-specific siRNA, indicating that IFN-α and/or IFN-γ-induced APOBEC3G plays a key role as innate immunity against retroviral infection.
Results
Expression of APOBEC3 family genes in the human CNS
We initiated our studies by examining the mRNA levels and protein expression of different APOBEC3 family members in the human CNS, including primary human BMVECs, primary human astrocytes and post-mitotically differentiated neurons. As controls in our studies we also included H9 cells, which are known to express high-levels of APOBEC3G and APOBEC3F, as well as 293T cells (negative control) (Sheehy et al., 2002). All CNS-based cell systems were cultured, maintained, and passaged by using specially designed media and following standard procedures in our research group(Acheampong et al., 2007; Argyris et al., 2003; Fang et al., 2005; Moses et al., 1993; Mukhtar et al., 2000; Mukhtar et al., 2002; Mukhtar and Pomerantz, 2000; Zhou et al., 2003). CNS-cells were well characterized for the expression of specific markers. Primary human BMVECs were positive for expressing von Willebrand factor (Fig. 1A), and Zonula Occludens-1 (ZO-1) (Fig. 1B). Primary human brain astrocytes were monitored for expressing glial fibrillary acidic protein (GFAP), (Fig. 1C), as well as for cytoskeletal structural proteins (Fig. 1D). The morphology of post-mitotically differentiated neurons was monitored carefully by examining the expression of neuroepithelial marker microtubule-associated protein (MAP-2) (Fig. 1E).
Figure 1. Primary human CNS-based cell systems used in our studies.
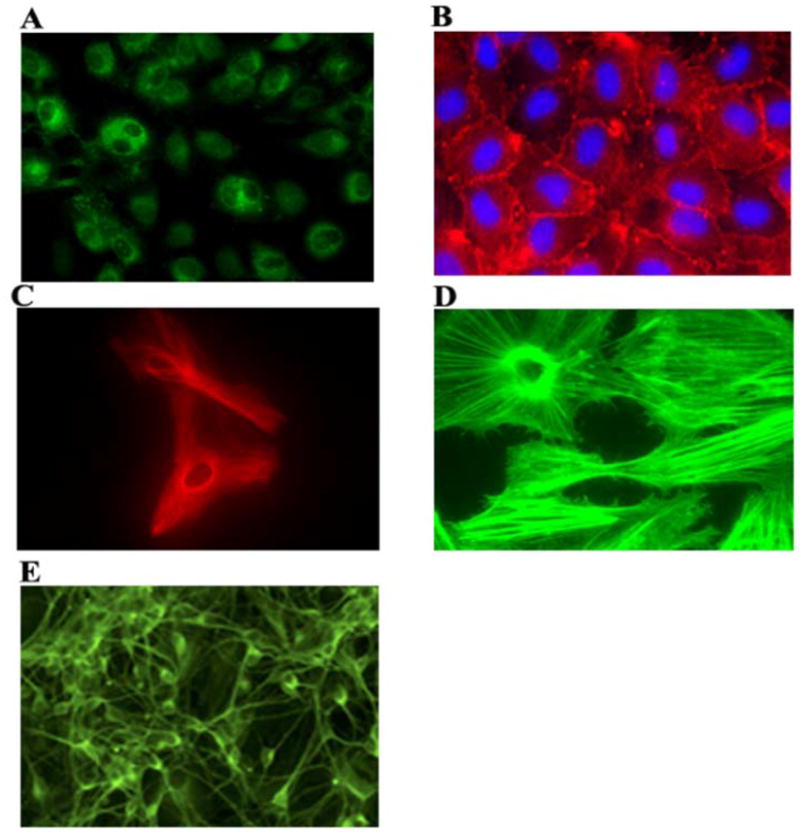
All CNS-based cell systems were cultured and maintained as described in Materials and Methods. Shown are: Primary human BMVECs expressing von Willebrand Factor (A), and ZO-1 (B). Primary human brain astrocytes expressing glial fibrillary acidic protein (C), and stained for cytoskeletal structural proteins (D). Post-mitotically differentiated neurons immunostained for MAP-2 (E).
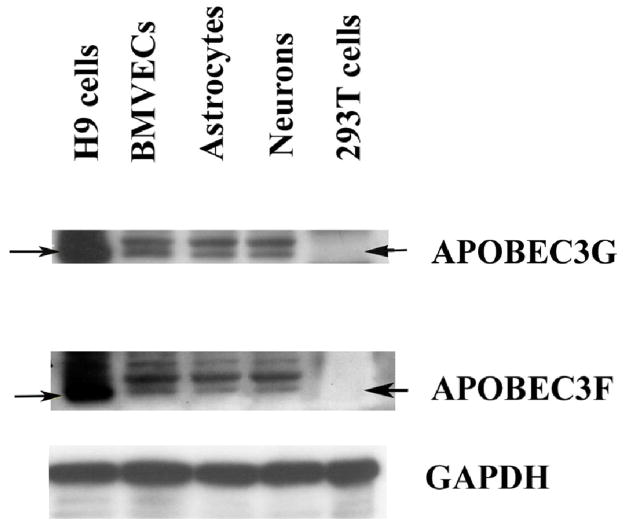
In order to evaluate the mRNA levels of various APOBEC3 family members in CNS, we analyzed BMVECs, astrocytes and mature neurons via semi-quantitative RT-PCR by employing specifically designed APOBEC3A-G primer-pairs. GAPDH was used as an input control, H9 cells were used as positive control, while NIH 3T3 murine fibroblast cells served as negative control. The results, as shown in Fig. 2A, indicate strong mRNA expression of APOBEC3B, APOBEC3C, APOBEC3F and APOBEC3G in all CNS-cells. On the contrary, there was no detection of APOBE3A, and APOBEC3D mRNA in all CNS cells, namely BMVCs, astrocytes and post-mitotically differentiated mature neurons. We further evaluated the protein expression levels of APOBEC3G and APOBEC3F, which are shown to represent powerful anti-retroviral host-restriction factors, by Western Blot analysis of CNS-cells. In our studies we used well-characterized rabbit polyclonal antibodies against APOBEC3G and APOBEC3F, which were obtained from AIDS Research and Reference Reagent Program, NIH. As shown in Fig. 2B, our findings clearly demonstrate strong protein expression of APOBEC3G and APOBEC3F (~46 kD) in all CNS-based cells. As shown, the detected APOBEC3G and APOBEC3F protein levels in BMVECs, astrocytes and mature neurons are somehow lower, yet clearly identified, as compared to the stronger signal detected in the positive control, H9 cells. As expected, neither APOBEC3G, nor APOBEC3F protein expression was detected in 293T cells, which served as a negative control. Taken together, the Western Blot findings confirm the RT-PCR evaluation and demonstrate strong protein expression of APOBEC3G and APOBEC3F in the CNS, including primary BMVCs and astrocytes, and mature neurons. Of note, as anti-APOBEC3B and anti-APOBEC3C antibodies are not yet available, we cannot detect these two proteins in this study.
Figure 2. Expression of APOBEC3 family proteins in human CNS-based cell cultures.
(A). Reverse transcriptase (RT)-PCR was employed to detect the presence of APOBEC-3A/3B/3C/3D/3F and 3G mRNA isolated from primary human brain microvascular endothelial cells (BMVECs), astrocytes and differentiated post-mitotic mature neuronal cells which are key components of the blood-brain barrier (BBB) and the CNS, as well as control H9 cells. 3T3 cells served as negative control. Arrows indicate the specifically amplified APOBEC3 products: Apobec-3B, -3C, -3F and –3G were detected (“+” positive), while Apobec-3A and –3D were not detected (“−” negative). GAPDH served as a positive control.
(B). Cell lysates from primary human BMVECs, astrocytes and differentiated post-mitotic neuronal cells, as well as H9 (positive control) and 293T cells (negative control) were analyzed via Western blot using rabbit polyclonal antibodies against APOBEC3G (~46 kD) and APOBEC3F (~46 kD). GAPDH (~36 kD) served as a positive control.
IFN-α significantly upregulates APOBEC3G and APOBEC3F protein expression in BMVECs
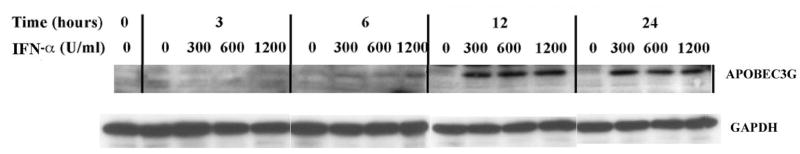
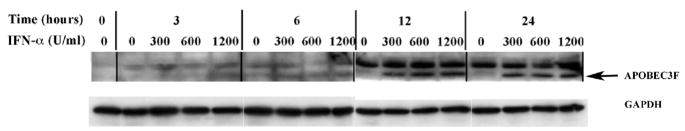
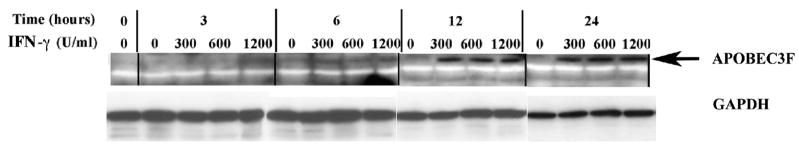
In recent years, our research group focused on HIV-1 entry into BMVECs, which by forming tight junctions, along with astrocytes, represent the most important component of BBB (Argyris et al., 2003). Herein we demonstrate that BMVECs express APOBEC3G and APOBEC3F, which via their antiviral properties are key elements of the host defense system. We and other investigators have recently demonstrated that IFN-α directly induces APOBEC3G expression in primary human resting CD4+ T cells, macrophages, pDCs and other cell systems (Chen et al., 2006; Peng et al., 2006; Stopak et al., 2007; Tanaka et al., 2006). We thus, expanded our current studies by examining the direct effect of exogenously added IFN on APOBEC3G and –3F protein expression in primary human BMVECs. In order to determine whether IFN-α directly influences APOBEC3G and –3F protein expression, primary human BMVECs were treated with single-dose increasing concentrations (0, 300, 600 and 1200 U/ml) of IFN-α and cell lysates were collected and analyzed via Western blotting at different time points (0-, 3-, 6-, 12-and 24-hrs) post-IFN-α treatment. A dose-dependent increase in both APOBEC3G (Fig. 3A), and APOBEC3F (Fig. 3B) protein expression was detected as early as 6-hrs following a single treatment with 300 U/ml IFN-α. Apparently IFN-α exerts its maximum enhancing effect on APOBEC-3G and –3F protein expression in BMVECs at a concentration of 300 U/ml, since no further protein induction was detected upon treatment with higher IFN-α concentrations, such as 600 U/ml and 1200 U/ml. Thus, a plateau was reached at a treatment of 300 U/ml IFN-α. In addition, as shown in Fig. 3A and 3B, a time-course analysis revealed that 300 U/ml IFN-α enhanced APOBEC-3G and –3F protein expression within 6 hrs, which increased further at 12 hrs by reaching a plateau (no further protein induction was detected at a 24-hour time-point). Therefore, our findings demonstrate that IFN-α can significantly induce the protein expression of APOBEC3G and APOBEC3F in primary human BMVECs.
Figure 3. APOBEC3G and APOBEC3F expression is upregulated by IFN-α in human primary BMVECs.
(A). Time course and dose-dependent studies on the upregulation of APOBEC3G by IFN-α. IFN-α (0, 300, 600 and 1200 U/ml) was added into cultures of primary human BMVECs. The intracellular expression of APOBEC3G protein was assessed by Western-blot analysis of cell lysate samples collected at various time points: 0-, 3-, 6-, 12- and 24-hrs post IFN-α treatment.
(B). Time course and dose-dependent studies on the upregulation of APOBEC3F by IFN-α. IFN-α (0, 300, 600 and 1200 U/ml) was added into cultures of primary human BMVECs. The intracellular expression of APOBEC3F protein was assessed by Western-blot analysis of cell lysate samples collected at various time points: 0-, 3-, 6-, 12- and 24- hrs post IFN-α treatment.
IFN-γ also upregulates the expression of APOBEC3G and APOBEC3F in human primary BMVECs
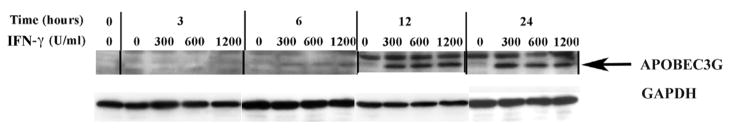
Recent studies have shown that in addition to IFN-α, other IFN-family members, such as IFN-γ as well as IFN-β, also directly upregulate APOBEC3G expression in macrophages (Peng et al., 2006; Stopak et al., 2007). We thus tested the direct effect of exogenously added IFN-γ on APOBEC3G and –3F protein expression in primary human BMVECs. To determine whether IFN-γ affects APOBEC-3G and –3F protein expression, BMVECs were similarly treated with single-dose increasing concentrations (0, 300, 600 and 1200 U/ml) of IFN-γ and cell lysates were collected and analyzed via Western blotting at different time points (0-, 3-, 6-, 12- and 24- hrs) post-IFN-γ treatment. Likewise to IFN-α treatment, a dose-dependent increase in both APOBEC3G and APOBEC3F (Fig. 4A and 4B respectively) protein expression was detected as early as 6 hrs upon a single treatment with 300 U/ml IFN-γ. IFN-γ also exerted its maximum inducing effect on APOBEC-3G and –3F protein expression in BMVECs at 300 U/ml and no further protein induction was detected upon treatment with higher concentrations. Moreover, similarly to IFN-α studies, a kinetic analysis revealed that a single-dose treatment of 300 U/ml IFN-γ enhances APOBEC-3G and –3F protein expression within 6 hrs, which increased further at 12 hrs, but not at 24 hrs (Fig. 4A and 4B respectively). It is important to mention that, upon treatment of primary MVECs even with high IFN-doses, such as 1200 U/ml, we did not observe any toxic effects on the cells and the morphology and cell numbers remained normal. Thus, our data indicate that both cytokines IFN-α and IFN-γ can induce expression of APOBEC3G and APOBEC3F in primary human BMVECs.
Figure 4. IFN-γ also upregulates the expression of APOBEC3G and APOBEC3F in human primary BMVECs.
(A). Time course and dose-dependent studies on the upregulation of AOBEC3G by IFN-γ. IFN-γ (0, 300, 600 and 1200 U/ml) was added into cultures of primary human BMVECs. The intracellular expression of APOBEC3G protein was assessed by Western-blot analysis of cell lysate samples collected at various time points: 0-, 3-, 6-, 12- and 24- hrs post IFN-γ treatment.
(B). Time course and dose-dependent studies on the upregulation of APOBEC3F by IFN-γ. IFN-γ (0, 300, 600 and 1200 U/ml) was added into cultures of primary human BMVECs. The intracellular expression of APOBEC3F protein was assessed by Western-blot analysis of cell lysate samples collected at various time points: 0-, 3-, 6-, 12- and 24-hrs post IFN-γ treatment.
IFN-α and IFN-γ inhibit HIV-1 replication in primary human BMVECs
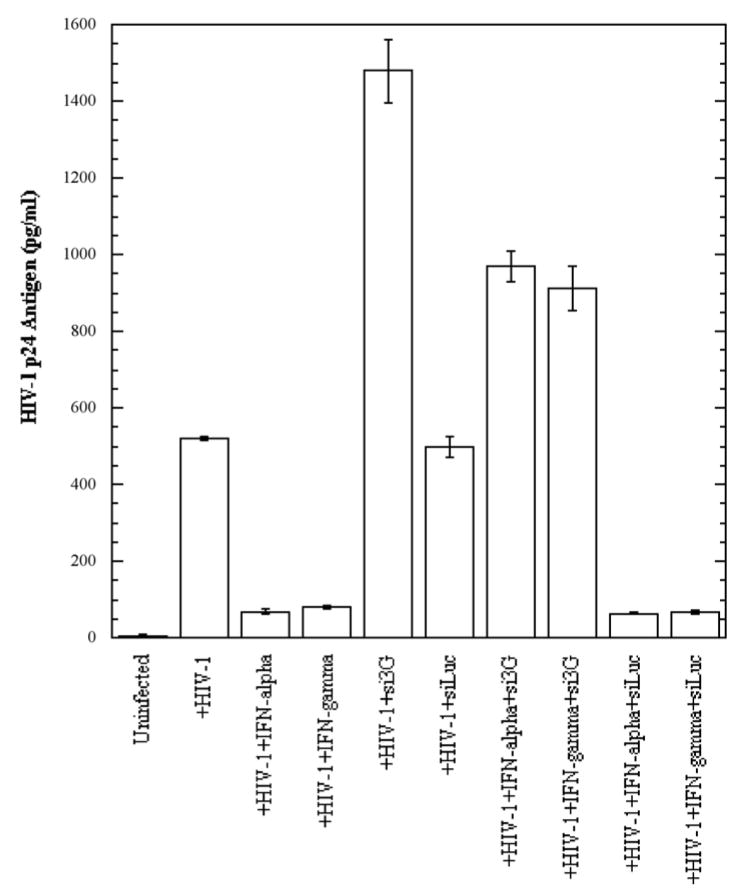
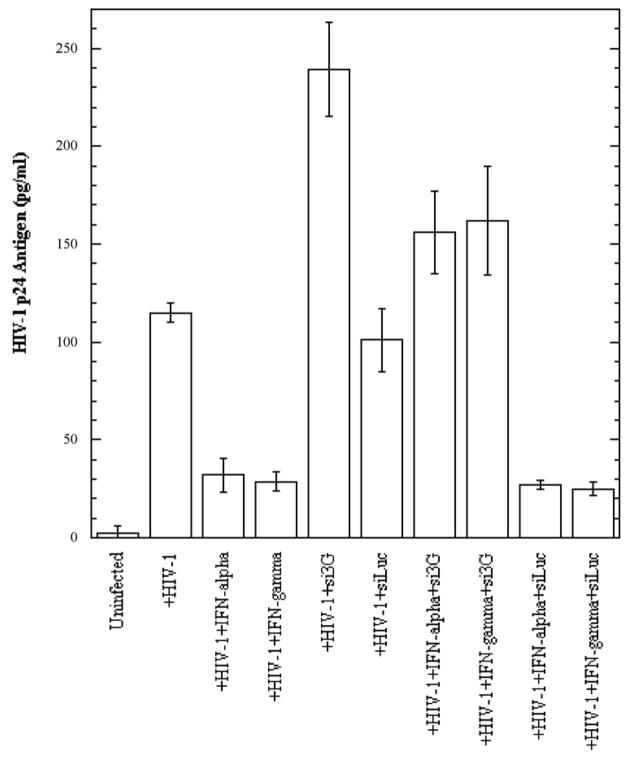
We demonstrated that both IFN-α and IFN-γ exert an enhancing effect on APOBEC3G and –3F expression in BMVECs. Recent reports have shown that these cytokines possess antiviral properties by inhibiting HIV-1 replication (Berglund et al., 1991; Coccia, Krust, and Hovanessian, 1994; Poli et al., 1989; Samuel, 2001; Shirazi and Pitha, 1993; Tissot and Mechti, 1995). It was also demonstrated that IFN-α strongly inhibits infection and replication of human herpesvirus 8 (HHV-8) in primary human dermal microvascular endothelial cells (Krug et al., 2004). To test whether these cytokines are able to inhibit HIV-1 replication in primary human BMVECs, cells were treated with exogenous IFN-α, or IFN-γ at a concentration of 300 U/ml, and concomitantly infected with HIV-1/VSV pseudotyped virus, or with the neurotropic HIV-strain YU-2 (First four left lanes in Fig. 5A and 5B, respectively). Three days post-infection cells were collected, lysed, normalized for protein content and analyzed for the presence of intracellular HIV-1 p24 antigen via ELISA. As shown in Fig. 5A and 5B, both cytokines at 300 U/ml exerted a dramatic several-fold inhibitory effect on HIV-1 replication. The effect was more profound on HIV-1/VSV pseudotyped virus (~10-fold inhibition), were the overall viral replication level was higher, probably due to bypassing the entry restriction, as compared to R5-tropic YU-2 (4-fold inhibition). Treatment with higher cytokine concentrations did not result in more efficient HIV-1 inhibition (data not shown). In addition, as detected by Western blot analysis, the IFN-α and IFN-γ inhibitory effects on viral replication correlated with strongly increased APOBEC3G and APOBEC3F protein expression in BMVECs at day 3 post-infection (data not shown), thus suggesting an APOBEC3 antiviral mechanism implication.
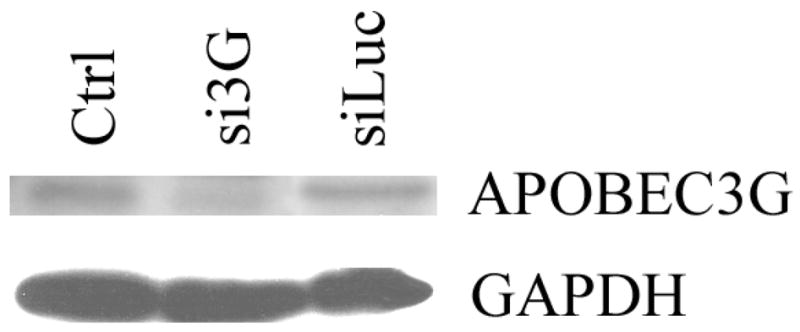
Figure 5. siRNA against APOBEC3G blocks IFN-α and IFN-γ anti-HIV-1 activity in human primary BMVECs.
(A). Primary human BMVECs cultured at 50% confluence in 12-well plates were transfected with and without APOBEC3G-specific siRNA (si3G) or luciferase-specific siRNA (siLuc) (100 nmol/ml). After 72 hrs, the transfected or untransfected BMVECs were infected with HIV-1-VSV-GFP virus (20 ng of p24 equivalents) for 6 hrs. Simultaneously, the infected cells were treated with and without IFN-α or IFN-γ (300 U/ml) for 72 hrs. HIV-1 p24 antigen in cell lysates normalized for protein content was detected by ELISA at day three post-infection (3 d.p.i.). The data represent two independent experiments.
(B). Primary human BMVECs cultured at 50% confluence in 12-well plates were transfected with or without si3G or siLuc (100 nmol/ml). After 72 hrs, the transfected or untransfected BMVECs were infected with the R5-tropic HIV-1 strain YU2 (20 ng of p24 equivalents) for 6 hrs. Simultaneously, the infected cells were treated with or without IFN-α or IFN-γ (300 U/ml) for 72 hrs. HIV-1 p24 antigen in cell lysates normalized for protein content was detected by ELISA at day three post-infection (3 d.p.i.). The data represent two independent experiments.
(C). Western blot for APOBEC3G protein 6 days post-transfection of primary human BMVECs with DharmaFECT 1 transfection reagent (Ctrl), APOBEC3G-specific siRNA (si3G) or luciferase-specific siRNA (siLuc).
The siRNA against APOBEC3G results in increased susceptibility of primary human BMVECs to HIV-1 infection
In order to determine whether down-regulation of intracellular APOBEC3G expression would result in increased susceptibility of BMVECs to HIV-1 infection, and possibly in at least a partial block of IFN-α and IFN-γ antiviral activity, cells were transfected with specific siRNAs against APOBEC3G, or luciferase, or simply treated with transfection reagent (negative control), followed by cytokine treatment along with HIV-1/VSV, or YU-2 infection. Three days post-infection the infected cells were collected, lysed and analyzed for the presence of intracellular HIV-1 p24 antigen via ELISA. As shown in Fig. 5C, transfection of specific siRNA against APOBEC3G into BMVECs resulted in efficient APOBEC3G protein reduction, while siRNA against luciferase did not alter APOBEC3G expression level. Our findings demonstrate a significant increase of HIV-1 p24 antigen in APOBEC3G-specific siRNA-transfected BMVECs 3 days post-infection (Fig. 5A and 5B, compare lane 5 versus 6), indicating that APOBEC3G is an important anti-HIV restriction factor in CNS. Furthermore, as shown in Fig. 5A and 5B, lanes 7 and 8 versus lanes 9 and 10) cells depleted of APOBEC3G by siRNA and then treated with 300 U/ml IFN-α, or IFN-γ, could still produce a relatively high amount of HIV-1 p24 antigen, as compared to cells transfected with siRNA against luciferase, indicating that induction of APOBEC3G by these cytokines competes with the APOBEC3G siRNA knockdown effect and provides strong evidence that IFN-induced APOBEC3G is a potent restriction factor of HIV-1 infection in primary human BMVECs and the CNS. Similar were our findings in HIV-1 p24 measurements in the supernatants of the samples examined, although at relatively lower overall levels (data not shown). While in our current experiments we did not test the effects of specific siRNA against APOBEC3F, we do not exclude that this molecule may also be important in restricting HIV-1 infection in primary brain MVECs.
Discussion
In the present study we evaluated the expression of different APOBEC3 family members in the CNS and more importantly, we also examined the regulation and the functional properties of these proteins with regard to restricting HIV-1 infection in this system. Recent studies in macaques have shown that APOBEC3G is widely distributed in the brain of these animals, yet its expression seems to be restricted in neurons, while brain astrocytes and microglia either do not express APOBEC3G or expression is too low to be detected by immunohistochemical approaches. Interestingly, Western blot analysis of fetal human astrocytes in these studies, by employing a monoclonal antibody against APOBEC3G, similarly did not provide evidence for APOBEC3G expression in these cells (Hill et al., 2006). In our studies, by using specifically designed primers in RT-PCR detection assays we were able to provide clear evidence on mRNA expression of different APOBEC3G family members, known to possess antiviral activity, such as APOBEC3G, APOBEC3F, as well as APOBEC3B and APOBEC3C, in all CNS-based cell systems, namely primary human BMVECs, primary human astrocytes as well as post-mitotically differentiated mature neurons. Western Blot analysis confirmed our findings on APOBEC3G and APOBEC3F protein expression in the CNS, by means of well-characterized rabbit polyclonal antibodies, that we obtained from AIDS Research and Reference Reagent Program, NIH. Interestingly, the use of monoclonal antibodies against APOBEC3G and –3F, obtained from the same source, did not produce satisfactory results in our Western blot assays (data not shown), thus possibly explaining the discrepancies between the findings of other investigators as compared to ours.
Recent data from different investigators, including ours, on the expression and regulation of intracellular APOBEC3 family members demonstrate that certain cytokines, including IFN-α, -β and -γ, as well as other cytokines, via a unique and direct pathway can upregulate the expression of APOBEC3G in various primary cell systems, which results in a potent anti-HIV-1 mechanism. We, and others have demonstrated this phenomenon in resting CD4+ T cells, pDCs, macrophages and other primary cells (Chen et al., 2006; Peng et al., 2006; Stopak et al., 2007; Tanaka et al., 2006). Our recent findings in resting CD4+ T cells also suggest that IFN-α upregulates APOBEC3G at transcriptional level through the ISRE-like cis-element in APOBEC3G promoter (Chen et al., 2006). In addition, it seems that in cytokine-stimulated CD4+ T cells, induction of APOBEC3G gene expression correlates with HMM (inactive) APOBEC3G complex formation, while in MDCs the increase in APOBEC3G levels associated with maturation appears to result in the increased appearance of intermediate and LMM (active) forms of APOBEC3G (Stopak et al., 2007).
The anti-HIV-1 activity of IFN-α through diverse mechanisms, including PKR, GBP-2, MxA, RANTES, and OAS, is well documented (Samuel, 2001). Interestingly, IFN-α induction of an RNA-specific adenosine deaminase, which catalyzes adenosine to inosine in both viral RNA and cellular pre-mRNA to cause hypermutation, may also antagonize infection and suggests a potential influence on other deaminases (Samuel, 2001). Notably, the use of IFN-α in the clinics, in combination with other drugs against HIV-1 infection, as well as other viral infections is also well documented, although its toxicity represent a clinical concern (Lane et al., 1990). In addition, IFN-α/β is widely used for the treatment of hepatitis C virus (HCV) or hepatitis B (HBV) infections (Davis, 2000; Lai et al., 2003). IFN-γ was also shown to function as an immune stimulant and possess antiviral properties (Samuel, 2001).
Here for the first time, we demonstrate that exogenously added IFN-α and IFN-γ can upregulate the expression of two key anti-HIV-1 host restriction factors, APOBEC3G and –3F, in primary human BMVECs, which represent the major component of BBB. Although, at this point we did not expand our studies on other CNS-based systems, it would be very interesting to further examine the effects of these, or other cytokines on APOBEC3 family members regulation in primary astrocytes and mature neurons. Nevertheless, our findings strongly suggest that host factors (APOBEC3 family, and possibly other) may represent important elements in the innate immunity against viral infections, especially during the development of AIDS dementia complex. Given that CNS represents a key site for HIV-1 invasion and a viral reservoir, elucidation of the molecular defense mechanisms against viral infection, especially at early stages, is of great importance. HIV-1 does not replicate productively in either neurons, or BMVECs (Argyris et al., 2003; Mukhtar et al., 2000; Mukhtar et al., 2002; Mukhtar and Pomerantz, 2000), possibly due to restriction at the level of viral entry and although certain studies have shown that astrocytes become infected, other studies indicate that astrocyte infection is restricted mainly due to viral entry restriction (Canki et al., 2001; Schweighardt and Atwood, 2001). Our current findings demonstrate that APOBEC3G may represent another viral restriction factor at least in BMVECs and the BBB. The expression of various APOBEC3 family members in all CNS-based cells may have implications for the restricted replication of HIV-1 in this system. Our data show that downregulation of APOBEC3G by siRNA in BMVECs resulted in increased viral replication, which suggests the identification of a novel component in the complex repertoire of antiviral mechanisms in CNS. Evidently, as our findings indicate, the protein expression level of APOBEC3G and APOBEC3F in all CNS-based cells is low as compared to expression in H9 cells. Upregulation of APOBEC3G and APOBEC3F via the IFN-pathway, as we show here, may be important, especially in vivo, in order to overcome persistent infection by HIV-1 in the CNS. This could include the administration of exogenous IFN-α and/or IFN-γ, or alternatively, the secretion by IFN-producing cells stimulated with certain reagents or cytokines (Soumelis et al., 2002). With regard to the mechanistic aspects, as we have recently reported in other cell systems (Chen et al., 2006), we suggest that in brain MVECs IFN-induced APOBEC3G (LMM-associated) can significantly inhibit the reverse transcription and irreversibly inactivate HIV-1 viruses in the pre-integration stage in a Vif-independent manner.
The molecular mechanisms of viral entry, infection, and spread in the CNS and its cellular components remain unclear. Despite extensive research on HIV-1 neuroinvasion, the mechanisms of initial entry into the CNS, and the precise causes of the AIDS dementia complex, which leads to neurological impairment in many HIV-1-seropositive patients, remain enigmatic. One of the hypotheses regarding how HIV-1 enters the CNS suggests direct infection of BMVECs as a major route of viral entry into the CNS, followed by low level replication of the virus in CNS-based cells (Moses et al., 1993; Poland, Rice, and Dekaban, 1995). Another hypothesis suggests cell-associated HIV-1 entry into the CNS via CD4+ T cells and monocytes that traffic across the BBB, potentially transferring the infection to other CNS-based cells (Bell et al., 1993; Kure et al., 1990; Liu et al., 2000; Persidsky, 1999; Persidsky et al., 1997; Pumarola-Sune et al., 1987). It has also been suggested that certain cytokines, HIV-1-specific proteins, and various cellular factors may also induce alterations in the BBB, creating a breach in the tight junctions of BMVECs. Consequently, this breach may assist the virus in gaining entry into CNS-based cells (Fiala et al., 1997; Lossinsky et al., 1999; Persidsky et al., 1999). Further work is required in order to fully characterize the pathways of HIV-1 replication into the CNS, involving not only BMVECs but also other critical CNS cellular components, such as microglia, neurons, and astrocytes. Understanding the infection of the human brain by HIV-1 will be critical in targeting this potential viral reservoir site. The IFN/APOBEC3 signal pathway(s) exert potent antiviral activity, and represent an important molecular tool in our efforts to eradicate residual HIV-1 replication in CNS in post-HAART era.
Materials and Methods
Reagents
Anti-human von Willebrand factor antibody was purchased from Sigma (St. Louis, Mo.), anti-ZO-1 antibody was purchased from Zymed (South San Francisco, Calif.). Rabbit anti-hu APOBEC3G and Rabbit Anti-hu APOBEC3F polyclonal antibodies were obtained from NIH AIDS Research and Reference Reagent Program (cat# 10084 and cat# 11226 respectively) and secondary antibodies conjugated with horseradish peroxidase from Santa Cruz Biotechnology, Inc. Recombinant human IFN-α and IFN-γ were purchased from Sigma (St. Louis, Mo.).
Primary human brain cell cultures
Primary isolated human fetal brain microvascular endothelial cells (BMVECs) were obtained from Cell Systems Corp. (Kirkland, Wash.). The cells were initially seeded into 75-cm2 flasks in supplemented endothelial cell basal medium 2 (Biowhittaker, Walkersville, Md.) and were maintained and passaged in vitro under strict conditions established in our laboratory (37°C, 5% CO2, 100% humidity in human endothelial growth medium). For all experiments, BMVECS were seeded either in 12-well tissue culture plates or 6-well plates at a density of 0.5 × 106 and 2 × 106 cells/well respectively. The purity of BMVECs (>95%) was analyzed by immunofluorescent staining and microscopy with antibodies against von Willebrand factor and ZO-1 (Zonula Occludens-1, a tight junction-associated protein characteristic of endothelium). Culture conditions for primary human BMVECs and immunofluorescent staining were optimized previously (Argyris et al., 2003; Mukhtar et al., 2000; Mukhtar et al., 2002; Mukhtar and Pomerantz, 2000). Primary human brain astrocytes (>95% purity) were obtained from Dr. B. Wigdahl (Drexel University), and maintained in culture as described previously (Acheampong et al., 2007; Fang et al., 2005; Mukhtar and Pomerantz, 2000; Zhou et al., 2003). NT2 precursor cells, obtained from the American Tissue Culture Collection (ATCC), have the capability of differentiating in vitro into post-mitotic CNS neurons by induction with retinoic acid (RA) (Acheampong et al., 2007; Moses et al., 1993; Mukhtar et al., 2000; Mukhtar and Pomerantz, 2000; Zhou et al., 2003). NT2 precursor cells were grown in T75 tissue culture flask, in Dulbecco’s modified Eagles medium (DMEM) supplemented with Fetal Calf Serum (FCS) and glutamine in a humidified environment at 37°C, 5% CO2. The differentiation of NT2 precursor cells to post-mitotic neurons involves growing these cells in RA supplemented DMEM over a period of 5–6 weeks. Briefly, actively growing NT2 precursor cells were passaged in T75 tissue culture flask to a final concentration of 2 × 106 cells per flask. After 24 hours the cells were fed with RA supplemented DMEM followed by feeding these cells with this media at 48 hrs intervals for six weeks. After six-weeks post-mitotic neurons isolation was performed as described previously (Acheampong et al., 2007; Mukhtar and Pomerantz, 2000). Mature neurons were treated with mitotic inhibitor medium for selectively enriching post-mitotic cells (Acheampong et al., 2007; Mukhtar and Pomerantz, 2000). Finally, the neurons were removed from the mitotic inhibitor medium and grown on poly-D-lysine and MATRIGEL-coated 6-well plates. The purity of each differentiated neuronal batch was confirmed by immunostaining with neuroepithelial markers microtubule-associated protein (MAP-2) (Pharmingen, San Diego, CA) utilized to identify neurons.
Preparation of HIV-1 viral stocks and infections
HIV-1/VSV pseudotyped viruses were generated by transfection of 293T cells with 10 μg of pNL4-3-ΔE-EGFP plasmid containing the HIV-1 proviral genome and a gfp expression cassette at the defective env region (Zhang et al., 2004), and 10 μg of plasmid encoding vesicular stomatitis virus envelope protein (VSV-G), with calcium phosphate transfection kit (Promega, Madison, WI). The supernatants were collected at 48 hrs post-transfection, filtered through a 0.45-μm size filter and stored in aliquots at −80°C till further use. The R5-tropic HIV-1 strain YU2 was produced by transfection of 293T cells with the respective HIV-1 infectious clone, as described previously (Argyris et al., 2003; Ohagen et al., 1999). Briefly, at 48 hrs post-transfection of HIV-1 infectious clone via the calcium phosphate coprecipitation method, supernatant was collected, filtered, quantified by HIV-1 p24 antigen enzyme-linked immunosorbent assay (NEN Life Science Products, Inc., Boston, Mass.), and stored at −80°C until further use. BMVECs (0.5 to 2 × 106) were infected with HIV-1/VSV pseudotyped viruses or R5-tropic HIV-1 YU2 at an input of 20 ng/ml of HIV-1 p24 antigen equivalents. In indicated experiments IFN-α or IFN-γ (300 U/ml) were added in the BMVEC cell cultures at the same time point with the viral input. Six hrs later the cells were washed three times with 1 × PBS and replenished with fresh media, with or without IFN-α or IFN-γ. 72-hrs post-infection the cells and supernatants were collected. The cells were washed three times with 1 × PBS, followed by 0.2% trypsin/EDTA treatment for 10 min at 37°C to remove non-internalized viral particles. After two additional washes, including once with the serum containing buffer, cell lysis buffer was added and the cell lysates were further processed for intracellular HIV-1 p24 antigen detection (Perkin Elmer).
RNA isolation and analysis
Total RNA from BMVECs, astrocytes, differentiated post-mitotic mature neurons grown in 6-well plates (confluent), as well as H9 and 3T3 cells was extracted using Trizol reagent (Invitrogen). A total of 200–300 ng RNA from each sample was reverse-transcribed using iScriptt™ cDNA Synthesis Kit (Bio-Rad). The cDNAs of APOBEC3A, APOBEC3B, APOBEC3C, APOBEC3D, APOBEC3F and APOBEC3G were PCR-amplified using specifically designed primers. GAPDH was used as a control. All PCR reactions were performed by 35-cycle amplification (94°C for 3 mins, followed by 35 cycles at 94°C for 45 s, 55°C for 45 s, 72°C for 1.5 min and finally extension at 72°C for 7 mins.) Primers were as follows: APOBEC3A: 5′-GAAGGGACAAGCACATGGAAGC-3′ (forward), 5′-ATCTACTTGATCGGGAGCATAC-3′ (reverse); APOBEC3B: 5′-TCGAGGCCAGGTGTATTTCAAG-3′ (forward), 5′-CTCATAGCACAAGTAGGTCTGG-3′ (reverse); APOBEC3C: 5′-AACCTATGGGAAGCCAACGATC-3′ (forward), 5′-CCTCCTGGTAACATGGATACTG-3′ (reverse); APOBEC3D: 5′-ACGTCAGTCGAATCACAGGCAG-3′ (forward), 5′-CTGGTCTCCTGGCTGTCAGTTG-3′ (reverse); APOBEC3F: 5′-CGGCCTGTCTTTATCAGAGGTC-3′ (forward), 5′-CAGGTGAGTGGTGCTTTACAAC-3′ (reverse); APOBEC3G: 5′-GGTGTATTCCGAACTTAAGTAC-3′ (forward), 5′-CAAGGAAACCGTGTTTATGTGG-3′ (reverse); GAPDH: 5′-AAGAGCACAAGAGGAAGAGAGAGAC-3′ (forward), 5′-GTCTACATGGCAACTGTGAGGAG-3′ (reverse).
The siRNA synthesis and transfections of BMVECs
The short interfering RNAs (siRNAs) were chemically-synthesized by Dharmacom. The APOBEC3G-specific siRNA was siGENOME SMART pool (CAT No: M-013072). The luciferase-specific siRNA served as negative control. Primary human MVECs cultured at 50% confluence in 12-well plates were transfected with and without APOBEC3G-specific siRNA or luciferase-specific siRNA (100 nmol/ml), using the DharmaFECT 1 transfection reagent (Dharmacon) and following the experimental procedures suggested by the manufacturer. In indicated experiments, after 72 hrs, the transfected or non-transfected MVECs were infected with HIV-1/VSV pseudotyped viruses or HIV-1 YU-2 viruses (20 ng of p24 equivalents) for 6 hrs. In certain experiments the infected cells simultaneously were treated with and without IFN-α or IFN-γ (300 U/ml) for 72 hrs. HIV-1 p24 antigen in cell lysates with normalized protein content was detected by ELISA at day three post-infection (3 d.p.i.).
Western blot Analysis
Human primary CNS-based cells were treated or untreated with indicated concentrations of IFN-α or IFN-γ at various time-points. Cells were lysed and total protein was extracted using CytoBuster protein extraction reagent (Novagen) and then quantified by a bicinchoninic acid (BCA) protein assay reagent kit (Pierce). Up to 25 μg of total protein was used for electrophoresis (10% polyacrylamide gel) and transferred onto PVDF membranes. After blocking with 10% milk in phosphate-buffered saline (PBS) with 0.05% Tween-20, membranes were probed with rabbit polyclonal anti-APOBEC3G or anti-APOBEC3F antibodies (NIH AIDS Research and Reference Reagent Program) at 1:1,000 for 2 hrs at room temperature. Loading control was detected with anti-GAPDH monoclonal antibody (Sigma, St. Louis, MO). Membranes were washed with PBS three times, followed by incubation with secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc.) for 1 hr at room temperature, and visualized by chemiluminescence (Pierce Chemical Co.).
Acknowledgments
We thank AIDS Research and Reference Reagent Program, NIH for supplying the rabbit anti-APOBEC3G and anti-APOBEC3F polyclonal antibodies as well as the pNL4-3-ΔE-EGFP plasmid. This work was supported by NIH grants AI058798 and AI052732 to H.Z and MH075686 to E.G.A.
Keywords
- HIV-1
human immunodeficiency virus type 1
- BMVECs
brain microvascular endothelial cells
- CNS
central nervous system
- BBB
blood brain barrier
- siRNA
short-interfering RNA
- IFN
interferon
- APOBEC
apolipoprotein B mRNA-editing enzyme catalytic polypeptid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheampong E, Parveen Z, Mengistu A, Ngoubilly N, Wigdahl B, Lossinsky AS, Pomerantz RJ, Mukhtar M. Cholesterol-depleting statin drugs protect postmitotically differentiated human neurons against ethanol- and human immunodeficiency virus type 1-induced oxidative stress in vitro. J Virol. 2007;81(3):1492–501. doi: 10.1128/JVI.01843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyris EG, Acheampong E, Nunnari G, Mukhtar M, Williams KJ, Pomerantz RJ. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J Virol. 2003;77(22):12140–51. doi: 10.1128/JVI.77.22.12140-12151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. Aids. 1996;10(6):573–85. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Bell JE, Busuttil A, Ironside JW, Rebus S, Donaldson YK, Simmonds P, Peutherer JF. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J Infect Dis. 1993;168(4):818–24. doi: 10.1093/infdis/168.4.818. [DOI] [PubMed] [Google Scholar]
- Berglund O, Engman K, Ehrnst A, Andersson J, Lidman K, Akerlund B, Sonnerborg A, Strannegard O. Combined treatment of symptomatic human immunodeficiency virus type 1 infection with native interferon-alpha and zidovudine. J Infect Dis. 1991;163(4):710–5. doi: 10.1093/infdis/163.4.710. [DOI] [PubMed] [Google Scholar]
- Bissel SJ, Wiley CA. Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations. Brain Pathol. 2004;14(1):97–108. doi: 10.1111/j.1750-3639.2004.tb00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobardt MD, Salmon P, Wang L, Esko JD, Gabuzda D, Fiala M, Trono D, Van der Schueren B, David G, Gallay PA. Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. J Virol. 2004;78(12):6567–84. doi: 10.1128/JVI.78.12.6567-6584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvin M, Achermann F, Greeve I, Stroka D, Keogh A, Inderbitzin D, Candinas D, Sommer P, Wain-Hobson S, Vartanian JP, Greeve J. Interferon-inducible expression of APOBEC3 editing enzymes in human hepatocytes and inhibition of hepatitis B virus replication. Hepatology. 2006;43(6):1364–74. doi: 10.1002/hep.21187. [DOI] [PubMed] [Google Scholar]
- Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J Virol. 2001;75(17):7925–33. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Huang J, Zhang C, Huang S, Nunnari G, Wang FX, Tong X, Gao L, Nikisher K, Zhang H. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J Virol. 2006;80(15):7645–57. doi: 10.1128/JVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435(7038):108–14. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- Coccia EM, Krust B, Hovanessian AG. Specific inhibition of viral protein synthesis in HIV-infected cells in response to interferon treatment. J Biol Chem. 1994;269(37):23087–94. [PubMed] [Google Scholar]
- Dang Y, Wang X, Esselman WJ, Zheng YH. Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family. J Virol. 2006;80(21):10522–33. doi: 10.1128/JVI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GL. Current therapy for chronic hepatitis C. Gastroenterology. 2000;118(2 Suppl 1):S104–14. doi: 10.1016/s0016-5085(00)70009-6. [DOI] [PubMed] [Google Scholar]
- Delebecque F, Suspene R, Calattini S, Casartelli N, Saib A, Froment A, Wain-Hobson S, Gessain A, Vartanian JP, Schwartz O. Restriction of foamy viruses by APOBEC cytidine deaminases. J Virol. 2006;80(2):605–14. doi: 10.1128/JVI.80.2.605-614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Acheampong E, Dave R, Wang F, Mukhtar M, Pomerantz RJ. The RNA helicase DDX1 is involved in restricted HIV-1 Rev function in human astrocytes. Virology. 2005;336(2):299–307. doi: 10.1016/j.virol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, Graves MC, Witte M, Kim KS. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3(8):553–64. [PMC free article] [PubMed] [Google Scholar]
- Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113(6):803–9. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Hill MS, Mulcahy ER, Gomez ML, Pacyniak E, Berman NE, Stephens EB. APOBEC3G expression is restricted to neurons in the brains of pigtailed macaques. AIDS Res Hum Retroviruses. 2006;22(6):541–50. doi: 10.1089/aid.2006.22.541. [DOI] [PubMed] [Google Scholar]
- Jarmuz A, Chester A, Bayliss J, Gisbourne J, Dunham I, Scott J, Navaratnam N. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics. 2002;79(3):285–96. doi: 10.1006/geno.2002.6718. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takaori-Kondo A, Shindo K, Abudu A, Fukunaga K, Uchiyama T. APOBEC3G targets specific virus species. J Virol. 2004;78(15):8238–44. doi: 10.1128/JVI.78.15.8238-8244.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug LT, Pozharskaya VP, Yu Y, Inoue N, Offermann MK. Inhibition of infection and replication of human herpesvirus 8 in microvascular endothelial cells by alpha interferon and phosphonoformic acid. J Virol. 2004;78(15):8359–71. doi: 10.1128/JVI.78.15.8359-8371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure K, Lyman WD, Weidenheim KM, Dickson DW. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990;136(5):1085–92. [PMC free article] [PubMed] [Google Scholar]
- Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362(9401):2089–94. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- Lane HC, Davey V, Kovacs JA, Feinberg J, Metcalf JA, Herpin B, Walker R, Deyton L, Davey RT, Jr, Falloon J, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann Intern Med. 1990;112(11):805–11. doi: 10.7326/0003-4819-112-11-805. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78(14):7319–28. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, Roberts J, Pushkarsky T, Bukrinsky M, Witte M, Weinand M, Fiala M. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76(13):6689–700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78(8):4120–33. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tang XP, McArthur JC, Scott J, Gartner S. Analysis of human immunodeficiency virus type 1 gp160 sequences from a patient with HIV dementia: evidence for monocyte trafficking into brain. J Neurovirol. 2000;6 Suppl 1:S70–81. [PubMed] [Google Scholar]
- Lossinsky AS, Buttle KF, Pluta R, Mossakowski MJ, Wisniewski HM. Immunoultrastructural expression of intercellular adhesion molecule-1 in endothelial cell vesiculotubular structures and vesiculovacuolar organelles in blood-brain barrier development and injury. Cell Tissue Res. 1999;295(1):77–88. doi: 10.1007/s004410051214. [DOI] [PubMed] [Google Scholar]
- Ludwig E, Silberstein FC, van Empel J, Erfle V, Neumann M, Brack-Werner R. Diminished rev-mediated stimulation of human immunodeficiency virus type 1 protein synthesis is a hallmark of human astrocytes. J Virol. 1999;73(10):8279–89. doi: 10.1128/jvi.73.10.8279-8289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Wang T, Liu B, Tian C, Xiao Z, Kappes J, Yu XF. Cytidine deaminases APOBEC3G and APOBEC3F interact with HIV-1 integrase and inhibit proviral DNA formation. J Virol. 2007 doi: 10.1128/JVI.02584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424(6944):99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114(1):21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- Marin M, Rose KM, Kozak SL, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med. 2003;9(11):1398–403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, Engelman A, Pathak VK. HIV-1 cDNAs Produced in the Presence of APOBEC3G Exhibit Defects in Plus-Strand DNA Transfer and Integration. J Virol. 2007 doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses AV, Bloom FE, Pauza CD, Nelson JA. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc Natl Acad Sci U S A. 1993;90(22):10474–8. doi: 10.1073/pnas.90.22.10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar M, Duke H, BouHamdan M, Pomerantz RJ. Anti-human immunodeficiency virus type 1 gene therapy in human central nervous system-based cells: an initial approach against a potential viral reservoir. Hum Gene Ther. 2000;11(2):347–59. doi: 10.1089/10430340050016076. [DOI] [PubMed] [Google Scholar]
- Mukhtar M, Harley S, Chen P, BouHamdan M, Patel C, Acheampong E, Pomerantz RJ. Primary isolated human brain microvascular endothelial cells express diverse HIV/SIV-associated chemokine coreceptors and DC-SIGN and L-SIGN. Virology. 2002;297(1):78–88. doi: 10.1006/viro.2002.1376. [DOI] [PubMed] [Google Scholar]
- Mukhtar M, Pomerantz RJ. Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis and neurovirologic disorders induced by human immunodeficiency virus type 1 infection. J Hum Virol. 2000;3(6):324–34. [PubMed] [Google Scholar]
- Navarro F, Bollman B, Chen H, Konig R, Yu Q, Chiles K, Landau NR. Complementary function of the two catalytic domains of APOBEC3G. Virology. 2005;333(2):374–86. doi: 10.1016/j.virol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19(6):517–24. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr Biol. 2005;15(2):166–70. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J Virol. 1999;73(2):897–906. doi: 10.1128/jvi.73.2.897-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OhAinle M, Kerns JA, Malik HS, Emerman M. Adaptive evolution and antiviral activity of the conserved mammalian cytidine deaminase APOBEC3H. J Virol. 2006;80(8):3853–62. doi: 10.1128/JVI.80.8.3853-3862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med. 2006;203(1):41–6. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y. Model systems for studies of leukocyte migration across the blood - brain barrier. J Neurovirol. 1999;5(6):579–90. doi: 10.3109/13550289909021287. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155(5):1599–611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158(7):3499–510. [PubMed] [Google Scholar]
- Pion M, Granelli-Piperno A, Mangeat B, Stalder R, Correa R, Steinman RM, Piguet V. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J Exp Med. 2006 doi: 10.1084/jem.20061519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland SD, Rice GP, Dekaban GA. HIV-1 infection of human brain-derived microvascular endothelial cells in vitro. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(5):437–45. doi: 10.1097/00042560-199504120-00002. [DOI] [PubMed] [Google Scholar]
- Poli G, Orenstein JM, Kinter A, Folks TM, Fauci AS. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989;244(4904):575–7. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987;21(5):490–6. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- Rosler C, Kock J, Kann M, Malim MH, Blum HE, Baumert TF, von Weizsacker F. APOBEC-mediated interference with hepadnavirus production. Hepatology. 2005;42(2):301–9. doi: 10.1002/hep.20801. [DOI] [PubMed] [Google Scholar]
- Russell RA, Wiegand HL, Moore MD, Schafer A, McClure MO, Cullen BR. Foamy virus Bet proteins function as novel inhibitors of the APOBEC3 family of innate antiretroviral defense factors. J Virol. 2005;79(14):8724–31. doi: 10.1128/JVI.79.14.8724-8731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkis PT, Ying S, Xu R, Yu XF. STAT1-independent cell type-specific regulation of antiviral APOBEC3G by IFN-alpha. J Immunol. 2006;177(7):4530–40. doi: 10.4049/jimmunol.177.7.4530. [DOI] [PubMed] [Google Scholar]
- Sasada A, Takaori-Kondo A, Shirakawa K, Kobayashi M, Abudu A, Hishizawa M, Imada K, Tanaka Y, Uchiyama T. APOBEC3G targets human T-cell leukemia virus type 1. Retrovirology. 2005;2(1):32. doi: 10.1186/1742-4690-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighardt B, Atwood WJ. HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res Hum Retroviruses. 2001;17(12):1133–42. doi: 10.1089/088922201316912745. [DOI] [PubMed] [Google Scholar]
- Seppen J. Unedited inhibition of HBV replication by APOBEC3G. J Hepatol. 2004;41(6):1068–9. doi: 10.1016/j.jhep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418(6898):646–50. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9(11):1404–7. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- Shirazi Y, Pitha PM. Interferon alpha-mediated inhibition of human immunodeficiency virus type 1 provirus synthesis in T-cells. Virology. 1993;193(1):303–12. doi: 10.1006/viro.1993.1126. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Scott I, Liu YJ, Levy J. Natural type 1 interferon producing cells in HIV infection. Hum Immunol. 2002;63(12):1206–12. doi: 10.1016/s0198-8859(02)00760-7. [DOI] [PubMed] [Google Scholar]
- Spencer DC, Price RW. Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol. 1992;46:655–93. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- Stopak KS, Chiu YL, Kropp J, Grant RM, Greene WC. Distinct patterns of cytokine regulation of APOBEC3G expression and activity in primary lymphocytes, macrophages, and dendritic cells. J Biol Chem. 2007;282(6):3539–46. doi: 10.1074/jbc.M610138200. [DOI] [PubMed] [Google Scholar]
- Suspene R, Guetard D, Henry M, Sommer P, Wain-Hobson S, Vartanian JP. Extensive editing of both hepatitis B virus DNA strands by APOBEC3 cytidine deaminases in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(23):8321–6. doi: 10.1073/pnas.0408223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Marusawa H, Seno H, Matsumoto Y, Ueda Y, Kodama Y, Endo Y, Yamauchi J, Matsumoto T, Takaori-Kondo A, Ikai I, Chiba T. Antiviral protein APOBEC3G is induced by interferon-alpha stimulation in human hepatocytes. Biochem Biophys Res Commun. 2006;341(2):314–9. doi: 10.1016/j.bbrc.2005.12.192. [DOI] [PubMed] [Google Scholar]
- Tardieu M, Boutet A. HIV-1 and the central nervous system. Curr Top Microbiol Immunol. 2002;265:183–95. doi: 10.1007/978-3-662-09525-6_9. [DOI] [PubMed] [Google Scholar]
- Tissot C, Mechti N. Molecular cloning of a new interferon-induced factor that represses human immunodeficiency virus type 1 long terminal repeat expression. J Biol Chem. 1995;270(25):14891–8. doi: 10.1074/jbc.270.25.14891. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44(3 Pt 1):481–7. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Ying S, Zhang X, Sarkis PT, Xu R, Yu X. Cell-specific Regulation of APOBEC3F by Interferons. Acta Biochim Biophys Sin (Shanghai) 2007;39(4):297–304. doi: 10.1111/j.1745-7270.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302(5647):1056–60. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424(6944):94–8. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhou Y, Alcock C, Kiefer T, Monie D, Siliciano J, Li Q, Pham P, Cofrancesco J, Persaud D, Siliciano RF. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol. 2004;78(4):1718–29. doi: 10.1128/JVI.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol. 2004;78(11):6073–6. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Fan X, Mukhtar M, Fang J, Patel CA, DuBois GC, Pomerantz RJ. Cell-cell fusion and internalization of the CNS-based, HIV-1 co-receptor, APJ. Virology. 2003;307(1):22–36. doi: 10.1016/s0042-6822(02)00021-1. [DOI] [PubMed] [Google Scholar]