Abstract
Neurons are submitted to an exceptional variety of stimuli and are able to convert these into high-order functions, such as storing memories, controlling behavior, and governing consciousness. These unique properties are based on the highly flexible nature of neurons, a characteristic that can be regulated by the complex molecular machinery that controls gene expression. Epigenetic control, which largely involves events of chromatin remodeling, appears to be one way in which transcriptional regulation of gene expression can be modified in neurons. This review will focus on how epigenetic control in the mature nervous system may guide dynamic plasticity processes and long-lasting cellular neuronal responses. We outline the molecular pathways underlying chromatin transitions, propose the presence of an “epigenetic indexing code,” and discuss how central findings accumulating at an exponential pace in the field of epigenetics are conceptually changing our perspective of adult brain function.
The ability to store information over long periods of time lies at the heart of cellular identity. This cellular “memory” is encoded in the specific pattern of expressed genes and allows a cell to ensure that it “remembers” who it is and how it should move along elaborate pathways during cellular development and differentiation. During development, germ cells or totipotent stem cells give rise to a diverse array of specialized cell types, including nerve cells, which become more hard-wired. These changes allow specialized cells to appropriately function in their specific niche—and, in the case of nerve cells, allows them to properly control cognitive and behavioral functions. Once cellular differentiation processes are established, postmitotic nerve cells become committed to a variety of highly specialized functions that collectively determine our responses to external stimuli. Yet, insults, injury, and neurodegenerative diseases can dramatically affect nerve cells, calling into place a poorly understood “reprogramming process” that may be able to erase previously established cellular settings and, possibly, dedifferentiate or revert these cells to a more primitive pluripotent state. Thus, it seems that developmental processes require “forward” differentiation with a built-in memory component as well as a “reversible” reprogramming capability, allowing for plasticity at many levels (anatomical, electrical, synaptic, etc.). How could one relatively fixed genetic blueprint permit this flexibility to accommodate variability resulting from signals originated from environmental, dietary, and other influences?
How are cellular memories shaped by past experiences and environmental cues? Does a molecular “sculpturing” process exist during development and adult life that takes adaptive cues from the environment (i.e., epigenetic mechanisms), or is this molding process purely stochastic in nature with selection doing the rest (i.e., genetic mechanisms)? (Figure 1) The nervous system is characterized by a vast spectrum of cell types as well as a staggering number of reinforcing connections (synapses) that collectively shape and translate our daily experiences into complex thoughts and behaviors. Can ∼25,000 genes in our relatively fixed human genome explain who we are and how we act? A wealth of accumulating evidence suggests that there is much more to the genome than DNA sequence, permitting variability beyond the Watson-Crick DNA double helix. One way that such additional variability can be established is through epigenetic mechanisms (Figure 1). In this review, we explore the evidence that suggests that these mechanisms play a critical role in regulating neuronal function in the adult brain.
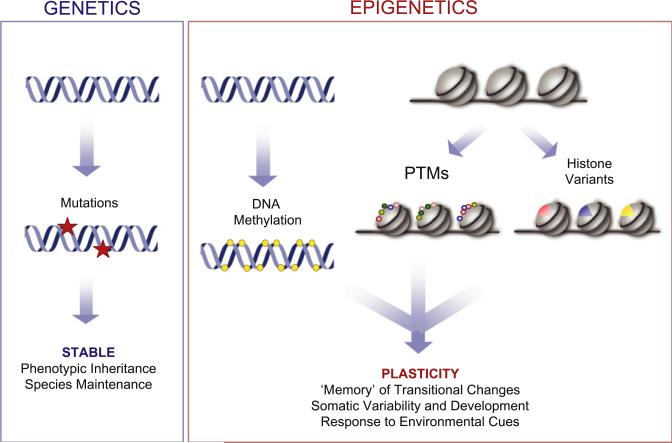
Figure 1. Genetic versus Epigenetic Control.
Regulation of biological processes can be achieved via genetic and epigenetic programs. Variation in genetic information is obtained by mutagenesis of the DNA sequence that irreversibly changes the encoded message. Epigenetic control operates either on DNA, via DNA methylation, or on chromatin. Variation in the chromatin template can be brought about by posttranslational modifications (PTMs; colored beads) added to histones, exchange and replacement of major histones with specialized variants (colored wedges), or ATP-dependent nucleosome remodeling (not depicted), which alters histone:DNA contacts. All of these mechanisms, along with DNA methylation and potential interactions with noncoding RNAs (not depicted), likely act together to bring about the plasticity that helps to define epigenetic phenomena. PTMs of histones occur at highly conserved residues of the N-terminal tails of the core histones (see Figure 3) and include acetylation, methylation, phosphorylation, ubiquitination, etc. Examples of combined DNA methylation and histone modifications have been reported.
Epigenetics: An Old Word Takes on New Meaning and Renewed Interest
A wealth of recent work from many laboratories has rekindled an interest in an old word: epigenetics. The general concept of an “epigenetic landscape” was first articulated by developmental biologist Conrad Waddington, who used it to explain how identical genotypes could unfold a wide collection of phenotypes as development proceeded (Waddington, 1957). With time, Waddington's concept of a phenotypic landscape took on additional meaning: “potentially heritable changes in gene expression that do not involve changes in DNA sequence” (Holliday and Pugh, 1975; Chambon, 1978; Jaenisch and Bird, 2003). While important questions remain, Waddington's landscape has taken a clearer molecular form with the documentation of a remarkable number of multisubunit complexes that act to remodel chromatin—to exchange specific histones (histone variants) in and out of assembled chromatin—or to enzymatically modify DNA and histones to bring about downstream events. It is not fully clear how these dedicated machines are guided to their target sequences, but it is likely to involve constellations of cis-acting regulatory proteins and noncoding RNAs that engage the DNA template directly (Bernstein and Allis, 2005). Some epigenetic marks, such as DNA methylation, appear to provide more stable, if not permanent, indexing marks that extend over long chromosomal domains, giving rise to “memorized” states of gene expression that may be inherited from one cell generation to the next (Figure 1). Other modifications, such as histone acetylation, may be more labile and mediate regulation of gene expression over shorter-term periods. Considering the staggering complexity of neurogenesis, to what extent do changes in synaptic connections, guided by experiences, environment, diet, etc., influence the epigenomes of postmitotic nerve cells that underlie animal behavior, normal or abnormal? Chromatin remodeling in the nervous system would not be directly heritable. Rather, heritability at this level could be through the reproducibility of behavioral patterns from a parent on its offspring.
In this review, we concentrate on emerging findings that tie epigenetic pathways to the special requirements exhibited by nerve cells, specifically of having seemingly opposing mechanisms allowing for cellular “memory” as well as cellular “plasticity.” Somewhat a reflection of our interests in chromatin remodeling, we focus our remarks on specific examples of how histone posttranslational modifications (PTMs) are “written,” “erased,” and “interpreted” in ways that might contribute to both stable and plastic neuronal properties (Figure 2). Of course it is expected that many variations on this general theme exist and that a multitude of interconnected molecular signaling pathways dictates the elaborate gene expression networks that direct the complexities of nerve cell function.
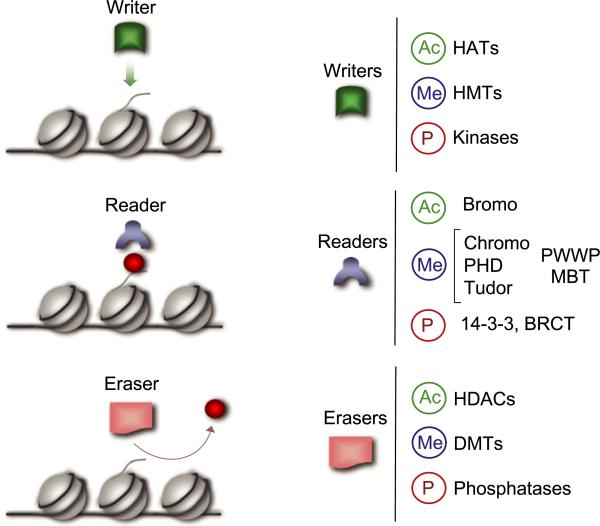
Figure 2. Distinct Classes of Chromatin Remodeling Molecules.
Specific marks on the N-terminal tails of core histones are PTMs elicited by chromatin remodeling machineries that include a large variety of regulatory molecules, many of which interact physically and functionally. Conceptually, the regulators may be indicated as follows. (1) Writers. These are enzymes such as kinases, HATs, and HMTs that modify specific substrates adding phosphate, acetyl, or methyl groups. (2) Readers. These include a large variety of regulatory proteins that share unique domains implicated in recognizing acetyl or methyl groups. Some other domains, such as BRCT (Manke et al., 2003) and a specific region in 14−3−3 (Macdonald et al., 2005), can be considered as readers of phosphate. Often, “readers” recruit to chromatin additional epigenetic effectors. (3) Erasers. These enzymes include phosphatases, HDACs, and DMTs, which directly remove PTMs.
Several broad questions can be posed at this juncture. Are PTMs suited for on-off behavior of short-lived, binary switches (Fischle et al., 2003) belonging to a different set from the PTMs compatible with more lasting, graded responses? Are some PTMs, or the protein complexes that engage them, more compatible with short-term versus long-term memory, and is there enough combinatorial readout of PTMs to deal with the diversity and plasticity of neuronal cells? A wealth of histone PTMs has been uncovered in a variety of cell models. Our goal here was not to provide a compilation of them but to illustrate general principles as they might apply to the nervous system, by specifically highlighting distinct examples for further discussion, including synaptic transmission, behavioral memory, drug addiction, circadian rhythms, and mental retardation-autism syndromes. Numerous reviews on chromatin biology (Berger, 2007; Lee and Workman, 2007), DNA methylation (Miranda and Jones, 2007), and noncoding RNAs (Bernstein and Allis, 2005) have appeared in the literature (see Allis et al., 2007a, for a textbook on epigenetics). Finally, a comprehensive nomenclature has been proposed for chromatin-modifying enzymes (Allis et al., 2007b).
Chromatin Remodeling: Defining Lasting States of Gene Expression in the Nervous System
Chromatin contributes in ensuring that the storage, organization, and readout of the genetic information occurs in a proper spatial and temporal sequence during cellular differentiation and organismal development. The fundamental repeating unit of chromatin, the nucleosome core particle, consists of 147 bp of DNA organized in approximately two superhelical turns of DNA wrapped around an octamer of core histone proteins (two copies each of H2A, H2B, H3, and H4 or variants thereof). When associated with other components, higher-order nucleosomal structures are formed. Variations introduced into nucleosome array structures by a variety of mechanisms (see below) cause subtle, but meaningful, differences in chromatin compaction that correlate closely with more “open” versus “closed” states. These states loosely correlate with “euchromatin” versus “heterochromatin” states that often, but not always, align with “active” versus “inactive” states of gene expression, respectively (Berger, 2007). Covalent histone modifications, histone variants, or chromatin remodeling complexes work together to alter the chromatin fiber (Cheung et al., 2000a; Strahl and Allis, 2000). For example, histone acetylation, a charge-altering modification that negates the positive charge on the ε-amino groups of lysine, has long been correlated with transcriptional activation (Cheung et al., 2000a), likely due to weakening of histone:DNA contacts. In contrast, histone hypoacetylation correlates closely with gene silencing (Lee and Workman, 2007).
For the purposes of this review, several well-studied PTMs serve to illustrate paradigms emerging in present-day chromatin biology, with neurobiologists only beginning to consider how these modifications contribute to neuronal functions (Crosio et al., 2003; Levenson and Sweatt, 2005; Tsankova et al., 2007). For example, acetylation and methylation result from the addition or removal of acetyl and methyl or groups enzymatically donated from respective high-energy donors (acetyl coenzymeA [acetyl-CoA] and S-adenosyl methionine [SAM]; see Table 1). Histones are acetylated by histone acetyltransferases (HATs), which comprise a large family of enzymes, and deacetylated by histone deacetylases (HDACs), which are divided in different families. Class I HDACs are believed to provide the major HDAC catalytic activity present in brain, which is then modified by class II HDACs through direct binding interactions. Class III HDACs represent a distinct subfamily, as discussed under circadian regulation below. Both histone H3 and H4 undergo polyacetylation at nearby lysine residues in the proteins’ N termini; however, still much more needs to be uncovered about the targeted recruiting and specificity of individual HATs and HDACs involved in these reactions.
Table 1.
Linking Histone Modifications to Metabolism
| Phosphorylation | ATP/ADP |
|---|---|
| Methylation | SAM/SAH, FAD/FADH2 |
| Acetylation | Acetyl-CoA/CoA, NAD/NADH, Acetyl-ADP-ribose |
| Ubiquitylation/sumoylation | glucose? |
| Glycosylation | UDP-GlcNAc/UDP |
Posttranslational modifications are elicited by specific enzymes whose activity depends on the intracellular levels of essential metabolites, thus sensing cellular metabolism, nutrients, and energy levels in the cell. PTMs target specific sites on histones, indicating that transient states of chromatin remodeling are under dynamic regulation of cellular physiology.
Methylation affects DNA, RNA, and histone and nonhistone proteins, at least to varying degrees in different organisms. In some cases, intriguing links have emerged between histone modifications, and specifically methylation, and DNA methylation (Mutskov et al., 2002; Ooi et al., 2007). Second, within any potential histone or nonhistone protein that is methylated, multiple lysines or arginines can be modified, often existing together in localized regions of the same or different histone domains. Thus, at least for lysine- and arginine-rich proteins like histones, a wealth of biological readouts may be possible. Third, and expanding on the complexity of methylation, individual lysine residues can be mono-, di-, or trimethylated; similarly, arginine residues can be mono- or dimethylated, and these can be dimethylated in a symmetric or asymmetric fashion. These various methylation reactions are mediated by distinct subtypes of histone methyltransferases and demethylases, as discussed in the next section. Note that, unlike acetylation, methylation does not alter the positive charge of the targeted lysine or arginine, suggesting potential differences in regulatory outputs. Fourth, methylation of distinct lysine residues has opposite functional consequences on gene activation (see below). Fifth, all of the core histones (Shi and Whetstine, 2007) are methylated depending upon the physiological setting. Moreover, in keeping with studies on acetylation, methylation is not limited to histone proteins. Nonhistone proteins, such as the tumor suppressor p53 (Huang et al., 2007; Shi et al., 2007), are physiological targets of methylation reactions, a growing list likely to cross over into neuronal-specific proteins. Thus, methylation alone provides a remarkable number of regulatory options to the cell (Ruthenburg et al., 2007a). Combining methylation with other PTMs, such as acetylation, causes a remarkable array of possibilities. As discussed in the next paragraph, we favor a scenario where the controlled addition and removal of specific PTMs results into unique combinations that constitute a sort of “epigenetic indexing code” that corresponds to distinct physiological states and genomic functions.
Decoding the “Epigenetic Indexing Code”: Writing, Reading, and Erasing Key Marks
Remarkable progress has been made in characterizing what is often being referred to as “writers, readers, and erasers” of PTMs in histone and nonhistone proteins (Figure 2). Thus, considerable attention has been placed in documenting the global distribution (or “patterns”) of histone PTMs using a variety of high-resolution genomic profiling methods (Bernstein et al., 2005; Barski et al., 2007). It emerges from these studies that “epigenomes” are highly organized and strikingly nonrandom with respect to histone and DNA modifications (reviewed in Bernstein et al., 2007). Evidence discussed in this review indicates that the enzymatic machinery that elicits these PTMs operates under the control of a variety of neuronal stimuli, which link physiological variations to modulated chromatin remodeling and thereby controlled gene expression (Figure 3). One important consideration relates to the intracellular pathways involved in the marking of these PTMs. Interestingly, all use physiological metabolites, thereby indicating that the dynamic process of chromatin remodeling may “sense” cellular metabolism and changes in energy levels (Table 1), which are highly controlled and functionally essential in neuronal responses.
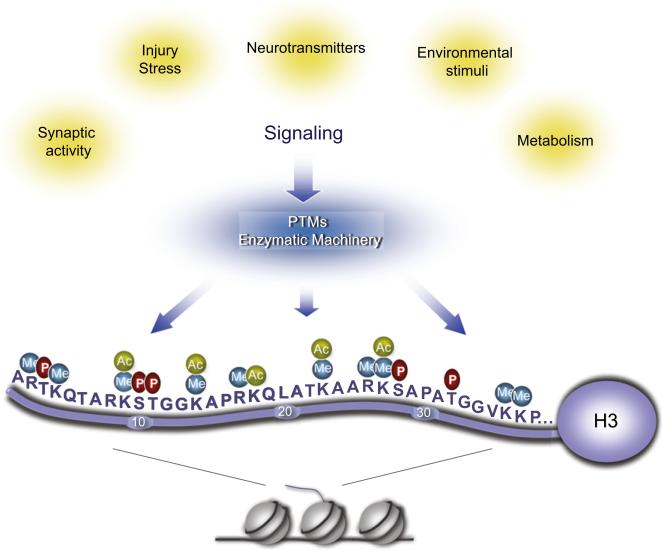
Figure 3. Multiple Posttranslational Modifications on Histone Tails.
The H3 N-terminal tail, here presented as a paradigm of all histone tails, can undergo numerous modifications. Here, only phosphorylation, acetylation, and methylation are indicated. Methylation can be mono-, di-, or trimethyl. The enzymatic machinery that elicits these PTMs is believed to be under the physiological control of neuronal stimuli. Specific combinations of PTMs correspond to selective states of chromatin, either permissive or not for transcription, responsive to damage and stress, or modulated by physiological changes in cellular metabolism.
Selected combinations of numerous PTMs occur at specific residues of the histone H3 N-terminal tail (Figure 3). For example, high levels of H3 and H4 acetylation and H3 lysine 4 methylation (H3K4me) are generally present in promoter regions of active genes (Ruthenburg et al., 2007b). In contrast, elevated levels of H3 lysine 27 (H3K27me) correlate with gene repression mediated by the protein Polycomb (Trojer and Reinberg, 2006). Interestingly, the “degree” of histone lysine methylation matters, with significant differences in large-scale patterns of mono-, di-, and trimethylation at specific lysine residues, whether activating or repressing (Barski et al., 2007). These sorts of epigenetic indexing patterns vary in different cell types or during different stages of development (Marin-Husstege et al., 2002; Lessard et al., 2007; Putignano et al., 2007). For example, a “bivalent domain,” characterized by a configuration of marking genes with both “ON” (H3K4me) and “OFF” (H3K27me), was noted in key developmental genes in embryonic stem cells (Bernstein et al., 2006). How bivalent domains are resolved during development is not known. As well, the extent to which neuronal cells use the strategies of histone PTMs as part of a mechanism for establishing “epigenomes” underlying neuronal diversity is not clear. How does DNA methylation and noncoding RNA enter into the above equation? Recent findings suggest that all of these components work together to bring about a “language” that greatly exceeds that of DNA alone (Ooi and Henikoff, 2007).
If histone methylation does not alter the charge of target lysines or arginines, how does histone methylation function? Unlike acetylation, where charge-based mechanisms are likely to apply, histone:DNA contacts might not be affected by histone methylation in what can be referred to as “cis” (structural) effects on nucleosome structure. Likely, histone methylation is “read” by effector proteins that bring about meaningful downstream events by “trans” mechanisms (Figure 2). Effector proteins contain “reader” modules that structural and functional evidence identify as relatively short (50−100 aa) binding motifs, such as chromodomains or PHD fingers, which read histone methyl-lysine marks with remarkable and elegant precision (Ruthenburg et al., 2007a; Lee and Workman, 2007). Similarly, as suggested in early articulations of the histone and epigenetic code hypotheses (Cheung et al., 2000a; Strahl and Allis, 2000; Jenuwein and Allis, 2001; Turner, 2002), specific acetyl-lysine marks are also read in histone and nonhistone proteins by bromodomains. Also, interplays exist between adjacent or neighboring modifications that may serve to govern the binding and interactions of the effector proteins (Fischle et al., 2003). Understanding the extent to which such repeated modules read PTMs, either on a single histone tail or on multiple tails or even distinct nucleosomes, remains a challenge for future studies.
The general stability of DNA and histone methylation marks, as compared to rapid turnover kinetics PTMs such as acetylation and phosphorylation (Figure 4), prompted early speculation that methylation might be the ideal epigenetic indexing system (Jenuwein and Allis, 2001; Bannister and Kouzarides, 2005). While the situation with DNA demethylation remains unclear, with some reports of rapid DNA demethylation occurring in brain (Fan et al., 2001; Miller and Sweatt, 2007), progress has been made in the area of histone demethylation. Demethylase activities are being described for essentially all of the well-known lysine methyl sites in histones, not to mention those that are selective for mono-, di-, or trimethylation states (Trojer and Reinberg, 2006), promising to carry this family of enzymes into the “celebrity status” of other chromatin modifiers, such as HATs and HDACs. Recent findings indicate that some of these enzymes turn out to be critical in neuronal functions. For example, the histone H3K4 tridemethylase SMCX links repression of target genes (e.g., the sodium channel type 2A and synapsin 1) to mental retardation and epilepsy (Tahiliani et al., 2007). These studies underscore several important points. First, they point to a general model wherein failure to remove, or most certainly add, “ON” epigenetic marks, such as H3K4me3 (or likely “OFF” marks as well), impairs downstream neuronal gene regulation leading to disease states by yet undefined mechanisms. Second, disease phenotypes are not always associated with alterations in enzyme activity. For example, H3K4me3 demethylase activity was examined in a small series of SMCX mutations, which interestingly correlated roughly with the severity of mental retardation in human patients affected with these mutations. In some cases, the mutations studied affected enzymatic activity, while in other cases the mutations affected the intracellular location of the protein or its association with other SMCX complex components. Third, SMCX contains multiple modules, including two PHD fingers, suggestive of a yet unknown methylation “reading” function. Stabilization of the protein in the remodeling complexes within the chromatin template may occur in part through this type of binding module (Wysocka et al., 2006; Taverna et al., 2006; Li et al., 2007). Interestingly, several of the mutations in the mental retardation-associated SMCX demethylase fall within some of its reading modules (Jensen et al., 2005). Similarly, the intriguing interaction between ATRX, a DNA helicase/ATPase mutated in the ATRX syndrome (α-thalassemia/mental retardation, X-linked) and MeCP2, a regulator of DNA methylation causally linked to Rett's syndrome (see later), is disrupted by mutations responsible for the pathologies associated with these two epigenetic regulators (Nan et al., 2007). As a footnote, we point out that many chromatin remodeling activities and histone-modifying enzymes contain one or more modules, such as bromodomains, chromodomains, and PHD fingers, linked in various combinations (Lee and Workman, 2007). Thus, while it is convenient to discuss “writers” and “readers” of PTMs separately (Figure 1), many writers and erasers contain their own reading modules whose functions remain unclear (Figure 2). In mixed lineage leukemia (MLL), for example, the writer of H3K4 methyl marks (Ruthenburg et al., 2007a) contains a series of PHD fingers and an atypical bromodomain whose function is unknown. We predict that deciphering the “epigenetic indexing code” will help reveal the pathways responsible for the timing, intensity, and precision of “memorized” gene expression.
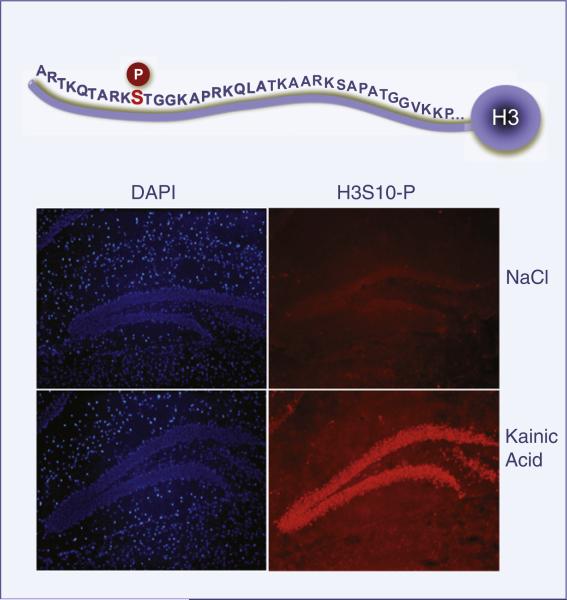
Figure 4. Dynamic Changes in Histone Modifications in the Hippocampus.
Chromatin remodeling occurs in hippocampal neurons of the dentate gyrus 30 min after administering kainic acid (35 mg/kg) to a mouse. Kainate receptors are involved in epileptogenesis and synaptic plasticity. H3S10 phosphorylation in these neurons is induced by stimulation of dopamine, acetylcholine, and glutamate receptors and is often associated with acetylation at H3K14 (Crosio et al., 2003). These events are coupled to transcriptional activation in hippocampal neurons. An antibody that recognizes specifically H3 P-S10 was used.
This evolving knowledge of chromatin remodeling mechanisms has already begun to inform our understanding of the regulation of brain function under normal conditions and certain pathophysiological states, as will be seen in the sections that follow.
Does Chromatin Remodeling Influence Synaptic Plasticity?
Repeated patterns of synaptic transmission lead to diverse forms of synaptic plasticity at excitatory and inhibitory synapses, e.g., long-term potentiation (LTP) or long-term depression (LTD), whereby the efficacy of synaptic transmission is up- or downregulated, respectively. Certain forms of LTP and LTD are long lived and thought to be dependent on lasting changes in gene expression. Based on the critical role that chromatin remodeling plays in dictating a transcription-permissive or silencing state of the genome (Felsenfeld and Groudine, 2003), it is notable that growing evidence suggests that histone PTMs may be involved in these processes. For example, H4 acetylation at specific promoters in Aplysia is altered after LTP and LTD (Guan et al., 2002), and HDAC inhibitors promote LTP in mammalian neurons (Levenson et al., 2004). Additionally, during synaptic transmission, neurotransmitters trigger responses in target neurons by activating two major families of receptors, ligand-gated ion channels and G protein-coupled receptors. Likewise, growth factors and cytokines are released from neurons in an activity-dependent manner and act on target neurons through receptor-mediated signaling. Triggering signaling cascades in target neurons leads to more long-lasting effects, including changes in gene expression via control of transcription and thereby chromatin remodeling (Figures 3 and 4).
One paradigmatic example involves the transcription factor CREB, which, once activated by signaling-induced phosphorylation, recruits CREB-binding protein (CBP), a coactivator with intrinsic HAT activity (Lonze and Ginty, 2002). In addition, an increase in cellular Ca2+ levels in muscle activates Ca2+/calmodulin-dependent kinases, which phosphorylate class II HDACs. This phosphorylation provides a docking site for the “reader” protein 14−3−3, which mediates the export of the phosphorylated HDACs from the nucleus (McKinsey et al., 2000). This pathway operates in hippocampal, striatal, and cerebellar granule neurons (Chawla et al., 2003; Renthal et al., 2007) and could represent a widespread mechanism of Ca2+-mediated histone acetylation and gene expression.
Independent of histone deacetylation, class II HDACs can recruit cyclin-dependent kinase-5 (Cdk5) to phosphorylate myocyte enhancing factor 2 (MEF2), a protein that mediates activity-dependent changes in synapse formation in neurons, and repress its transcriptional activity (Gong et al., 2003; Pulipparacharuvil et al., 2008). These studies reveal mechanisms by which synaptic activity may mediate long-lasting changes in brain, such as those associated with drug addiction. As discussed later, intriguing similarities indicate that some common pathways of epigenetic control may be relevant to memory and neurodegenerative disease.
Synaptic activity is also reported to influence DNA methylation. This is unexpected, as it suggests that DNA methylation, classically thought as a very stable modification, undergoes rapid and dynamic regulation in the nervous system (Levenson et al., 2006). An important example is the activity-dependent control of Bdnf gene expression that has been correlated with reduced DNA methylation and release of a repressor complex comprising methyl-CpG binding protein (MeCP2; a protein that binds to and represses methylated DNA; Chen et al., 2003; Martinowich et al., 2003; Chang et al., 2006). It is proposed that neural activity, via increases in cellular Ca2+ levels and activation of Ca2+/calmodulin kinases, leads to the phosphorylation of MeCP2 and its release from the Bdnf promoter. This induces Bdnf expression and attendant dendritic outgrowth (Chen et al., 2003).
In addition to direct mediators of these synaptic changes, various immediate-early genes seem to be particular targets of synaptic-plasticity-regulated epigenetic control. For example, the induction of c-fos is associated with dramatic increases in H4 acetylation and H3 phospho-acetylation (Figure 4; Crosio et al., 2003; Li et al., 2004; Brami-Cherrier et al., 2005; Kumar et al., 2005). Perturbation of dopamine signaling triggers the dramatic induction of c-fos and other immediate-early genes in distinct subsets of striatal neurons in response to psychostimulant drugs of abuse (e.g., cocaine and amphetamine) and in response to antipsychotic drugs that block D2 dopamine receptors (Welter et al., 2007). The nuclear accumulation of DARPP32, a protein phosphatase 1 inhibitor, has been recently invoked in cocaine-induced H3 S-10 phosphorylation (Stipanovich et al., 2008). Another study implicated mitogen and stress activated kinase 1 (MSK1) in cocaine stimulation of H3 P-S10 and c-fos induction (Brami-Cherrier et al., 2005), although other kinases may be also implicated in response to various stimuli (Sassone-Corsi et al., 1999; Nowak and Corces, 2004). Likewise, induction of c-fos by seizure activity or memory paradigms in other brain regions such as hippocampus is associated with H3 phosphoacetylation and ERKs (extracellular signal regulated kinases) activation (Crosio et al., 2003; Tsankova et al., 2004; Levenson and Sweatt, 2005; Chwang et al., 2006). Several other kinases can phosphorylate H3 S10 in nonneuronal cells, but their actions in brain are unexplored.
Histone Modifications Affect Behavioral Memory
Consistent with the coupling of histone modifications with synaptic plasticity and correlations between behavioral plasticity and epigenetic control of immediate-early gene transcription, there are numerous reports of the importance of histone modifications in behavioral memory. Mice deficient in CBP exhibit memory deficits, and administration of an HDAC inhibitor can restore normal long-term memory formation in the mutants, and even enhance it in normal animals (Alarcon et al., 2004; Korzus et al., 2004; Levenson et al., 2004). Contextual fear conditioning, or activation of the ERK pathway that is thought to contribute to memory formation, increases levels of H3S10-K14 phospho-acetylation in the CA1 area of hippocampus, without affecting H4 acetylation (Levenson and Sweatt, 2005; Chwang et al., 2006). Interestingly, changes in histone acetylation and methylation in hippocampus have also been implicated in depression and antidepressant action, where HDAC inhibitors exert antidepressant-like effects (Tsankova et al., 2006; Schroeder et al., 2007).
Recent work has implicated changes in DNA methylation in learning and memory as well. Contextual fear conditioning induces the expression of Dnmt3A and -3B in CA1 of hippocampus, and administration of the DNMT inhibitors, zebularine and 5-aza-2-deoxycytidine, blocks the induction of both contextual fear conditioning (Miller and Sweatt, 2007) and hippocampal LTP (Levenson et al., 2006). However, it remains unknown how these drugs, which are thought to regulate DNA methylation in dividing cells only, affect gene expression in mature neurons. Fear conditioning causes rapid methylation and silencing of the protein phosphatase 1 (Pp1) gene promoter (Miller and Sweatt, 2007), a gene important for LTP and memory formation. Interestingly, fear conditioning also induces demethylation of the Reelin promoter (Dong et al., 2007), indicating that both DNA methylation and demethylation may be highly regulated in the adult brain.
Chromatin Remodeling Mechanisms in Drug-Induced Plasticity and Addiction
Drug addiction can be viewed as a form of drug-induced neural plasticity, whereby repeated exposure to drugs of abuse leads to long-lasting changes in the brain's natural reward centers and associated memory circuits, which underlie the addiction phenotype (Hyman et al., 2006). One major site for these lasting changes is the nucleus accumbens (NAc)—the ventral portion of the striatum, although many other regions are also involved. Drug-induced changes in gene expression, largely driven by dopaminergic signaling, occur in the NAc and other relevant regions (Tsankova et al., 2007). Some of these changes persist even after months of abstinence (Grimm et al., 2003), consistent with the persisting behavioral abnormalities. These observations have driven research into chromatin remodeling as one molecular basis of sustained, even life-long, alterations in gene expression in brain reward regions that underlie an addicted state (Tsankova et al., 2007).
As stated earlier, an acute dose of cocaine induces the expression of fos family immediate-early genes in the NAc and dorsal striatum, an event associated with a rapid and transient increase in H4K5 acetylation (Brami-Cherrier et al., 2005; Kumar et al., 2005). CBP, with its intrinsic HAT activity, has been implicated in these effects (Levine et al., 2005). Acute cocaine also induces Ser10/Lys14 H3 phospho-acetylation at the c-fos promoter (Crosio et al., 2003; Kumar et al., 2005), an event that is mediated by activation of ERKs and could require the protein kinase MSK1 (Lu et al., 2006; Brami-Cherrier et al., 2005). In contrast, chronic exposure to cocaine activates or represses many distinct genes compared to acute treatment. The c-fos gene, for instance, desensitizes in the NAc during a course of chronic drug exposure, while the fosB gene continues to be induced, and these changes are associated with decreased and increased histone acetylation at these genes, respectively. In addition, chronic cocaine increases H3K9 methylation at the c-fos gene, a modification tightly associated with gene repression. This effect could be mediated by the induction in the NAc of SUV39H1, a Lys9 histone methyltransferase (Renthal et al., 2008). Interestingly, several genes that are selectively induced in the chronic state, such as Cdk5 and Bdnf (Bibb et al., 2001; Grimm et al., 2003), are associated with increased H3 acetylation. Cocaine induction of H3 acetylation at the Bdnf promoter builds over a week of withdrawal (Kumar et al., 2005), and this change precedes the progressive increase in Bdnf expression in this brain region (Grimm et al., 2003). Little is known about the specific HATs and HDACs that may mediate these changes in histone acetylation at cocaine-regulated genes. Yet these results highlight the importance of exploring genome-wide chromatin alterations, by use of ChIP on chip (Lee et al., 2003) or SACO (Impey et al., 2004) or related techniques, to identify many other genes whose dysregulation contributes to cocaine addiction. Genome-wide epigenetic approaches have yielded exciting results in the fields of developmental (Lessard et al., 2007) and cancer biology (Lee et al., 2003).
The transcription factor ΔFosB is implicated in the transition to an addicted state in chronic drug treatment (Nestler, 2008). ΔFosB is a truncated product of the fosB gene, which accumulates uniquely during chronic drug treatment due to its unique protein stability (Ulery et al., 2006). Gene expression array experiments indicate that ΔFosB accounts for >25% of all changes in steady-state mRNA levels induced in the NAc by chronic cocaine administration (McClung and Nestler, 2003). ChIP assays have shown that the induction by cocaine of one of these mRNAs, Cdk5, represents a direct, activating effect of ΔFosB on the Cdk5 gene (Kumar et al., 2005). By contrast, Bdnf is not a direct target of ΔFosB, consistent with a different mechanism being involved in its induction (McClung and Nestler, 2003). Induction of Cdk5, in turn, partly mediates the effects of chronic cocaine and of ΔFosB on dendritic remodeling in the NAc (Norrholm et al., 2003; Lee et al., 2006). ΔFosB is also responsible for c-fos repression after chronic cocaine, where it recruits HDAC1, leading to the deacetylation of nearby histones (Renthal et al., 2008). These findings support a model in which the accumulating ΔFosB interacts with distinct chromatin remodeling factors at specific promoters of genes that control reward neurons in this brain region.
Cocaine-induced chromatin remodeling is mediated in part via regulation of the enzymatic machineries that control histone acetylation and methylation. For example, phosphorylation of HDAC5, a class II HDAC enriched in the NAc, is induced by chronic cocaine exposure (Renthal et al., 2007), consistent with activation of Ca2+/calmodulin-dependent kinases in this brain region (Mattson et al., 2005). Another example is the transcriptional regulator nucleus accumbens 1 (NAC-1), which is highly induced by cocaine in the NAc and interacts with HDAC3 and HDAC4 (Korutla et al., 2005). Chronic cocaine also induces the HMT SUV39H1 in the NAc (Renthal et al., 2008).
Drug-induced chromatin remodeling is behaviorally relevant. Systemic or intra-NAc administration of HDAC inhibitors, or knockout of HDAC5, potentiates behavioral responses to cocaine, whereas viral-mediated overexpression of HDAC5 specifically in the NAc has the opposite effect (Kumar et al., 2005; Renthal et al., 2007). Interestingly, overexpression of HDAC4, but not HDAC9, has a similar effect, suggesting some specificity of HDAC action, the basis of which is still poorly understood. Moreover, CBP-deficient mice show reduced cocaine-induced locomotor activity (Levine et al., 2005).
There are also several reports of chromatin changes after chronic ethanol administration (Mahadev and Vemuri, 1998; Bonsch et al., 2006; Bardag-Gorce et al., 2007). Interestingly, maternal ethanol consumption has been associated with lasting changes in DNA methylation in the fetal heart, which may contribute to increased risk of ischemic injury (Zhang et al., 2007). Identification of chromatin changes in blood and other peripheral tissues as a consequence of chronic ethanol administration raises the possibility of using such epigenetic markers to monitor drug use in vulnerable human populations.
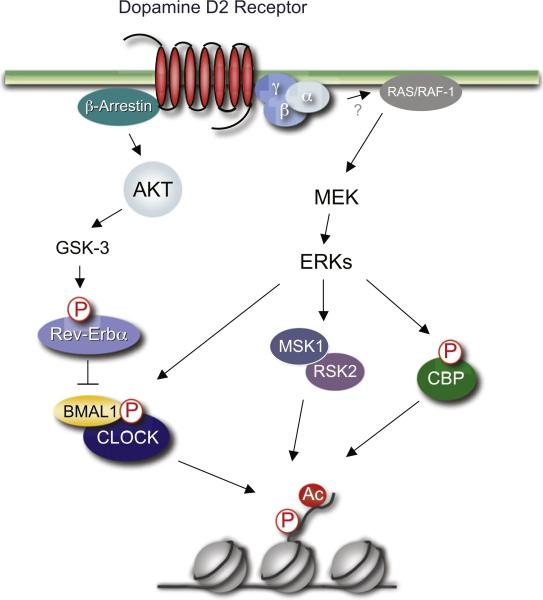
Intriguing connections have also been found between dopamine neurotransmission and the circadian clock machinery, via D2 receptors (Yujnovsky et al., 2006; Doi et al., 2006b; Yan et al., 2006). Dopamine is the major catecholamine in retinal neurons and plays a central role in neural adaptation to light (Iuvone et al., 1978), mostly through D2 receptors highly expressed in these neurons (Doi et al., 2006b). Light stimulates the synthesis, turnover, and release of retinal dopamine, and dopaminergic activity is higher during the day than at night. Thereby, dopamine is a likely mediator of light signaling to the retinal circadian clock. Activation of D2 receptors stimulates CLOCK:BMAL1 function through the MAPK signaling pathway. Since CLOCK is a HAT (see the next section; Doi et al., 2006a), dopamine may exert its function by signaling directly to the HAT potential of CLOCK (Figure 5). Another tempting speculation includes the involvement of the AKT/GSK-3 transduction pathway, which is implicated in D2 receptor-dependent signaling (Beaulieu et al., 2007). GSK-3 kinase function is tightly regulated by lithium, an antimanic and antidepressant agent (Beaulieu et al., 2008), and shaggy, the GSK-3 Drosophila homolog, plays an essential role in circadian control (Martinek et al., 2001). Thus, this regulatory pathway may link circadian rhythms, depression cycles, and chromatin remodeling.
Figure 5. Signaling Pathways Linking Dopamine to the Circadian Clock.
Dopamine controls many brain functions, including roles in behavior and cognition, motor activity, motivation, and reward. Signaling mediated by D2 receptor is envisaged to control chromatin remodeling via modulation of various enzymatic functions. By using either the ERK transduction pathway or the alternative AKT/GSK-3 signaling system, dopamine could control kinases that phosphorylate H3, such as Rsk2 and MSK1, or the enzymatic function of HATs, such as CBP or CLOCK. The GSK-3 kinase is inhibited by lithium and thereby is implicated in the treatment of mood disorders. These regulatory pathways may integrate circadian control, dopamine signaling, and chromatin remodeling.
The Circadian Clock: How to “Time” Periodic Chromatin Remodeling
Unlike memory and addiction behaviors, the neuronal dynamic response that underlies circadian regulation is peculiar, as it is characterized by an oscillatory and recurrent pattern of gene expression. About 15,000 neurons within the hypothalamic suprachiasmatic nucleus (SCN) constitute the mammalian master pacemaker, a highly organized structure that synchronizes peripheral oscillators to ensure temporally coordinated physiology (Saper et al., 2005). In SCN neurons, a number of clock genes display the unique pattern of oscillatory gene expression that respects a rhythm of about 24 hr (Dunlap, 1999). Similarly, almost all peripheral cells in the organism show circadian rhythmicity and oscillatory expression of clock-controlled genes. Remarkably, at least 10% of all mammalian transcripts oscillate with circadian rhythmicity (Panda et al., 2002), revealing that a highly efficient molecular machinery must thereby operate to insure periodic chromatin remodeling. Several studies have described that histone modifications occur at promoters of clock-controlled genes and that these—specifically acetylation—show a rhythmic pattern (Etchegaray et al., 2003; Naruse et al., 2004; Ripperger and Schibler, 2006). These correlative analyses indicated that transcription-permissive chromatin states are dynamically established in a circadian-time-specific manner but did not demonstrate whether unique chromatin remodeling events are required for clock control, i.e., cause versus effect. Additional studies indicated that H3K27 methylation also oscillates at mammalian clock gene promoters (Etchegaray et al., 2006), whereas ATP-dependent chromatin remodeling operates within the circadian machinery, at least in Neurospora (Belden et al., 2007). This collection of reports underscored the complexity of the circadian machinery, which is constituted in large part of a variety of transcription factors whose structural features have been analyzed during the past 10 years. The finding that a master clock regulator, the protein CLOCK, is an enzyme with HAT activity provided the key to unlock the regulatory code that causally couples circadian chromatin plasticity and transcription (Doi et al., 2006a).
CLOCK shares a number of structural features with ACTR, a HAT previously shown to function as a coactivator for some nuclear receptors (Doi et al., 2006a). In addition to the carboxyl-terminal glutamine-rich region—the domain where the HAT function resides—similarities include the highly conserved bHLH-PAS domain at the N13 termini, a nuclear receptor interaction domain (NRID), as well as serine-rich regions within the middle portion of both proteins. Yet, some features show that CLOCK is a unique HAT. Indeed, while the overall organization resembles the SRC-type of HAT, the acetyl-coenzyme A (CoA) binding motif is more similar to the MYST-family of HAT proteins, a combination not found in other HATs. In addition, when dimerized with its partner BMAL1, CLOCK binds DNA at E box promoter elements of clock-controlled genes, thereby functioning as a classical bHLH type of transcription factor.
CLOCK acetylates histones H3 and H4, with specific preference for H3K14 (Doi et al., 2006a), an event associated with transcriptional activation. Acetylation at K14 is enhanced by prior phosphorylation at Ser10 in fibroblasts (Cheung et al., 2000b; Lo et al., 2000). Importantly, light-induces phosphorylation at H3S10 in SCN's neurons (Crosio et al., 2000), indicating that light-mediated signaling influences the state of higher chromatin organization and suggesting that coordinated PTMs of histones may contribute to the integration of light signaling and clock function (Figure 2).
As other HATs (Sterner and Berger, 2000), CLOCK also acetylates nonhistone proteins. CLOCK acetylates its own partner, BMAL1, at position Lys537, an event that is regulated in a rhythmic manner and that is critical for circadian control (Hirayama et al., 2007). This finding suggests that CLOCK may have several putative targets and that their identification is likely to provide significant clues about the neuronal pathways influenced by the circadian clock. In this respect, another protein may play a relevant regulatory function: NPAS2. This is an alternative partner of BMAL1, whose structure is loosely similar to CLOCK (Reick et al., 2001). Interestingly, NPAS2 displays a neuronal-specific distribution, being abundant in the forebrain areas, including the cortex, hippocampus, striatum, amygdala, and thalamus (Garcia et al., 2000) and appears to have a role in sleep and behavioral adaptability (Dudley et al., 2003). While it is yet unclear whether NPAS2 may have acetyltransferase activity, its dimerization with BMAL1 confers to it a potential role in indirectly regulating CLOCK's HAT activity.
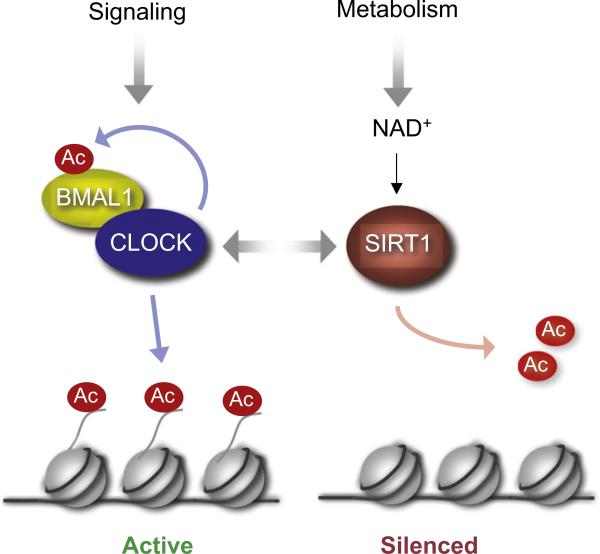
Interestingly, CLOCK expression is not rhythmic (Gekakis et al., 1998), whereas CLOCK's enzymatic function is, indicating that its chromatin remodeling activity is critical for circadian physiology (Doi et al., 2006a). The finding of a circadian HAT opened the search for a counterbalancing HDAC. Recent reports indicate that SIRT1 may play this role (see Nakahata et al., 2008; and Figure 3). SIRT1 belongs to the family of sirtuins that constitutes the so-called class III of HDACs. These are the only HDACs whose enzymatic activity is NAD+ dependent and that has been intimately linked to the control of metabolism and aging (Bishop and Guarente, 2007). SIRT1 directly associates with CLOCK and functions as a rheostat in modulating the acetylation state of histone H3 and BMAL1 (Nakahata et al., 2008). These results are relevant in establishing a direct molecular coupling between circadian control and energy metabolism (Figure 6). The CLOCK-SIRT1 complex is in fact regulated by the NAD+/nicotinamide balance in the cell (Nakahata et al., 2008; Asher et al., 2008), providing a novel perspective to the links between circadian rhythms, metabolism, and cellular reduction-oxidation pathways (Wijnen and Young, 2006). Intriguingly, SIRT1 has been found to regulate aging and neurodegeneration (Gan and Mucke, 2008). For example, inhibitors of SIRT1 rescue α-synuclein-mediated toxicity in animal models of Parkinson's disease (Outeiro et al., 2007). As the role of dopamine in neurotoxicity and neuroprotection is established (Bozzi and Borrelli, 2006), this may represent an intriguing link between dopamine signaling and SIRT1-mediated metabolic control in neurons. Indeed, SIRT1 has also been found to contribute to the redox-dependent fate of neural progenitors (Prozorovski et al., 2008).
Figure 6. CLOCK-Mediated Acetylation and Regulation by SIRT1, an NAD+-Dependent HDAC.
CLOCK acetylates H3 and its dimerization partner BMAL1 to regulate clock-controlled genes. SIRT1 associates with CLOCK and, in response to metabolic changes in intracellular NAD+ levels, modulates clock-controlled genes by virtue of its HDAC enzymatic activity. Thus, metabolic, nutritional, and environmental cues modulate the circadian machinery via chromatin remodeling.
Getting “Nervous” about Chromatin Remodeling: Disease Links
Given the involvement of epigenetic mechanisms in nervous system function, it is not surprising that a growing number of disorders, in particular mental retardation and autism spectrum syndromes, have been linked to chromatin remodeling defects. This likely relates to the elegant control pathways that exist to regulate activation of neuronal gene programs as well as repression of nonneuronal gene sets in neuronal tissues (Feng et al., 2007). Well-studied, for example, is the REST/coREST repressor complex that serves to silence numerous neuronal genes in nonneuronal cells (Ballas and Mandel, 2005). Interestingly, REST/coREST contains HDACs and other chromatin regulators often associated with gene silencing. Thus, a neuronal differentiation program, from stem or progenitor cell to a well-connected postmitotic neuron, requires “undoing” the above-mentioned epigenetic silencing program. Taking clues from other well-studied “ying-yang” chromatin relationships (Ruthenburg et al., 2007a), it seems likely that reversal of silencing includes hyperacetylation of the H3 and H4 N tails along with hypermethylation of H3K4. Interestingly, the finding that HATs (such as MOF, for maless on the first) and HMTs (for histone methyltransferases such as MLL) coexist in large complexes suggests that this machinery operates to bring about concerted gene-activating reactions (Dou et al., 2006; Taverna et al., 2006; Li et al., 2007). The H3K4 tridemethylase complex SMCX (Tahiliani et al., 2007), for example, contains the repressive HMT G9a, a writer of repressive H3K9me marks, and a H3K9me reader known as HP1ã, a heterochromatin-associated protein, which uses its chromodomain module to “dock” onto H3K9me marks. These findings are paralleled by previous observations on the concerted function of kinases and HATs (Lo et al., 2001; Merienne et al., 2001) and of interdependent trans-modifications on distinct histone tails (Sun and Allis, 2002).
How might disruption of the concerted action of chromatin modifiers translate into pathological conditions? Some important examples exist (Ausio et al., 2003; Levenson and Sweatt, 2005; Tsankova et al., 2007). The most well-studied “epigenetic disease” associated with altered neurological function, is Rett's syndrome, an X-linked postnatal autism spectrum disorder characterized by stereotypical motor, learning, and social abnormalities that generally worsen over time (Moretti and Zoghbi, 2006). Candidate gene analyses identified MeCP2 as the causative gene (Amir et al., 1999). MeCP2 was identified on the basis of binding selectively to methylated CpG dinucleotides in heterochromatic regions and functioning in a methylation-dependent repressive fashion (Nan et al., 1997). Thus, MeCP2 can be described as a DNA “reader” in analogy to readers of histone methylation (Figure 2). In keeping with the general theme developed above for REST/coREST and SXMC repressive complexes, engagement with additional corepressors and HDACs provide the enzymatic “punch” to the silencing activity elicited by MeCP2. Interestingly, MeCP2 may switch partners and thereby its activity, for example by interacting with CREB and thus eliciting activation (Chahrour et al., 2008).
In Rett's syndrome, MeCP2 stands as the key DNA-binding “hook” to bring the repressive chromatin remodeling machinery to target loci. In Rubinstein-Taybi syndrome (RSTS), characterized by mental retardation and developmental abnormalities, the DNA-binding hook is provided by CREB. Phosphorylation of CREB leads to CBP recruitment and activation of target promoters. Causative mutations in RSTS map to the CBP gene and may result in impairment of HAT activity (Murata et al., 2001). Mice haploinsufficient for CBP display impaired cognitive function, altered neuronal plasticity, and aberrant histone acetylation at target promoters (Alarcon et al., 2004; Korzus et al., 2004). Interestingly, the behavioral symptoms can be ameliorated by administration of HDAC inhibitors (Vo and Goodman, 2001).
Additional examples show how HAT activity may be modulated in pathological conditions because of unique interaction between epigenetic regulators. A polyglutamine-expanded protein, spinocerebellar ataxia-7, regulates several HAT complexes (McMahon et al., 2005; Palhan et al., 2005). The degree of polyglutamine expansion correlates with the impairment of HAT activity, which may, in turn, correlate with disease progression.
Docking of effector proteins, especially proteins containing modules that bind to more stable methyl marks, is a rapidly expanding area of chromatin biology (Ruthenburg et al., 2007a). Equally exciting are “cross-talk” mechanisms wherein an adjacent or nearby modification can affect histone modifications on “cis” or “trans” tails (Briggs et al., 2002; Fischle et al., 2003). For example, binding of HP1 to H3K9 methyl marks is regulated by phosphorylation at the adjacent H3S10 (Figure 3) in what has been referred to as “methyl/phos switching” (Fischle et al., 2003). Interestingly, H3S10 phosphorylation can be induced during mitosis or during immediate-early gene activation, elicited by distinct kinases (Nowak and Corces, 2004). One kinase that induces H3S10 phosphorylation during gene activation is Rsk2, a kinase causally linked to Coffin-Lowry syndrome, a type of human mental retardation (Sassone-Corsi et al., 1999). Chromatin changes, in part brought about by H3S10 phosphorylation, have been directly demonstrated in hippocampal neurons (Crosio et al., 2003), leading to wider speculations that chromatin remodeling could contribute to learning and memory. Similar conclusions have been reached using HDAC inhibitors (Fischer et al., 2007). These studies cast new light on using “epigenetic therapies” to develop strategies for neuronal dysfunctions, an approach that has proved effective in the treatment of cancer (Jones and Baylin, 2007).
Summary, Conclusions, and Future Challenges
Remarkable progress has been made in documenting marks, writers, readers, and erasers of a still incompletely defined epigenetic histone code. While many questions remain, several trends and conclusions can be reached. An elaborate series of concerted enzymatic reactions remodel chromatin, leading to the generation and maintenance of quasi-stable epigenetic states. For example, HDAC recruitment to chromatin targets could be considered a late step in a silencing pathway wherein removal of acetyl groups from histone lysines, leading to the re-establishment of positive charge, causes condensation of the chromatin fiber in what is considered “cis mechanisms.” However, HDACs might also act earlier in a silencing pathway, “resetting” a particular lysine for subsequent methylation by HMTs that, in turn, serve as docking surfaces for methyl-binding effectors in what is being referred to as “trans mechanisms.” Other variations on this general theme may apply. For example, acetylation may be used to recruit downstream machinery (activating or repressive) through bromodomain recognition or other mechanisms. The general concept of multivalency in chromatin, the combinatorial association of multiple “Velcro” modules to bring about distinct effector:chromatin interactions, promises to be an exciting area of future work (Ruthenburg et al., 2007a). Early glimpses of this strategy at work have already been reported (Li et al., 2007).
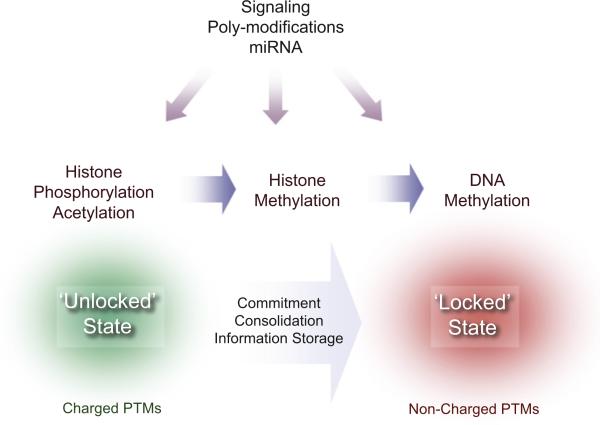
How do epigenetic signals translate into short-term versus long-term memory effects in the nervous system? We favor a scenario where neuronal-specific transcription factors recruit chromatin remodeling activities that then lock in positive or negative epigenetic states. We like to speculate that more stable, noncharge-altering modifications, such as methylation of DNA or histones, participate in “lock-down” activities by engaging chromatin-opening or chromatin-closing effectors. In this view, highly reversible modifications, such as acetylation and phosphorylation, may act as the dynamic “on-off switches” in response to changing conditions and environmental cues (Figure 7). However, much of biology, particularly that displayed by multicellular organisms, requires less “black versus white” outputs and rather gradual, “gray” responses. Here, we propose that “polymodifications,” like polyubiquitylation, etc., may be more in keeping with graded responses. While well-documented in pathways of protein degradation, polymodifications should receive more attention in chromatin biology (Verhey and Gaertig, 2007), not only because of their biological significance, but also since they may identify novel avenues of pharmacological intervention.
Figure 7. Consolidation of Epigenetic Information by Progressive Enzymatic Modifications.
Scheme of the proposed step-wise consolidation process of epigenetic information, in which successive and interconnected histone PTMs elicit transition from an “unlocked” chromatin state to a “locked,” fully committed state to either gene activation or silencing. The unlocked state is characterized by dynamic, transient, and charged PTMs, such as phosphorylation and acetylation. The locked state is achieved through noncharged, stable modification of both histones and DNA by methylation. Graded modulation of this process is under the control of intracellular signaling, polymodifications (polyubiquitylation, etc.), and microRNA pathways.
Considerable progress has been made in identifying chromatin-binding modules that function to read selective chromatin marks. Less clear are functions of adding (or removing) chromatin-binding modules to nonhistone proteins. We note that many chromatin-modifying enzymes (writers) also contain reading modules, suggesting that the writing and reading of epigenetic marks may work together in bringing about defined chromatin states. Along this line, multiple prion-like domains (glutamine- and asparagine-rich domains) have been noted in Polycomb, leading to provocative suggestions that “self-aggregation” properties may exist in chromatin regulators to carry or to propagate long-term “memory formation and transcriptional memory” through altered chromatin conformational states (Shorter and Lindquist, 2005).
Finally, is there an epigenetic-based chromatin remodeling process that serves to “shape” or to “mold” the landscape of our genome as it deals with environmental cues? It may not be a coincidence that many well-studied chromatin-modifying enzymes are regulated by, or use directly as substrates, key intermediates from central metabolism. Acetyl-coenzyme A is required for most known HAT activities, while certain HDACs are regulated by nicotinamide. S-adenyosyl methonine is required for HMTs, and FAD is required for certain classes of the histone demethylases. We predict that other “exotic” enzyme systems will be uncovered that function to regulate chromatin output following instructions from the environment. It follows that diet, vitamin intake (or depletion), calorie restriction, oxidative stress, aging, etc., will contribute to and impact epigenetic signatures (DNA and histone) in ways that have yet to be fully determined (Denu, 2007). Moreover, nonhistone proteins, in some cases, those of cytosolic origin, will be critical substrates that are only beginning to receive much-needed attention. Determining how this overall regulation impinges directly on neuronal function, survival, and regeneration remains a worthwhile challenge for future studies. Finding the “writers,” “erasers,” “readers,” and “interpreters” of this “epigenetic language” will keep neurobiologists, as well as many other biologists, busy for years to come.
ACKNOWLEDGMENTS
We would like to thank all the members of our laboratories for help and stimulating discussions. We also wish to apologize to all the colleagues whose work could not be cited because of space limitations.
REFERENCES
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/ mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Allis CD, Jenuwein T, Reinberg D, Caparros ML. Epigenetics. Cold Spring Harbor Laboratory Press; New York: 2007a. [Google Scholar]
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, et al. New nomenclature for chromatin modifying enzymes. Cell. 2007b;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Asher G, Garfield D, Stratman M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through Per2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Ausio J, Levin DB, De Amorim GV, Bakker S, Macleod PM. Syndromes of disordered chromatin remodeling. Clin. Genet. 2003;64:83–95. doi: 10.1034/j.1399-0004.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 2005;15:500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;436:1103–1106. doi: 10.1038/nature04048. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, French BA, Joyce M, Baires M, Montgomery RO, Li J, French S. Histone acetyltransferase p300 modulates gene expression in an epigenetic manner at high blood alcohol levels. Exp. Mol. Pathol. 2007;82:197–202. doi: 10.1016/j.yexmp.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J. Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A betaarrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Belden WJ, Loros JJ, Dunlap JC. Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol. Cell. 2007;25:587–600. doi: 10.1016/j.molcel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Allis CD. RNA meets chromatin. Genes Dev. 2005;19:1635–1655. doi: 10.1101/gad.1324305. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ, 3rd, Gingeras TR, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Duncan EM, Masui O, Gil J, Heard E, Allis CD. Mouse polycomb proteins bind differently to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol. Cell. Biol. 2006;26:2560–2569. doi: 10.1128/MCB.26.7.2560-2569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen JS, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat. Rev. Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Lenz B, Fiszer R, Frieling H, Kornhuber J, Bleich S. Lowered DNA methyltransferase (DNMT-3b) mRNA expression is associated with genomic DNA hypermethylation in patients with chronic alcoholism. J. Neural Transm. 2006;113:1299–1304. doi: 10.1007/s00702-005-0413-2. [DOI] [PubMed] [Google Scholar]
- Bozzi Y, Borrelli E. Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci. 2006;29:167–174. doi: 10.1016/j.tins.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Hervé D, Darragh J, Corvol JC, Pages C, Arthur SJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J. Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs SD, Xiao T, Sun ZW, Caldwell JA, Shabanowitz J, Hunt DF, Allis CD, Strahl BD. Gene silencing: trans-histone regulatory pathway in chromatin. Nature. 2002;418:498. doi: 10.1038/nature00970. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. The molecular biology of the eukaryotic genome is coming of age. Cold Spring Harb. Symp. Quant. Biol. 1978;42:1209–1234. doi: 10.1101/sqb.1978.042.01.122. [DOI] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Chawla S, Vanhoutte P, Arnold F, Huang C, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 2003;85:151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000a;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol. Cell. 2000b;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD. ERK/ MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn. Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. Light induces chromatin modification in cells of the mammalian circadian clock. Nat. Neurosci. 2000;3:1241–1247. doi: 10.1038/81767. [DOI] [PubMed] [Google Scholar]
- Crosio C, Heitz E, Allis CD, Borrelli E, Sassone-Corsi P. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippo-campal neurons. J. Cell Sci. 2003;116:4905–4914. doi: 10.1242/jcs.00804. [DOI] [PubMed] [Google Scholar]
- Denu JM. Vitamins and aging: pathways to NAD+ synthesis. Cell. 2007;129:453–454. doi: 10.1016/j.cell.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006a;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Doi M, Yujnovsky I, Hirayama J, Malerba M, Tirotta E, Sassone-Corsi P, Borrelli E. Impaired light masking in dopamine D2 receptor-null mice. Nat. Neurosci. 2006b;9:732–734. doi: 10.1038/nn1711. [DOI] [PubMed] [Google Scholar]
- Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc. Natl. Acad. Sci. USA. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol. 2006;13:713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2- deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Yang X, DeBruyne JP, Peters AH, Weaver DR, Jenuwein T, Reppert SM. The polycomb group protein EZH2 is required for mammalian circadian clock function. J. Biol. Chem. 2006;281:21209–21215. doi: 10.1074/jbc.M603722200. [DOI] [PubMed] [Google Scholar]
- Fan G, Beard C, Chen RZ, Csankovszki G, Sun Y, Siniaia M, Biniszkiewicz D, Bates B, Lee PP, Kuhn R, et al. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J. Neurosci. 2001;21:788–797. doi: 10.1523/JNEUROSCI.21-03-00788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–453. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr. Res. 2007;61:58R–63R. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis CD. Binary switches and modification cassettes in histone biology and beyond. Nature. 2003;425:475–479. doi: 10.1038/nature02017. [DOI] [PubMed] [Google Scholar]
- Gan L, Mucke L. Paths of convergence: Sirtuins in aging and neurodegeneration. Neuron. 2008;58:10–14. doi: 10.1016/j.neuron.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, Reick M, Scott K, Diaz-Arrastia R, McKnight SL. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288:2226–2230. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, Blair LA, Marshall J, Mao Z. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. [PubMed] [Google Scholar]
- Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T, Berger SL. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- Hyman S, Malenka R, Nestler E. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;26:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jensen LR, Amende M, Gurok U, Moser B, Gimmel V, Tzschach A, Janecke AR, Tariverdian G, Chelly J, Fryns JP, et al. Mutations in the JARID1C gene which is involved in transcriptional regulation and chromatin remodeling cause X-linked mental retardation. Am. J. Hum. Genet. 2005;76:227–236. doi: 10.1086/427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korutla L, Wang PJ, Mackler SA. The POZ/BTB protein NAC1 interacts with two different histone deacetylases in neuronal-like cultures. J. Neurochem. 2005;94:786–793. doi: 10.1111/j.1471-4159.2005.03206.x. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn't fit all. Nat. Rev. Mol. Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee EK, Cho YW, Matsui T, Kang IC, Kim TS, Han MH. ProteoChip: a highly sensitive protein microarray prepared by a novel method of protein immobilization for application of protein-protein interaction studies. Proteomics. 2003;3:2289–2304. doi: 10.1002/pmic.200300541. [DOI] [PubMed] [Google Scholar]
- Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem. Biol. 2006;13:563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA, Crabtree GR. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron. 2007;55:201–215. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat. Rev. Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J. Biol. Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc. Natl. Acad. Sci. USA. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Guo Y, Schroeder FA, Youngs RM, Schmidt TW, Ferris C, Konradi C, Akbarian S. Dopamine D2-like antagonists induce chromatin remodeling in striatal neurons through cyclic AMP-protein kinase A and NMDA receptor signaling. J. Neurochem. 2004;90:1117–1131. doi: 10.1111/j.1471-4159.2004.02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gogol M, Carey M, Lee D, Seidel C, Workman JL. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science. 2007;316:1050–1054. doi: 10.1126/science.1139004. [DOI] [PubMed] [Google Scholar]
- Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu JY, Allis CD, Marmorstein R, Berger SL. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol. Cell. 2000;5:917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- Lo WS, Duggan L, Emre NC, Belotserkovskya R, Lane WS, Shiekhattar R, Berger SL. Snf1—a histone kinase that works in concert with the histone acetyltransferase Gcn5 to regulate transcription. Science. 2001;293:1142–1146. doi: 10.1126/science.1062322. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Macdonald N, Welburn JP, Noble ME, Nguyen A, Yaffe MB, Clynes D, Moggs JG, Orphanides G, Thomson S, Edmunds JW, et al. Molecular basis for the recognition of phosphorylated and phosphoacetylated histone h3 by 14−3−3. Mol. Cell. 2005;20:199–211. doi: 10.1016/j.molcel.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Vemuri MC. Effect of ethanol on chromatin and nonhistone nuclear proteins in rat brain. Neurochem. Res. 1998;23:1179–1184. doi: 10.1023/a:1020778018149. [DOI] [PubMed] [Google Scholar]
- Manke IA, Lowery DM, Nguyen A, Yaffe MB. BRCT repeats as phosphopeptide binding modules involved in protein targeting. Science. 2003;302:636–639. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J. Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, Young MW. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell. 2001;105:769–779. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Mattson B, Bossert J, Simmons D, Nozaki N, Nagarkar D, Kreuter J, Hope B. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocainesensitized rats is enabled by enhanced activation of extra-cellular signal-related kinase, but not protein kinase A. J. Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DFosB. Nat. Neurosci. 2003;11:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McKinsey T, Zhang C, Olson E. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinasestimulated binding of 14−3−3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SJ, Pray-Grant MG, Schieltz D, Yates JR, Grant PA. Polyglutamine-expanded spinocerebellar ataxia-7 protein disrupts normal SAGA and SLIK histone acetyltransferase activity. Proc. Natl. Acad. Sci. USA. 2005;102:8478–8482. doi: 10.1073/pnas.0503493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merienne K, Pannetier S, Harel-Bellan A, Sassone-Corsi P. Mitogen-regulated RSK2-CBP interaction controls their kinase and acetylase activities. Mol. Cell. Biol. 2001;21:7089–7096. doi: 10.1128/MCB.21.20.7089-7096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J. Cell. Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr. Opin. Genet. Dev. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Murata T, Kurokawa R, Krones A, Tatsumi K, Ishii M, Taki T, Masuno M, Ohashi H, Yanagisawa M, Rosenfeld MG, et al. Defect of histone acetyltransferase activity of the nuclear transcriptional coactivator CBP in Rubinstein-Taybi syndrome. Hum. Mol. Genet. 2001;10:1071–1076. doi: 10.1093/hmg/10.10.1071. [DOI] [PubMed] [Google Scholar]
- Mutskov VJ, Farrell CM, Wade PM, Wolffe AP, Felsenfeld G. The barrier function of an insulator couples high histone acetylation levels with specific protection of promoter DNA from methylation. Genes Dev. 2002;16:1540–1554. doi: 10.1101/gad.988502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente L, Sassone-Corsi P. The NAD+ dependent deacetylase SIRT1 acts as a rheostat of CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- Nan X, Hou J, Maclean A, Nasir J, Lafuente MJ, Shu X, Kriaucionis S, Bird A. Interaction between chromatin proteins MECP2 and ATRX is disrupted by mutations that cause inherited mental retardation. Proc. Natl. Acad. Sci. USA. 2007;104:2709–2714. doi: 10.1073/pnas.0608056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse Y, Oh-hashi K, Iijima N, Naruse M, Yoshioka H, Tanaka M. Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol. Cell. Biol. 2004;24:6278–6287. doi: 10.1128/MCB.24.14.6278-6287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of addiction: role of delta-FosB. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Green-gard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuro-science. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak SJ, Corces V. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–220. doi: 10.1016/j.tig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Ooi SL, Henikoff S. Germline histone dynamics and epigenetics. Curr. Opin. Cell Biol. 2007;19:257–265. doi: 10.1016/j.ceb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Ooi SK, Qiu C, Bernstein E, Li K, Jia D, Yang Z, Erdjument-Bromage H, Tempst P, Lin SP, Allis CD, et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell M, Rochet JC, McLean PJ, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Palhan VB, Chen S, Peng G, Tjernberg A, Gamper AM, Fan Y, Chait BT, La Spada AR, Roeder RG. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc. Natl. Acad. Sci. USA. 2005;102:8472–8477. doi: 10.1073/pnas.0503505102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller B, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schröter F, Ninnemann O, Siegert E, Bendix I, Brüstle O, Nitsch R, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao GH, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Reick M, Garcia J, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, Xiao G, Kumar A, Russo S, Graham A, Tsankova N, Kippin TE, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, III, Truong H, Alibhai I, Kumar A, Olson EN, Nestler EJ. DFosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine. J. Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat. Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat. Rev. Mol. Cell Biol. 2007a;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthenburg AJ, Allis CD, Wysocka J. Methylation of Lysine 4 on Histone H3: Intricacy of writing and reading a single epigenetic mark. Mol. Cell. 2007b;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, Hanauer A, Allis CD. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol. Cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Shi X, Kachirskaia I, Yamaguchi H, West LE, Wen H, Wang E, Dutta S, Appella E, Gozani O. Modulation of p53 function by SET8 mediated methylation at lysine 382. Mol. Cell. 2007;27:636–646. doi: 10.1016/j.molcel.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat. Rev. Genet. 2005;6:435–450. doi: 10.1038/nrg1616. [DOI] [PubMed] [Google Scholar]
- Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn J, Maroteaux M, Bertran-Gonzalez J, Brami-Cherrier K, Enslen H, Corbillé AG, et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Sun ZW, Allis CD. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002;418:104–108. doi: 10.1038/nature00883. [DOI] [PubMed] [Google Scholar]
- Tahiliani M, Mei P, Fang R, Leonor T, Rutenberg M, Shimizu F, Li J, Rao A, Shi Y. The histone H3K4 demethylase SMCX links REST target genes to Xlinked mental retardation. Nature. 2007;447:601–605. doi: 10.1038/nature05823. [DOI] [PubMed] [Google Scholar]
- Taverna SD, Ilin S, Rogers RS, Tanny JC, Lavender H, Li H, Baker L, Boyle J, Blair LP, Chait B, et al. Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol. Cell. 2006;24:785–796. doi: 10.1016/j.molcel.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojer P, Reinberg D. Histone lysine demethylases and their impact on epigenetics. Cell. 2006;125:213–217. doi: 10.1016/j.cell.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J. Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Berton O, Renthal W, Kumar A, Neve R, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Turner B. Cellular memory and the histone code. Cell. 2002;111:285–291. doi: 10.1016/s0092-8674(02)01080-2. [DOI] [PubMed] [Google Scholar]
- Ulery PG, Rudenko G, Nestler EJ. Regulation of DeltaFosB stability by phosphorylation. J. Neurosci. 2006;26:5131–5142. doi: 10.1523/JNEUROSCI.4970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The Strategy of the Genes; a Discussion of Some Aspects of Theoretical Biology. Allen & Unwin; London: 1957. [Google Scholar]
- Welter M, Vallone D, Samad TA, Meziane H, Usiello A, Borrelli E. Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc. Natl. Acad. Sci. USA. 2007;104:6840–6845. doi: 10.1073/pnas.0610790104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen H, Young MW. Interplay of circadian clocks and metabolic rhythms. Annu. Rev. Genet. 2006;40:409–448. doi: 10.1146/annurev.genet.40.110405.090603. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett A, Chait BT, Badenhorst P, et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- Yan L, Bobula JM, Svenningsson P, Greengard P, Silver R. DARPP-32 involvement in the photic pathway of the circadian system. J. Neurosci. 2006;26:9434–9438. doi: 10.1523/JNEUROSCI.2538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc. Natl. Acad. Sci. USA. 2006;103:6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Darwanto A, Linkhart TA, Sowers L, Zhang L. Maternal cocaine administration causes an epigenetic modification of protein kinase Cå gene expression in fetal rat heart. Mol. Pharmacol. 2007;71:1319–1328. doi: 10.1124/mol.106.032011. [DOI] [PubMed] [Google Scholar]