Abstract
The cardiac outflow tract (OFT) of birds and mammals undergoes complex remodeling in the transition to a dual circulation. We have previously suggested a role of myocardial hypoxia and hypoxia inducible factor (HIF)-1 in the apoptosis-dependent remodeling of the OFT. In the present study we transduced recombinant adenovirus mediated HIF-1α in embryonic chick OFT myocardium to test its role in OFT remodeling. HIF-1α reduced the prevalence of apoptosis in OFT cardiomyocytes at stages 25 and 30 as determined by lysosome accumulation and Caspase-3 activity. Associated conotruncal defects included mal-rotation of the aorta and excessive infundibular myocardium. HIF-1 targets induced in these gain-of-function experiments included vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS), and stromal cell-derived factor (SDF)-1. To test the role of VEGF in this context, an adenovirus expressing secreted Flk1 (VEGFR2) that binds and blocks VEGF signaling was targeted to the OFT myocardium. This caused increased cell death in the OFT myocardium at stages 25 and 30. Associated conotruncal heart defects included mal-rotation of the aorta, defects in the subpulmonic infundibulum associated with a small right ventricle, and increased OFT mesenchyme with failure of semi-lunar valve formation. We conclude that hypoxia signaling through HIF-1 and VEGF provides an autocrine survival signal in the developing cardiac OFT, and perturbation in this pathway causes OFT defects that model congenital human conotruncal heart defects.
Keywords: HIF-1, VEGF apoptosis, OFT remodeling
The arterial pole of the heart in birds and mammals, referred here as the cardiac outflow tract (OFT), undergoes extensive remodeling in the developmental transition to a dual series circulation1. Spatially and temporally regulated elimination of OFT cardiomyocytes by apoptosis from Hamburger-Hamilton (HH) stages 25–32 of development is required for the proper shortening and rotation of the OFT2;3. Inhibition or widespread activation of apoptosis in OFT cardiomyocyte results in failure of the aorta to rotate to its posterior position and thus double outlet right ventricle (DORV), as well as excess or absence of the sub-pulmonic myocardial infundibulum, respectively. The sub-pulmonic infundibulum in the mature heart is the remnant of the embryonic OFT myocardium.
A critical question is why, of all the cardiac chambers, does the OFT myocardium undergo apoptosis-dependent remodeling during normal development? Our previous studies suggested that myocardial hypoxia may be involved based on 1) increased binding of the hypoxia indicator EF5 to the OFT myocardium at HH stages 25–324;5 2) expression of the hypoxia-inducible transcription factor (HIF)-1α specifically in the OFT myocardium at the same stages6 3) approximately synchronous recruitment of endothelial progenitor cells to the OFT and coronary vasculogenesis6 and 4) incubation of eggs under hyperoxic conditions perturbed OFT remodeling4. In the current study we have tested the role of HIF-1 in apoptosis-dependent remodeling of the chick OFT.
HIF-1 is a hetero-dimer composed of a ubiquitously expressed β subunit (also known as ARNT) and oxygen regulated α subunit7;8. As oxygen concentrations decrease HIF-1α accumulates due to reduced prolyl hydroxylase-dependent degradation of the protein. HIF binding to its consensus DNA binding sequence A/GCGTG regulates the transcription of the hypoxia-dependent gene program. Inactivation of the HIF-1α or β subunits in the mouse results in severe cardiovascular compromise and demise of the embryo by embryonic day 119–11. However, the role of hypoxia and HIFs in specific morphogenic processes in the embryonic heart and vascular system remains to be elucidated.
Hypoxia/HIF may function to regulate cell death or cell survival. HIF activates the transcription of pro-death genes such as Bcl family members BNip3 and BMF12 and the death ligand FasL13. HIF also activates the pro-survival genes including VEGF, insulin-like growth factor and erythropoietin14;15, therefore enhance survival under low oxygen through vasculogenesis and erythropoiesis. Other hypoxia/HIF responsive genes, e.g. inducible nitric oxide synthase (iNOS), have pro-survival or pro-death effects depending on the biological context16.
In this study we used recombinant adenovirus to express a stabilized HIF-1α in the embryonic chick OFT myocardium. Forced expression of HIF-1α reduced the incidence of apoptosis and increased VEGF expression. Interfering with VEGF activity by adenoviral-mediated expression of Flk1-Fc increased apoptosis, suggesting that HIF-1 acting through VEGF provides a survival signal to the cardiomyocytes of the developing OFT. Interference with the hypoxia-dependent gene program in the embryonic OFT through specific gain-and-loss of function strategies results in OFT defects that model congenital human heart defects.
Materials and Methods
Experimental details can be found online in Supplemental Methods.
In ovo injection of chicken heart
Stage 17–18 chicken embryos were injected with recombinant adenovirus including AdHIF-1α17, AdFlk1-Fc (AdFlk1)18 and AdGFP as previously described19.
Assays for apoptosis
Lyso Tracker Red (LTR) accumulation and immunohistochemical detection of active Caspase-3 were performed as previously described20. Caspase-3/7 activity was measured with OFT protein lysates using Caspase-Glo 3/7 Assay kit. Image J and Cell Counter software were used to quantify LTR signal and Caspase-3 immunostaining.
Morphological and histological analyses
Stage 34–35 hearts were photographed with a Leica FLIII stereomicroscope and SpotRT digital camera. De-paraffinized serial frontal sections were stained with hematoxylin and eosin (H&E) as previously described4.
Gene expression analysis
Total RNA was isolated using PicoPure RNA Isolation Kit from OFT tissues of stage 25 hearts injected with AdHIF-1α or AdGFP. Ten ng of total RNA was used to perform real time RTPCR by standard methods. Data were first normalized to GAPDH, and then to the GFP+ group.
Statistical analysis
Data are expressed as Mean±SEM. All statistics were done with Prism software (GraphPad software Inc., San Diego, CA). One-way ANOVA followed by Bonferroni post test or t-test was used for comparison among experimental groups. P<0.05 was considered statistically significant.
Results
Forced expression of stabilized HIF-1α reduces apoptosis in OFT myocardium
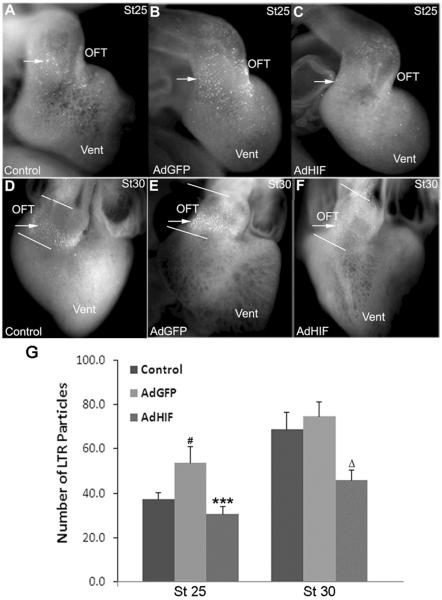
LTR was used as one of the most sensitive assays for apoptosis in the developing chick heart3;20;21. In control (un-injected) hearts a low level of LTR staining was detected in the OFT at stage 25 and increased to a peak at stage 30 (Figure 1A, D, G), with minimal staining in the remainder of the heart, as we previously reported6;20. In embryos injected with the control virus AdGFP, LTR staining was modestly increased compared to un-injected embryos at stage 25 but was not different at stage 30 (Figure 1B, E, G). Hearts transduced with AdHIF-1α expressed stabilized HIF-1α in the nuclei of the OFT cardiomyocytes (Supplemental Figure 1). The LTR staining in AdHIF-1α transduced hearts was reduced at stages 25 and 30 as compared to AdGFP transduced hearts, and at stage 30 as compared to un-injected embryos (Figure 1C, F, G). The reduction of LTR staining was observed in 16/21 AdHIF-1α hearts at stage 25 and 14/21 AdHIF-1α hearts at stage 30, respectively, compared to the AdGFP transduced hearts, as judged by two independent observers using a 5-point scoring system for the number of LTR particles (data not shown). These scores correlated well with the quantitative measures of LTR particle numbers (Figure 1G). Caspase-3 activity was used as a second indicator of apoptosis. Activated Caspase-3 was detected at low levels in the stage 25 OFT myocardium of AdGFP injected embryos (Figure 2A, B) and increased at stage 30 (Figure 2E, F), consistent with our previous observation22. There was no or minimal staining in the remainder of the heart. At stages 25 and 30 some cells showed co-localization of GFP and Caspase-3 signals (Figure 2B, F, arrowheads), indicating GFP labeled cardiomyocytes were executing apoptosis. At both stages 25 (Figure 2C, D, I) and 30 (Figure 2G, H, I) there was a marked reduction in the number of active Caspase-3 staining cells in the OFT myocardium of AdHIF-1α injected hearts compared to AdGFP injected stage matched controls. Cells expressing GFP in AdHIF-1α treated hearts were not active Caspase-3 positive.
Figure 1.
Adenoviral mediated expression of HIF-1α reduces LTR staining in the cardiac OFT. LTR staining (arrows) is significantly reduced in the OFT of AdHIF-1α injected hearts (C and F) as compared to AdGFP (B and E) at both stages 25 and 30, and un-injected embryos at stage 30 (D). (G) Number of LTR particles in the OFT in whole mount as quantified by Image J. # P<0.01, compared to control; *** P<0.001 compared to AdGFP group; Δ P<0.001 compared to both control and AdGFP group. OFT, outflow tract; Vent, ventricles.
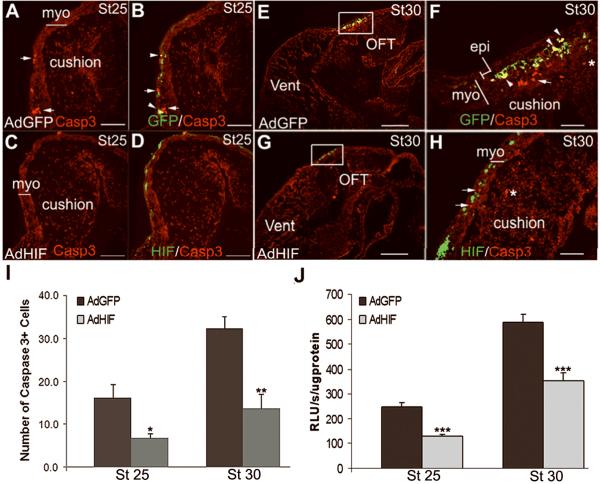
Figure 2.
Caspase-3 immunostaining and activity are reduced in the OFT myocardium of AdHIF-1α injected embryos. The active Caspase-3 staining (arrows) was significantly reduced in AdHIF-1α treated hearts (C, D, G and H) compared to AdGFP-injected embryos (A, B, E and F) in the OFT myocardium at stages 25 and 30. GFP positive cells were stained with active Caspase-3 (yellow cells, arrowheads in B and F) at stages 25 and 30 in the control group but not AdHIF-1α group. The boxed areas in E, G are shown at higher magnification in F, H. Asterisk denotes active Caspase-3 staining in the OFT cushion mesenchyme. (I) Number of active Caspase-3 positive cells in the OFT myocardium per section as quantified by Image J program. Scale bar, 50μm in A-D and F, H; 500μm in E and G. Casp3, active Caspase-3 staining; Myo, myocardium; Vent, ventricles. (J) The Caspase-3 activity was significantly decreased in AdHIF-1α OFT compared to AdGFP OFT at stages 25 and 30. * P<0.05, **P<0.01, *** P<0.001 compared to AdGFP group. RLU, Relative luminescent units.
Caspase-3 activity measured in tissue homogenates increased 2.4-fold in the OFT of AdGFP injected embryos between stages 30 and 25 (Figure 2J), consistent with our previous reports2;22. Forced expression of HIF-1α reduced the activity of Caspase-3 in the OFT homogenates by 40–50% as compared to AdGFP injected embryos at stages 25 and 30. This is concordant with the reduction in staining for active Caspase-3 observed in sections. In total, these observations indicate that forced expression of HIF-1α reduces the incidence of apoptosis in OFT cardiomyocytes.
Forced expression of HIF-1α causes OFT defects
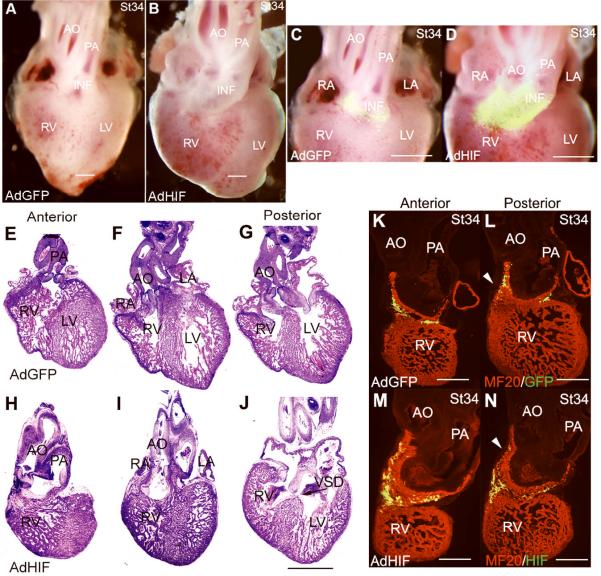
A separate group of embryos injected with AdHIF-1α were examined at stages 34–35 when OFT remodeling is nearly complete and effects on morphology can be assessed. Thirty-two AdHIF-1α embryos survived to stages 34–35 and 23 had abnormal heart and OFT morphology as observed in whole mount. In 19 out of 23 embryos, the aorta is mal-positioned anterior and to the right and coursing parallel to the pulmonary artery (Figure 3B and D), whereas it is normally posterior to and spirals about the pulmonary artery (Figure 3A and C). Also evident is abnormal persistence of a stalk of GFP labeled OFT myocardium beneath the aorta. In control, the GFP labeled OFT myocardium has committed to the right heart where it forms the infundibular connection between the right ventricle and pulmonary artery. In the remaining abnormal hearts (4/23), the aorta is mal-positioned almost directly posterior to the pulmonary artery (not shown). In frontal sections from anterior to posterior, the abnormal anterior and rightward position of the aorta and its connection to the right ventricle in a DORV morphology is evident (Control: Figure 3E-G vs AdHIF Figure 3H-J). The extended nature of the infundibulum is also evident by 1) the position of the semilunar valves well above the base of the ventricles; 2) staining of sections with the MF20 antibody that specifically recognizes cardiac myosin (AdGFP: Figure 3K and L vs AdHIF Figure 3M and N). Also noted in sections of the AdHIF-1α hearts is thickening of the semilunar valves with increased mesenchyme in the OFT, and a muscular ventricular septal defect (VSD, Figure 3J). The trabeculation of the ventricles appears normal while there is variable thinning of the ventricular compact myocardium likely secondary to the hemodynamic alterations. Thus, forced expression of HIF-1α blocks the normal shortening and rotation of the OFT that is required for the transition to a dual circulation.
Figure 3.
Adenoviral mediated expression of HIF-1α causes cardiac OFT defects. Representative AdGFP (A, C, E-G, K, L) and AdHIF-1α hearts (B, D, H-J, M, N) from stage 34-5 embryos are shown in whole mount, H&E stained frontal sections and MF20 stained frontal sections to delineate the myocardium. The whole mount and H&E stained sections are from the same embryo. The MF20 stained sections are from another set of embryos. The morphological defects are described in the text. The OFT myocardium is identified by the GFP label in C and D. Abbreviations: AO, aorta; PA, pulmonary artery; RV, right ventricle; LV, left ventricle; RA, right atrium; LA, left atrium; VSD, ventricular septal defect (arrow in J). Arrowheads point to OFT myocardium (L and N). Scale bar, 500μm in A-D and K-N; 1mm in E-J.
HIF-1α activation of hypoxia-responsive genes
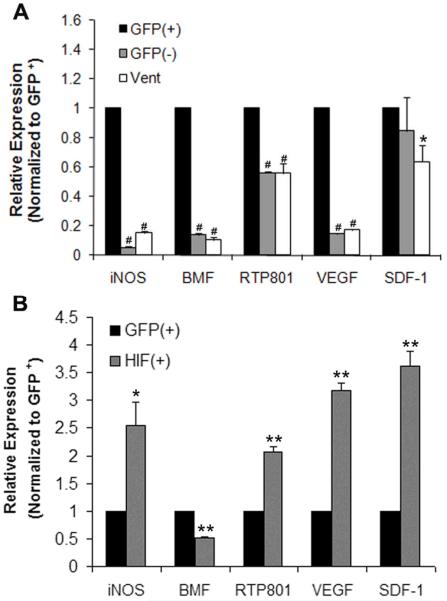
To investigate which genes downstream of hypoxia/HIF-1α regulated cell survival or cell death in this context, transcript abundance was measured by real time PCR in GFP positive and negative cells sorted by flow cytometry. Given the limiting supply of RNA with this approach, the analysis was restricted to transcripts that appeared to be differentially expressed in the developing heart and embryos (data not shown). In control hearts, the expression of VEGF, BMF, RTP801 and iNOS were increased in the GFP+ cells (cardiomyocytes) of the OFT as compared to: 1) the GFP− cells, which are predominately cushion mesenchymal cells, and 2) ventricular myocardium from the same embryos (Figure 4A). Forced expression of HIF-1α in the OFT cardiomyocytes caused a 2–4 fold increase in the abundance of the iNOS, RTP801, VEGF and SDF-1 transcripts as compared to the GFP only OFT cardiomyocytes, while the abundance of BMF was reduced (Figure 4B).
Figure 4.
Expression of hypoxia-responsive pro-survival and pro-death genes in the OFT of stage 25 chicken hearts. The abundance of iNOS, BMF, RTP801, VEGF were higher in OFT cardiomyocytes (GFP+) as compared to OFT non-cardiomyocytes (GFP−) and ventricular myocardium (A). SDF-1 was higher in the GFP+ OFT cardiomoyctes than in the ventricular myocardium. HIF-1α increased the expression of iNOS, RTP801, VEGF and SDF-1, and reduced the expression of BMF in OFT cardiomyocytes as compared to the GFP only group (B). The data is from three separate experiments in which cells were pooled from multiple hearts and measured in duplicate. The data were first normalized to GAPDH then to AdGFP GFP+ OFT cells. *P<0.05, **P<0.01, # P<0.001 compared to GFP+ group.
Blockade of VEGF induces apoptosis in OFT myocardium
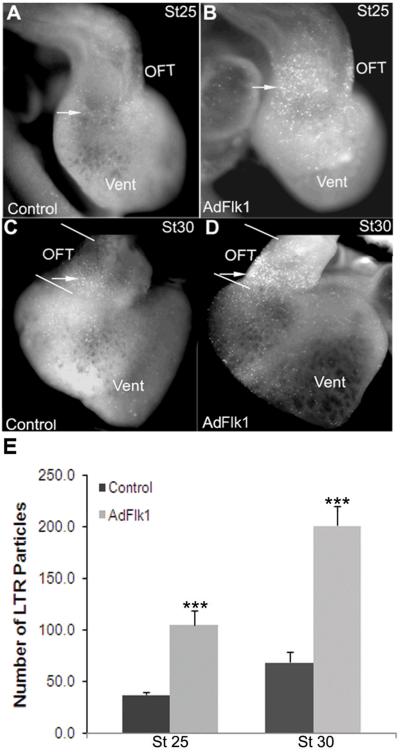
We hypothesized that the ability of HIF-1α to promote OFT cardiomyocyte survival may be through VEGF signaling based on the ability of VEGF to function as an autocrine survival factor in other contexts14;15;23; and the selective expression of the VEGFR2 in the stage 25–32 OFT cardiomyocytes concordant with tissue hypoxia6. To test this hypothesis, we transduced the embryonic hearts with AdFlk1-Fc (AdFlk1) to trap VEGF and block VEGF signaling18. Embryonic hearts transduced with AdFlk1 expressed the truncated Flk1 throughout the OFT myocardium (Supplemental Figure 2). There was increased LTR staining in the OFT myocardium of these hearts as compared to stage-matched controls (Figure 5). Eleven of 11 and 11 of 13 AdFlk1 injected hearts showed increased LTR staining at stages 25 and 30, respectively, in whole mount, as judged by 5-point scoring system (data not shown) and quantitative measurement. In sections, LTR staining located in the OFT myocardium (Figure 6). No significant increase in LTR staining was observed in other chambers of the heart. The staining for active Caspase-3 was also increased in the OFT myocardium of AdFlk1 injected hearts at these stages (Figure 6A-I). There was a corresponding increase in Caspase-3 activity measured in OFT homogenates of AdFlk1 injected embryos as compared to controls (Figure 6J). Thus, blockade of VEGF signaling increases apoptosis in the OFT myocardium during OFT remodeling.
Figure 5.
Adenoviral mediated expression of Flk1 increases LTR staining in the cardiac OFT. LTR staining in chicken hearts transduced with AdFlk1 (B, D) is significantly higher than that in control hearts (A, C) at stage 25 (A and B) and 30. Arrow indicated LTR positive cells. (E) Number of LTR particles in the OFT in whole mount as quantified Image J. *** P<0.001 compared to control group.
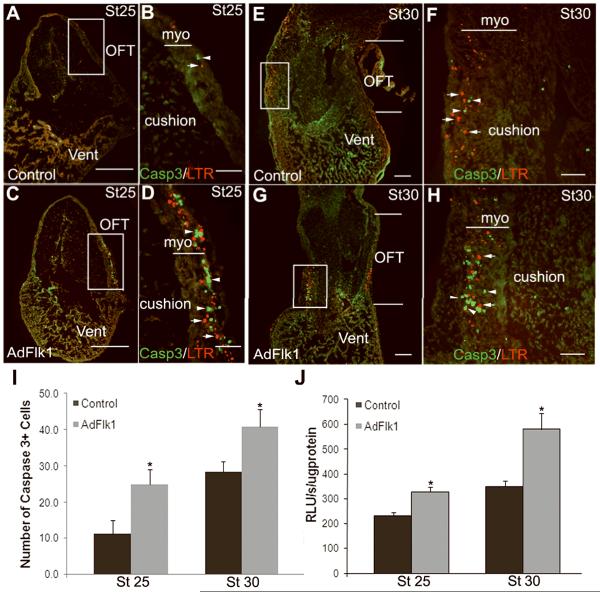
Figure 6.
Caspase-3 immunostaining and activity are increased in the OFT myocardium of AdFlk1 injected embryos. At stages 25 and 30, LTR (arrows) and active Caspase-3 staining (arrowheads) are markedly increased in the OFT myocardium of AdFlk1 embryos (C, D; G, H) as compared to control embryos (A, B; E, F). In control embryos LTR and active Caspase-3 staining was higher in the stage 30 (E, F) compared to stage 25 myocardial OFT (A, B). The Caspase-3 and LTR signals did not co-localize. (I) Number of active Caspase-3 positive cells in the OFT myocardium per section as quantified by Image J. Scale bar, 250μm in A, C, E, G; 10μm in B, D; 50μm in F, H. (J) Caspase-3 activity was significantly increased by expression of Flk1 in the OFT at stages 25 and 30. * P<0.05 compared to control group.
Blockade of VEGF causes OFT defects
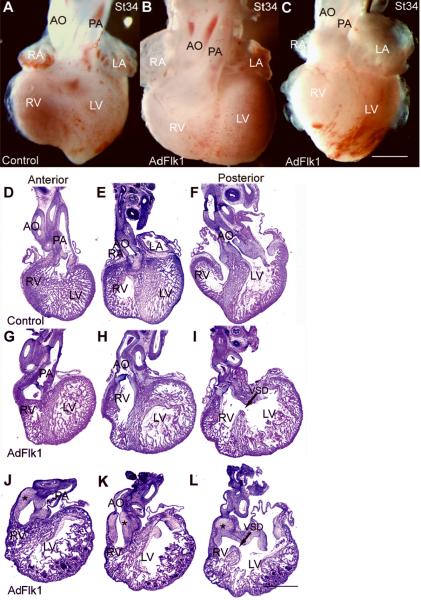
A separate group of embryos injected with AdFlk1 were examined at stages 34–35 for morphological assessment. Thirty-eight AdFlk1 injected embryos survived to this stage and 28 had abnormal heart and OFT morphology. The OFT dys-morphology was more variable than that of the AdHIF-1α hearts, with all of the hearts showing combinations of defects in the OFT and right ventricle. A heart representative of 13/28 abnormal hearts is shown in whole mount in Figure 7B, and in section in Figure 7G-I. In these hearts, there is a diminutive or absent subpulmonic infundibulum, such that the pulmonic valve is situated at a level below the base of the ventricles. In this heart, as in all of the abnormal AdFlk1 hearts, there is a defect in the positioning of the aorta. It is transposed rightward so that it originates from the right ventricle and overrides a more posterior located muscular VSD. The pulmonic and aortic valves are not markedly abnormal. A heart representative of 15/28 abnormal hearts is shown in whole mount in Figure 7C and in section in Figure 7J-L. In these hearts, a bulbous appearing infundibular stalk giving rise to both the aorta and pulmonary artery is evident above the base of the ventricles, and is not incorporated into the right ventricle as occurs in normal heart development. In section the infundibulum can be seen to be composed of myocardial tissue and large amounts of mesenchyme. This large amount of mesenchyme is in the position where the sculpted semi-lunar valves would normally reside. In these hearts the right ventricles are markedly diminutive, and there is again a more posterior VSD with over-riding aorta. All of the abnormal hearts have thinned myocardial walls with reduced trabeculation as evident in these representative embryos.
Figure 7.
Interference of VEGF pathway causes cardiac OFT defects. Embryos injected with AdFlk1 were harvested at stages 34–5 to assess OFT morphology and compared to stage matched controls. Shown are representative Control (A, D-F) and two different AdFlk1 hearts in whole mount (B, C) and each in section (G-I, J-L). The morphological defects are described in the text. Abbreviations are as in prior figures. Scale bar, 1mm. Arrow indicates VSD in I and L; the increased OFT mesenchyme is indicated by stars in J-L.
Discussion
In the current study we utilized an adenoviral gene delivery system to test the effects of HIF-1α gain-of-function targeted to the OFT myocardium during apoptosis-dependent remodeling (stages 25–32). HIF-1α was appropriately expressed in the nuclei of the OFT cardiomyocytes, and enhanced the transcription of HIF target genes, indicating that the gain-of-function was achieved. HIF gain-of-function reduced the prevalence of apoptosis as indicated by both LTR staining and Caspase-3 activity. Thus, in the context of the developing OFT, hypoxia and HIF signaling promote the survival of the cardiomyocytes. This result is consistent with a study in which transgenic forced expression of HIF-1α in the adult mouse heart reduced myocardial injury in a model of ischemia24 . In contrast, in vitro studies have suggested that HIF-1α triggers cardiomyocyte apoptosis through death ligand or mitochondrial pathways12;13. One important difference between in vitro and in vivo studies may be the strength of the stimulus. Incubation of stage 25 embryos in 5–7.5% oxygen results in the demise of the embryo within 3–6 hours. Even with in vitro studies, a second stimulus, e.g. acidosis or re-oxygenation, appears to be required for the severe hypoxia to trigger a BNip-3 and mitochondrial-dependent apoptosis program12;25. What triggers the cardiomyocyte apoptosis during the normal development of the OFT? One possibility is that tissue hypoxia conditions the myocardium such that a second stimulus later in development triggers apoptosis. What that second stimulus might be is uncertain. One suggestion is that invasion of the OFT by endothelial progenitor cells carrying FasL and their formation of the coronary arteries and coronary orifices is a trigger26. Neural crest cells that migrate to the heart have a high prevalence of apoptosis27, are also candidates for triggering the apoptosis-dependent remodeling of the OFT.
HIF-1α may trigger a pro-survival pathway through the induction of VEGF, a well described hypoxia-dependent signaling pathway. The increased expression of VEGF in the OFT cardiomyocytes, and its induction by AdHIF-1α, suggested VEGF as a candidate pro-survival factor. Loss-of-function of VEGF signaling using AdFlk1-Fc significantly increased the prevalence of apoptosis in the OFT cardiomyocytes. These results suggest that HIF-1 induction of VEGF functions in autocrine pro-survival signaling in the developing OFT myocardium. Consistent with this interpretation, co-transduction of the embryos with AdHIF-1α and AdFlk1-Fc resulted in an intermediate level of LTR staining and morphological defects similar to that of AdFlk-Fc treatment (Supplemental Figure 3 and 4). A role for receptor tyrosine kinase signaling in the survival of the OFT cardiomyocytes was supported by our previous study, in which gain-and-loss of function of Akt increased and decreased, respectively, the survival of the OFT cardiomyocytes6. VEGF/Akt may exert pro-survival signals through inactivation of the pro-apoptotic Bcl-2-family member Bad, or through the suppression of the FOXO and p53-dependent induction of pro-apoptotic Bcl-2 family members Bim, Puma and Noxa, and the death ligand FasL28. Which of these or other gene targets of VEGF or Akt signaling are operative in this developmental context requires further study.
The morphological defects of the OFT observed with the perturbations of hypoxia-dependent signaling are consistent with the effects on apoptosis in the OFT myocardium. Forced expression of HIF-1α reduced apoptosis and resulted in a failure of resorption of the myocardium from under the aorta, in conjunction with a failure of rotation of the aorta to its posterior position. This DORV morphology with an extended conal myocardium is similar to that observed with direct delivery of caspase inhibitors to the embryonic OFT myocardium3. In these and other gain-and-loss of function experiments there is variability in the phenotype. This could reflect inter-embryo variability in the efficiency of adenoviral gene delivery19, and/or variability in the tissue response to these perturbations. In a few of the AdHIF-1α embryos the aorta, rather than being side-by-side the pulmonary artery, was abnormally positioned directly posterior to the pulmonary artery, and again overriding a muscular VSD. We interpret this to represent a spectrum of rotational defects in aortic positioning, though other interpretations may also be plausible. This is not dissimilar to human congenital conotruncal heart defects, that while neatly categorized into DORV, Tetralogy of Fallot, and Transposition of the Great Arteries, in actuality represent a spectrum of defects in the conal myocardium and the positioning of the great vessels with respect to the conus and ventricles29.
Cardiomyocytes are added to the OFT during its normal development from the anterior (or secondary) heart field (AHF)30–32prior to apoptosis-dependent remodeling. Abnormal addition of cardiomyocytes to the OFT may also cause conotruncal heart defects33, and the addition of myocytes and subsequent remodeling may be linked by, e.g. FGF834. Whether hypoxia signaling through HIF and VEGF also regulate these multiple aspects of OFT development and remodeling requires further study.
The variability in morphological defects in AdFlk1 embryos was greater than that of HIF-1α. In some embryos, the myocardial infundibulum was diminutive, associated with defects in the posterior rotation of the aorta similar to that observed when apoptosis is directly triggered through the delivery of the death ligand2. However, in other embryos, OFT myocardium appeared to be “hung-up”, a protuberant stalk above the base of the ventricles, associated with a mass of mesenchyme, again with a defect in the position of the aorta. We speculate that temporally coordinated remodeling of the OFT myocardium and cushion mesenchyme may be required for the transition to a dual in-series circulation. Interference with either one of these processes may contribute to the conotruncal heart defects with mal-position of the aorta. Interestingly, these embryos also had a markedly diminutive right ventricle. Cell marking and fate analyses have indicated that cardiomyocytes from AHF contribute to the formation of both the OFT and right ventricle35;36. We suggest that the OFT cardiomyocytes are hung-up by the dense mesenchyme and cannot contribute to the right ventricle in these AdFlk1 embryos, leading to its small size. Alternatively, it is possible that VEGF, like FGF834, provides a direct growth stimulus to the right ventricular myocardium which is abrogated by AdFlk1. In this and other models of loss-of-VEGF function thinned ventricular myocardium is noted, but it remains to be established if this is a direct or secondary effect.
A dramatic increase in OFT cushion mesenchyme was observed in the AdFlk1 embryos, and to a lesser extent than AdHIF-1α embryos. Mesenchymal cell proliferation was modestly but significantly increased as assessed by BrdU incorporation in the AdFlk1 embryos (data not shown). In vitro studies have shown that VEGF, depending on the dose, positively or negatively regulates endothelial-to-mesenchymal transformation37;38. The increased mesenchyme couldreflect a defect in later remodeling of the cushions to form the semi-lunar valves. It is well established that myocardial signaling to the endocardium through the release of TGF-β and other molecules affects these processes38;39. Further study is indicated to define the cell and molecular basis of the cushion and valve defects with VEGF loss-of-function.
Experiments in mouse models also support a role of VEGF in OFT development. Exclusive expression of the VEGF120 (soluble) isoform resulted in a spectrum of conotruncal defects, some of which resembled Tetralogy of Fallot40. Interestingly, increased apoptosis in the ED12.5 sub-pulmonic myocardium and hyperplasia of the OFT cushions were noted, quite similar to the effects of trapping VEGF in chick in the current study. The VEGF gene has been proposed as a disease modifying locus for Tbx1 in DiGeorge syndrome and OFT defects in human and animal models41. In contrast, conditional inactivation of HIF-1α and VEGF in the embryonic mouse myocardium have not induced significant morphologic defects42;43(and data not shown). Loss-of-function studies of HIF family members in the diverse cell types of the OFT are required to further define its role in the complex morphogenesis of this structure.
In conclusion, the current targeted HIF-1α gain-of-function study suggests that hypoxia signaling though HIF-1α and VEGF exerts a pro-survival effect on the OFT myocardium. Interference with the hypoxia-dependent signaling alters the timing and prevalence of apoptosis in the OFT. Perturbations in the hypoxia-dependent signaling pathway may contribute to conotruncal heart defects in warm blooded animals.
Supplementary Material
Acknowledgment
The authors thank Dr. Gregg Semenza for generously providing AdHIF-1α and Drs. Kenneth Walsh and Calvin Kuo for providing AdFlk1-Fc. We thank Michael Payne for technical assistance and Dr. Michiko Watanabe for helpful discussions.
Sources of Funding This research was supported by NIH R01 HL65314
Footnotes
Disclosures None
Reference
- 1.Gittenberger-de Groot AC, Bartelings MM, DeRuiter MC, Poelmann RE. Basics of cardiac development for the understanding of congenital heart malformations. Pediatr Res. 2005;57:169–176. doi: 10.1203/01.PDR.0000148710.69159.61. [DOI] [PubMed] [Google Scholar]
- 2.Sallee D, Qiu Y, Liu J, Watanabe M, Fisher SA. Fas ligand gene transfer to the embryonic heart induces programmed cell death and outflow tract defects. Dev Biol. 2004;267:309–319. doi: 10.1016/j.ydbio.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Jafri A, Fisher SA. Apoptosis is required for the proper formation of the ventriculo-arterial connections. Dev Biol. 2001;240:274–288. doi: 10.1006/dbio.2001.0466. [DOI] [PubMed] [Google Scholar]
- 4.Sugishita Y, Watanabe M, Fisher SA. Role of myocardial hypoxia in the remodeling of the embryonic avian cardiac outflow tract. Dev Biol. 2004;267:294–308. doi: 10.1016/j.ydbio.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Wikenheiser J, Doughman YQ, Fisher SA, Watanabe M. Differential levels of tissue hypoxia in the developing chicken heart. Dev Dyn. 2006;235:115–123. doi: 10.1002/dvdy.20499. [DOI] [PubMed] [Google Scholar]
- 6.Sugishita Y, Leifer DW, Agani F, Watanabe M, Fisher SA. Hypoxia-responsive signaling regulates the apoptosis-dependent remodeling of the embryonic avian cardiac outflow tract. Dev Biol. 2004;273:285–296. doi: 10.1016/j.ydbio.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 8.Simon MC, Ramirez-Bergeron D, Mack F, Hu CJ, Pan Y, Mansfield K. Hypoxia, HIFs, and cardiovascular development. Cold Spring Harb Symp Quant Biol. 2002;67:127–132. doi: 10.1101/sqb.2002.67.127. [DOI] [PubMed] [Google Scholar]
- 9.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 10.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 11.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster KA, Graham RM, Bishopric NH. BNip3 and signal-specific programmed death in the heart. J Mol Cell Cardiol. 2005;38:35–45. doi: 10.1016/j.yjmcc.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Yaniv G, Shilkrut M, Lotan R, Berke G, Larisch S, Binah O. Hypoxia predisposes neonatal rat ventricular myocytes to apoptosis induced by activation of the Fas (CD95/Apo-1) receptor: Fas activation and apoptosis in hypoxic myocytes. Cardiovasc Res. 2002;54:611–623. doi: 10.1016/s0008-6363(02)00264-x. [DOI] [PubMed] [Google Scholar]
- 14.Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, Madri JA, Ment LR. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, Lin CS, Nikodem VM, Hempstead B, Flanders KC, Costantini F, Noguchi CT. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129:505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- 16.Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ Res. 2000;86:319–325. doi: 10.1161/01.res.86.3.319. [DOI] [PubMed] [Google Scholar]
- 17.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 18.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D'Amato R, Folkman J, Mulligan RC. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher SA, Watanabe M. Expression of exogenous protein and analysis of morphogenesis in the developing chicken heart using an adenoviral vector. Cardiovasc Res. 1996;31 Spec No:E86-E95. [PubMed] [Google Scholar]
- 20.Schaefer KS, Doughman YQ, Fisher SA, Watanabe M. Dynamic patterns of apoptosis in the developing chicken heart. Dev Dyn. 2004;229:489–499. doi: 10.1002/dvdy.10463. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M, Hitomi M, van der WK, Rothenberg F, Fisher SA, Zucker R, Svoboda KK, Goldsmith EC, Heiskanen KM, Nieminen AL. The pros and cons of apoptosis assays for use in the study of cells, tissues, and organs. Microsc Microanal. 2002;8:375–391. doi: 10.1017/S1431927602010346. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, Choudhry A, Berlan M, Singal A, Siwik E, Mohr S, Fisher SA. Developmental remodeling and shortening of the cardiac outflow tract involves myocyte programmed cell death. Development. 1998;125:3809–3820. doi: 10.1242/dev.125.19.3809. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF Signaling Is Required for Vascular Homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 25.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–231. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 26.Eralp I, Lie-Venema H, DeRuiter MC, van den Akker NM, Bogers AJ, Mentink MM, Poelmann RE, Gittenberger-de Groot AC. Coronary artery and orifice development is associated with proper timing of epicardial outgrowth and correlated Fas-ligand-associated apoptosis patterns. Circ Res. 2005;96:526–534. doi: 10.1161/01.RES.0000158965.34647.4e. [DOI] [PubMed] [Google Scholar]
- 27.Poelman RE, Mikawa T, Gittenberger-de Groot AC. Neural crest cells in outflow tract septation of the embryonic chicken heart: differentiation and apoptosis. Dev Dyn. 1998;212:373–384. doi: 10.1002/(SICI)1097-0177(199807)212:3<373::AID-AJA5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Praagh R, Weinberg PM, Matsuoka R, Van Praagh S. Malposition of the heart. In: Adams FH, Emmanouilides GC, editors. Heart disease in infants, children and adolescents. Williams and Wilkins Co.; Baltimore: 1983. [Google Scholar]
- 30.Abu-Issa R, Waldo K, Kirby ML. Heart fields: one, two or more? Dev Biol. 2004;272:281–285. doi: 10.1016/j.ydbio.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Bajolle F, Zaffran S, Meilhac SM, Dandonneau M, Chang T, Kelly RG, Buckingham ME. Myocardium at the base of the aorta and pulmonary trunk is prefigured in the outflow tract of the heart and in subdomains of the second heart field. Dev Biol. 2008;313:25–34. doi: 10.1016/j.ydbio.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward C, Stadt H, Hutson M, Kirby ML. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Dev Biol. 2005;284:72–83. doi: 10.1016/j.ydbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rana MS, Horsten NCA, Tesink-Taekema S, Lamers WH, Moorman AFM, van den Hoff MJB. Trabeculated Right Ventricular Free Wall in the Chicken Heart Forms by Ventricularization of the Myocardium Initially Forming the Outflow Tract. Circulation Research. 2007;100:1000–1007. doi: 10.1161/01.RES.0000262688.14288.b8. [DOI] [PubMed] [Google Scholar]
- 36.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 37.Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–1538. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- 38.Lambrechts D, Carmeliet P. Sculpting heart valves with NFATc and VEGF. Cell. 2004;118:532–534. doi: 10.1016/j.cell.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Huang JX, Potts JD, Vincent EB, Weeks DL, Runyan RB. Mechanisms of cell transformation in the embryonic heart. Ann N Y Acad Sci. 1995;752:317–330. doi: 10.1111/j.1749-6632.1995.tb17441.x. [DOI] [PubMed] [Google Scholar]
- 40.van den Akker NM, Molin DG, Peters PP, Maas S, Wisse LJ, van Brempt R, van Munsteren CJ, Bartelings MM, Poelmann RE, Carmeliet P, Gittenberger-de Groot AC. Tetralogy of fallot and alterations in vascular endothelial growth factor-A signaling and notch signaling in mouse embryos solely expressing the VEGF120 isoform. Circ Res. 2007;100:842–849. doi: 10.1161/01.RES.0000261656.04773.39. [DOI] [PubMed] [Google Scholar]
- 41.Stalmans I, Lambrechts D, De Smet F, Jansen S, Wang J, Maity S, Kneer P, von der OM, Swillen A, Maes C, Gewillig M, Molin DG, Hellings P, Boetel T, Haardt M, Compernolle V, Dewerchin M, Plaisance S, Vlietinck R, Emanuel B, Gittenberger-de Groot AC, Scambler P, Morrow B, Driscol DA, Moons L, Esguerra CV, Carmeliet G, Behn-Krappa A, Devriendt K, Collen D, Conway SJ, Carmeliet P. VEGF: a modifier of the del22q11 (DiGeorge) syndrome? Nat Med. 2003;9:173–182. doi: 10.1038/nm819. [DOI] [PubMed] [Google Scholar]
- 42.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, Gu Y, Nath AK, Huang Y, Hickey R, Dalton N, Peterson KL, Ross J, Jr., Chien KR, Ferrara N. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Hickey RP, Yeh JL, Liu D, Dadak A, Young LH, Johnson RS, Giordano FJ. Cardiac myocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J. 2004;18:1138–1140. doi: 10.1096/fj.04-1510fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.