Abstract
Background
Cardiopulmonary complications are common after endoscopy for upper gastrointestinal (UGI) hemorrhage in the intensive care unit (ICU)
Objective
To evaluate the practice and outcome of elective prophylactic endotracheal intubation prior to endoscopy for UGI hemorrhage in the ICU
Design
Retrospective, propensity matched case-control study
Setting
A 24-bed medical ICU in a tertiary center.
Patients
ICU patients who underwent endoscopy for UGI hemorrhage
Main Outcome Measurements
Cardiopulmonary complications, ICU and hospital length of stay and mortality. In a propensity analysis, patients who were intubated for airway protection prior to UGI endoscopy were matched by probability of intubation to controls not intubated prior to UGI endoscopy.
Results
Fifty-three out of 307 patients underwent elective prophylactic intubation prior to UGI endoscopy. Probability of intubation depended on APACHE III score (OR 1.4, 95%, CI 1.2 to 1.6), age (OR 0.97, 95%CI 0.95 to 0.09), presence of hematemesis (OR 1.9, 95%CI 0.8 to 5.1), prior lung disease (OR 2.1, 95%CI 0.8 to 4.9) and number of transfusions (OR 1.1 95%CI 1.0 to 1.1 per unit). Non-intubated matched controls were identified for all but 4 patients with active massive hematemesis who were excluded from matched analysis. Cumulative incidence of cardiopulmonary complications (53% vs 45%, p=0.414), ICU (median 2.2 days vs. 1.8 days, p=0.138) and hospital length of stay (6.9 vs. 5.9, p=0.785), and hospital mortality (14% vs. 20%, p=0.366) were similar.
Conclusions
Cardiopulmonary complications are frequent after endoscopy for acute UGI bleeding in ICU patients, and are largely unaffected by the practice of prophylactic intubation.
Keywords: endotracheal intubation, upper gastrointestinal hemorrhage, cardiopulmonary complications, airway protection, esophagogastroduodenoscopy
Introduction
Since approximately half of all complications related to upper gastrointestinal (UGI) endoscopy are cardio-pulmonary in nature,1,2 one of the major goals during UGI endoscopy in the intensive care unit (ICU) is preventing these cardio-pulmonary complications. The overall incidence of adverse outcomes with gastrointestinal (GI) endoscopy is low, with the reported incidence rates of serious cardiorespiratory complications and death of 5.4 and 0.3 cases per 1,000 procedures, respectively.3 However emergency UGI endoscopy is associated with a greater risk of serious cardiorespiratory events. It has been shown that 20% of ICU patients undergoing UGI endoscopy for GI bleeding develop new radiographic pulmonary infiltrates, most of which are accompanied by fever, leukocytosis and hypoxemia.4
Prophylactic endotracheal intubation for airway protection during upper GI hemorrhage is performed with variable frequency5 with the assumption that it may help prevent cardiorespiratory complications. However, there is minimal clinical data on the effectiveness of intubation in this group of patients.
In this retrospective study, we aimed to determine whether prophylactic endotracheal intubation prior to UGI endoscopy improved cardio-pulmonary outcomes, length of stay and mortality in patients admitted to a medical ICU for management of upper gastrointestinal hemorrhage.
Patients and Methods
We reviewed the medical records of patients that were admitted for upper GI hemorrhage to our 24 bed medical ICU at the Mayo Clinic, Rochester, Minnesota, from March 2002 to August 2006. All patients received sedation during UGI endoscopy, regardless of the extent of the endoscopic examination. The Institutional Review Board approved the study protocol. Inclusion criteria included the presence of known cirrhosis, hematemesis or shock. Patients were excluded if they refused research authorization, refused intubation, were intubated for reasons other than airway protection, or were intubated prior to transfer to the ICU. The rationale for intubation prior to initial UGI endoscopy had to be clearly stated in the medical records as being necessary for airway protection. Cirrhosis was defined based on histological confirmation or known portal hypertensive complications (i.e. varices, recurrent ascites). Hematemesis was defined as witnessed or reported bloody or coffee-ground emesis. Shock was defined as either mean arterial blood pressure (MAP) <65 mm Hg or systolic blood pressure (SBP) <90 mm Hg for greater than 1 hour or the presence of lactic acidosis (serum lactate >4 mmol/L) with the evidence of organ hypoperfusion (urine output <0.5cc/kg/h or documented mental status changes) prior to UGI endoscopy. Chronic lung disease was defined as a documented history of chronic obstructive or restrictive pulmonary disease.
The primary outcome was a cumulative incidence of cardiopulmonary complications: myocardial infarction, cardiac arrest, aspiration, pneumonia, acute respiratory distress syndrome (ARDS), or cardiogenic pulmonary edema.
Myocardial infarction was defined according to the consensus conference,6 and had to occur within 12 hours post-endoscopy. Cardiac arrest was defined as pulseless arrest (asystole, pulseless electrical activity, ventricular fibrillation or pulseless ventricular tachycardia) within 12 hours post-endoscopy. Aspiration was witnessed or suspected abnormal entry of secretions, fluid or particles into lower respiratory airways within 48 hours post-endoscopy. Pneumonia was defined as a new infiltrate on chest radiograph with two of the following within 48 hours post-endoscopy: fever, leukocytosis, purulent sputum.7 ARDS was defined per consensus definition within 48 hours post-endoscopy.8 Cardiogenic pulmonary edema was defined as bilateral pulmonary infiltrates with systolic or diastolic myocardial dysfunction based on echocardiography, elevated brain natriuretic peptide (BNP >250 ng/dL) or pulmonary capillary wedge pressure (PCWP >18 mm Hg). Blood products (e.g. packed red blood cells, platelets, fresh frozen plasma, cryoprecipitate) transfused within the first 24 hours after ICU admission were recorded.
Monitoring data reflecting cardiopulmonary function, chest radiographs and arterial blood gases were independently reviewed.
Univariate and multivariate logistic regression determined significant risk factors for prophylactic intubation that were present before the decision was made. The probability of intubation was calculated for each patient based on a logistic regression model that included age, severity of illness (APACHE III scores), hematemesis, number of transfusions (either red cells, platelets or fresh frozen platelets [FFP]) prior to endoscopy, and pre-existing chronic lung disease. In a propensity analysis, cases (patients who were prophylactically intubated prior to endoscopy) were matched by probability of intubation ± 0.1 to controls who were not electively intubated prior to endoscopy. Categorical and continuous variables were compared between cases and controls using Mc Nemar's and Wilcoxon sign rank test, respectively. JMP statistical software (version 6.0, SAS, Cary, NC) was used for all data analyses. Our sample size was limited by the timing of introduction of detailed electronic medical records (2002). We calculated that 50 patients per group would provide a power of 0.8 to detect a 25% difference (50% to 25%) in cumulative rate of cardiopulmonary complications post UGI endoscopy using a 1:1 matched pairs design.
Results
We retrospectively identified 489 patients who had an admitting diagnosis of GI hemorrhage and underwent an UGI endoscopy, 182 of whom were excluded (Figure 1) because they did not meet inclusion criteria (161 patients without shock, cirrhosis or hematemesis), intubated for other reasons (n=10), denied research authorization (n=2) or fulfilled other exclusion criteria (n=9 patients intubated prior to inter-hospital transfer). The cumulative incidence of cardiopulmonary complications was 31%.
Figure 1.
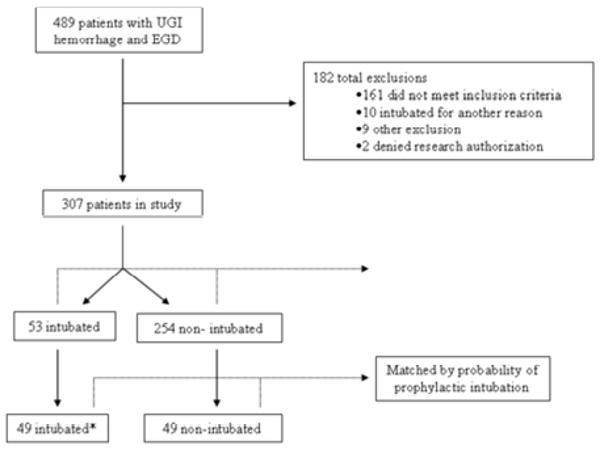
Outline of the study. UGI – upper gastrointestinal, APACHE – acute physiology and chronic health evaluation score. *Four patients with massive hematemesis and the probability of >0.9 could not be matched to non-intubated controls
Of 307 patients included in the data analysis, 53 were intubated for airway protection. Probability of prophylactic intubation depended on severity of illness (OR 1.4, 95%CI 1.2 to 1.6), age (OR 0.97, 95%CI 0.95 to 0.09) presence of hematemesis (OR 1.9, 95%CI 0.8 to 5.1), history of chronic lung disease (OR 2.1, 95%CI 0.8 to 4.9) and number of transfusions (OR 1.1 95%CI 1.0 to 1.1)
Non-intubated matched controls were identified for all but 4 patients with massive hematemesis who were excluded from matched analysis APACHE III scores, transfusion requirements, age, gender and probability for prophylactic intubation were similar between 49 cases and 49 matched controls (Table 1). Endoscopic interventions (such as cautery, banding, epinephrine injection) were performed in 17 controls (35%) vs. 18 cases (37%), p= 0.695. Endoscopically confirmed peptic ulcer disease (39% in controls vs. 35% in cases) and varices (35% vs. 43%) were the most common causes of upper gastrointestinal hemorrhage in the ICU. Other etiologies included Mallory-Weiss tears, gastritis, esophagitis and other causes (such as polyps or previous biopsy sites).
Table 1.
Baseline characteristics of patients who underwent prophylactic intubation and matched controls
| Variable | Patients intubated prior to UGI endoscopy (cases, n=49) | Patients not intubated prior to UGI endoscopy (controls, n=49) | P value |
|---|---|---|---|
| Age - median (IQR) | 62.0 (48.0, 75.0) | 68.0 (51.0, 74.0) | 0.236 |
| Female | 19 (39%) | 18 (37%) | 0.819 |
| Caucasian | 43 (92%) | 47 (96%) | 0.750 |
| APACHE III median (IQR) | 77.0 (58.0, 96.0) | 78.0 (57.0, 96.0) | 0.562 |
| Predicted Hospital Death median (IQR) | 0.3 (0.1, 0.6) | 0.3 (0.2, 0.5) | 0.619 |
| Shock | 23 (47%) | 26 (53%) | 0.564 |
| Cirrhosis | 24 (49%) | 21 (43%) | 0.532 |
| Hematemesis | 41 (84%) | 43 (88%) | 0.564 |
| History of CAD | 15 (31%) | 10 (20%) | 0.251 |
| History of CHF | 8 (16%) | 7 (14%) | 0.739 |
| History of lung disease | 9 (18%) | 13 (27%) | 0.371 |
| History of alcohol abuse | 16 (33%) | 17 (35%) | 0.827 |
| Number of transfusions median (IQR) | 6.0 (3.0, 10.0) | 5.0 (2.0, 11.0) | 0.621 |
| Probability of intubation, median (IQR) | 22.8 (14.9, 46.6) | 22.6 (14.8, 44.6) | 0.866 |
IQR – interquartile range, APACHE – acute physiology and chronic health evaluation score, CAD - coronary artery disease, CHF – congestive heart failure, UGI – upper gastrointestinal
Cumulative incidence of cardiopulmonary complications (45% vs 53%, p=0.414), ICU and hospital length of stay, and hospital mortality were similar (Table 2). Post-endoscopy cardiorespiratory arrest occurred in 1 case (2%) and 4 control patients (8%, p=0.180). ICU mortality was 6% in cases vs 16% in controls (p=0.059).
Table 2.
Outcome of patients who underwent prophylactic intubation and matched controls
| Outcome | Patients intubated prior to UGI endoscopy (cases, n=49) | Patients not intubated prior to UGI endoscopy (controls, n=49) | P value |
|---|---|---|---|
| Cardiac Arrest | 1 (2%) | 4 (8%) | 0.180 |
| Myocardial Infarction | 7 (14%) | 4 (8%) | 0.366 |
| Aspiration | 10 (20%) | 9 (18%) | 0.739 |
| ALI/ARDS | 8 (16%) | 4 (8%) | 0.157 |
| Pneumonia | 9 (18%) | 5 (10%) | 0.102 |
| Pulmonary edema | 15 (31%) | 8 (16%) | 0.071 |
| ICU LOS | 2.2 (1.7, 3.7) | 1.8 (1.1, 2.6) | 0.138 |
| Hospital LOS | 6.9 (4.9, 12.7) | 5.9 (4.0, 12.3) | 0.785 |
| ICU mortality | 3 (6%) | 8 (16%) | 0.059 |
| Hospital mortality | 7 (14%) | 10 (20%) | 0.366 |
ALI – acute lung injury, ARDS- acute respiratory distress syndrome, ICU – intensive care unit, LOS- length of stay, UGI – upper gastrointestinal
Discussion
We observed variation in the practice of prophylactic intubation for airway protection prior to UGI endoscopy in patients with cirrhosis, shock and/or hematemesis. The probability of intubation depended on age, severity of illness, number of transfusions and the presence or absence of preexisting lung disease. In a matched propensity analysis, the cumulative incidence of cardiopulmonary complications did not differ significantly between patients who were or were not intubated for airway protection.
Patients are intubated for airway protection when a physician considers the endoscopy to be hazardous to perform without a secure airway. However, physicians vary as to which patients should be endotracheally intubated. Only 20-25% of gastroenterologists believe a patient should be intubated because of unstable vital signs5 and only 60% believe ongoing hematemesis is an indication for intubation.5 We found that patients with higher severity of illness scores, hematemesis, need for large volume transfusion and preexisting pulmonary disease were more likely to be endotracheally intubated. However, in matched patients our analysis showed similar baseline clinical characteristics and the decision to proceed with intubation was made subjectively and individually applied by involved physicians, leading to practice variability.
Pulmonary complications including pulmonary edema, pneumonia, aspiration, and acute lung injury can occur in patients undergoing UGI endoscopy. One study showed that an intraoral lipoidal solution was aspirated and seen on pulmonary radiographs following UGI endoscopy in 25% of non-ICU patients.9 The clinical significance and outcomes of these infiltrates are unknown. Another study showed that 20% of ICU patients undergoing UGI endoscopy for GI bleeding develop new radiographic pulmonary infiltrates, most of which are accompanied by fever, leukocytosis and hypoxemia.4 Our data showed even higher rates of pulmonary complications which were not altered by prophylactic endotracheal intubation.
Cardiac arrest is the most feared cardiorespiratory complication of UGI endoscopy. A previous study compared two time periods in a medical ICU: one in which endotracheal intubation was rarely performed prior to UGI endoscopy and the other when endotracheal intubation was routinely performed for hematemesis, altered mentation, unstable cardiopulmonary status, large amount of blood in the proximal GI tract, or prior to endoscopic treatment of lesions felt to be at high-risk for bleeding.10 There was no difference in the number of cardiopulmonary complications, ICU length of stay or mortality between the two groups. However, the group of patients who underwent prophylactic endotracheal intubation suffered fewer in-hospital cardiac arrests. We also observed fewer cardiac arrests within 12 hours of UGI endoscopy in patients who received prophylactic intubation (4 patients in control vs. 1 in cases), but a small sample size precluded finding a statistically significant difference.
Retrospective observational studies have the potential limitation of not being able to control for unknown confounding variables. Matched propensity analysis may have not completely eliminated the indication bias. The examples of unaccounted imbalances include the preference and skill of individual gastroenterologist and the differences in sedation management used in cases in controls. A combination of midazolam and fentanyl is routinely used for both upper GI endoscopy and endotracheal intubation in our institution, however, endotracheal intubation is usually facilitated by the addition of etomidate with or without succinyl choline. Although we had examined 489 patient medical records over a 5-year period, the number of patients who were prophylactically intubated was small. The small sample size did not allow for detection of more subtle differences between the groups. The study was conducted in a single medical ICU in tertiary care center and thus external validity is limited.
In conclusion, we found that endotracheal intubation for airway protection in ICU patients undergoing UGI endoscopy for GI hemorrhage is variably performed. Cardiopulmonary complications were common and largely unaltered by prophylactic endotracheal intubation. Prospective studies are needed to determine if any patient subgroups may benefit from airway protection prior to emergency UGI endoscopy for management of upper GI bleeding.
Acknowledgments
Supported in part by NIH grant K23 HL78743-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisen GM, Baron TH, Dominitz JA, et al. Complications of upper GI endoscopy. Gastrointest Endosc. 2002;55:784–93. doi: 10.1016/s0016-5107(02)70404-5. [DOI] [PubMed] [Google Scholar]
- 2.Freeman ML. Sedation and monitoring for gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 1994;4:475–99. [PubMed] [Google Scholar]
- 3.Arrowsmith JB, Gerstman BB, Fleischer DE, et al. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37:421–7. doi: 10.1016/s0016-5107(91)70773-6. [DOI] [PubMed] [Google Scholar]
- 4.Lipper B, Simon D, Cerrone F. Pulmonary aspiration during emergency endoscopy in patients with upper gastrointestinal hemorrhage. Crit Care Med. 1991;19:330–3. doi: 10.1097/00003246-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Waye JD. Intubation and sedation in patients who have emergency upper GI endoscopy for GI bleeding. Gastrointest Endosc. 2000;51:768–71. doi: 10.1016/s0016-5107(00)70104-0. [DOI] [PubMed] [Google Scholar]
- 6.Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31:347–82. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Prout BJ, Metreweli C. Pulmonary aspiration after fibre-endoscopy of the upper gastrointestinal tract. Br Med J. 1972;4:269–71. doi: 10.1136/bmj.4.5835.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph SJ, Landsverk BK, Freeman ML. Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage. Gastrointest Endosc. 2003;57:58–61. doi: 10.1067/mge.2003.46. [DOI] [PubMed] [Google Scholar]
- 11.Koch DG, Arguedas MR, Fallon MB. Risk of aspiration pneumonia in suspected variceal hemorrhage: the value of prophylactic endotracheal intubation prior to endoscopy. Dig Dis Sci. 2007;52:2225–8. doi: 10.1007/s10620-006-9616-0. [DOI] [PubMed] [Google Scholar]


