Abstract
Experimental and simulated ESR data are in good agreement with a biradical mechanism for the intramolecular pericyclic reactions of bicyclo[1.1.0]butanes.
Introduction
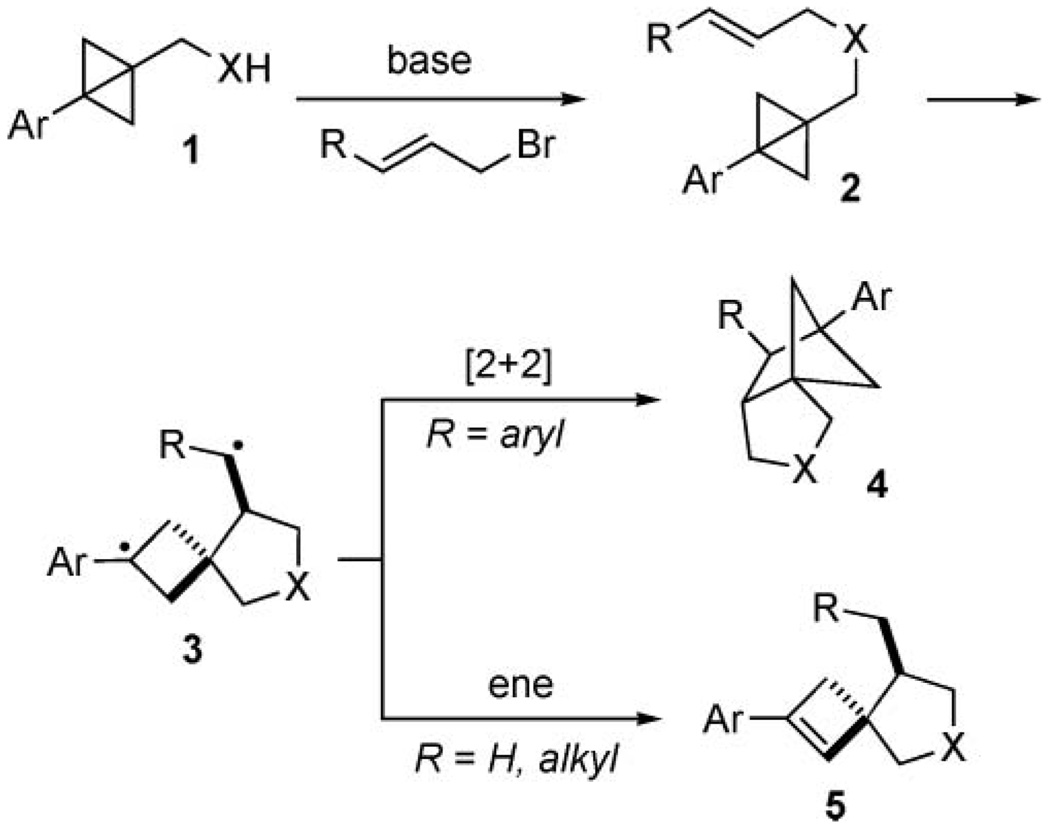
The utility of strained organic molecules for facile carbon–carbon bond cleavage and formation processes has been documented by a large number of useful chemical transformations.1 During our studies on the unique reactivity of bicyclo[1.1.0]butanes,2,3 we found that the course of the diastereoselective intramolecular formal Alder-ene and [2 + 2] cycloadditions was dependent on the electronic nature of the alkene moiety (Scheme 1). Specifically, cinnamyl substituents on 2 (R = Ph) afforded as rearrangement products the functionalized tricyclic pyrrolidines 4. Conversely, reactions with non-conjugated allyl amides 2 (R = H, alkyl) resulted in the exclusive formation of pyrrolidines 5.
Scheme 1.
Selective formation of intramolecular Alder-ene and [2 + 2] cycloaddition products from bicyclo[1.1.0]butane 1; X = NTs or NP(O)Ph2.
The mild reaction conditions—ambient temperature—and the selectivity of these transformations raised questions about the reaction mechanism. Our initial proposal for the formal Alder-ene and [2 + 2] processes was based on the notion that the central bond in the bicyclo[1.1.0]butane ring may undergo a facile hemolytic cleavage,4 and that the formation of 4 and 5 could be explained by the biradical intermediate 3. With R = aryl, the resonance stabilized 3 would be able to undergo an intramolecular radical recombination, resulting in the exclusive formation of tricycle 4. Alternatively, a more reactive 3 with R = alkyl or hydrogen would undergo an intramolecular endo-hydrogen atom transfer5 from the cyclobutyl methylene group, leading to spirocyclic pyrrolidine 5.
The formation of a biradical intermediate in the pericyclic reactions of bicyclo[1.1.0]butanes was supported by earlier studies of ene and cycloaddition processes in strained systems.5 Intermolecular reactions of bicyclo[1.1.0]pentanes with electron-deficient alkenes and alkynes proceeded to a cycloadduct without showing a substantial solvent effect, suggesting that an ionic mechanism did not represent a major pathway. Also, reactions of bicyclo[1.1.0]butane with benzyne afforded a mixture of ene and other cycloaddition products.6 Early studies by Gassman and Richmond provided some evidence that deuterium labelled bicyclo[1.1.0]butane and benzyne afforded a tetracyclic product that could derive from a rearrangement of a biradical intermediate.7 However, the intermolecular character of these reactions and the unique electronic nature of benzyne could lead to erroneous conclusions for less activated alkenes and alkynes.8 Since there was no direct evidence in favor of (bi)radical intermediates and because chemical studies were inconclusive,3a we used electron spin resonance (ESR) spectroscopy9 to further elucidate the process shown in Scheme 1.
Results and discussion
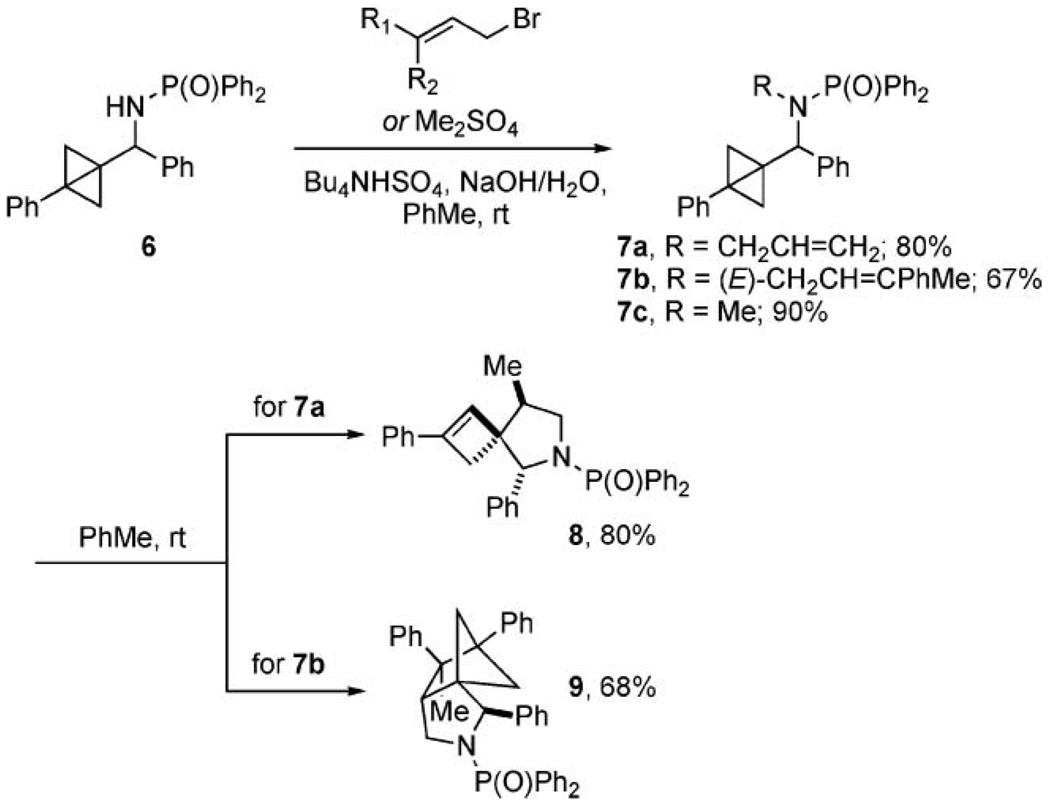
Our initial attempts to directly detect the putative radical intermediate 3 by ESR were inconclusive. When allyl amide 7a was prepared under phase transfer conditions, the transient intermediate underwent a clean conversion at room temperature to the trisubstituted pyrrolidine 8 in 80% yield and high diastereoselectivity (97 : 3, Scheme 2). The reaction rate measured by 1H NMR indicated first-order kinetics for the disappearance of starting material with k = 0.056 h−1 (25 °C, chloroform-d). When a concentrated solution of 7a in chloroform-d (1.5 M) was subjected to ESR measurements, a weak signal was observed; but, due to a low signal-to-noise ratio, we were unable to assign the spectra conclusively. The intensity of the signal changed over time and finally decreased to zero after ca. 4 h at room temperature.10
Scheme 2.
Synthesis and rearrangement reactions of N-allylated and N-methylated bicyclo[1.1.0]butylmethylamides 7.
For a further analysis of the mechanism of the cycloaddition reactions of bicyclo[1.1.0]butanes, the cinnamyl derivative 7b was prepared in 67% yield, and spontaneously rearranged to 9 within 12 h in 68% yield. Due to the faster reaction rate observed in the reactions with cinnamyl amides,3a an additional terminal methyl group in the allyl chain was added to allow for an isolation of 7b. The rate for the rearrangement of 7b (k = 0.24 h−1, 20 °C, chloroform-d) indicated that this reaction was ca. four times faster than the analogous rearrangement of 7a. The same reaction performed in the ESR tube showed a weak signal, but the intensity was again too low for a conclusive interpretation. However, these experiments suggested that transient radical species were indeed generated in the course of the reactions of allyl amides with bicyclo[1.1.0]butanes. In a control experiment using N-methyl amide 7c, no signals corresponding to the radical intermediates generated in the pericyclic reactions of 7a,b could be observed.
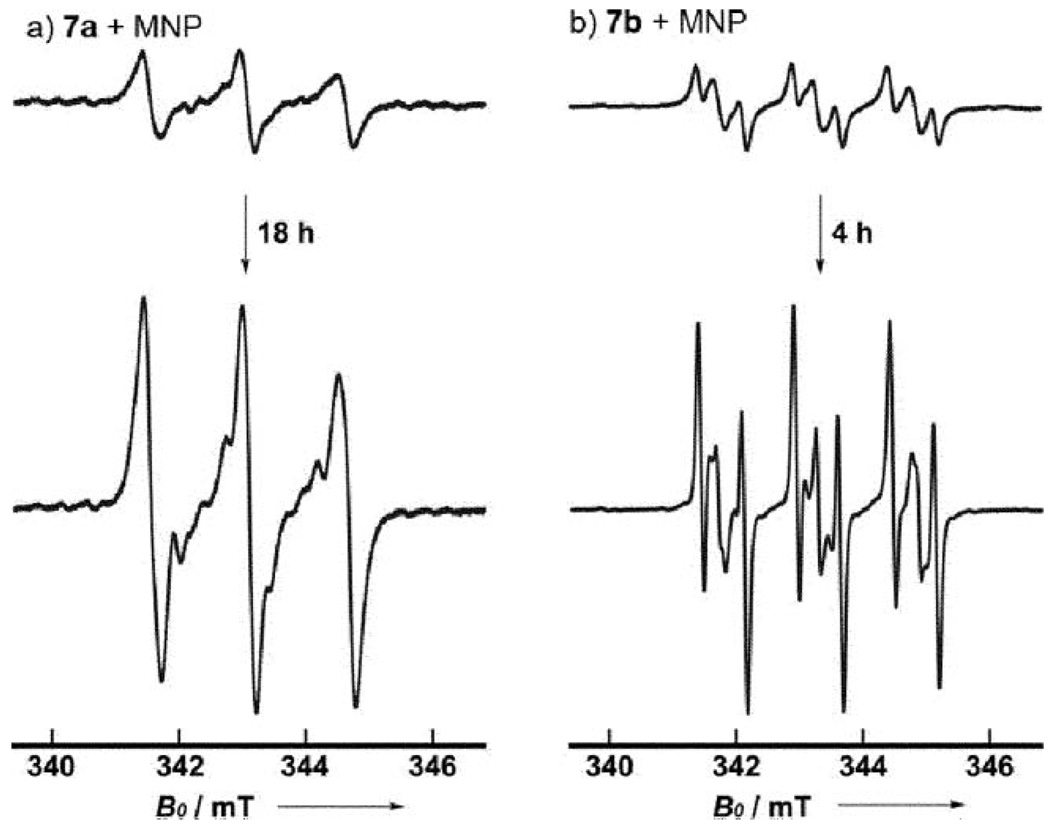
Although the weak ESR signals suggested that radical intermediates were present in the reaction mixtures, the transient nature of these species and their low concentration prevented precise spectral analyses. Furthermore, it was also conceivable that the ESR signals arose from oxidation products of the phosphamide moiety (Fig. S1†). Accordingly, we decided to carry out the intramolecular rearrangement of 7 in the presence of a spin trapping agent.11 Gratifyingly, long-lived radical species were formed in the presence of 5–6 molar equivalents12 of 2-methyl-2-nitrosopropane (MNP). The results of these trapping experiments are shown in Fig. 1.
Fig. 1.
ESR spectra for reactions of 7a and 7b recorded in the presence of MNP.
Monitoring the progress of these reactions over time revealed that the ESR signal underwent significant amplification. Analysis of the fine structure demonstrated that more than one radical species (spin adducts) were present in the reactionmixture.10 These observations were consistent with the MNP trapping mechanism, and it was likely that the ESR signals from the transient radical species were obscured by the much stronger signal from the stable spin adducts. Finally, there was strong evidence that the radical adducts detected in the reactions of 7a and 7b were derived from transient radical species—ESR analysis of a mixture of MNP and N-methylated 7c showed no signals corresponding to the spin adducts. For the intramolecular rearrangement of 7a in the presence of MNP, the major component was characterized by aN = 1.56 mT and this value was consistent with the literature spectra of MNP adducts.11,13
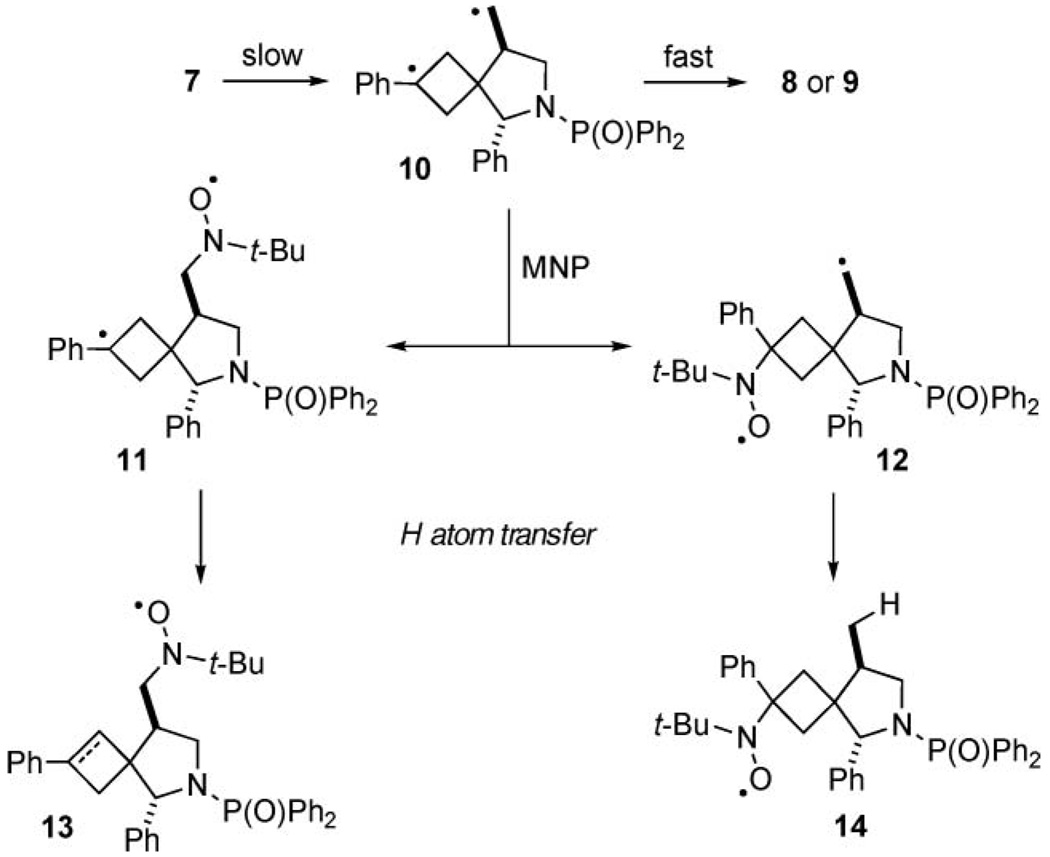
In order to explain the entirety of the observed signal patterns in the intramolecular rearrangement of 7 in the presence of MNP and assign them to possible products of these reactions, we considered the formation of two major spin adducts, 13 and 14 (Scheme 3). The biradical intermediate 1014 could be trapped by MNP to give monoadducts 11 and/or 12, and these compounds were considered to be the major reaction constituents. Subsequently, for example by an intramolecular hydrogen atom transfer from 11 to 12, the reactive biradical intermediates were quenched to afford 13 and 14. Either 11 or 12, or both, could also react with MNP to form a stable biradical trapping product, which would also be consistent with the mechanistic hypothesis. However, while we see m/z for 13 and 14, as both saturated and dehydrogenated species, we did not detect any double spin trapping products in the MS analysis of the reaction mixture.
Scheme 3.
Radical trapping mechanisms in the rearrangements of N-allylated bicyclo[1.1.0]butylmethylamides 7.
We also briefly explored the possibility that solvent radicals and solvent-derived spin adducts might be formed. These could derive from a reaction between solvent radicals and MNP, the spin trapping agent, and could dominate the ESR spectrum. However, a change of solvent from chloroform-d to benzene-d6 only led to a slight change in hyperfine coupling constants.10 Since these solvents have significantly different structures, the similarity in hyperfine coupling constants rules out the possibility of solvent-derived spin adducts being major contributors to the spectrum. In addition, the spectrum of 7b and MNP in chloroform-d is different from the spectrum of 7a and MNP in the same solvent, which is also in agreement with this hypothesis.10
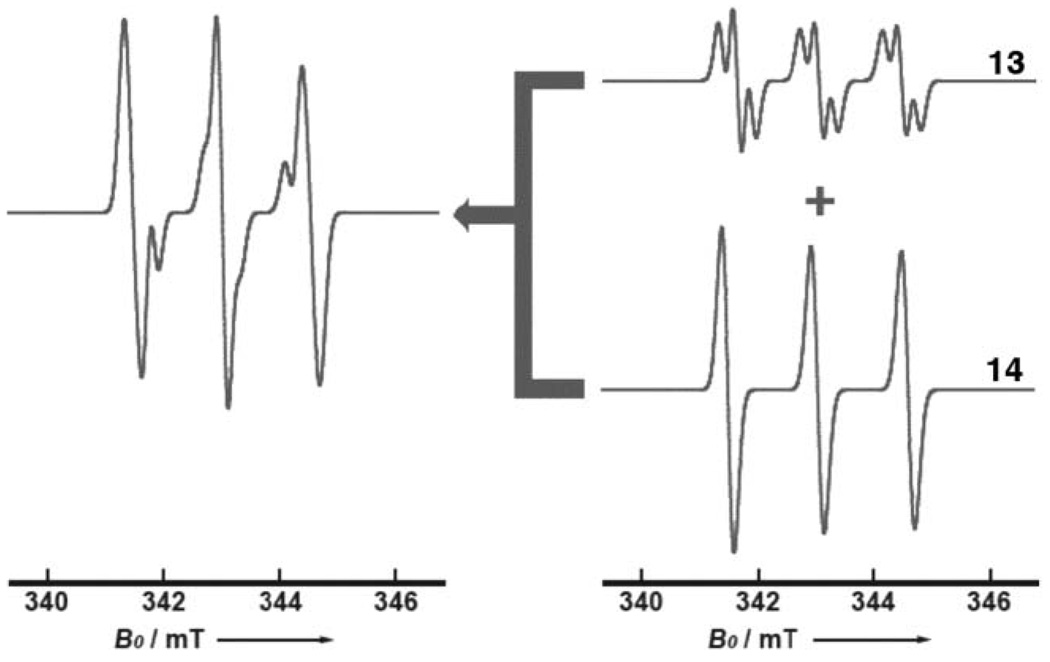
Based on the proposed radical structures, we performed a series of spectral simulations. The results of one of these calculations, for a reaction time of 18 h, are presented in Fig. 2. For 13 and 14, the dominant coupling would arise from the hyperfine interactions of the electron spin with the nitrogen nuclear spin, and in the simulated spectrum, we found the best fit with aN = 1.43 mT (ΔH = 0.23 mT) and 1.56 mT (ΔH = 0.23 mT) for 13 and 14, respectively. The methylene radical adduct 13 gave rise to additional non-equivalent hyperfine couplings. While methyne hyperfine couplings are expected to be small, they were included for completeness. The best fit of the simulated spectrum with the experimental data was obtained with aH1 = 0.31 mT, aH2 = 0.01 mT, where H1 and H2 corresponded to methylene and methyne protons respectively. Taking these intermediates into account, we found that a satisfactory correlation between observed and simulated spectra (Fig. 2) could be obtained for a 1 : 1 ratio of 13 and 14.
Fig. 2.
Simulated ESR spectra of MNP spin adducts formed from 7a. The simulated spectrum corresponding to two radical moieties showed a good agreement with the experimental data.
The hyperfine coupling constant of the methylene protons (0.31 mT) in 13 is smaller than comparable literature values for the hydrogen of a methylene group attached to a 5-membered ring, which generally range from 0.8 mT to 2.0 mT. However, when the α-position (with respect to the nitroxide) is substituted with two alkyl groups, an O-alkyl group, or an N-alkyl group, the hyperfine coupling constant for the α-hydrogen can be as small as 0.1–0.3 mT.15,16 Thus, while the hyperfine coupling constant value is clearly not sufficient to ascertain the proposed structure 13, it strongly depends on adjacent functional groups and a value of 0.31 mT is not unreasonable.
The presence of two different types of radical moieties strongly supported the hypothesis that the intramolecular cycloadditions of bicyclo[1.1.0]butanes proceeded via a biradical mechanism. Although the nature of the spin adduct was deduced indirectly from the ESR studies and a detailed NMR product evaluation was prevented by the low efficiency of the trapping experiments, MS-TOF analyses were in agreement with the presence of the monoadducts: for 13 or 14 (C36H40N2O2P, M+ m/z calc 563.69 and [2M + K]+ calc 1166.53), we observed m/z 563.54 and 1166.50. We were also able to detect m/z 561.53, which might correspond to a dehydro derivative of 13/14 ([M − 2H]+ m/z calc 561.67). The ESR simulations carried out for the reaction of 7b with MNP were in agreement with analogous assumptions about the nature of the radical intermediates. Accordingly, we propose that the reaction with MNP affords two major radical species that can be explained from the biradical intermediate.
Conclusions
We have demonstrated that the experimental ESR data are in good agreement with a biradical mechanism for the intramolecular pericyclic reactions of bicyclo[1.1.0]butanes. Our spectral simulations show that there are two major spin adduct species originating from 7, thus corroborating the hypothesis that the biradical mechanism accounts for the rearrangement and the product selectivity of both N-allylated chemotypes.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIGMS P50 grant GM067082 (the CMLD program).
Footnotes
Electronic supplementary information (ESI) available: ESR experiments in the absence of a spin trapping agent; ESR experiments in the presence of a spin trapping agent; ESR spectra without a spin trapping agent; ESR intensity change with time; ESR experiment and simulation of 7b; solvent-derived spin adducts; ESR signals from MNP.
Notes and references
- 1.(a) For recent applications of cyclopropanes and cyclobutanes, see: Brandi A, Cicchi S, Cordero FM, Goti A. Chem. Rev. 2003;103:1213–1270. doi: 10.1021/cr010005u. Nakamura M, Isobe H, Nakamura E. Chem. Rev. 2003;103:1295–1326. doi: 10.1021/cr0100244. Namyslo JC, Kaufmann DE. Chem. Rev. 2003;103:1485–1538. doi: 10.1021/cr010010y. Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117–3179. doi: 10.1021/cr050988l.
- 2.(a) Hoz S. In: The Chemistry of the Cyclopropyl Group. Rappoport Z, editor. Chichester, New York: J. Wiley & Sons; 1987. [Google Scholar]; (b) Wiberg KB, Lampman GM, Ciula LP, Connor DS, Schertler P, Lavanish J. Tetrahedron. 1965;21:2749–2769. [Google Scholar]
- 3.(a) Wipf P, Walczak MAA. Angew. Chem. 2006;118:4278–4281. [Google Scholar]; Wipf P, Walczak MAA. Angew. Chem., Int. Ed. 2006;45:4172–4175. doi: 10.1002/anie.200600723. [DOI] [PubMed] [Google Scholar]; (b) Walczak MAA, Wipf P. J. Am. Chem. Soc. 2008;130:6924–6925. doi: 10.1021/ja802906k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ueda M, Walczak MAA, Wipf P. Tetrahedron Lett. 2008;49:5986–5989. doi: 10.1016/j.tetlet.2008.07.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Jung Y, Head-Gordon M. Chem Phys Chem. 2003;4:522–525. doi: 10.1002/cphc.200200668. [DOI] [PubMed] [Google Scholar]; (b) Jung Y, Head-Gordon M. J. Phys. Chem. A. 2003;107:7475–7481. [Google Scholar]
- 5.(a) Gassman PG, Mansfield KT. J. Chem. Soc., Chem. Commun. 1965:391–391. [Google Scholar]; (b) Gassman PG, Mansfield KT. J. Am. Chem. Soc. 1968;90:1517–1524. [Google Scholar]; (c) Gassman PG, Mansfield KT. J. Am. Chem. Soc. 1968;90:1524–1526. [Google Scholar]; (d) Gassman PG, Mansfield KT, Murphy TJ. J. Am. Chem. Soc. 1968;90:4746–4748. [Google Scholar]; (e) Gassman PG, Richmond GD. Chem. Commun. 1968:1630–1632. [Google Scholar]; (f) Pomerantz M, Wilke RN. Tetrahedron Lett. 1969;10:463–465. [Google Scholar]; (g) Gassman PG, Mansfield KT, Murphy TJ. J. Am. Chem. Soc. 1969;91:1684–1689. [Google Scholar]; (h) Gassman PG. Acc. Chem. Res. 1971;4:128–136. [Google Scholar]
- 6.(a) Pomerantz M, Gruber GW, Wilke RN. J. Am. Chem. Soc. 1968;90:5040–5041. [Google Scholar]; (b) Pomerantz M, Wilke RN, Gruber GW, Roy U. J. Am. Chem. Soc. 1972;94:2752–2758. [Google Scholar]
- 7.(a) Gassman PG, Richmond GD. J. Am. Chem. Soc. 1968;90:5637–5639. [Google Scholar]; (b) Gassman PG, Richmond GD. J. Am. Chem. Soc. 1970;92:2090–2096. [Google Scholar]
- 8.(a) For pericyclic reactions of bicyclo[1.1.0]butanes with ketones and diazocompounds, see: Blanchard EP, Jr, Cairncross A. J. Am. Chem. Soc. 1966;88:487–495. Cairncross A, Blanchard EP., Jr J. Am. Chem. Soc. 1966;88:496–504. Amey AB, Smart BE. J. Org. Chem. 1981;46:4090–4092.
- 9.Gerson F, Huber W. Electron Spin Resonance Spectroscopy of Organic Radicals. Weinheim: Wiley-VCH; 2003. [Google Scholar]
- 10.For detailed information, see the ESI.
- 11.(a) For selected ESR studies using spin trapping agents, see: Mackor A, Wajer AJW, de Bohr ThJ, van Voorst JDW. Tetrahedron Lett. 1966;7:2115–2123. Janzen EG. Acc. Chem. Res. 1971;4:31–40. Pou S, Halpern HJ, Tsai P, Rosen GM. Acc. Chem. Res. 1999;32:155–161. Usuki T, Mita T, Lear MJ, Das P, Yoshimura F, Inoue M, Hirama M, Akiyama K, Tero-Kubota S. Angew. Chem. 2004;116:5361–5364. doi: 10.1002/anie.200454133. Usuki T, Mita T, Lear MJ, Das P, Yoshimura F, Inoue M, Hirama M, Akiyama K, Tero-Kubota S. Angew. Chem., Int. Ed. 2004;43:5249–5253. doi: 10.1002/anie.200454133. Usuki T, Nakanishi K, Ellestad GA. Org. Lett. 2006;8:5461–5463. doi: 10.1021/ol062061t.
- 12.Even in the presence of an excess of MNP, the efficiency of the trapping process was low and mostly rearranged product 8 was observed.
- 13.(a) Madden KP, Taniguchi H. J. Am. Chem. Soc. 1991;113:5541–5547. [Google Scholar]; (b) Bentley J, Madden KP. J. Am. Chem. Soc. 1994;116:11397–11406. [Google Scholar]
- 14.For ESR studies on cyclobutanediyl biradicals, see: Jain R, Sponsler MB, Coms FD, Dougherty DA. J. Am. Chem. Soc. 1988;110:1356–1366.
- 15.Mash EA, Korth HG, DeMoss SM. Tetrahedron. 1997;53:15297–15320. [Google Scholar]
- 16.Rees MD, Hawkins CL, Davies MJ. J. Am. Chem. Soc. 2003;125:13719–13733. doi: 10.1021/ja0370591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.