Abstract
Estrogens are potent neuroprotective hormones and mitochondria are the site of cellular life-death decisions. As such, it is not surprising that we and other have shown that estrogens have remarkable effects on mitochondrial function. Herein we provide evidence for a primary effect of estrogens on mitochondrial function, achieved in part by the import of estrogen receptor β (ERβ) into the mitochondria where it mediates a number of estrogen actions on this vital organelle. ERβ is imported into the mitochondria, through tethering to cytosolic chaperone protein and/or through direct interaction with mitochondrial import proteins. In the mitochondria, ERβ can affect transcription of critical mitochondrial genes through the interaction with estrogen response elements (ERE) or through protein-protein interactions with mitochondrially imported transcription factors. The potent effects of estrogens on mitochondrial function, particularly during mitochondrial stress, argues for a role of estrogens in the treatment of mitochondrial defects in chronic neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD) and more acute conditions of mitochondrial compromise, like cerebral ischemia and traumatic brain injury.
Key Works: Mitochondria, estrogen, estradiol, estrogen receptorβ, apoptosis, neurodegeneration, neuroprotection
1. Introductions
The role of estrogens in cell viability is now well known and some of the mechanisms have been described. A new area of active investigation is the action of estrogens on mitochondria, the respiratory center of neurons as well as the site of major life-death decisions. In this treatise, we described what is currently known about the effects of estrogens on the mitochondria and potential mechanisms by which these effects are exerted. Our focus is those effects of this amazing steroid that may be related to its potent neuroprotective activities. Additionally, we discuss the mechanisms of trafficking of ERβ into the mitochondria, since ERβ cellular localization is likely to play a major role in the effects of estrogens on mitochondrial function. Further, the potential role of ERβ as a mitochondrial transcription factor is considered. Finally, the clinical implications of these data on neurological condition in which mitochondrial compromise had been demonstrated are discussed.
2. Mitochondria and Cell Death
Acute conditions that compromise neurons, such as a stroke or traumatic brain injury also compromise mitochondrial function and contribute to cell death. Neurons are dependent on mitochondrial ATP production for their high energy demand. Damage to mitochondria causes disruptions in ATP production and an increase in reactive oxygen species (ROS) that compromise antioxidant defense systems of the cell (Dykens, 1997; Lemasters et al., 1999) and are key events leading to both necrosis and apoptosis (Kroemer and Reed, 2000; Zou et al., 1997). Oxidative stress, coupled with excessive Ca2+ loading, causes mitochondria to undergo a catastrophic loss of the impermeability of the inner mitochondrial membrane. This causes a collapse of the mitochondrial membrane potential (Δψm) (Green and Kroemer, 2004). This collapse of Δψm is usually accompanied by mitochondrial swelling and release of cytochrome c and Apaf-1 (Murphy et al., 1999) into the cytoplasm where they activate caspases and induce apoptotic cell death (Zou et al., 1997; Dykens, 1999). Excessive Ca2+ from glutamate receptor activation can lead to mitochondrial Ca2+ loading, interruption of ATP production, generation of ROS and collapse of the Δψm, ultimately leading to neuronal death (Lemasters et al., 1999; Dykens, 1994). As such, mitochondria are believed to be the key modulator of neuronal viability during excitotoxicity (Dykens, 1997).
The CNS is more susceptible to mitochondrial impairment than many other tissues because of its extraordinary aerobic poise; the human brain comprises only 5% of the body, yet is responsible for 20% of the organism’s respiration. Thus, the brain is at risk when mitochondrial function decline below the level that would otherwise be tolerated by less metabolically demanding tissues. Selective CNS susceptibility is because mitochondria from different tissues respond to stresses differently. For example, conditions that do not elicit free radical production from mitochondria isolated from liver cause brain mitochondria to produce copious amounts of oxygen- and carbon-centered radicals (Dykens, 2007). In addition, mitochondrial impairment that reduce organelle transport within neurons puts them at increased risk of injury (Chang and Reynolds, 2006; von Lewinski and Keller, 2005).
3. Mitochondrial Function in Neurodegenerative Diseases
Mitochondrial DNA (mtDNA) is an intronless, circular genome of 16.5 kb encoding 37 genes of the approximately 3000 proteins in the mitochondrial proteome. Of these mitochondrial genes, 13 code for proteins that serve in the electron transport system, with the remainder encoding for elements required for expression. Most mitochondriopathies are associated with deficits in the electron transport system that increase free radical production and reduce energy production and have a chronic, slowly progressive course with multiorgan involvement (Finsterer, 2004). Organ systems at particular risk in mitochondriopathies are metabolically active tissues, such as the peripheral and central nervous systems, where dementias, epilepsy and ataxias are frequently present; the eyes, where glaucoma, retinopathy and optic atrophy occur; and the heart (Betts et al., 2004).
Other diseases caused by mitochondrial failure are due to mutations in nuclear genes encoding proteins that are imported into the mitochondria. For example, Friedreich’s ataxia (FRDA) is a recessively inherited early onset disease that affects children between 5 and 15 years old. It is characterized by progressive deterioration of the CNS, resulting in debilitating muscle weakness and heart disease, and most patients succumb in early adulthood. Friedreich’s ataxia is caused by large expansions of a GAA repeat in the first intron of the gene for the protein called frataxin (Monticelli et al., 2004). Frataxin is involved in iron homeostasis, and these repeats impede its translocation into the mitochondria, causing excessive iron availability in mitochondria. Iron is one of several transition metals that can serve as Fenton catalysts to accelerate production of OH from H2O2. Excessive iron in FRDA mitochondria exacerbates oxidative stress, and promotes membrane lipid peroxidation reactions that can directly undermine mitochondrial function by degrading the impermeability of the inner membrane (Schapira and Lodi, 2004). Other chronic diseases with bioenergetic and oxidative etiologies that implicate mitochondrial dysfunction include Alzheimer’s and Parkinson’s diseases, as well as amyotrophic lateral sclerosis (ALS), where a mutation in superoxide dismutase is associated with the familial disease (Gurney et al., 1996).
Although the etiology of Alzheimer’s disease (AD) is multifactoral (Prasad et al., 2002), a consistent finding is hypometabolism of glucose in those brain regions affected by the disease (Bosetti et al., 2002) that can be detected very early in the disease, even before cognitive symptoms are reported (Hirai et al., 2001; Blass, 2003). Although hypometabolism may simply reflect neuronal loss in the effected regions, mitochondrial dysfunction can be more directly implicated in AD. β-Amyloid undermines mitochondrial stability, inducing both oxidative and bioenergetic crises (Muller et al., 2001; Casley et al., 2002; Canevari et al., 2004), and such mitochondrial impairment in turn enhances the production of Aβ (Busciglio et al., 2002). Similarly, normal distribution of mitochondria in neurons could participate in AD progression secondarily to mitochondrial failure. Peri-nuclear mitochondria are more actively replicating than those elsewhere in the neuron, after which many are distributed to the synapses. Impairment of normal axonal transport of mitochondria is likely due to the breakdown of microtubules from the hyperphosphorylation of the microtubule-associated protein, tau (Cash et al., 2002; Swerdlow, 2002).
Mitochondria from AD subjects are hypofunctional (Bosetti et al., 2002; Cottrell et al., 2001), produce excessive reactive oxygen species (ROS) (Aliev et al., 2002; Smith et al., 2000), and show a defect in respiratory complex IV (C-IV) (Kish, 1997; Gibson et al., 1998). When inserted into transformed cells depleted of their endogenous mtDNA, mtDNA from AD patients produces a phenotype in the resulting cytoplasmic hybrids (cybrids) of increased oxidative stress, propensity towards apoptosis and C-IV impairment (Ghosh et al., 1999; Trimmer et al., 2000), suggesting that many of the cellular defects found in AD reflect mitochondrial defects. Although such mitochondrial impairment could be interpreted as a consequence of the disease, not as a primary causal factor (Shoffner, 1997), mitochondrial dysfunction is clearly involved in progressive neuronal death and as such represents a viable therapeutic target.
The evidence for mitochondrial defects in Parkinson’s disease (PD) is very consistent with impairment in respiratory complex I (C-I) documented from brain and peripheral tissues (Haas et al., 1995; Orth and Schapira, 2001; Beal, 2003). Cybrids containing mtDNA from PD patients not only show comparable mitochondrial impairment in complex I activity and ensuing oxidative stress (Veech et al., 2000), but also Lewey bodies in these cybrids react positively with cytochrome c antibodies suggesting a mitochondrial origin (Trimmer et al., 2004). Mitochondrial involvement in the etiology of PD is also supported by toxin models where selective inhibitors of respiratory C-I, such as N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone and 6-OH-dopamine, yield degeneration of substantia nigra neurons and PD symptoms in animals and humans (Schapira et al., 1998; Schapira, 1998).
4. Estrogens and Neuroprotection
Both estrogen receptor-dependent and -independent forms of neuroprotection have been described (Green and Simpkins, 2000a, 2000b). Many genes under the regulation of estrogen are believed to have anti-apoptotic effects, including Bcl2 (Dong et al. 1999), Bcl-xl (Pike, 1999), BDNF (Singh et al. 1999). These studies argue for a receptor involvement in mediating the neuroprotection by estrogens. The neuroprotective effect of E2 in in vitro models is attenuated by the ER antagonists tamoxifen or ICI 182,780 in some studies (Singer et al, 1996, 1999; Pike, 1999, Wilson et. al., 2000) but not in others (Green et al., 1997; Sawada et al, 1998; Weaver et al., 1997; Moosmann and Behl, 1999;. Regan and Guo, 1997). An emerging concept is that neuroprotection afforded by estrogens is ER-mediated at low physiological concentrations of the steroid, but ER-independent at pharmacological concentrations of estrogens (Green and Simpkins, 2000a, 2000b; Wise et al., 2001; McEwen, 2001).
We have now extensively assessed the neuroprotective effects of estrogens in an animal model of cerebral ischemia. Following our first report of neuroprotection with estrogens in an animal model of ischemia (Simpkins et al., 1997a, 1997b), we and other have demonstrated that estrogens protects the brain from ischemic damage induced by transient cerebral ischemia (Alkayed et al., 1998; Rusa et al., 1999; Hurn and Macrae, 2000; Shi et al., 2001, Sampei et al., 2000), permanent cerebral ischemia (Dubal et al., 1998, 2001; Yang et al., 2001), subarachnoid hemorrhage (Yang et al., 2001), and global ischemia (He et al., 2002). The protective effects of estrogens are seen with 17β-estradiol, as well as non-feminizing estrogens, such as 17α-estradiol (Simpkins et al., 1997b), ENT-estradiol (Green et al., 2001), and 2-adamantyl-estrone (Liu et al., 2002). Additionally, the protection afforded by 17β-estradiol can be observed for up to 3 hours following the onset of cerebral ischemia (Yang et al., 2000). Dubal et al. (2001) reported that ERαKO, but not ERβKO mice were resistant to the neuroprotective effects of 17 β-E2 administered chronically at low concentrations and concluded that ERα is a necessary mediator of estrogen neuroprotection. Later, however, McCullough et al (2001) demonstrated estrogen neuroprotection in ERαKO mice using a pharmacological pretreatment paradigm.
A variety of studies have demonstrated antioxidant activity of estrogen, which does not appear to require the classical receptor-dependent mechanism. Our laboratory has shown that estradiol at physiological concentrations can block membrane oxidation (Green et al., 1996). E2 treatment has been shown to reduce lipid peroxidation induced by glutamate and further attenuate the increase in intracellular peroxide induced by H2O2 (Green et al., 2000). In agreement, in vitro studies reported that estrogen inhibited formation of lipid peroxyls and oxidation of low-density lipoproteins (Mukai et al., 1990; Rifici and Khachadurian, 1992). In in vivo studies, estrogen replacement therapy with the transdermal patch reduces low-density lipoproteins (Sack et al., 1994). These effects of estrogen do not require estrogen receptors (Green et al., 1997; Behl et al., 1997; Gridley et al., 1998), indicating that estrogen exerts antioxidant activities through estrogen receptor-independent mechanisms. Indeed, we have described an estrogen redox cycle that enables estrogens to tap into the large reducing potential of the cell through interaction with NADPH (Prokai et al, 2003).
5. Estrogen Effects on Mitochondria
We and other have shown that estrogens have substantial effects on mitochondrial function, particularly during insults that might contribute substantially to the neuroprotective effects of estrogens. First, estrogen binding sites have been described in the mitochondria, including the F0/F1 ATPase (Zheng and Ramirez, 1999a,b) and we have demonstrated that the estrogen receptor (ER) beta (ERβ) localizes to the mitochondria (Yang et al., 2004). Furthermore, estrogens have been shown to affect concentrations and localization of anti-apoptotic proteins (Pike, 1999; Singer et al., 1998; Yang et al., 2004; Zhao et al., 2004; Wise et al., 2000; Nilsen and Brinton, 2003), which appear to exert their anti-apoptotic effects through maintenance of mitochondrial membrane potential in the face of stresses. Based upon the aforementioned role of mitochondria in cell death decisions, it is reasonable that the mitochondria are a major site of the neuroprotective effects of estrogens. To assess this possibility, we undertook a series of studies to determine if estrogens affect mitochondrial functions, particularly in the face of stresses that are known to compromise this vital organelle and neuronal health.
We assessed the effects of estrogens on the production of ATP under two condition of stress to the mitochondria (Wang et al., 2001, 2003a, 2006). We first determined the effects on ATP production of 3-nitropropionic acid (3NPA), a succinate dehydrogenase inhibitor that uncouples oxidative phosphorylation, and the capacity of estrogens to ameliorate the effect on ATP of this mitochondrial toxin. Second, we induced a compromise in mitochondrial function through the exogenous administration of the H2O2, and assessed the effects of estrogens on this response. 3NPA effectively suppresses succinate dehydrogenase activity and caused a dose- and time-dependent decrease in cellular ATP levels (Wang et al., 2001). Inhibition of complex II by 50% or greater caused a marked and sustained reduction in the ability of the mitochondria to produce ATP (Wang et al., 2001). Neuronal cultures pre-treated with 17 β-estradiol (E2) for 6 h, followed by 3NPA treatment (10 mM, a concentration that reduced ATP by 50% at 12 h), dose-dependently attenuated the 3NPA-induced decrease in ATP production (Wang et al., 2001). E2, in the absence of 3NPA, had no effect on ATP level, suggesting that under basal, non-stress conditions, E2 has little effect on ATP production (Wang et al., 2001). Recently however, Irwin et al. (2008) have reported a modest increase in state 3 respiration in mitochondria isolated from E2 treated rats. Administration of H2O2 to neuronal cells in culture caused a dose- and time-dependent decline in ATP production (Wang et al., 2003b, 2006), demonstrating that exogenous ROS severely compromises mitochondrial oxidative phosphorylation. Pre-treatment with E2 ameliorated the H2O2-induced decline in cellular ATP (Wang et al., 2003b, 2006). This ability of E2 to prevent pro-oxidant-induced declines in cellular ATP appears to be a general property of E2, as we have demonstrated a similar effect of E2 against H2O2-induced decline in cellular ATP in a non-neuronal, human lens cell type (Wang et al., 2003a). Furthermore, non-feminizing estrogens share this ability to protect ATP production (Wang et al., 2006). Collectively, these data indicate that, while estrogens have little effect on mitochondrial ATP production under basal condition, they are potent stabilizers of ATP production during oxidative stress. This ability to maintain oxidative phosphorylation in the face of compromising stresses may explain the ability of estrogens to potently protect neurons from a variety of insults, both in vitro and in vivo.
Estrogens could affect mitochondrial function by directly or indirectly influencing mitochondrial loading with Ca2+. Brinton’s laboratory (Zhao et al., 2004; Nilsen et al., 2002) has demonstrated that with mild glutamate stimulation, estrogens enhance Ca2+ flux into cells, an effect that may be involved in estrogen’s ability to increase memory function through this NMDA receptor mediated mechanism (Brinton, 2001; Foy et al., 1999). We have recently shown that estrogens also potentiate Ca2+ influx through L-type Ca2+ channels (Sarkar et al., 2006). At high levels of excitotoxic stimulation, however, estrogens prevented both cytosolic and mitochondrial influx of Ca2+ (Wang et al., 2001; Nilsen and Brinton, 2003, Nilsen et al., 2002; Wang et al., 2006), presumable providing a protection from neurotoxic Ca2+ influx. Comparable effects on mitochondrial stability and function have been reported by Morin et al. (2002) who studied the ability of 17 α-estradiol, an isomer of 17 β-estradiol that is equipotent as a cytoprotectant yet at least 200-fold less active than as a hormone (Littlefield et al., 1990) to maintain respiratory coupling after imposed ischemia reoxygenation.
Diminution of NMDA-mediated Ca2+ influx could also be due to allosteric regulation via the redox status of the receptor per se (Choi and Lipton, 2000). In any event, we assessed the effects of mitochondrial toxins on cytosolic and mitochondrial loading of Ca2+ and determined the dose dependence of estrogen protection from these effects (Wang et al., 2001). 3-NPA caused a rapid and profound increase in cytosolic Ca2+ concentrations. Pretreatment with E2 dose-dependently reduced the influx of Ca2+ into the cytosol. Similarly, 3NPA caused a rapid and 3-fold influx of Ca2+ into the mitochondria, an effect that was dose dependently reduced by E2. We have observed essentially the same protection of cytosolic and mitochondrial Ca2+ levels by E2 when H2O2 was used as a pro-oxidant mitochondrial toxin (Wang et al., 2006). Furthermore, non-feminizing estrogens were as effective as E2 in preventing mitochondrial Ca2+ influx (Wang et al., 2006). To some extent, such repression of Ca2+ mobilization could be attributable to the aforementioned preservation of ATP, which would serve to fuel ER uptake and cellular extrusion via Ca2+-ATPases. In view of the observation that sustained increases in mitochondrial Ca2+ impair oxidative phosphorylation and cause an increase in ROS, our observations suggest that the Ca2+ modulating effects of estrogens may serve to protect ATP production and thereby neuronal viability.
Mitochondrial membrane potential (Δψm) collapse is a critical event in the life-death decision of neurons (Dykens, 1997, 1995; Kroemer and Reed, 2000; Zou et al., 1997; Murphy, 1999). We used two methods to determine the effects of mitochondrial toxins and of E2 on Δψm in neuronal cultures. First, analysis of rhodamine 123, a mitochondrial specific dye, demonstrated that 3NPA caused mitochondrial depolarization and this effect of the mitochondrial toxin was antagonized by E2 pre-treatment (Wang et al., 2001). Similarly, using a FRET assay to measure Δψm (Dykens and Stout, 2001), we observed that treatment with either E2 or its diasteriomer, 17 α-estradiol (17 α-E2) increased the Ca2+ concentration required to cause Δψm collapse (Dykens et al., 2003). This increase in the EC50 of Ca2+ could be due to a partial resistance of all mitochondria to Ca2+ or complete resistance of a subpopulation of mitochondria in the presence of estrogens. Collectively, these data indicate that estrogens protect mitochondria by preventing mitochondrial membrane potential collapse. This could explain the described ability of estrogens to prevent the release from mitochondria of apoptotic factors (Green and Kroemer, 2004) that is dependent on Δψm collapse. This role of estrogens is even more important, given our preliminary studies that suggest that ERβ could function as a mitochondrial component regulating membrane potential maintenance (Simpkins and Yang, unpublished data).
A critical test of the role played by mitochondrial actions of estrogens in their ability to neuroprotect is to define a correlation between the potency of compounds in assays of neuroprotection and mitoprotection. If the neuroprotective effects of estrogens are mediated by a mitochondrial action, the two parameters ought to correlate strongly. We tested the relationship between the neuroprotective activity of estrogens and their ability to moderate Δψm collapse induced by Ca2+ loading of HT-22 cells in culture. Ten estrogen analogues that ranged in neuroprotective potency (ED50) from 20 nM to 8.6 μM (essentially ineffective in cytoprotection assays) were selected for comparison (Dykens et al, 2003). The correlation between ED50 values for neuroprotection and the ED50 values for Ca2+- induced Δψm collapse was highly significant (r2=0.73, Spearman r= −0.9387, p<0.0001) (Dykens et al., 2003). The significant correlation between neuroprotection and mitochondrial protection is particularly impressive in view of the fact that the ED50 values for the two parameters were derived from data generated in two separate laboratories. This strong correlation suggests that the stabilizing effects of these estrogen-like compounds on mitochondrial function explain much of their neuroprotective activity.
6. Mitochondrial Localization of ERβ
Estrogens are known as the major female steroid hormones, which play fundamental role in the female reproductive system. In recently years, estrogens have been appreciated as pleiotropic hormones that play roles in a wide variety of nonreproductive functions as cardiovascular function (Stevenson, 2000), memory and cognition (Sherwin, 1999), bone and mineral metabolism (Compston, 2001), and immune function (Ahmed et al., 1999). As indicated above, there is accumulating evidence suggesting that mitochondria are also important targets for the actions of estrogens (Chen et al., 2005; Felty and Roy, 2005)). Mitochondria play a fundamental role in cell respiration, through oxidative phosphorylation. It also controls ion homeostasis and the synthesis of heme, lipids, amino acids, and nucleotide. Estrogens are highly hydrophobic molecules and are endogenously synthesized in the mitochondria. Given the high hydrophobicity of estrogen molecules and the bilayer membrane structure of mitochondria, it will not be surprising that exogenously added estrogen molecules are also mainly transported to mitochondria (Moats and Ramirez, 1998; Felty and Roy, 2005). Thus, the targeting of estrogen molecules to mitochondria and their identified role on mitochondrial function warrant further research to determine the mechanism underlying the role of estrogens in mitochondrial function.
It is generally accepted that majority of the biological effects of estrogens are mediated via two estrogen receptors: ERα and ERβ (Greene et al., 1986; Kuiper et al., 1996; Mosselman et al., 1996; Tata, 2002). Consistent with their wide biologic role in the variety of system, both ERα and ERβ have been found to be widely distributed in different systems and tissues, including, but not limited to, reproductive system, central nervous system, cardiovascular system, gastrointestinal tract, urogenital tract, bone, and liver (Gustafsson, 1999). Both ERα and ERβ have been widely accepted as transcriptional factors belong to nuclear receptor super-family. Classically, it is believed that estrogens could modulate the expression of nuclear estrogen responsive genes through both ERs. Also, estrogen could elicit rapid, non-nuclear action on a number of biological processes via non-genomic mechanisms mediated by ERs (Levin, 2005). Consistently, extranuclear localization of both ERα and ERβ has been indicated (McEwen et al., 2001; Milner et al., 2005; Herrick et al., 2006). In fact, increasing evidence has demonstrated that ERβ are mainly localized extranuclearly (Milner et al., 2001; Cammarata et al., 2004; Chen et al., 2004a; Yang et al., 2004; Chen et al., 2005; Herrick et al., 2006).
It became clear, not long after its identification, that ERβ has biological roles distinct form those of ERα (Gustafsson, 1999). ERα and ERβ are encoded by separate genes found at different chromosomal locations. While sharing a high homology in both DNA binding domain and ligand binding domain, ERα and ERβ have very low sequence identity in both N- (12%) and C- terminals (9%), correspond to AF1 and AF2 domain, respectively (Ascenzi et al., 2006). Hence, it is not surprising that ERβ has very low classic transcriptional activity, when compared with ERα (Lubahn et al., 1993; Cowley et al., 1997; Pettersson et al., 1997; Ogawa et al., 1998; Cowley and Parker, 1999; Curtis et al., 2000; Yi et al., 2002). There is accumulating evidence suggesting that mitochondria are also important targets for the actions of estrogens (Chen et al., 2005; Felty and Roy, 2005). Mitochondria play a fundamental role in cell respiration and oxidative phosphorylation. It also controls ion homeostasis and the synthesis of heme, lipids, amino acids, and nucleotide. We and several other laboratories have recently reported the localization of ERβ in mitochondria in varies cells, including rat primary neuron (Yang et al., 2004; Chen et al., 2005; Mehra et al., 2005), rat primary cardiomyocyte (Yang et al., 2004), a murine hippocampal cell line (HT-22), neurons and glia in rat hippocampus (Milner et al., 2005; Herrick et al., 2006), human breast cancer lines (MCF-7, MCF-10F) (Chen et al., 2004a; Chen et al., 2005), immortal human breast epithelial cells (HBEC) (Chen et al., 2005), human lens epithelial cell lines (nHLE and HLE-B3) (Cammarata et al., 2004; Cammarata et al., 2005), human osteosarcoma cells (SaOS-2) (Solakidi et al., 2005b), hepatocarcinoma cells (HepG2) (Solakidi et al., 2005a), human sperm (Solakidi et al., 2005b), and periodontal ligament cells (Jonsson, 2007). Similar perinuclear punctate staining of ERβ has been reported in a murine mammary epithelial cell line (HC11) and human fetal cortical neurons (Fried et al., 2004; Helguero et al., 2005). Notably, the localization of ERβ in mitochondria has been demonstrated by immunocytochemistry, immunohistochemistry, immunoblots, using a large group of diversified antibodies. Furthermore, the localization of ERβ in mitochondria has also been verified by proteomics.
7. Mitochondrial Trafficking of ERβ
Mitochondrial activity is dependent on the import and assembly of proteins (Pfanner et al, 2004; Cannino et al, 2007; Neupert and Herrmann, 2007). Nuclear-coded protein expression, including transcription factors and nuclear coactivators, regulates mitochondria biogenesis perhaps through nuclear-mitochondria cross-talk (Cannino et la, 2007). Most mitochondrial proteins are nuclear encoded, synthesized in the cytosol (preproteins), then directed to import receptors on the outer mitochondria surface through NH2-terminal 20–50 amino-acid residues (presequences) (Rapaport, 2003; Pfanner et al, 2004). Others are carrier proteins that contain internal sequences (Endo and Kohda, 2002). Presequence-less proteins are recognized through chaperone recognition sequences. These recognized amino acid sequences are the mechanism of mitochondrial protein trafficking. Import receptors are outer-membrane proteins that span the mitochondria membrane. They are characterized as N-terminally anchored (Tom20, Tom70), tail-anchored (Tom5, Tom6, Tom22, Bcl-2, Bcl-xL, Fis1, VAMP1B), Two TMDs (Fzo1), and beta-barrel (porins, Tom40) (Rapaport, 2003).
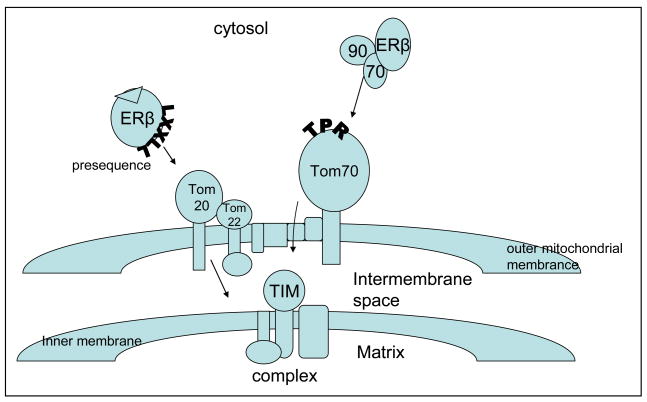
The major protein transport pathways into the mitochondria are the presequence/matrix proteins and the carrier/import of hydrophobic inner membrane proteins (Pfanner et al, 2004; Rapaport, 2005). Most known mitochondrial proteins are imported through the translocase of the outer membrane of mitochondria (TOM) complex. The TOM complex recognizes presequences with both trans and cis binding sites, and translocates prepoteins into/across the mitochondria. This complex is comprised of at least 7 units that include import receptors Tom20, Tom70, Tom22, channel protein Tom40, and small Tom6–7 (Lill et al, 1996; Rapaport, 2005; Neuport and Herrmann, 2007). Tom20 and Tom70 are the principal receptors, and as such differ in their binding functions. The remaining Tom components form the translocation pore (Neuport and Herrmann, 2007). Human Tom22 can complex with Tom20 (Yano, 2000; Rapaport, 2003). Tom20 binds preferentially to presequences of preproteins, while Tom70 binds to internal sequences in membrane proteins (Brix et al, 1997; Ellis, 2003; Neupert and Herrmann, 2007) (Figure 1).
Figure 1. Tom20/22 and Tom70 receptors potential targeting of ERβ.
A simplistic diagram of potential ERβ mitochondrial import through Tom receptor mediated activity. The Tom complex can recognize protein such as ERβ through two major receptor transport pathways, Tom20/Tom22 or Tom70. Tom20 with Tom22 can recognize a LXXLL presequences available in ligand bound ERβ (N-terminal or internal), then bind and transport ERβ across the outer membrane to the inner membrane or TIM complex. Alternatively, unliganded ERβ can be recognized by Tom70 through its chaparone complex, Hsp90/70. Hsp90 and Hsp70 can target proteins without a presequence by docking to tetraticopeptide repeat motifs (TRP) of Tom20 before ultimate transport to the TIM complex.
Tom20 and 22 function as preprotein receptors and contain domains that are exposed to the cytosol. While both are involved in translocation of proteins with classical N-terminus presequences, Tom20 also recognizes internal targeting signals (Brix et al, 1997; Neupert and Herrmann, 2007). When Tom20 recognizes a targeting sequence on the precursor proteins it binds them, then transfers them to a core complex. The core complex in turn translocates them across the outer membrane (Neupert and Herrmann, 2007). From there, proteins are translocated to sorting and assembly pathway/outer membrane proteins (SAM complex) or translocases of the inner membrane (TIM complex) (Pfanner et al, 2004; Neupert and Herrman, 2007). Studies indicate that both Tom20 and 22 recognize a consensus motif or presequence represented by ϕXXϕϕ (where ϕ is a hydrophobic amino acid such as leucine, isoleucine, phenylalanine, tryptophan, valine, and tyrosine, and × is any amino acid) (Pfanner, 2000; Mukhopadhyay et al, 2006; Saitoh et al, 2007). While peptide mobility within the binding grove of Tom20 also allows binding of alternative sequences, there is a hydrophobic preference for LXXLL (Chou et al, 2006; Mukhopadhyay et al, 2006; Neupert and Herrmann, 2007; Saitoh et al, 2007).
Tom70 is considered the receptor for presequence-less inner membrane proteins and cytosolic chaperone Hsp70. Studies suggest that Tom70 may be both a preprotein receptor and a cochaperone in mitochondria protein targeting (Young et al, 2003a, 2003b; Ellis, 2003). Some mitochondria directed proteins must adopt conformations, and then easily unfold to allow for movement through outer and inner membranes (Ellis, 2003). Heat shock proteins or molecular chaperones (Hsp90, Hsp70) are constitutively expressed and assist in (i) normal folding of polypeptides, (ii) miss-folded proteins attaining or regaining native status, (iii) regulating protein degradation and/or (iv) protein translocation to different cellular compartments (Hartl and Hayer-Hartl, 2002; Arya et al, 2007). They have vital roles in both intrinsic and extrinsic pathways involved in cell survival and/or death responses (Arya et al, 2007). Both Hsp90 and Hsp70 bind unfolded or hydrophobic preproteins and deliver them to Tom70 through tetratricopeptide repeat (TPR) motifs (Young et al, 2003b; Neupert and Herrmann, 2007). TPR are similar to cofactors of Hsp90 and Hsp70 (Young et al, 2003b; Smith, 2004). Hsp90 may facilitate translocation in addition to targeting (Young et al, 2003a; Fan et al, 2006). The Tom70 receptor recognizes preproteins with internal targeting sequences, including multiple targeting signals throughout a polypeptide sequence (Wiedemann et al, 2001; Neuport and Herrmann, 2007; Bolender et al, 2008).
Studies support mitochondrial trafficking of an increasing number of nuclear receptors and transcription factors including ERα, ERβ, thyroid hormone receptor, glucocorticoid receptor, Nur 77, PPARgamma 2, RXR, RAR, AR, A-RAF, telomerase, Connexin 43, AP1, CREB, NF-kB, p53, c-Myc, HMGA1, TFAM, TFB1M, and TFB2M (Rodrigues-Sinovas et al, 2006; Yuryev et al, 2000; Santos et al, 2006; Hammes and Levin, 2007; Lee et al, 2007; Psarra and Sekeris, 2008). How they are targeted is not discerned, but most contain at least one F/LXXLL motif and/or are bound by Hsp90/70 in the cytosol. Unliganded ERβ, as a member of the nuclear receptor family, is regulated through a molecular chaperone-complex that includes Hsp90 and Hsp70 (Cheung and Smith, 2000; Gougelet et al, 2005). As a client protein, Hsp90 helps maintain the receptor in a quiescent state by binding the ligand binding domain, and thereby assists in normal folding to maintain its native status and prevents degradation (Smith et al, 1998; Cheung and Smith, 2000; Pratt and Toft, 2003). Hsp90/70 complex through dynein or cytokeratins can facilitate cytoplasmic movement of ERβ to the mitochondria membrane where either Hsp can dock through a TPR of Tom70 (Pratt and Toft, 2003).
ERβ contains three LXXLL and one FXXLL motifs in the ligand binding domain that mediate binding to co-activator proteins through the same motifs. Interaction between bound ERβ and co-activator can be strict and ligand dependent (Chang et al, 1999; Lee et al, 2007; Psarra and Sekeris, 2008). Saitoh et al propose that even though these peptide sequences or motifs are similar, their recognition modes are different. Based on structural comparisons using an ERα LXXLL motif, they show a strict recognition due to lock-and-key mechanism, while Tom20 likely uses an induced fit mechanism (Saitoh et al, 2007). However, they acknowledge that their analysis could not fully account for the hydrophobic preference at the three Ls, and proposed a multiple-mode recognition model involving Tom20 with Tom22 (Saitoh et al, 2007). NMR and mutation studies with rat liver precursor aldehyde dehydrogenase (pALDH) leader sequence (LSRLL), precursor ornithine transcarbamoylase (pOTC) leader sequence (LRILL), and COX8 leader sequences (LTPLLLRGL) determined that LXXLL motifs with leusine residues are necessary to Tom20 binding (Haggie and Verkman, 2002; Mukhopadhyay et al, 2006; Saitoh et al, 2007). Further, COX8 and pOTC leader sequences are use to directly target expression of certain proteins to the mitochondria for functional studies (Psarra and Sekeris, 2008). Computer analysis has also identified a putative internal targeting sequence in ERβ that included a reverse LXXLL motif (LLDAL) (Chen et al, 2004a). Interestingly, many recognized mitochondrial proteins such as p53, Bcl2, CREB, and connexin 43 have their own possible presequence-like motifs (LWKLL). Hsp90 and 70 also contain motifs based on human peptide sequences. Our studies indicate ERβ, along with Tom20 and/or Hsp90 are localized to the mitochondria (Figure 2). Since studies purport that ER functions in the mitochondria, then ERβ should be targeted to either Tom20 or Tom70 receptors (Hammes and Levin, 2007). Receptor preference may depend on ligand induced conformational change in the receptor, and studies show that estrogen increases mitochondria localization of ERβ (Chen et al, 2004a). At the mitochondria, ERβ has at least three alternative ways of targeting; (i) through Tom70 at Hsp70/Hsp90 when unbound, (ii) through Tom20/Tom22 at the LXXLL motif (a putative presequence or internal sequence) when ligand bound, and (iii) Tom70 at Hsp70 through an internal sequence when unbound. The dynamic state of ERβ with and without ligand may facilitate its trafficking based on hydrophobic interactions to and/or into mitochondria. Perhaps the small-world properties of protein-protein complexes based on hydrophobic interactions are mitigated by oxidative stress, whereby one interaction is promoted, while another is abated during normal or stress conditions (Chang et al, 2008).
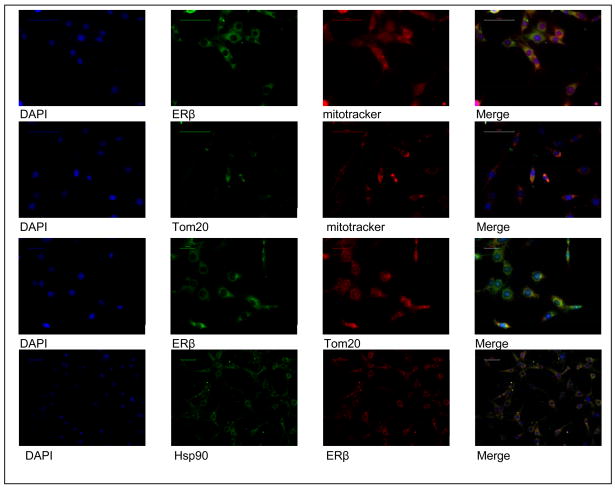
Figure 2. The mitochondrial distribution of ERβ shows co-localization with Tom20 and/or Hsp90.
Dual immunofluorescent staining was performed in HT22 cells using mitotracker with either anti-ERβ, Tom20, or Hsp90. Fluorescent microscopy of HT22 cells stained for anti-Tom20, Hsp90 and/or ERβ also indicated that both Tom20 and Hsp90 associated with each other and with ERβ at the mitochondria.
8. ERβ as a Mitochondrial Transcriptional Factor
The localization of ERβ and the targeting of estrogen molecules to mitochondria suggest that estrogen could modulate mitochondrial function through a mitochondrial genomic mechanism mediated by ERβ. The notion that mitochondrial genes as sites of action of steroid hormones is not new, the effects of steroids on mitochondria energy metabolism have been the object of intensive research back to 1970s.
Given its own genome in mitochondrial, we should be not surprise that some of the nuclear receptors have also been found in mitochondria, including ERβ (Psarra and Sekeris, 2008). The crystal structure of ERβ has been well demonstrated. Without any doubt, ERβ shares a highly conserved structure with other nuclear receptors, which has a typical nuclear receptor structure with DNA binding domain and ligand binding domain. Indeed, recently studies demonstrated that ERβ is localized in the mitochondrial matrix, hence enables its access to the mitochondrial genome. Therefore, both its structure and matrix localization provide ERβ the capacity to regulate mitochondrial gene expression.
Mitochondrial DNA (mtDNA) an intronless, circular genome of 16.5 kb, which is maternally inherited and encodes 37 genes of the approximately 3000 proteins in the mitochondrial proteome. Of these 13 codes for proteins that serve in the electron transport system, with the remainder encoding for elements required for expression. In addition to mRNA molecules, the mitochondrial genome also encodes 2 ribosomal RNAs and 22 transfer RNAs (Falkenberg et al., 2007).
Although ERα and ERβ have nearly identical DNA-binding domains, increase evidence has indicated that they regulate a total distinct set of gene expression (Katzenellenbogen and Katzenellenbogen, 2000; O’Lone et al., 2007). Currently, most of the studies have been focused on the nuclear transcription regulation. Consistently, most of the genes regulated by ERβ are mitochondrial structure proteins related to oxidative phosphorylation (O’Lone et al., 2007). This distinction could be partly due to the recruitment of different coactivators and adaptor proteins, which play roles both in ERs binding and transcriptional activation. Furthermore, different compartment of ERα and ERβ could also contribute to this distinction. The mitochondrial localization could enable ERβ to directly regulate a total different set of mitochondrial gene from the nuclear gene regulate by ERα, or indirectly affect nuclear-coded mitochondrial target genes by its action on mitochondrial function.
The stimulation of target gene expression in response to the action of ERs is thought to be mediated through the direct binding of ERs to a specific sequence called an estrogen response element (ERE) and interacts directly with coactivator proteins and components of the RNA polymerase II transcription initiation complex. Both ERα and ERβ bind with high affinity to EREs. Consistently, sequences showing partial similarity to ERE consensus sequence have been detected in the mitochondrial genome (Demonacos et al., 1996). In addition, ERs have been shown to mediate transcription through non-classical mechanisms via other DNA binding elements and potentially involving other transcription factors such as AP-1, NF-κB, and cAMP response element-binding protein, which have been found in mitochondria (Demonacos et al., 1996). Transcription factor search (TF search, Heinemeyer, 1998) of human mitochondrial genome (GenBank no. 1705226) revealed that there are at least two 86% homologous CREB sequences present in the genome (Sarkar et al, 2008, unpublished observation). Consistently, estrogens have been found to increase expression of mitochondrial coded cytochrome c oxidase subunits I, II, and II (Chen et al., 2004b; Chen et al., 2005; Hsieh et al., 2006; Nilsen et al., 2007; Yager and Chen, 2007).
The biogenesis of the mitochondrial oxidative phosphorylation system depends not only on the mitochondrial genomes, but also the nuclear genomes. In fact, mitochondrial genome only encodes 13 genes of the mitochondrial oxidative phosphorylation system, with all others encoded by nuclear genome. Therefore, the biogenesis of mitochondria depends on the coordinated expression of two genomes, nuclear and mitochondrial (Garesse and Vallejo, 2001). The structure of ERβ could enable it to track between nucleus and mitochondria, hence orchestra the function of mitochondria through its genomic action. This notion is further suggested by a recent study, which found shuttling of ERβ between mitochondria and nucleus (Chen et al., 2007).
Transgenic mice are a powerful tool to delineate function of various proteins. Five mouse lines lacking ERβ have been produced, but there are discrepancies over phenotypes (Harris, 2007; Antal et al., 2008). The βERKOs produced in Chapel Hill (βERKOCH), Strasbourg (βERKOST), and Wyeth (βERKOWYE) observed similar phenotype, whereas the colony of βERKOCH mice that were subsequently established at the Karolinska Institute exhibited a different phenotype (Harris, 2007). Even more remarkably, the most recently generated βERKO mice demonstrate sterility in both male and females, which is not consistent with previous reports describing the βERKO phenotype (Antal et al., 2008). Although the different phenotypes could be due to the different transcript variants, encoding putative truncated forms of ERβ in these βERKO mice lines, the effect of ERβ on embryonic development can not be ruled out due to the conventional knockout approach.
9. Conclusions and Future Research Directions
Estrogens are potent neuroprotective and mitoprotective agents. In addition to being produced locally in the mitochondria, estrogens are transported to mitochondria, where they can interact with imported ERβ, to effect gene transcription through yet unknown mechanisms. The relative proportion of estrogen’s mitoprotective effects that are mediated by an antioxidant redox cycling of the steroid (Prokai et al., 2003) versus through ERβ mediated mitochondrial transcriptional effects is currently unknown and the subject of future research. In either case, estrogen use is indicated for the treatment of mitochondrial related chronic neurodegenerative diseases and more acute nerve cell insults that compromise mitochondrial function.
Further studies using reversible knockout or knockdown approaches could provide valuable information to decipher the function of ERβ. Given the potent effects of estrogens on mitochondrial function, the localization of ERβ in this vital organ, and the role of mitochondria in life-death decisions of neurons, there is strong evidence to support the need for the further development of reagents, animal models to enhance future ERβ research with a focus on mitochondrial function.
Acknowledgments
This work was supported in part by NIH grants P01 AG010485, P01 AG022550 and P01 AG027956.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- Ahmed SA, Hissong BD, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E. Environ Health Perspect. 1999;107 Suppl 5:681–686. doi: 10.1289/ehp.99107s5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev G, Smith MA, Seyidov D, Neal ML, Lamb BT, Nunomura A, Gasimov EK, Vinters HV, Perry G, LaManna JC, Friedland RP. Brain Pathol. 2002;12:21–35. doi: 10.1111/j.1750-3639.2002.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M. Proc Natl Acad Sci U S A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya R, Mallik M, Lakhotia SC. J Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- Ascenzi P, Bocedi A, Marino M. Mol Aspects Med. 2006;27:299–402. doi: 10.1016/j.mam.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Beal MF. Ann NY Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- Betts J, Lightowlers RN, Turnbull DM. Neurochem Res. 2004;29:505–511. doi: 10.1023/b:nere.0000014821.07269.8d. [DOI] [PubMed] [Google Scholar]
- Blass JP. Neurol Res. 2003;25:556–566. doi: 10.1179/016164103101201995. [DOI] [PubMed] [Google Scholar]
- Bolender N, Sickmann A, Wagner R, Meisinger C, Pfanner EMBO Reports. 2008;9:42–49. doi: 10.1038/sj.embor.7401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Neurobiol Aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Learn Mem. 2001;8:121–33. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Brix J, Dietmeier K, Pfanner N. J Biol Chem. 1997;33:20730–20735. doi: 10.1074/jbc.272.33.20730. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Neuron. 2002;33:677–688. doi: 10.1016/s0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- Cammarata PR, Chu S, Moor A, Wang Z, Yang SH, Simpkins JW. Exp Eye Res. 2004;78:861–871. doi: 10.1016/j.exer.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Cammarata PR, Flynn J, Gottipati S, Chu S, Dimitrijevich S, Younes M, Skliris G, Murphy LC. Exp Eye Res. 2005;81:165–175. doi: 10.1016/j.exer.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Canevari L, Abramov AY, Duchen MR. Neurochem Res. 2004;29:637–650. doi: 10.1023/b:nere.0000014834.06405.af. [DOI] [PubMed] [Google Scholar]
- Cannino G, Di Liegro CM, Rinaldi AM. Mitochondrion. 2007;7:359–366. doi: 10.1016/j.mito.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Cash AD, Perry G, Ogawa O, Raina AK, Zhu X, Smith MA. Neuroscientist. 2002;8:489–496. doi: 10.1177/107385802236968. [DOI] [PubMed] [Google Scholar]
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Prog Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Chang CY, Norris JD, Gron H, Paige LA, Hamilton PT, Kenan DJ, Fowlkes D, McDonnell DP. Mol Cell Biol. 1999;19:8226–8239. doi: 10.1128/mcb.19.12.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Jiao X, Li C-H, Gong X-Q, Chen W-Z, Wang C-X. Biophysical Chem. 2008 doi: 10.1016/j.bpc.2007.12.005. BIOCHEM-05052. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Delannoy M, Cooke C, Yager JD. Am J Physiol Endocrinol Metab. 2004a;286:E1011–E1022. doi: 10.1152/ajpendo.00508.2003. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Eshete M, Alworth WL, Yager JD. J Cell Biochem. 2004b;93:358–373. doi: 10.1002/jcb.20178. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Yager JD, Russo J. Biochim Biophys Acta. 2005;1746:1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Russo PA, Cooke C, Russo IH, Russo J. Biochim Biophys Acta. 2007;1773:1732–1746. doi: 10.1016/j.bbamcr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Cheung J, Smith DF. Mol Endo. 2000;14:939–946. doi: 10.1210/mend.14.7.0489. [DOI] [PubMed] [Google Scholar]
- Choi YB, Lipton SA. Cell Mol Life Sci. 2000;11:1535–1541. doi: 10.1007/PL00000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CH, Lee RS, Yang-Yen HF. Mol Biol Cell. 2006;17:3952–3963. doi: 10.1091/mbc.E06-04-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston JE. Physiol Rev. 2001;81:419–447. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- Cowley SM, Parker MG. J Steroid Biochem Mol Biol. 1999;69:165–175. doi: 10.1016/s0960-0760(99)00055-2. [DOI] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- Cottrell DA, Blakely EL, Johnson MA, Ince PG, Turnbull DM. Neurology. 2001;57:260–264. doi: 10.1212/wnl.57.2.260. [DOI] [PubMed] [Google Scholar]
- Curtis HS, Couse JF, Korach KS. Breast Cancer Res. 2000;2:345–352. doi: 10.1186/bcr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonacos CV, Karayanni N, Hatzoglou E, Tsiriyiotis C, Spandidos DA, Sekeris CE. Steroids. 1996;61:226–232. doi: 10.1016/0039-128x(96)00019-0. [DOI] [PubMed] [Google Scholar]
- Dong L, Wang W, Wang F, Stoner M, Reed JC, Harigai M, Samudio I, Kladde MP, Vyhlidal C, Safe S. J Biol Chem. 1999;274:32099–32107. doi: 10.1074/jbc.274.45.32099. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens JA. J Neurochem. 1994;63:584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- Dykens JA. In: The Oxygen Paradox. Davies KJA, Ursini F, editors. Cleup Press; U. of Padova: 1995. pp. 453–467. [Google Scholar]
- Dykens JA. In: Neurodegenerative Diseases: Mitochondria and Free Radicals in Pathogenesis. Beal MF, Bodis-Wollner I, Howell N, editors. John Wiley & Sons; 1997. pp. 29–55. [Google Scholar]
- Dykens JA. In: Cell Death and Diseases of the Nervous System. Koliatos VE, Ratan VV, editors. Humana Press; New Jersey: 1999. pp. 45–68. [Google Scholar]
- Dykens JA. RedOx targets: enzyme systems and drug development strategies for mitochondrial dysfunction. In: Triggle DJ, Taylor JB, editors. Comprehensive Medicinal Chemistry II. Elsevier; Oxford: 2007. pp. 1053–1087. [Google Scholar]
- Dykens JA, Stout AK. Methods Cell Biol. 2001;65:285–309. doi: 10.1016/s0091-679x(01)65018-0. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Simpkins JW, Wang J, Gordon K. Exp Gerontol. 2003;38:101–107. doi: 10.1016/s0531-5565(02)00162-6. [DOI] [PubMed] [Google Scholar]
- Ellis JR. Nature. 2003;421:801–802. doi: 10.1038/421801a. [DOI] [PubMed] [Google Scholar]
- Endo T, Kohda D. Biochim Biophys Acta. 2002;1592:3–14. doi: 10.1016/s0167-4889(02)00259-8. [DOI] [PubMed] [Google Scholar]
- Falkenberg M, Larsson NG, Gustafsson CM. Ann Rev Biochem. 2007;76:679–699. doi: 10.1146/annurev.biochem.76.060305.152028. [DOI] [PubMed] [Google Scholar]
- Fan ACY, Bhangoo MK, Young JC. J Biol Chem. 2006;281:33313–33324. doi: 10.1074/jbc.M605250200. [DOI] [PubMed] [Google Scholar]
- Felty Q, Roy D. J Carcinog. 2005;4:1. doi: 10.1186/1477-3163-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J. Eur J Neurol. 2004;11:163–186. doi: 10.1046/j.1351-5101.2003.00728.x. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Fried G, Andersson E, Csoregh L, Enmark E, Gustafsson JA, Aanesen A, Osterlund C. Eur J Neurosci. 2004;20:2345–2354. doi: 10.1111/j.1460-9568.2004.03693.x. [DOI] [PubMed] [Google Scholar]
- Garesse R, Vallejo CG. Gene. 2001;263:1–16. doi: 10.1016/s0378-1119(00)00582-5. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Swerdlow RH, Miller SW, Sheeman B, Parker WD, Jr, Davis RE. Ann NY Acad Sci. 1999;893:176–191. doi: 10.1111/j.1749-6632.1999.tb07825.x. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP. J Neural Transm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- Gougelet A, Bouclier C, Marsaud V, Maillard S, Mueller SO, Korach KS, Renoir JM. J Steroid Biochem Mol Biol. 2005;94:71–81. doi: 10.1016/j.jsbmb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Int J Develop Neurosci. 2000a;18:347–358. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Ann NY Acad Sci. 2000b;924:93–98. doi: 10.1111/j.1749-6632.2000.tb05566.x. [DOI] [PubMed] [Google Scholar]
- Green PS, Gridley KE, Simpkins JW. Neurosci Let. 1996;218:165–168. doi: 10.1016/s0304-3940(96)13148-7. [DOI] [PubMed] [Google Scholar]
- Green PS, Bishop J, Simpkins JW. J Neurosci. 1997a;17:511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Gordon K, Simpkins JW. J Steroid Biochem Mol Biol. 1997b;63:229–235. doi: 10.1016/s0960-0760(97)00124-6. [DOI] [PubMed] [Google Scholar]
- Green PS, Perez EJ, Calloway T, Simpkins JW. J Neurocytology. 2000;29:419–423. doi: 10.1023/a:1007173509470. [DOI] [PubMed] [Google Scholar]
- Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. Endocrinol. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Gridley KE, Green PS, Simpkins JW. Mol Pharm. 1998;54:874–880. doi: 10.1124/mol.54.5.874. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Cutting FB, Zhai P, Andrus PK, Hall ED. Pathol Biol (Paris) 1996;44:51–56. [PubMed] [Google Scholar]
- Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW. Ann Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- Haggie PM, Verkman AS. J Biol Chem. 2002;277:40782–40788. doi: 10.1074/jbc.M207456200. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Endocrine Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Harris HA. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- He Z, He Y-J, Day AL, Simpkins JW. J Neurol Sci. 2002;193:79–87. doi: 10.1016/s0022-510x(01)00648-7. [DOI] [PubMed] [Google Scholar]
- Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, Ignatieva EV, Podkolodnaya OA, Kolpakov FA, Podkolodny NL, Kolchanov NA. Nucleic Acids Res. 1998;26:364–370. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helguero LA, Faulds MH, Gustafsson JA, Haldosen LA. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Brain Res. 2006;1121:46–58. doi: 10.1016/j.brainres.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YC, Yu HP, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. J Mol Cell Cardiol. 2006;41:511–521. doi: 10.1016/j.yjmcc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Yao J, Hamilton R, Cadenas E, Brinton RD, Nilsen J. Endocrinol. 2008 doi: 10.1210/en.2007–1227. [DOI] [Google Scholar]
- Jonsson D, Nilsson J, Odenlund M, Bratthall G, Broman J, Ekblad E, Lydrup ML, Nilsson BO. Arch Oral Biol. 2007;52:669–676. doi: 10.1016/j.archoralbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Katzenellenbogen JA. Breast Cancer Res. 2000;2:335–344. doi: 10.1186/bcr78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ. Ann NY Acad Sci. 1997;826:218–228. doi: 10.1111/j.1749-6632.1997.tb48473.x. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sharma S, Kim J, Ferrante RJ, Ryu H. J Neuro Res. 2007;86:961–971. doi: 10.1002/jnr.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ, Qian T, Bradham CA, Brenner DA, Cascio WE, Trost LC, Nishimura Y, Nieminen AL, Herman B. J Bioenerg Biomembranes. 1999;31:305–319. doi: 10.1023/a:1005419617371. [DOI] [PubMed] [Google Scholar]
- Levin ER. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R, Nargang FE, Neupert W. Current Opin Cell Biol. 1996;8:505–512. doi: 10.1016/s0955-0674(96)80028-7. [DOI] [PubMed] [Google Scholar]
- Littlefield BA, Gurpide E, Markiewicz L, McKinley B, Hochberg RB. Endocrinology. 1990;127:2757–2762. doi: 10.1210/endo-127-6-2757. [DOI] [PubMed] [Google Scholar]
- Liu R, Yang SH, Perez E, Yi KD, Wu SS, Eberst K, Prokai L, Prokai-Tatrai K, Cai ZY, Covey DF, Day AL, Simpkins JW. Stroke. 2002;33:2485–2491. doi: 10.1161/01.str.0000030317.43597.c8. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- McEwen BS. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Proc Natl Acad Sci U S A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Moats RK, 2nd, Ramirez VD. Biol Reprod. 1998;58:531–538. doi: 10.1095/biolreprod58.2.531. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. Proc Natl Acad Sci U S A. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli A, Giacchetti M, De Biase I, Pianese L, Turano M, Pandolfo M, Cocozza S. Hum Genet. 2004;114:458–463. doi: 10.1007/s00439-004-1089-7. [DOI] [PubMed] [Google Scholar]
- Morin C, Zini R, Simon N, Tillement JP. Neurosci. 2002;115:415–424. doi: 10.1016/s0306-4522(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Mukai K, Daifuku K, Yokoyama S, Nakano M. Biochim Biophys Acta. 1990;1035:348–352. doi: 10.1016/0304-4165(90)90099-i. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Yang Chun-song, Weiner H. Protein Sci. 2006;15:2739–2748. doi: 10.1110/ps.062462006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WE, Kirsch C, Eckert GP. Biochem Soc Trans. 2001;29:617–623. doi: 10.1042/bst0290617. [DOI] [PubMed] [Google Scholar]
- Murphy AN. Ann NY Acad Sci. 1999;893:19–32. doi: 10.1111/j.1749-6632.1999.tb07815.x. [DOI] [PubMed] [Google Scholar]
- Murphy AN, Fiskum G, Beal MF. J Cereb Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Neupert W, Herrmann JM. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Proc Natl Acad Sci U S A. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Irwin RW, Gallaher TK, Brinton RD. J Neurosci. 2007;27:14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, Schapira AH. Am J Med Genet. 2001;106:27–36. doi: 10.1002/ajmg.1425. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Endocrinol. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Mol Endocrinol. 2007;21:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- Psarra AM, Sekeris CE. Biochim Biophys Acta. 2008;1783:1–11. doi: 10.1016/j.bbamcr.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Pfanner N. Curr Biol. 2000;10:R412–R415. doi: 10.1016/s0960-9822(00)00507-8. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Wiedeman N, Meisinger C, Lithgow T. Nat Struct Mol Biol. 2004;11:1044–1048. doi: 10.1038/nsmb852. [DOI] [PubMed] [Google Scholar]
- Pike CJ. J Neurochem. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Cole WC, Prasad KC. J Am Coll Nutr. 2002;21:506–522. doi: 10.1080/07315724.2002.10719249. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova AD, Perez E, Liu R, Simpkins JW. Proc Natl Acad Sci. 2003;100:11741–11746. doi: 10.1073/pnas.2032621100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psarra AMG, Sekeris CE. Biochim Biophys Acta. 2008;1783:1–11. doi: 10.1016/j.bbamcr.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Rapaport D. Embo Reports. 2003;4:948–952. doi: 10.1038/sj.embor.embor937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D. J Cell Biol. 2005;171:419–423. doi: 10.1083/jcb.200507147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan RF, Guo Y. Brain Res. 1997;764:133–140. doi: 10.1016/s0006-8993(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Rifici VA, Khachadurian AK. Metabolism. 1992;4:1110–1114. doi: 10.1016/0026-0495(92)90295-l. [DOI] [PubMed] [Google Scholar]
- Rodriques-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, Konietzka I, Miro E, Totzeck A, Heusch G, Schulz R, Garcia-Dorado D. Circulation Res. 2006:93–101. doi: 10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sack MN, Rader DJ, Cannon RO. Lancet. 1994;343:269–270. doi: 10.1016/s0140-6736(94)91117-7. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Igura M, Obita T, Ose T, Kojima R, Maenaka K, Endo T, Kohda D. The Embo J. 2007;26:4777–4787. doi: 10.1038/sj.emboj.7601888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampei K, Goto S, Alkayed NJ, Crain BJ, Korach KS, Traystman RJ, Demas GE, Nelson RJ, Hurn PD. Stroke. 2000;31:738–743. doi: 10.1161/01.str.31.3.738. [DOI] [PubMed] [Google Scholar]
- Santos JH, Meyer JN, Houten BV. Human Molecular Genetics. 2006;15:1757–1768. doi: 10.1093/hmg/ddl098. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Huang RQ, Lodan S, Dillon GH, Simpkins JW. Soc Neurosci. 2006 Abstracts.-update. [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. J Neurosci Res. 1998;54:707–719. doi: 10.1002/(SICI)1097-4547(19981201)54:5<707::AID-JNR16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Biochim Biophys Acta. 1998;1366:225–233. doi: 10.1016/s0005-2728(98)00115-7. [DOI] [PubMed] [Google Scholar]
- Schapira A, Lodi R. Methods Mol Biol. 2004;277:293–307. doi: 10.1385/1-59259-804-8:293. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Gu M, Taanman JW, Tabrizi SJ, Seaton T, Cleeter M, Cooper JM. Ann Neurol. 1998;44:S89–S98. doi: 10.1002/ana.410440714. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. J Psychiatry Neurosci. 1999;24:315–321. [PMC free article] [PubMed] [Google Scholar]
- Shi J, Bui JD, Yang SH, Lucas TH, Buckley DL, Blackband SP, King MA, Day AL, Simpkins JW. Stroke. 2001;32:987–992. doi: 10.1161/01.str.32.4.987. [DOI] [PubMed] [Google Scholar]
- Shoffner JM. Neurogenetics. 1997;1:13–19. doi: 10.1007/s100480050002. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CH, Bodor N, Day AL. J Neurosurg. 1997b;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Dorsa DM. NeuroReport. 1998;9:2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Strickland TM, Dorsa DM. Neurosci Lett. 1996;212:13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF. Cell Stress & Chapersones. 2004;09:109–121. doi: 10.1379/CSC-31.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Katsanis E. Pharmacol Rev. 1998;50:493–514. [PubMed] [Google Scholar]
- Smith MA, Nunomura A, Zhu X, Takeda A, Perry G. Antioxid Redox Signal. 2000;2:413–420. doi: 10.1089/15230860050192198. [DOI] [PubMed] [Google Scholar]
- Solakidi S, Psarra AM, Sekeris CE. Biochim Biophys Acta. 2005a;1745:382–392. doi: 10.1016/j.bbamcr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE. Hum Reprod. 2005b;20:3481–3487. doi: 10.1093/humrep/dei267. [DOI] [PubMed] [Google Scholar]
- Stevenson JC. J Steroid Biochem Mol Biol. 2000;74:387–393. doi: 10.1016/s0960-0760(00)00117-5. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Arch Pathol Lab Med. 2002;126:271–280. doi: 10.5858/2002-126-0271-MDRMDI. [DOI] [PubMed] [Google Scholar]
- Tata JR. Nat Rev Mol Cell Biol. 2002;3:702–710. doi: 10.1038/nrm914. [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Jr, Miller SW, Davis RE, Parker WD., Jr Exp Neurol. 2000;162:37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Borland MK, Keeney PM, Bennett JP, Jr, Parker WD., Jr J Neurochem. 2004;88:800–812. doi: 10.1046/j.1471-4159.2003.02168.x. [DOI] [PubMed] [Google Scholar]
- Veech GA, Dennis J, Keeney PM, Fall CP, Swerdlow RH, Parker WD, Jr, Bennett JP., Jr J Neurosci Res. 2000;61:693–700. doi: 10.1002/1097-4547(20000915)61:6<693::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- von Lewinski F, Keller BU. Trends Neurosci. 2005;28:494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Green PS, Simpkins JW. J Neurochem. 2001;77:804–811. doi: 10.1046/j.1471-4159.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Simpkins JW, Dykens JA, Cammarata PR. Invest Ophthalmol Vis Sci. 2003a;44:2067–2075. doi: 10.1167/iovs.02-0841. [DOI] [PubMed] [Google Scholar]
- Wang X, Dykens JA, Perez EJ, Zhang X, Simpkins JW. Toxic Soc Neurosci Abstr. 2003b;29:635. [Google Scholar]
- Wang X, Dykens JA, Perez EJ, Liu R, Yang SH, Covey DF, Simpkins JW. Mol Pharmacol. 2006;70:395–404. doi: 10.1124/mol.106.022384. [DOI] [PubMed] [Google Scholar]
- Weaver CE, Jr, Park-Chung M, Gibbs TT, Farb DH. Brain Res. 1997;761:338–341. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- Wiedemann N, Pfanner N, Ryan MT. EMBO J. 2001;20:951–960. doi: 10.1093/emboj/20.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Dubal DB, Wise PM. Brain Res. 2000;873:235–242. doi: 10.1016/s0006-8993(00)02479-3. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW. J Neurocytol. 2000;29:401–410. doi: 10.1023/a:1007169408561. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M, Rosewell KL. Brain Res Brain Res Rev. 2001;37:313–319. doi: 10.1016/s0165-0173(01)00136-9. [DOI] [PubMed] [Google Scholar]
- Yager JD, Chen JQ. Trends Endocrinol Metab. 2007;18:89–91. doi: 10.1016/j.tem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Yang SH, Day AL, Simpkins JW. Stoke. 2000;31:745–749. doi: 10.1161/01.str.31.3.745. [DOI] [PubMed] [Google Scholar]
- Yang SH, He Z, Wu SS, He YJ, Cutright J, Millard WJ, Day AL, Simpkins JW. J Cerebral Blood Flow and Metab. 2001;21:174–181. doi: 10.1097/00004647-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Proc Natl Acad Sci U S A. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Hoogenraad N, Terada K, Mori M. Mol Cell Biol. 2000;20:7205–7213. doi: 10.1128/mcb.20.19.7205-7213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi P, Bhagat S, Hilf R, Bambara RA, Muyan M. Mol Endocrinol. 2002;16:1810–1827. doi: 10.1210/me.2001-0323. [DOI] [PubMed] [Google Scholar]
- Young JC, Barral JM, Hartl FU. Trends Biochem Science. 2003a;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU. Cell. 2003b;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- Yuryev A, Ono M, Goff SA, Macaluso F, Wennogle LP. Mol Cell Biol. 2000;20:4870–4878. doi: 10.1128/mcb.20.13.4870-4878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. J Steroid Biochem Mol Biol. 1999a;68:65–75. doi: 10.1016/s0960-0760(98)00161-7. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. Eur J Pharmacol. 1999b;368:95–102. doi: 10.1016/s0014-2999(99)00012-6. [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]