Abstract
Introduction
Hemorrhagic transformation (HT) is a major factor limiting the use of tissue plasminogen activator (tPA) for stroke patients. This study examined the role of deferoxamine (DFX) in brain injury and HT in a rat model of transient focal ischemia with hyperglycemia.
Methods
Rats received an injection of 50% glucose (6 mL/kg, i.p.) 15 minutes before undergoing transient middle cerebral artery occlusion (tMCAO; two hours occlusion) with reperfusion. Rats were treated with DFX (100mg/ kg, i.m.) or vehicle immediately after tMCAO. Rats were killed at 4, 8 and 24 hours later and used for brain edema, blood-brain barrier permeability, hemorrhage volume, hemoglobin content, and infarct volume measurements. Mortality rate was also evaluated.
Results
DFX treatment reduced mortality at 24 hours (4% vs. 24% in the vehicle-treated group, p<0.05). DFX also reduced infarct volume (85.1±56.3 vs. 164.3±93.4mm3 in vehicle, p<0.05) and swelling in the basal ganglia (p<0.05) 24 hours after tMCAO. The total hemorrhage volume in the ipsilateral hemisphere at 8 hours post-tMCAO was less in DFX treated animals (p<0.05). However, blood-brain barrier permeability was same in DFX- and vehicle-treated groups.
Conclusions
DFX attenuates death rate, hemorrhagic transformation, infarct volume, brain swelling in a rat transient focal ischemia with hyperglycemia model, suggesting that DFX could be potential treatment to reduce the hemorrhagic transformation for stroke patients.
Section
Disease-Related Neuroscience
Keywords: Cerebral ischemia, deferoxamine, hyperglycemia, transient middle cerebral artery occlusion, rats
1. Introduction
Currently there are no effective treatments for stroke except thrombolytic recanalization with tissue plasminogen activator (tPA) within three hours (The NINDS rt-PA stroke study group, 1995). A major factor limiting the use of tPA to reduce ischemic brain damage in patients is the risk of hemorrhagic transformation (HT) (Lyden and Zivin, 1993; Wang and Lo, 2003).
In recent years, the role of hyperglycemia in the pathophysiology and outcome of acute ischemic stroke has gained significant attention. Hyperglycemia has a major impact on post-ischemic HT. In experimental transient middle cerebral artery occlusion (tMCAO) models, acute hyperglycemia induced by glucose administration augments ischemic injury and increases HT (de Courten-Myers et al., 1992; Kawai et al., 1997; Venables et al., 1985). More importantly, human data shows that hyperglycemia is a predictor of HT in patients undergoing tPA therapy (Garg et al., 2006).
Experimental studies suggest that deferoxamine (DFX), an iron chelator, has neuroprotective properties in association with brain ischemia (Palmer et al., 1994). In our previous studies, we found that DFX reduces intracerebral hemorrhage-induced brain edema, neuronal death, neurological deficits and brain atrophy (Hua et al., 2006; Okauchi et al., 2009; Song et al., 2007; Xi et al., 2006). However, it is not clear whether DFX can reduce brain injury and HT after cerebral ischemia with hyperglycemia.
The present study investigated the effects of DFX on mortality rate, HT, brain edema formation, BBB permeability and brain infarct volume after transient focal cerebral ischemia in a hyperglycemic rat model.
2. Results
2.1 Physiological Parameters and Hemorrhagic Transformation
Physiological variables were measured immediately before the introduction of MCAO. The levels of pH, pO2, and pCO2 were controlled in the normal range (Table 1). Blood glucose levels just before tMCAO were not significantly different between vehicle and DFX treated rats (386 ± 74 vs. 380 ± 87 mg/dL, respectively, p>0.05).
Table 1.
Physiological Parameters
| Variables | Vehicle | DFX |
|---|---|---|
| Body weight (g) | 291±17 | 295.26±21.53 |
| pH | 7.38±0.07 | 7.39±0.08 |
| pO2 (mmHg) | 100.3±8.1 | 99.6±8.9 |
| pCO2 (mmHg) | 35.8±3.5 | 35.4±3.7 |
| Hematocrit (%) | 35.7±5.7 | 34.7±6.0 |
| Glucose (mg/dl) | 385.6±73.7 | 379.7±87.4 |
| MABP (mmHg) | 80.2±17.1 | 75.7±13.3 |
Values are expressed as the mean ± SD.
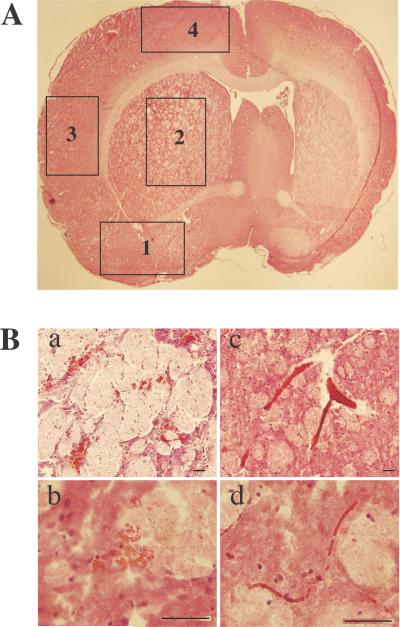
Either petechial or confluent petechial hemorrhage was found in the ipsilateral brain after tMCAO in hyperglycemic rats (Figure 1), occurring mostly in the ipsilateral basal ganglia (91%) and lateral cortex (59%), and occasionally in the ipsilateral cingulated cortex (14%) and pre-optic area (4.5%). Red blood cells were contained in pockets of hemorrhage and but were also found within congested blood vessels in the ipsilateral hemisphere.
Figure 1.
Hematoxylin and eosin brain sections captured at magnification of 4x demonstrating the four portions: 1, pre-optic area, 2, basal ganglia, 3, lateral cortex and 4, cingulate cortex. B. Red blood cells contained in pockets of hemorrhage in the lower (a, scale bar= 100μm) and higher (b, scale bar=50μm) magnification and red blood cells contained within congested blood vessels at lower (c, scale bar=100μm) and higher (d, scale bar=50μm) magnification.
2.2 Early Brain Injury (4-8 hours post tMCAO)
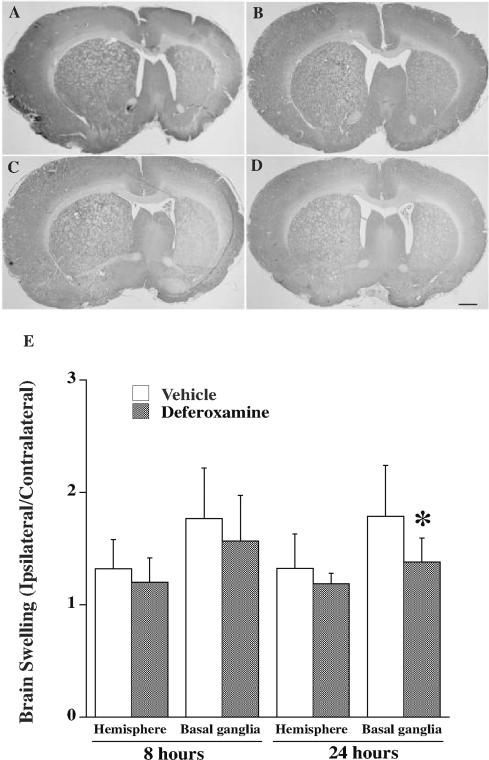
DFX treatment had no effect on mortality at 8 hours after tMCAO (7% in both DFX- and vehicle-treated groups). Hemispheric swelling (edema) developed in the ipsilateral hemisphere following tMCAO and DFX failed to reduce whole brain or basal ganglia swelling at 8 hours (Figure 2). In agreement, when brain edema was assessed by the wet/dry weight method, there was a marked increase in brain water content in the ipsilateral hemisphere (82.1 ± 1.3% vs. 79.4 ± 0.4% in the contralateral hemisphere, p<0.01) in vehicle-treated rats 8 hours after tMCAO and this was not significantly affected by treatment (81.2 ± 1.6 vs. 82.1 ± 1.3% in the vehicle-treated group). In addition, DFX did not significantly affect blood-brain damage as assessed by Evans blue extravasation at 4 hours post tMCAO. Evans blue content was increased in the ipsilateral compared to the contralateral hemisphere after tMCAO in both vehicle- (76 ± 50 vs. 8 ±8 μg/g, p<0.01) and DFX-treated rats (107 ± 47 vs. 7±6 μg/g, p<0.01), and the ipsilateral hemisphere values were not significantly different (p=0.3).
Figure 2.
Coronal rat brain sections 8 (A, B) and 24 (C, D) hours after 2 hours transient middle cerebral artery occlusion with hyperglycemia treated with vehicle (A, C) or deferoxamine (B, D) with H-E staining. (E) Brain swelling measurements. Values are mean ± SD, n=6 in 8 hours group and n=7 to 9 in 24 hours group.
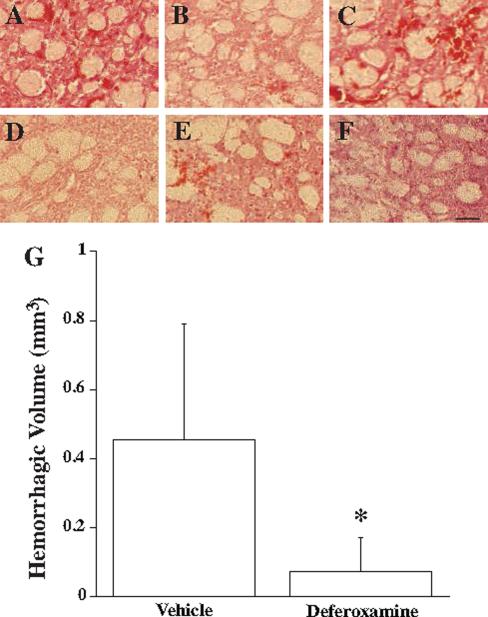
There was, however, a difference in the degree of HT as assessed at 8 hours after tMCAO with DFX treatment (Figure 3). DFX reduced the total volume of HT (0.07±0.1 mm3) compared to vehicle treated controls (0.46±0.33 mm3; p<0.05).
Figure 3.
Hemorrhagic transformation 8 hours after 2 hours transient middle cerebral artery occlusion with hyperglycemia treated with vehicle (A-C) or deferoxamine (D-F). Scale bar: 100μm. (G) Hemorrhagic volume measurements. Values are mean ± SD, n=6 per group. *p<0.05.
2.3 Delayed Brain Injury (24 hours post tMCAO)
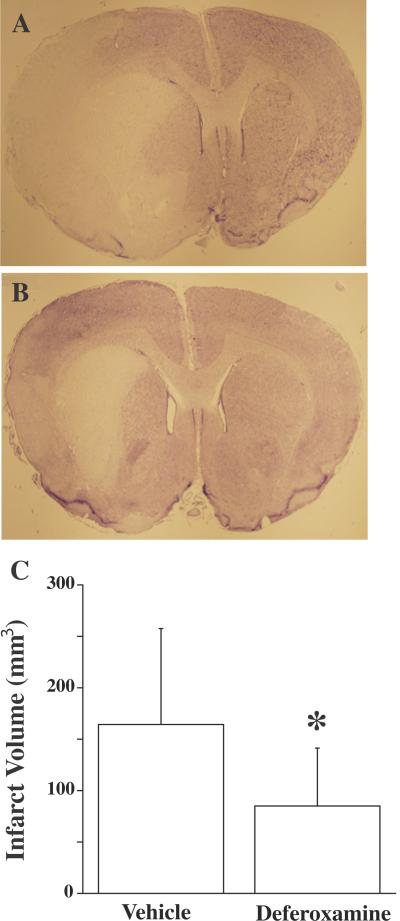
In contrast to the general lack of effect on early brain injury, DFX significantly attenuated mortality 24 hours after tMCAO (4% vs. 24% in vehicle-treated rats, p<0.05, Table 2). In addition, 24 hours post MCAO, brain infarct volume in the ipsilateral hemispheres of DFX treated animals was significantly smaller than in vehicle-treated rats (85±56 vs. 164±93 mm3, p<0.05; Figure 4). Brain swelling in the basal ganglia expressed as the ipsilateral/contralateral ratio was also smaller in DFX-treated rats (1.4 ± 0.2 vs. 1.8 ± 0.5 in the vehicle-treated group, p<0.05; Figure 2) 24 hours after tMCAO, although hemispheric swelling was not significantly affected.
Table 2.
Mortality Rate
| Mortality rate |
|||
|---|---|---|---|
| Group | Animal | # of Death | Mortality(%) |
| 8 Hour Vehicle | 15 | 1 | 7 |
| 8 Hour DFX | 15 | 1 | 7 |
| 24 Hour Vehicle | 29 | 7 | 24* |
| 24 Hour DFX | 27 | 1 | 4 |
Significance set at p<0.05.
Figure 4.
Brain coronal sections in rats 24 hours after 2 hours transient middle cerebral artery occlusion with hyperglycemia. Rats were treated with vehicle (A) or deferoxamine (B) with cresyl violet staining. (C) Infarct volume. Values are mean ± SD, n=7-9, *p<0.05.
Lysis of extravasated red blood cells occurred within the first 24 hours after tMCAO (i.e. in vehicle treated rats, hemorrhagic volume decreased from 0.46±0.33 mm3 at 8 hours to 0.05±0.06 mm3 at 24 hours, Figure 3). By 24 hours, there was only a tendency for the total volume of hemorrhage to be lower in DFX-treated than vehicle-treated animals (0.01±0.02 vs. 0.05±0.06 mm3, p>0.05). This was in accordance with results on hemoglobin content in the ipsilateral hemisphere (0.47 ± 0.22 vs. 0.6 ± 0.31mg in DFX and vehicle-treated groups, respectively; p>0.05).
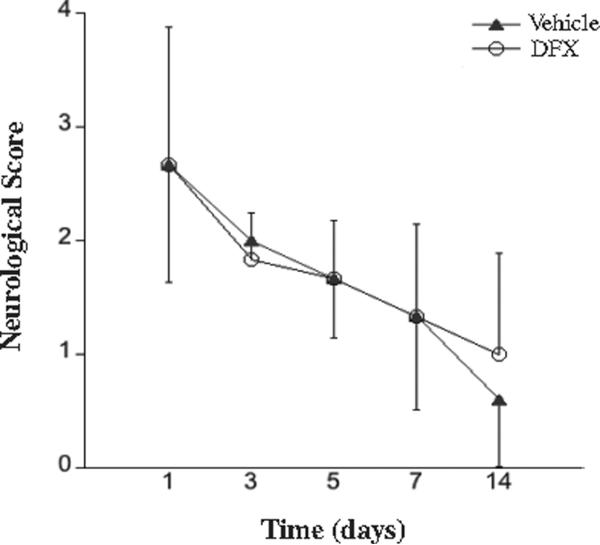
In rats that survived after the tMCAO, there was a gradual improvement in neurological deficit between 1 and 14 days. That improvement did not differ between DFX and vehicle treated rats (Figure 5). The most common residual deficits consisting of gait abnormalities or minor ataxia.
Figure 5.
Neurological deficits in rats subjected to 2 hours of transient middle cerebral artery occlusion with hyperglycemia. Rats were treated with vehicle (A) or deferoxamine (B). Animals that died within the 14 days of the experiment were excluded from the analysis. Values are mean ± SD, n=6-8. There were no significant differences between the two treatments.
3. Discussion
The major findings of our study using a hyperglycemic rat model of transient cerebral ischemia are: 1) DFX reduces mortality after tMCAO at 24 hours; 2) DFX attenuates brain swelling in the caudate at 24 hours, but has no effects on early Evans blue leakage and edema; 3) DFX reduces HT at 8 hours and brain infarct at 24 hours. These results suggest DFX may be useful for stroke patients especially these patients with hyperglycemia and treated with tPA. The underlying hypothesis of the current study was that since DFX can reduce brain injury following intracerebral hemorrhage (Hua et al., 2006; Okauchi et al., 2009; Song et al., 2007; Xi et al., 2006) it might also reduce injury associated with HT after tMCAO. Mortality and functional outcome are two major endpoints of stroke clinical trials. DFX did indeed reduce mortality rate at 24 hours after tMCAO in this hyperglycemic stroke model (24 vs 4% in vehicle treated rats). Excluding animals that died after tMCAO, there was no difference in neurological deficit between DFX- and vehicle-treated rats. It should be noted, however, that the greater death rate in the vehicle-treated rats may bias that analysis by causing a drop out of animals with more severe stroke. Although our current study showed that DFX can reduce hemorrhagic transformation in a rat model of cerebral ischemia with hyperglycemia, hyperglycemia is not the only reason causing hemorrhagic transformation after stroke. Future studies should examine whether or not DFX can reduce hemorrhagic transformation in other cerebral ischemic models with normoglycemia.
In experimental animal models of tMCAO, hyperglycemia induces HT (de Courten-Myers et al., 1989; de Courten-Myers et al., 1992). Although the precise mechanisms that enhance HT are unknown, acidosis may be involved in hyperglycemia-induced HT (de Courten-Myers et al., 1992). The resultant cellular acidosis include enzymatic dysfunction, enhanced free radical production through increased iron-catalyzed hydroxyl radical production (Siesjo et al., 1985), depressed mitochondrial function by inhibited ADP-stimulated respiratory activity (Hillered et al., 1984), induction of endonucleases that may initiate programmed cell death (Kalimo et al., 1981), increased intracellular Ca2+ accumulation (OuYang et al., 1994), and cellular swelling (Kraig et al., 1987). Recent studies indicate that hyperglycemia can increase production of matrix metalloproteinases, reactive oxygen species, and proinflammatory cytokines that might result in vascular damage and lead to hemorrhage (Garg et al., 2006). We studied hyperglycemia-induced hemorrhagic conversion in this ischemic model for two reasons. First, hyperglycemia is strongly associated with HT and tPA-associated intracerebral hemorrhage (Demchuk et al., 1999; Garg et al., 2006). Second, the model produces a consistent HT after 2 hours of tMCAO. In our previous study we have demonstrated this model of HT induced by acute hyperglycemia is consistent and reliable (Qin et al., 2007).
The results in this study indicate that the lower mortality in the DFX-treated group was associated with less hemorrhagic transformation (assessed at 8 hours), smaller infarcts and less brain swelling. The effect on hemorrhagic transformation was unexpected. Our prior results have shown an effect of DFX on the brain injury induced by a set size of intracerebral hematoma but have not examined whether DFX can affect intracerebral bleeding and this merits further investigation. In the current study, the difference in hemorrhagic transformation with DFX was not associated with a reduction in blood-brain barrier disruption (as assessed by Evans blue extravasation). It is possible that DFX may accelerate the clearance of red blood cells and hemoglobin after a hemorrhage. In a study on porcine intracerebral hemorrhage, we found that DFX reduced a red band found around the hematoma (Gu et al., 2009), suggesting an impact on the clearance of hemoglobin.
In this study, we examined hemorrhagic transformation by two methods: histology and hemoglobin assay. The study suggests that there may be potential problems with the latter. Although the animals were perfused well transcardially, brain pathology showed red blood cells contained within congested vessels besides red blood cells contained in pockets of hemorrhage. Red blood cells in congested vessels in the ischemic area complicate HT measurement. In addition, we recently found that hemoglobin is synthesized in neurons and is upregulated after ischemia (He et al., 2009). This can explain why hemoglobin content in the contralateral hemisphere is high (0.3±0.2 mg, n=6).
In the current study, DFX reduced brain infarct volume and brain swelling after tMCAO. It is well known that brain edema and tissue damage develops after an intracerebral hemorrhage, but the role of HT in the brain swelling and infarct that occurs in this model needs to be better defined. It has been reported by several groups that DFX can decrease infarct volume (Soloniuk et al., 1992) and brain edema after ischemia/reperfusion (Patt et al., 1990) in models where hemorrhagic transformation probably did not occur. It is, thus, possible that DFX affects both hemorrhage- and ischemia-induced brain damage in our model.
In general brain edema can be divided into two basic types, vasogenic and cytotoxic. Cytotoxic brain edema is the main form of edema after cerebral ischemia, but vasogenic edema occurs after BBB disruption. DFX may reduce both types of edema. We found that DFX reduces HT at 8 hours but not Evans blue leakage at 4 hours. At 24 hours but not earlier there was a difference in brain swelling. We examined Evans blue at 4 hours to see if there was a subtle change in BBB function that was not reflected in brain water. These results indicate DFX given after cerebral ischemia may be not able to limit BBB leakage at extremely early phase, but can prevent further BBB disruption and reduce HT later.
The molecular mechanisms by which DFX reduces HT and brain damage after tMCAO with hyperglycemia still remain to be fully elucidated. The mechanisms of DFX-induced brain protection may include: 1) DFX reduces iron-related brain injury through iron chelation; 2) DFX scavenges hydroxyl free radicals directly (Hurn et al., 1995; Liachenko et al., 2003); 3) DFX inhibits cell cycle transition (Farinelli and Greene, 1996; Wang and Semenza, 1995). Proliferating cells have an essential requirement for iron, and iron chelators block DNA synthesis and halt the cell cycle before the G1/S boundary which is defined as the “safe point” in the cell cycle. Thus DFX can suppress apoptotic death. 4) DFX increases hypoxia-inducible factor-1 (HIF-1) levels which affect many neuroprotective genes such as heme oxygenase-1 (Llesuy and Tomaro, 1994) and erythropoietin.
In conclusion, DFX reduces brain swelling, hemorrhagic transformation, infarct volumes, and mortality in a model of cerebral ischemia with hyperglycemia suggesting DFX treatment may be useful for stroke patients.
4. Experimental procedures
4.1. Animal Preparation and Middle Cerebral Artery Occlusion
Animal study protocols were approved by the University of Michigan Committee on the Use and Care of Animals. Male Sprague-Dawley rats (Charles River Laboratories; Portage, Michigan) weighing 275 to 300 g were used in this study. Rats were fasted for 12 hours before surgery but had free access to water. Anesthesia was induced by inhalation of 5% isoflurane in a nitrous oxide/oxygen mixture (70/30). Following induction of anesthesia, 1.5% isoflurane was maintained with mechanical ventilation. Rectal temperature was maintained at 37.5°C with use of a feedback-controlled heating pad. All rats had an injection of 50% glucose (6 mL/kg) intraperitoneally to induce acute hyperglycemia 15 minutes prior to MCAO.
Middle cerebral artery occlusion was induced using the filament model as previously described with some modification (Karabiyikoglu et al., 2004; Longa et al., 1989). In brief, under an operating microscope, the left common, external, and internal carotid arteries were dissected from connective tissue through a midline neck incision. The left external carotid artery and pterygopalatine artery of the internal carotid artery were separated and ligated by 5-0 silk sutures. A 23-mm segment of 3-0 nylon monofilament suture with the tip rounded by flame was inserted into the stump of the left common carotid artery and advanced into the internal carotid artery approximately 19 to 20 mm from the bifurcation to occlude origin of the middle cerebral artery. The suture was removed after 2 hours of occlusion. The animal was allowed to awaken and recover with free access to food and water.
The left femoral artery was catheterized for continuous blood pressure monitoring. Blood was obtained from the catheter for analysis of pH, PaO2, PaCO2, hematocrit and glucose. We measured blood gases before tMCAO to make sure the physiological conditions were the same in vehicle- and DFX-treated animals at baseline.
4.2. Experimental Groups
All rats had tMCAO after injected with 50% glucose (6 mL/kg I.P.). Rats were treated with DFX (100mg/ kg) or vehicle (equivalent volume of saline) directly after tMCAO. First, rats were killed at 8 (n=6 per group) or 24 hours (n=8 to 10) after MCAO. Rat brains were used for histological examination, including the measurements of infarct volume, brain swelling and hemorrhage volume. Second, rat brains were sampled (n=9 to 13) for hemoglobin content determination 24 hours after tMCAO. Third, rats (n=6 per group) were killed 4 hours after tMCAO and brains were used for Evans blue content measurement. Fourth, brain water content (n=9 per group) was determined 8 hours after tMCAO. Fifth, the neurological scores (n=8 per group) were determined at days 1, 3, 5, 7 and 14 after tMCAO.
4.3. Histopathology
Eight or twenty-four hours after tMCAO, the animals were reanesthetized and perfused intracardially with 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (pH 7.4). Brains were removed and kept in 4% paraformaldehyde for 6 hours and then immersed in 25% sucrose for 3 to 4 days at 4°C. The brains were embedded in the mixture of 25% sucrose and optimal cutting temperature compound (Sakura Finetek) and 20μm-thick coronal frozen sections were made on a cryostat. After disposing the 2-mm anterior part of the forebrain, slices for every 2mm-thick interval distance were stained with cresyl violet or hematoxylin-eosin (HE). The cresyl violet staining was used for measurements of infarct volume and the HE staining was used for measure the brain swelling and hemorrhage volume. In total, five slices of each brain were stained and analyzed.
(1) To quantify infarct volume, the areas of infarction at five coronal levels throughout the brain were identified and the infarct and hemispheric volumes were measured with National Institutes of Health Image by an investigator blind to the treatments. To avoid an artifact in volume measurement from brain edema within the infarct, infarct volume was calculated by measuring and subtracting the volume of the non-infarcted ipsilateral hemisphere from the volume of the contralateral hemisphere (Lin et al., 1993).
(2) The extent of brain swelling was assessed by measuring the ratio of ipsilateral to contralateral hemispheric volume. Swelling were measured and analyzed by whole hemisphere and by region (basal ganglia).
(3) HT formation measurements. First, four images (4x) (pre-optic area, basal ganglia, lateral cortex and cingulated cortex; Figure 3) of each tissue section were acquired by a microscope (Olympus BX51, Japan). Second, brain areas containing extravasated blood in the four areas were captured at magnification of 10x. Microscopic fields were identified at 40x magnification. The investigators need to pick out red blood cells contained within congested blood vessels from red blood cells contained in pockets of hemorrhage (Figure 3) until “all hemorrhages” and “only hemorrhages” were included in areas for quantization. All brains' regions in the four areas showing histological evidence of hemorrhage were measured in this fashion and summed to provide a quantitative estimate of the extent of bleeding in each animal.
4.4. Brain hemoglobin measurement
HT formation was also quantified by spectrophotometric assay of brain hemoglobin content (Choudhri et al., 1997). At 24 hours after MCAO, the animals were perfused transcardially with 0.1mol/L phosphate-buffered saline under deep anesthesia until the outflow fluid from the right atrium was colorless. The brain was rapidly removed and dissected into the left and right hemispheres. The hemispheric brain tissue was then homogenized in 0.1mol/L phosphate-buffered saline followed by 30-minute centrifugation (13 000 g). Then 200μL reagent (QuantiChrom Hemoglobin Assay Kit; BioAssay Systems) was mixed with 50μL supernatant. After 15 minutes, optical density was determined by a spectrophotometer (Ultrospec 3; Pharmacia LKB) at 400nm wave-length. The total hemispheric hemoglobin content was calculated as milligrams per hemisphere using a Hb standard curve.
4.5. Brain Water Content
Twenty-four hours after MCAO, animals were anesthetized and decapitated as described in our previous study (Xi et al., 1998). The brains were removed and dissected into three parts: the cerebellum and left and right hemispheres. Brain samples were immediately weighed on an electronic balance (model AE 100; Mettler Instrument) to obtain wet weight. Brain samples were then dried in a gravity oven (Blue M. Electric Co) at 100°C for 48 hours to obtain the dry weight. Brain water content was then calculated as (wet weight-dry weight)*100/wet weight.
4.6. BBB integrity
Blood-brain barrier disruption was assessed using the extravasation of Evans blue into brain tissue of the animals (n=6 each). Evans blue dye (2% in saline, 4 m/kg) was given intravenously 2 hours after tMCAO, immediately after of the intraluminal filament removal. Two hours after Evans blue injection, the chest wall was opened under lethal anesthesia and the brains were perfused with 0.1mol/L phosphate-buffered saline through the left ventricle to remove the intravascular localized dye until colorless perfusion fluid was obtained from the right atrium. After decapitation, the brain was removed and dissected into left and right hemispheres and each hemisphere weighed. Brain samples were then placed in 3 mL 50% trichloroacetic acid solution, homogenized and centrifuged (10, 000 rpm for 20 minutes). The supernatant absorbance was measured at 610 nm by spectrophotometer (Ultrospec 3; Pharmacia LKB). The concentration of Evans blue was quantified from a linear standard curve and was expressed as micrograms per gram of brain tissue.
4.7. Neurological Score
Two hours and 1, 3, 5, 7 and 14 days after MCAO, a neurological examination was performed as previously described with modifications (Menzies et al., 1992). Briefly, the scores were: 0, no apparent deficits; 1, contralateral forelimb flexion when suspended by tail; 2, decreased grip of the contralateral forelimb while tail pulled; 3, spontaneous movement in all directions or contralateral circling only if pulled by the tail; 4, spontaneous contralateral circling; and 5, death after recovery from the anesthesia. Animals that showed the features of the higher scores also showed all the features of the lower grades.
4.8. Statistical Analysis
Results are expressed as mean ± SD. Statistical significance was analyzed by two-tailed Student t test and Mann-Whitney U test for continuous variable and by x2 test for mortality and HT formation rate. Statistical significance was considered at p<0.05.
Acknowledgements
This study was supported by grants NS-017760, NS-039866, NS-047245 and NS-057539 from the National Institutes of Health (NIH) and 0755717Z and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
Abbreviations
- HT
Hemorrhagic transformation
- DFX
deferoxamine
- tMCAO
transient middle cerebral artery occlusion
- tPA
tissue plasminogen activator
References
- Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr., Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Wagner KR, Myers RE. Fatal strokes in hyperglycemic cats. Stroke. 1989;20:1707–15. doi: 10.1161/01.str.20.12.1707. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Holm P, DeVoe G, Schmitt G, Wagner KR, Myers RE. Hemorrhagic infarct conversion in experimental stroke. Ann Emerg Med. 1992;21:120–6. doi: 10.1016/s0196-0644(05)80144-1. [DOI] [PubMed] [Google Scholar]
- Demchuk AM, Morgenstern LB, Krieger DW, Linda Chi T, Hu W, Wein TH, Hardy RJ, Grotta JC, Buchan AM. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–9. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- Farinelli SE, Greene LA. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J Neurosci. 1996;16:1150–62. doi: 10.1523/JNEUROSCI.16-03-01150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37:267–73. doi: 10.1161/01.STR.0000195175.29487.30. [DOI] [PubMed] [Google Scholar]
- Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–3. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hua Y, Liu W, Hu H, Keep RF, Xi G. Effects of cerebral ischemia on neuronal hemoglobin. J Cereb Blood Flow Metab. 2009;29:596–605. doi: 10.1038/jcbfm.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillered L, Ernster L, Siesjo BK. Influence of in vitro lactic acidosis and hypercapnia on respiratory activity of isolated rat brain mitochondria. J Cereb Blood Flow Metab. 1984;4:430–7. doi: 10.1038/jcbfm.1984.62. [DOI] [PubMed] [Google Scholar]
- Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long- term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–12. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Koehler RC, Blizzard KK, Traystman RJ. Deferoxamine reduces early metabolic failure associated with severe cerebral ischemic acidosis in dogs. Stroke. 1995;26:688–94. doi: 10.1161/01.str.26.4.688. [DOI] [PubMed] [Google Scholar]
- Kalimo H, Rehncrona S, Soderfeldt B, Olsson Y, Siesjo BK. Brain lactic acidosis and ischemic cell damage: 2. Histopathology. J Cereb Blood Flow Metab. 1981;1:313–27. doi: 10.1038/jcbfm.1981.35. [DOI] [PubMed] [Google Scholar]
- Karabiyikoglu M, Hua Y, Keep RF, Ennis SR, Xi G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:159–66. doi: 10.1097/01.WCB.0000100062.36077.84. [DOI] [PubMed] [Google Scholar]
- Kawai N, Keep RF, Betz AL. Hyperglycemia and the vascular effects of cerebral ischemia. Acta Neurochir Suppl. 1997;70:27–9. doi: 10.1007/978-3-7091-6837-0_8. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Petito CK, Plum F, Pulsinelli WA. Hydrogen ions kill brain at concentrations reached in ischemia. J Cereb Blood Flow Metab. 1987;7:379–86. doi: 10.1038/jcbfm.1987.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liachenko S, Tang P, Xu Y. Deferoxamine improves early postresuscitation reperfusion after prolonged cardiac arrest in rats. J Cereb Blood Flow Metab. 2003;23:574–81. doi: 10.1097/01.WCB.0000057742.00152.3F. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–21. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Llesuy SF, Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994;1223:9–14. doi: 10.1016/0167-4889(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Zivin JA. Hemorrhagic transformation after cerebral ischemia: mechanisms and incidence. Cerebrovasc Brain Metab Rev. 1993;5:1–16. [PubMed] [Google Scholar]
- Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31:100–6. doi: 10.1227/00006123-199207000-00014. [DOI] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–63. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OuYang YB, Mellergard P, Kristian T, Kristianova V, Siesjo BK. Influence of acid-base changes on the intracellular calcium concentration of neurons in primary culture. Exp Brain Res. 1994;101:265–71. doi: 10.1007/BF00228746. [DOI] [PubMed] [Google Scholar]
- Palmer C, Roberts RL, Bero C. Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke. 1994;25:1039–45. doi: 10.1161/01.str.25.5.1039. [DOI] [PubMed] [Google Scholar]
- Patt A, Horesh IR, Berger EM, Harken AH, Repine JE. Iron depletion or chelation reduces ischemia/reperfusion-induced edema in gerbil brains. J Pediatr Surg. 1990;25:224–7. doi: 10.1016/0022-3468(90)90407-z. [DOI] [PubMed] [Google Scholar]
- Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, Xi G. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–7. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Bendek G, Koide T, Westerberg E, Wieloch T. Influence of acidosis on lipid peroxidation in brain tissues in vitro. J Cereb Blood Flow Metab. 1985;5:253–8. doi: 10.1038/jcbfm.1985.32. [DOI] [PubMed] [Google Scholar]
- Soloniuk DS, Perkins E, Wilson JR. Use of allopurinol and deferoxamine in cellular protection during ischemia. Surg Neurol. 1992;38:110–3. doi: 10.1016/0090-3019(92)90087-4. [DOI] [PubMed] [Google Scholar]
- Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. 2007;38:2861–3. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- The NINDS rt-PA stroke study group Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt- PA Stroke Study Group. N Eng J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Venables GS, Miller SA, Gibson G, Hardy JA, Strong AJ. The effects of hyperglycaemia on changes during reperfusion following focal cerebral ischaemia in the cat. J Neurol Neurosurg Psychiatry. 1985;48:663–9. doi: 10.1136/jnnp.48.7.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–7. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–44. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–6. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]