Abstract
The pathogenic protozoan Giardia lamblia is known to not synthesize membrane lipids de novo. Therefore, it is possible that lipids in the small intestine, where trophozoites colonize, play key roles in regulating the growth and differentiation of this important pathogen. The focus of the current study is to conduct a complete lipidomic analysis and to test the hypothesis that Giardia has some ability to generate new phospholipids (PLs). Using mass spectrometry, now we show that phosphatidylglycerols (PGs) are major PLs followed by phosphatidylcholines (PCs) and phosphatidylethanolamines (PEs) in non-encysting and encysting trophozoites, as well in cysts. The fatty acids attached to these PLs consist mostly of palmitate, palmitoleate, oleate, and linoleate. Results also indicate that PGs and PEs, unlike PCs, are not present in bovine bile and serum, the major sources of lipids of the culture medium, and that they could therefore be produced by fatty acid and headgroup remodeling reactions, circumventing the synthesis of entirely new PLs via de novo pathways. Genomic and transcriptional analyses show the presence of giardial phosphatidylglycerolphosphate synthase (gpgs) and phosphatidylserine decarboxylase (gpsd) genes, which are expressed throughout the life cycle. Bioinformatic and phylogenetic analyses further indicated that both genes are of prokaryotic origin and that they have undergone duplication in the course of the evolution. Our studies suggest that the abundance of PG in Giardia is unique among eukaryotes and that its synthesis thus could serve as a potential target for developing new therapies against this waterborne parasite.
Keywords: Giardia lamblia, lipidomics, genomics, phospholipid, transcription, base-exchange reactions
INTRODUCTION
Phospholipids are important constituents of cell membranes in both prokaryotes and eukaryotes. In parasitic protozoa, phospholipids not only are essential for the synthesis of membranes and organelles but also play key roles in energy production and in interactions with host cells. Many intracellular parasites can alter the membrane components of their hosts and exchange lipid moieties, allowing parasites to survive and multiply within the host cell. For example, Plasmodium falciparum-infected erythrocytes have been shown to have an altered lipid composition as compared with non-infected cells [1]. In bovine erythrocytes infected with Babesia bovis, phosphatidylcholine (PC) has been demonstrated to be a major phospholipid (PL), but in non-infected cells it comprises only a minor group [1]. In Entamoeba and trichomonads, the major fatty acids in the cell resemble those found in serum and are, therefore, likely obtained from the growth medium [2].
The intestinal protozoan Giardia lamblia colonizes the luminal surface of the human small intestine and commonly causes diarrhea, abdominal cramps, bloating, and vomiting syndromes [3]. Although giardiasis is commonly and effectively treated with nitroheterocyclic compounds like metronidazole, tinidazole, and furazolidone, such drugs can be problematic, resulting in unpleasant side effects and ultimately leading to drug resistance in Giardia [4]. Therefore, it is important to identify novel targets for developing new anti-giardial agents. One of these targets could be lipid metabolic pathways. It has been proposed that this protozoon is unable to synthesize its own lipid molecules de novo and that it depends upon exogenous lipids for its growth and differentiation [2]. The inability of Giardia to synthesize lipids de novo indicates that lipid remodeling pathways play a significant role in the organism and that it could, therefore, serve as a potential drug target. Recent studies from our laboratory indicate that blocking the synthesis of sphingolipids results in decreased growth and encystation of Giardia, further suggesting the importance of the role that lipids play in the giardial life cycle [5]. Previous reports from various laboratories indicate that the lipid composition of Giardia resembles that of its growth medium and that the major PLs in Giardia are PC, Phosphatidylethanolamine (PE), and sphingomyelin (SM) [6-8]. Ellis et al. [9] reported that the lipid compositions in Giardia are similar to those of the growth and encystation medium and that the amount of unsaturated fatty acids increases in encysting cells.
In the present study, we carried out a comprehensive lipidomic analysis of vegetative and encysting trophozoites as well as in-vitro-derived cysts of Giardia using electrospray ionization-quadrupole time-of-flight-mass spectrometry (ESI-QTOF-MS), and we compared these results with lipids from bovine serum and bile. We also analyzed the Giardia Genome Database (http://giardiadb.org/giardiadb/) [10] to identify lipid biosynthesis genes in this organism. Our results revealed the presence of two PE and phosphatidylglycerol (PG) biosynthesis-related genes in Giardia, which are expressed and transcribed in both stages of the giardial life cycle. This is the first report of a comprehensive lipidomic analysis of Giardia, a protozoon that infects many children worldwide, especially in developing countries.
2. MATERIALS AND METHODS
2.1. Material
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and were of the highest purity available. Serratia marcescens chitinase and α-N-acetyl-galactosaminidase were purchased from Sigma-Aldrich and Genway Biotech, Inc. (San Diego, CA), respectively. Phospholipid and fatty acid standards were obtained from Avanti Polar Lipids (Alabaster, AL) and Supelco (Bellefonte, PA), respectively.
2.2. Organisms
Giardia lamblia trophozoites (strain WB, ATCC No. 30957) were cultivated in TYI-S-33 medium supplemented with adult bovine serum and bile [11, 12]. The antibiotic piperacillin (100 μg/ml) was added during routine culture of the parasite [13], and parasites were detached by chilling in ice and harvested by centrifugation at 1,500 ×g for 10 min at 4 °C, followed by repeated washes. Encystation in culture was carried out by the method of Gillin et al. [13] by culturing trophozoites in TYI-S-33 medium (pH 7.8), supplemented with bovine serum (10%, v/v), lactic acid (5 mM), and porcine bile (250 mg/ml) for various time points, as described in the text and figure legends. In vitro-derived cysts were generated by culturing trophozoites in high-bile medium (TYI-S-33 medium, supplemented with 10% bovine serum and bile, pH 7.8) as described previously by Kane et al. [14].
2.3. Digestion of cysts
In vitro-derived giardial cysts and 48 hour (h) encysting cells were suspended in 0.1 M phosphate buffer (pH 7.0) and incubated overnight at 37 °C with chitinase (1.5 mg/ml) and α-N-acetyl-galactosaminidase (5.3 Units/ml). Digested cell extracts were subjected to lipid extraction as described below.
2.4. Lipid extraction
Non-encysting and encysting Giardia trophozoites were grown to confluency and harvested as described above (section 2.2). Lipids from giardial cell pellets (vegetative and encysting trophozoites), cysts, bovine serum, and bile were extracted 3 times in 10 volumes of each of the following chloroform (CHCl3)/methanol (CH3OH) solutions: CHCl3:CH3OH:H2O (1:2:0.8, v/v/v) and CHCl3:CH3OH (2:1, v/v). After adding each solvent solution, the tube was vortexed for approximately 1 min, then centrifuged at room temperature for 30 min at 2,000 ×g. Following centrifugation, the organic phase was transferred to a clean glass tube with Teflon/PTFE inner disks and stored at −20 °C until further use. The sample was dried under highly pure nitrogen stream after the last extraction in each step [15].
2.5. Phospholipid and sterol purification
Glass columns prepared in Pasteur pipettes were packed with fine glass wool and approximately 100 mg silica gel resin (pore size 60 Å, 200−400 μm mesh, Sigma-Aldrich). Columns were washed with CH3OH and acetone and equilibrated with CHCl3. Following the equilibration, the lipid sample dissolved in 1 ml CHCl3 was loaded onto the column. Neutral lipids (e.g., sterols, triglycerides etc.) and free fatty acids were eluted out with 2−3 ml CHCl3. This was followed by 2−3 ml acetone to elute glycolipids and ceramides, and 2−3 ml methanol to elute phospholipids [16]. All samples were dried under nitrogen stream and stored at −20 °C until further use.
2.6. Phospholipid analysis by ESI-QTOF-MS
MS spectra for fractionated lipids were acquired in an ESI-QTOF-MS (Micromass Qtof1, Waters). Samples analyzed in positive-ion mode were dissolved in CHCl3:CH3OH (1:1, v/v) containing 10 mM LiOH. For analysis in negative-ion mode, samples were dissolved in CHCl3: CH3OH: formic acid: NH4OH (1:1:0.1%:0.1%). Samples were injected by infusion at a flow rate of 0.5 μl/min, and the capillary voltage was set at 2.5 kV. Full-scan spectra were collected in the 200−2,000 m/z range, and MS/MS spectra were automatically collected for each parent ion with abundance higher than 20 counts using ramp-collision energy (20−65 eV) according to the mass range. Argon was used as the collision gas. For quantitative analysis, samples were normalized to 5000 cells/μl and spiked with an internal standard at a final concentration of 2.5 μM C11:0/C11:0-PC for positive-ion mode, or 5 μM C12:0/C12:0-PE for negative-ion mode. Peak height of the standards was normalized to 30% of the total peak height.
2.7. Gas chromatography-mass spectrometry (GC-MS) analysis
The analysis of the sterol fraction by GC-MS was carried out as described by Fridberg et al. [17]. For analysis of fatty acids by GC-MS, total lipids were isolated as described above. Alkaline hydrolysis of total fatty acids was carried out following a method adapted from Maldonado et al. [18]. Twenty-five-μl aliquots of the total lipid extracts were dried under nitrogen stream and re-suspended in 100 μl 13 N ammonium hydroxide: methanol (1:1, v:v), then incubated for 1 h at 37 °C and dried under nitrogen stream. The samples were then washed twice with 100 μl of dry methanol, with complete drying under nitrogen stream between each wash. For the methylation, 100 μl 0.5 N methanolic HCl (Supelco, Sigma-Aldrich) were added, and the reaction mixture was incubated for 1 h at 75 °C. The reaction mixture was allowed to cool to room temperature and then neutralized with 100 μl 0.5 N NaOH. To remove HCl from the reaction, samples were washed with 1 ml each deionized water and dichloromethane (DCM). The aqueous phase was extracted and samples were washed two more times with water. Finally, the organic phase was transferred to a fresh tube and briefly dried under nitrogen stream [18].
For GC-MS analysis, samples were redisolved in 100 μl DCM and 1 μl was used for analysis in a trace gas chromatographer (Thermo Fisher Scientific, Austin, TX) coupled to a mass spectrometer (Polaris Q, Thermo Fisher Scientific) (GC-MS). Samples were separated in a SP-2380 fused silica column (30 m × 250 μm × 0.20 μm, Supelco, Sigma-Aldrich). The injector was set at 200°C, and the following gradient was used: 70°C for 5 min, followed by 4°C/min up to 140°C, 2°C/min up to 185°C, and 185°C for 10 min. Helium was used as the carrier gas, with a flow rate of 1 ml/min. The molecules were ionized by electron impact at 70 eV and 200 °C. The spectra were collected in the 30−400 m/z range, and a chromatogram was generated by plotting the spectra of diagnostic fragment-ion species for m/z 41, 43, and 55. Fatty-acid species were identified by comparison with the FAME 37 methylated FA mix standard (Supelco, Sigma-Aldrich).
2.8. Bioinformatic, phylogenetic, and molecular analyses of putative phospholipid synthesis genes
Predicted open-reading frames (ORFs) were obtained from the Giardia genome database (http://giardiadb.org/giardiadb/) and were compared using BLASTP [10]. Sequences for PE and PG biosynthesis were identified, including the genes of phosphatidylserine decarboxylase (psd, accession no. XM_001707858, ORF no. 16495) and phosphatidylglycerolphosphate synthase (pgps, accession no. XP_769290, ORF no. 7259). PCR primers were designed using Primer3 software (http://frodo.wi.mit.edu) and were synthesized by MWG Biotech (High Point, NC) or Sigma Genosys (St. Louis, MO). The sequences of the primer pairs are as follows: gpgps: 5’- CTAACGGTCGGCATTTTCAT-3’ and 3’-GGAGAAAAGGATGGCACAGA-5’ gpsd: 5’-CACTGTCCGAAGCCACAATA-3’ and 3’-AGGCCTTTGCATGTGAGTGT-5’ α-tubulin: 5’-GAGTTCACAGAGGCCGAGTC-3’ and 3’-CTCCTCCTCCTCGAACTCCT-5’ Following harvest, cysts were resuspended in 4 ml TRI reagent (Sigma) and freeze-thawed at −20°C and 37°C three times. Following the last freeze thaw, 0.5-mm glass beads (BioSpec Products) were added, and the cyst wall was broken by vortexing for approximately 5 minutes. Total RNA was extracted from trophozoites and encysting cells using TRI reagent and was reverse transcribed using the ImProm-II Reverse Transcriptase Kit (Promega). Levels of PGPS (GenBank accession number XP_769290) transcription were quantified by quantitative real-time PCR. RT2 Real-Time SYBR Green/Fluroescein PCR Master Mix (SuperArray) was used in the reaction mixture, and all reactions were performed in triplicate. Primers targeting α-tubulin were used as a control to normalize the samples. Transcript levels were quantified using the relative standard curve method [19]. Statistical analysis was performed with one-way ANOVA with Dunnett's Post Test using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
In order to characterize the evolutionary relationships of the giardial sequences to those of other organisms, homologous PSD and PGPS amino acid sequences were found by performing BLAST [20] searches of the GenBank database. The BLAST searches were usually executed using the default BLOSUM62 scoring matrices, although for searches of more distantly related taxa (e.g., bacteria using eukaryotic queries) BLOSUM45 was also used. In general, only matches with significant local alignments, as indicated by e-values << 10−3, were considered. A sequential multiple alignment was performed using Clustal X [21] with the default settings for scoring matrices (Gonnet) and gap penalties (10 to open, 0.20 to extend). The aligned sequences were then used to construct phylogenetic trees using the PHYLIP software packages [22] — specifically, the maximum-parsimony (protpars) and maximum-likelihood (proml) modules for protein sequences. For maximum-likelihood reconstructions, both the Jones-Taylor and Dayhoff substitution models were used in the analyses for comparison. In most instances, the trees were rooted using sequences from Bacillus, which, as a Firmicute Gram-positive eubacterium, is thought to be an outgroup with respect to representative Proteobacteria and other prokaryotes [23]. The robustness of the results to errors in the sequences themselves or in the choice of alignments was investigated using standard bootstrap analysis, run over 100 replicates of re-sampled data from the original multiple alignments. The final trees, along with support values greater than 50% were plotted using the MrEnt tree visualization package [24].
3. RESULTS
3.1 Mass spectrometric analysis reveals phosphatidylglycerol (PG), phosphatidylethanolamine (PE) and phosphatidylcholine as major phospholipids in Giardia
Several reports suggest that dietary lipids (i.e., PLs, fatty acids, and bile acids) are important for giardial growth and encystation in the human small intestine [2]. Reports also indicate that many of these lipids are not synthesized de novo; instead, they are scavenged from outside sources. To further elucidate whether giardial lipid compositions are similar to the growth medium or Giardia has the capacity to synthesize new lipids, the total lipid composition of vegetative and encysting trophozoites, and water-resistant cysts was determined by ESI-QTOF-MS (Fig. 1A, B). The analysis in positive-ion mode identified multiple species of sphingomyelin (SM) and PC (Supplementary Fig. 1A, B). Giardial PCs were composed mostly of palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), and linoleic (C18:2) acids (Table 1). A few lipid species had the highest peak heights and were present in both vegetative and encysting trophozoites, as well as in in vitro-derived cysts — i.e., C16:0/d18:1-SM, C16:0/C18:1-PC, C18:1/C18:2-PC, C18:1/C18:1-PC, and C18:0/C18:1-PC. 18:1/18:1-PC appeared to be the major species (Supplementary Fig. 1A and B, Table 1). Supplemental Fig. 1A shows the fragmentation of the major PC species (C18:1/C18:1-PC) observed at m/z 792.6 (Fig. 1A). Loss of the choline head group is identified by the fragment ion at m/z 603.7, and loss of the acyl chains by ions at m/z 451.5 and 504.6 (Supplementary Fig. 1).
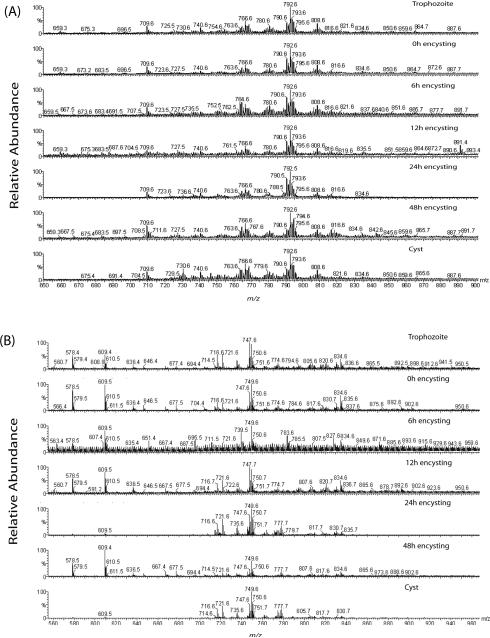
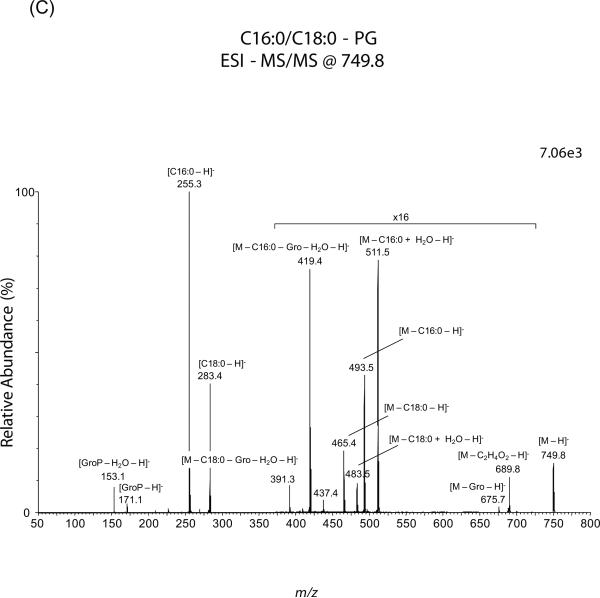
Fig. 1.
Lipid analysis by MS of G. lamblia. Total phospholipids were fractionated by silica-gel 60 and analyzed by ESI-QTOF-MS. (A and B) Positive- and negative-ion mode full-scan spectra of giardial phospholipids, respectively. Total lipids and phospholipids from vegetative, encysting and water-resistant cysts were isolated as described in the Materials and Methods. (C) MS-MS spectrum of C18:0/16:0-PG parent-ion at m/z 749.5, ionized in negative-ion mode. “x16” indicates that the portion of that spectrum was magnified sixteen times to make the peaks more visible. The number at the top right corner of each spectrum indicates signal strength, measured as ion intensity at 100% relative abundance. m/z, mass to charge ratio.
Table 1.
Lipid analysis and composition of the major phospholipids from differentiating Giardia lamblia.a
| PL | m/z | Ion Species | Proposed Structure sn-1/sn-2b | Relative Abundancec |
|---|---|---|---|---|
| Positive-Ion Mode | ||||
| PC | 502.4 | M + Li | lyso-C16:0 | + |
| 526.4 | M + Li | lyso-C18:2 | + | |
| 528.4 | M + Li | lyso-C18:1 | + | |
| 600.5 | M + Li | C11:0/C11:0 (IS) | N/A | |
| 740.8 | M + Li | C16:0/C16:0 | + | |
| 752.7 | M + Li | C16:0/C17:1 and/or C18:1/C15:0 | + | |
| 754.8 | M + Li | C16:0/C17:1 | + | |
| 764.8 | M + Li | C16:1/C18:1 | + | |
| 766.8 | M + Li | C16:0/C18:1 | ++ | |
| 768.8 | M + Li | C16:0/C18:0 | + | |
| 780.6 | M + Li | C17:0/C18:1 | + | |
| 788.6 | M + Li | C18:2/C18:2 | + | |
| 790.7 | M + Li | C18:1/C18:2 | + | |
| 792.7 | M + Li | C18:1/C18:1 | ++ | |
| 794.6 | M + Li | C18:0/C18:1 | ++++ | |
| 806.8 | M + Na | C18:1/C18:2 | + | |
| 808.8 | M + Na | C18:1/C18:1 | ++ | |
| 814.6 | M + Li | C20:4/C18:1 | ++ | |
| 816.6 | M + Li | C20:4/C18:0 | + | |
| SM | 709.9 | M + Li | C16:0/d18:1 | ++ |
| 725.7 | M + Na | C16:0/d18:1 | + | |
| 737.8 | M + Li | C18:0/d18:1 | + | |
| Negative-Ion Mode | ||||
| PC | 804.6 | M + formate | C18:1/C16:0 | + |
| 806.6 | M + formate | C18:0/C16:0 | + | |
| 820.6 | M + Cl | C18:1/C18:1 | + | |
| 828.6 | M + formate | C18:2/C18:1 | + | |
| 830.6 | M + formate | C18:1/C18:1 | + | |
| 832.6 | M - H | C18:0/C18:1 | + | |
| PE | 578.4 | M - H | C12:0/C12:0 (IS) | N/A |
| 636.4 | M + NaCl - H | C12:0/C12:0 (IS) | N/A | |
| 646.4 | M + Na + formate - H | C12:0/C12:0 (IS) | N/A | |
| 714.5 | M - H | C16:0/C18:2 and/or C16:1/C18:1 | ++ | |
| 716.5 | M - H | C18:1/C16:0 | +++ | |
| 834.6 | M - H | C22:6/C22:6 (IS) | N/A | |
| PG | 609.4 | M - H | C12:0/C12:0 (IS) | N/A |
| 707.5 | M - H | C16:0/15:0 and/or C14:0/C17:0 | + | |
| 719.6 | M - H | C16:0/C16:1 and/or C18:1/C14:0 | + | |
| 721.5 | M - H | C16:0/16:0 and/or C18:0/C14:0 | +++ | |
| 733.5 | M - H | C16:0/C17:1 and/or C18:1/C15:0 | + | |
| 735.5 | M - H | C16:0/C17:0 and/or C15:0/C18:0 | + | |
| 745.5 | M - H | C18:2/C16:0 and/or C16:1/C18:1 | + | |
| 747.5 | M - H | C18:1/C16:0 | ++++ | |
| 749.5 | M - H | C18:0/C16:0 | ++++ | |
| 761.5 | M - H | C18:1/C17:0 | + | |
| 763.6 | M - H | C18:0/C17:0 and/or C19:0/C16:0 | + | |
| 771.5 | M - H | C18:1/C18:2 | + | |
| 773.5 | M - H | C18:1/C18:1 | + | |
| 775.5 | M - H | C18:0/C18:1 | + | |
| 777.6 | M - H | C16:0/20:0 and/or C18:0/C18:0 | + | |
| 789.5 | M + Na + formate – H | C16:0/C16:0, C18:0/C14:0, and/or C15:0/C17:0 | + | |
| 817.5 | M + Na + formate – H | C18:0/C16:0 | + | |
| PI | 809.5 | M - H | C16:0/C16:0 | + |
| 835.5 | M - H | C18:1/C16:0 | + | |
Phospholipid species were identified by MS-MS analysis in positive- and negative-ion modes.
Relative abundance is designated by the peak height: ++++, up to 100%; +++, up to 75%; ++, up to 50%; +, 10% or less. IS, internal standard.
Relative abundances for each sample were similar and therefore only the peak heights for trophozoites are shown.
N/A, not applicable.
Negative-ion mode spectra revealed that PGs are predominant PLs in Giardia not only in vegetative trophozoites but also in encysting cells and cysts. The phospholipids found include primarily PG but also PE, PC, and two species of phosphatidylinositol (PI) (Fig. 1B, C, Table 1, and Supplementary Fig. 1C and D). Interestingly, no phosphatidylserine (PS) could be detected. The fatty acids covalently linked to these PLs were similar to those found in positive-ion mode, with C16:0, C16:1, C18:0, and C18:1 fatty acids being the most predominant ones. In both negative- and positive-ion modes, we found that some of these fatty acids included species with retention time similar or identical to odd-carbon number fatty acids (OCFAs) (i.e., C15:0, C17:0, C17:1, C19:0) (Table 1). Since the GC-MS analysis was carried out using internal standards containing only linear OCFAs and a column that was not appropriate for discriminating between linear and monomethyl-branched OCFAs, we could not determine here the true nature of giardial OCFAs; thus, further experiments are required.
Moreover, it is possible sometimes to misidentify PG as lysobisphosphatidic acid (LBPA) or even other glycerophospholipids, because they share the same dehydrated glycerophosphate fragment-ion at m/z 153. In the current study, however, some characteristic fragment ions led to the unambiguous identification of these ion species as PGs [26]. For instance, Figure 1C shows fragmentation of the parent-ion species at m/z 749.7, tentatively assigned as C18:0/C16:0-diacyl-PG. The phospholipid was unequivocally identified as PG by the presence of the daughter-ion at m/z 675.5 ([M – glycerol – H]−), which is derived from the neutral loss of the glycerol moiety linked to phosphate at the headgroup. The resulting fragment represents a phosphatidic acid with two fatty acids attached and, therefore, it is specific of PG and could not be generated from a lysobisphosphatidic acid (LBPA) species, because in the latter only one fatty acid is linked to each glycerol moiety. We also observed a fragment-ion at m/z 689.8, which most likely resulted from the internal fragmentation of the double-bond formed between C-2 and C-3 by dehydration of the glycerol backbone at the headgroup. Further fragmentation analysis of m/z 749.7 confirmed the presence of C18:0 (m/z 283.3) and C16:0 (m/z 255.3) at sn-1 and sn-2, respectively. The position of the sn-1 and sn-2 fatty acids was determined by their relative peak heights obtained by low collision energy dissociation, as reviewed by Pulfer and Murphy [25]. Other fragments corresponding to the neutral loss of these fatty acids were also observed at m/z 465.4 ([M – C18:0 – H]−), 483.4 ([M – C18:0 + H2O – H]−), 493.5 ([M – C16:0 – H]−), and 511.5 ([M – C16:0 + H2O – H]−). The glycerol head group was confirmed by the formation of daughter-ions at m/z 391.3 and 419.3, which represent the loss of the sn-1 and sn-2 fatty acids, respectively, plus the glycerol moiety. A similar pattern of fragmentation was observed for other PG species identified in this study (Tables 1 and 2).
Table 2.
Positive-ion mode MS-MS analysis of phospholipids from bile and serum.
| m/z | Ion Species | Proposed Structures sn-1/sn-2 |
|---|---|---|
| 526.4 | M + Li | lyso-C18:2-PC |
| 528.4 | M + Li | lyso-C18:1-PC |
| 530.5 | M + Li | lyso-C18:0-PC |
| 552.4 | M + Li | lyso-C20:3-PC |
| 653.7 | M + Li | C18:0/C20:3-DAG |
| 764.7 | M + Li | C16:0/C18:2-PC |
| 766.7 | M + Li | C16:0/C18:1-PC |
| 790.7 | M + Li | C18:1/C18:2-PC and/or C18:0/C18:3-PC |
| 792.7 | M + Li | 18:0/18:2-PC |
| 794.7 | M + Li | 18:0/18:0-PC |
| 816.7 | M + Li | 18:0/20:4-PC |
Earlier Ellis et al. [9] have reported that PC, SM, and PE were the major phospholipids in Giardia, and that PG comprises only 10% of the total PL content in trophozoites [9]. In the current study, however, the peak intensities and the number of PG species present in negative-ion mode indicate that PG could be a major phospholipid in this protozoan parasite (Fig. 1C). As far as PE is concerned, we have found that this particular phospholipid is bound to common fatty acids such as oleate, linoleate, and stearate. Furthermore, the peak heights for PE species are higher than 50% in the full-scan spectra, indicating that these are a fairly abundant species (Table 1, and Supplementary Fig. 1).
When a relative quantitative analysis of phospholipids was performed in positive-ion mode (Suppl. Fig. 3A), the major PC species observed was 18:1/18:1-PC (m/z 792.6), with higher (∼75%) relative abundance observed in non-encysting trophozoites, and lower (∼25%) in 12-h encysting cells. In cysts, nevertheless, the relative abundance of 18:1/18:1-PC returned to levels comparable to that of trophozoites. Another point of interest is the observation of C16:0/d18:1-SM at m/z 709.6 (Suppl. Fig. 3A). While the relative abundance of this sphingosine-based phospholipid in trophozoites was <10% or even undetectable in 12-h encysting cells, it increased to ∼25% in cysts, suggesting that SM might be important for cyst wall synthesis [5]. For negative-ion mode analysis, the most predominant PL species observed was C18:0/C16:0-PG (m/z 749.5), with a relative abundance of 85%, as compared to 30% for the C12:0/C12:0-PE internal standard (m/z 578.4). Nevertheless, 18:0/16:0-PG decreased to ∼45% in 12-h encysting cells and cysts. Other abundant PLs of interest in negative-ion mode, particularly in trophozoites, were 16:0/16:0-PG and 18:0/14:0-PG (both at m/z 721.5), with relative abundance of ∼25%, making it clear that while the most commonly bound fatty acids were C16:0, C18:0, and C18:1, shorter fatty acids such as C14:0 were also present in reasonable amounts.
Giardia is cultured in TYI-S-33 media supplemented with adult bovine serum as a source of lipids. Bovine bile is also added to the media and is thought to facilitate the growth of G. lamblia by a mechanism yet to be elucidated. Studies also suggest that bile acids are taken up by the parasite through carrier-dependent pathway and most likely involve transporting lipid molecules by forming mixed micelles [27]. In the current study, an attempt was made to analyze the PL composition of serum and bile to verify which lipids could be present in the culture medium, because previous reports suggest that the lipid composition of Giardia closely resembles that of its growth medium, especially the medium containing high bile content [6, 7-9]. Our mass spectrometric data, on the other hand, show that serum and bile are rich in PC, lyso-PC, and diacylglycerol (DAG) (Table 2), and do not contain other PLs. This difference could be due to the fact that thin-layer chromatography (TLC) was used to separate PLs [9], rather than a more sensitive and powerful resolving tool like mass spectrometry. Mass spectrometry has the advantage over TLC and other methodologies of being able to resolve complex lipids with much higher sensitivity. Because the growth medium lacks PG and PE, the current results support the idea that at least PG and PE are newly synthesized PLs in Giardia and most likely generated via remodeling reactions as proposed earlier [28, 2].
3.2. GC/MS analysis of fatty acids
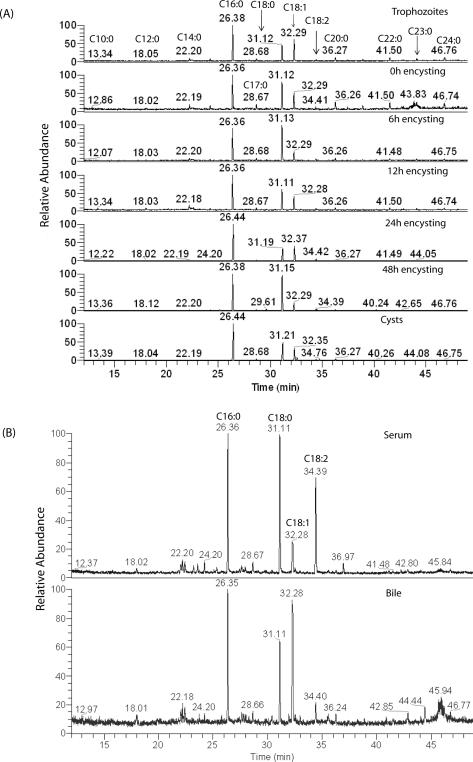
Like PLs, fatty acids are also important for giardial growth and encystation [28]. Although fatty acids trigger encystation in vitro [29], excess fatty acids are toxic and possess anti-giardial properties. It has been demonstrated that medium-chain fatty acids, especially dodecanoic acid (C12:0), kill Giardia in vitro with (LD50 ∼13.78 μg/ml) [30]. Previous reports [7-9] indicate that C16:0 is the major fatty acid in vegetative and encysting Giardia, followed by C18:0, C18:1, and C18:2. Traces of C14:0, C15:0, C17:0, C18:3, C19:0, C20:0, C22:0, C24:0, C26:0, and C28:0 have also been detected. Interestingly, no dramatic differences were observed in fatty-acid compositions between vegetative and encysting trophozoites. In the current study, the total fatty acid content of trophozoites, encysting cells, and cysts was analyzed by GC-MS, and the results revealed that C16:0, C18:0, and C18:1 were indeed the major fatty acids in all giardial lipid species, as evidenced by relative peak areas (Fig. 2A). However, less common acyl groups such as C10:0, C12:0 and C14:0 were also present. The fatty-acid composition remained unchanged in vegetative trophozoites, encysting cells (0−48 hours), and water-resistant cysts (Fig. 2A, Table 3). In serum and bile, the major fatty acids are C16:0, C18:0, C18:1, and C18: 2 (Fig. 2B, Table 3). Traces of other fatty acids such as C12:0, C14:0, C15:0, C17:0, C20:0, and C22:0, were also identified. Our results also suggest that some long chain fatty acids (i.e., C24:0, C24:1, C23:0, etc.), which are present in Giardia, could be synthesized de novo by the action of elongase(s). Although no attempt was made to measure the elongase activity, the BLAST search of the Giardia Genome Database (http://giardiadb.org/giardiadb/) [10] yielded the presence of fatty acid elongase 1 gene (accession no. XM_00170849.1, E-value 4e-81), suggesting that fatty acid elongation machinery may be present in this pathogen. Interestingly, in Dictyostelium discoideum, the same sequence was annotated as fatty acid elongase 3-ketoacyl-CoA synthase (NCBI accession no. XP_638938). Our GC-MS analysis also revealed that the sterol fractions purified from trophozoites, encysting cells and cysts contain only cholesterol (data not shown). However, a previous report suggests the presence of low levels of ergosterol along with the more abundant cholesterol in this protozoan parasite [9].
Fig. 2.
GC-MS spectra of fatty acid content. Total lipids from vegetative trophozoites, encysting cells, water-resistant cysts, bile and serum were isolated and processed as described in Materials and Methods. (A) Giardial fatty acids; (B) Fatty acids from the bile and serum. The retention times (min) of identified fatty acids and internal standards are indicated.
Table 3.
Fatty acid analysis by GC-MS.a
| Retention time (min) | Fatty acid | Relative Peak Area (%)b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vegetative Trophozoites | 0-h Encysting | 6-h Encysting | 12-h Encysting | 24-h Encysting | 48-h Encysting | In vitro Cysts | Bovine Serum | Bovine Bile | ||
| 13.3 | C10:0 | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 18.0 | C12:0 | 1.1 | 1.9 | 0.0 | 2.7 | 0.0 | 0.7 | 0.4 | 1.7 | 3.2 |
| 22.2 | C14:0 | 3.6 | 3.4 | 2.7 | 6.1 | 0.3 | 0.8 | 0.3 | 2.8 | 3.2 |
| 24.2 | C15:0 | 1.6 | 2.0 | 1.3 | 1.3 | 0.4 | 0.6 | 0.3 | 2.3 | trace |
| 26.3 | C16:0 | 33.5 | 25.7 | 32.2 | 33.3 | 51.7 | 38.2 | 47.7 | 27.4 | 30.2 |
| 28.6 | C17:0 | 2.6 | 3.6 | 2.7 | 2.0 | 1.1 | 0.9 | 1.5 | 2.7 | 2.1 |
| 31.1 | C18:0 | 15.9 | 22.3 | 39.1 | 23.4 | 19.1 | 45.0 | 27.6 | 30.9 | 22.2 |
| 32.2 | C18:1 | 22.7 | 12.2 | 10.8 | 16.1 | 23.7 | 9.2 | 18.2 | 7.7 | 31.4 |
| 34.4 | C18:2 | 1.3 | 1.5 | 2.0 | 1.8 | 2.7 | 2.3 | 1.3 | 24.5 | 5.7 |
| 36.2 | C20:0 | 3.5 | 6.6 | 2.7 | 2.7 | 0.9 | 1.3 | 2.1 | trace | 2.0 |
| 37.4 | C20:1 | 0.9 | 1.4 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 |
| 38.8 | C21:0 | 1.9 | 2.7 | 1.1 | 1.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 41.5 | C22:0 | 3.9 | 5.6 | 2.6 | 2.8 | 0.1 | 0.5 | 0.3 | 0.0 | 0.0 |
| 44.0 | C23:0 | 2.3 | 6.2 | 1.1 | 1.7 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 |
| 46.7 | C24:0 | 4.4 | 4.9 | 1.7 | 2.9 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 |
| 48.1 | C24:1 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
Fatty acids present in trophozoites, encysting cells, in vitro-derived cysts, and bovine bile and serum.
Relative abundance is designated by peak area and is shown as percentage of the total fatty acid content for each sample.
3.3. Genomic and phylogenetic analyses of PG and PE synthesis genes
Since PG and PE are not present in bile or serum (Table 2), we asked whether they could be newly synthesized by the parasite. Therefore, we searched the Giardia Genome Database (http://giardiadb.org/giardiadb/) [10] to identify PG- and PE-related synthesis/remodeling genes. The BLASTp search yielded giardial phosphatidylglycerolphosphate synthase (gpgps) and phosphatidylserine decarboxylase (gpsd) genes. Pfam analyses revealed that gpgps matched well with bacterial and trichomonads pgps (Table 4). The two closest matches were Opitutaceae bacterium and Trichomonas vaginalis, with E-values of 1.00e-32 and 5.00e-25, respectively. However, the open-reading frame (ORF) annotations (http://giardiadb.org/giardiadb/) [10] for these matches were CDP-DAG-glycerol-3-phosphate and 3-phosphatidyltransferase, rather than PGPS, and they belong to the CDP-alcohol phosphatidyltransferase family of enzymes. On the other hand, gpsd yielded matches of the eukaryotic phylum, with the closest being Plasmodium vivax (E-value 2.00e-22). Other protozoa with psd sequences that closely matched the giardial psd included Toxoplasma gondii and Plasmodium falciparum. For example, gpsd belongs to the phosphatidylserine decarboxylase family of enzymes (Table 4). To retrieve the predicted sub-cellular localization of gPGPS and gPSD, DNA sequences were translated into protein using the translation tool, available at the Expert Protein Analysis System (ExPaSy) proteomics server (www.expasy.ch/) [31]. The predicted sub-cellular localizations (using ExPasy) suggest that gPGPS is an ER-bound protein and that gPSD localizes in the cytoplasm in Giardia (not shown).
Table 4.
Predicted open reading frames and Pfam matches of giardial PGPS and PSD a
| Designation (gORFS) | GenBank Accession | Match to Pfam (motif location) | Species with best BLASTp match (E value) | Pfam family match |
|---|---|---|---|---|
| PGPS | XP_769290 | 2.3e−29 (aa 63−191) |
Opitutacaea bacterium (1e−32) Trichomonas vaginalis (5e−25) |
CDP-alcohol phosphatidyl transferase |
| PSD | XP_779868 | 1.1e−13 (aa 186−409) | Plasmodium vivax (2e-22) | Phosphatidylserine decarboxylase |
Putative genes encoding phosphatidylglycerolphosphate synthase (PGPS) and phosphatidylserine decarboxylase (PSD) were identified in Giardia using the NCBI and Protein Family (Pfam) database.
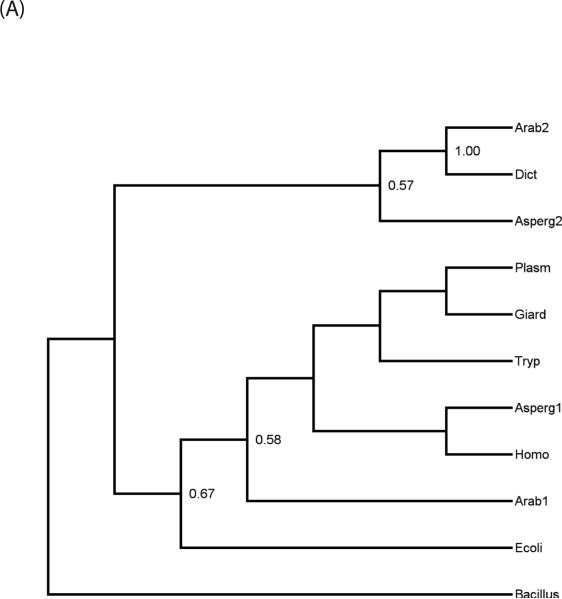
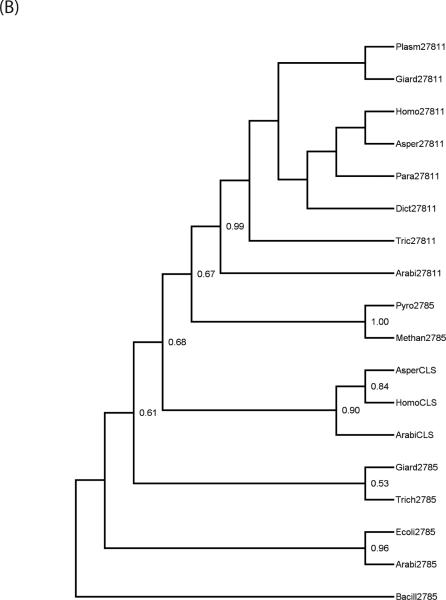
Since Giardia is evolutionarily basal [32] and PG and PE syntheses are more predominant in bacteria, we asked whether giardial PGPS and PSD are similar to bacterial enzymes. Phylogenetic analyses indicated that PGPS was found to be present in each of the major “domains” of living organisms: Eubacteria, Archae, and Eukaryotes. Furthermore, in the majority of Eukaryote, more than a single homologue was found, which suggests that gene duplication had played a role in the evolutionary history of this enzyme. PSD was found to have a somewhat more restricted distribution, being entirely absent from Archaebacteria, and while two paralogs of the gene were found in some Eukaryotes (namely plants and fungi) the majority of taxa assayed had only a single copy. The relationships of the paralogs and the positions of giardial sequences were revealed by the phylogeny reconstructions. Figure 3A shows the maximum-likelihood tree of PSD sequences based on a Jones-Taylor model of equitable substitution. Bootstrap support values of over 50% are indicated at each node in the trees. In the case of PSD, a fairly clear pattern emerges. There are two paralogs of the gpsd gene, as evidenced by the presence of both in Aspergillus (fungus) and Arabidopsis (plant). One form (referred to as gpsd-1 for convenience) is more closely allied to the bacterial sequences while another, gpsd-2, is unique to eukaryotic clades. The first form is present in Metazoa (Homo) as well as in most single-celled Eukaryotes assayed, including Giardia, Trypanosoma, and Plasmodium. However, the second paralog seems to be the form present in Dictyostelium, while PSD homologs of any kind are apparently absent from Trichomonas.
Fig. 3.
(A) Maximum likelihood phylogeny, replicated over 100 bootstrap samples, for PSD sequences. The numbers at each node represent the support values, with only values equal to or greater than 50% shown. An abbreviated name is followed by a number to designate first versus second paralogs, when more than one sequence is present in an organism. Taxon names are: Arab (Arabidopsis thaliana), Bacillus (Bacillus anthracis), Dict (Dictyostelium discoideum), Ecoli (Escherichia coli), Giard (Giardia lamblia), Homo (Homo sapiens), Plasm (Plasmodium falciparum), Tryp (Trypanosoma cruzi). (B) Maximum likelihood for PGPS and its paralogs. The support values are shown at each node for 100 bootstrap replicates. The taxon name is followed by the paralog designation, either E.C. 2.7.8.5, 2.7.8.11, or CLS (cardiolipin synthase). The taxon abbreviations are the same as in Fig. 3A, with the additional organisms: Asper (Aspergillus fumigatus), Methan (Methanococcoides burtonii), Para (Paramecium tetraurelia), Pyro (Pyrococcus furiosus), and Trich (Trichomonas vaginalis).
In the case of PGPS, the pattern is somewhat more complicated, as there are three distinct paralogous sequences. Two are respectively identified with KEGG identifiers E.C. 2.7.8.5 and E.C. 2.7.8.11. The third, annotated as cardiolipin synthase (completely unrelated to the bacterial enzyme with the same name) does not presently have an EC number. Curiously, plants have all three paralogs. Cardiolipin synthase (CLS) appears to be restricted to “higher” multicellular eukaryotes such as metazoa, fungi, and plants, while most eukaryotes have E.C. 2.7.8.11. The paralog of PGPS annotated as E.C. 2.7.8.5 seems to be the ancestral sequence, as it is the only form found in prokaryotes. The evolutionarily basal Metamonads, Giardia, and Trichomonas have both the bacterial form E.C. 2.7.8.5 and the characteristic eukaryotic form E.C. 2.7.8.11. In Fig. 3B and Supplementary Fig. 2, respectively, maximum likelihood and parsimony trees for the PGPS sequences are shown, with bootstrap support values greater than or equal to 50% indicated at every node. It is noted that EC 2.7.8.5 and CLS form a monophyletic subclade, with 99% support, and that the monophyly of CLS also has very high (90%) bootstrap support (while bootstrap support for nodes within each paralog subclade tends to be poor). In contrast to EC 2.7.8.11 and CLS, EC 2.7.8.5 is in a sense paraphyletic, in that a number of eukaryotic 2.7.8.5 sequences are more closely related to CLS than they are to Archaean 2.7.8.5.
There are a number of anomalies in the PGPS phylogeny, however. For example, the E. coli E.C. 2.7.8.5 sequence forms a subclade with Arabidopsis rather than with fellow eubacterium 0020 Bacillus, which may be due to a either breakdown of the substitution models used in likelihood analysis (similar problems occurred when a Dayhoff substitution matrix was used), or perhaps an independent origin of this paralog in plants through horizontal transfer. The maximum-parsimony analysis has all eubacterial EC 2.7.8.5 sequences as outgroups to those from Archaea and Eukaryotes (not shown). In spite of these caveats, both the likelihood and parsimony trees support the existence of three distinct paralogs, regardless of possible errors in the details of intra-paralog phylogeny.
3.4. Transcriptional analysis suggests that gpgps and gpsd genes are transcribed in Giardia
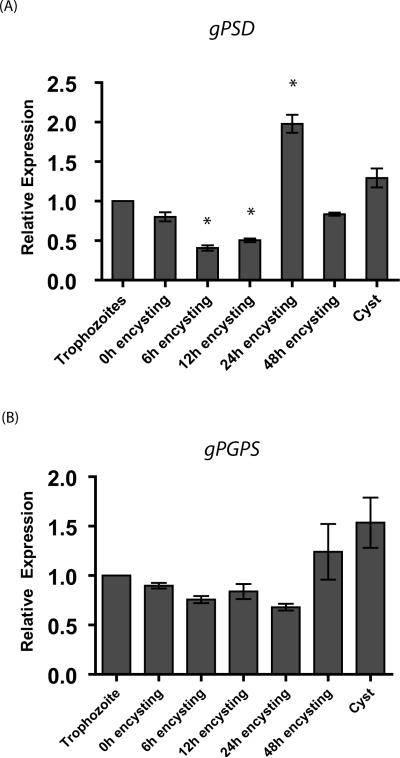
Recent results from our laboratory suggest that many lipid synthesis genes in Giardia are regulated differentially during encystation [5]. Here, we asked whether gpsd and gpgps are transcribed and are expressed in trophozoites and cysts. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) results indicate that both gpsd and gpgps are expressed in vegetative and encysting trophozoites as well as in water-resistant cysts. It is clear from Fig. 4 that these two genes display differential patterns of expression. For example, in 6-h and 12-h encysting cells, gpsd transcript levels decrease ∼60% and 50%, respectively, whereas in 24-h encysting cells the transcript level is two-fold higher than in the vegetative state and remains high (∼1.5 fold) in cysts. Transcript levels were found to be significantly different from vegetative trophozoites by one-way ANOVA with Dunnett's Post Test (p<0.05). In the case of gpgps, the transcript levels decrease by ∼30% in 6-, 12-, and 24-h encysting cells. In 48h encysting cells and cysts, expression increases about ∼1.5 fold (Fig. 4B). Interestingly, Dunnett's Post Test did not show that gpgps transcript levels in encysting cells or cysts were significantly different from trophozoites (p>0.05). Although it is not known whether these genes are translated into products, data indicate that both gPSD and gPGPS could play significant roles in giardial biology. For example, while gPSD is required for encystation, gPGPs might play precise roles in forming cysts and during excystation to trophozoites.
Fig. 4.
Differential expression of giardial phosphatidylserine decarboxylase (gpsd) and giardial phosphatidylglycerolphosphate synthase (gpgps) genes, relative to vegetative trophozoites. Experiments were carried out three times with three replicates each time. The data are the means and standard deviations of these replicates. An asterisk indicates significant difference compared to vegetative trophozoites with p<0.05. Panel A indicates the expression of gpsd, and Panel B denotes gpgps expression.
4. DISCUSSION
Because of its inability to synthesize membrane lipids de novo, it has been proposed that Giardia has evolved well-orchestrated mechanisms to import and utilize pre-formed lipids and fatty acids from the cell-exterior [28, 33, 34]. Many of these exogenous lipids/fatty acids undergo remodeling before they are incorporated into giardial membranes [2, 33]. Although, these reports provide some insights into the metabolism of exogenously acquired lipids, the detailed mechanisms of lipid metabolism in Giardia are yet to be delineated.
The current study was undertaken to address two fundamental questions related to the lipid metabolism by Giardia. First, we asked whether giardial lipid compositions are similar to that of the culture medium, as proposed by other investigators [see reference 2 for review], and second we asked whether any new membrane lipids are synthesized by this parasite.
We began our experiments by identifying membrane lipids and fatty-acid constituents through ESI-QTOF-MS. Lipid samples from trophozoites, encysting cells, and cysts were analyzed in positive- as well as negative-ion mode to retrieve overall information. Interestingly, significant differences in the lipid species present in any stage of the life cycle were not detected (Fig. 1). Furthermore, our results indicate that PG, PE, and PC are major phospholipids in Giardia and most likely PG and PE are synthesized de novo, since the analysis of phospholipid content in bovine bile and serum yielded no PG or PE (Tables 1 and 2, and Supplementary Fig. 3). In another experiment (not shown), we examined phospholipid compositions of the culture medium (containing peptone, yeast extract, salts, serum, and bile) by thin-layer chromatography, and again, no PG and PE could be identified. Although PG is abundant in bacteria, trace amounts of this acidic PL can also be found in mammalian and protozoan cells [35]. On the other hand, PE is present in both prokaryotic and eukaryotic membranes and is involved in the formation, fusion, and vesiculation of lipid bilayers [35]. Various biochemical and structural studies [35, 36] have indicated that PG interacts with other charged lipids (i.e., PC and PE) and that it acts as a membrane stabilizer in addition to regulating the lipid-protein interactions in bacterial membranes. PG-PE interactions are also important for vesicle morphology, membrane permeability, and overall maintenance of the membrane stability [37]. Based on these studies, and considering the challenges that Giardia meet in the human gut, it can be proposed that the interactions between PG and PE as well as PG and PC are important for this protozoan parasite to survive and complete its life cycle in the hostile environment of the human small intestine. Furthermore, the abundance of PG in Giardia could also be traced back to its evolutionary origin. It has been demonstrated that Giardia synthesizes a cryptic mitochondrion or mitosome as part of its putative mitochondrial ancestry [38, 39]. A recent report suggests that cardiolipin (a PG dimer), which is present in bacterial membrane, is also present in hydrogenosome (i.e., a mitosome-like organelle) in Tritrichomonas foetus [40]. Therefore, it can be hypothesized that the abundance PG in Giardia could be a signature of its endosymbiotic past and that it originated from alphaproteobacteria during symbiosis [39]. However, more in-depth experiments are required to support this notion.
To comprehend whether Giardia is able to synthesize its own PG and PE, we surveyed the Giardia genome database (http://giardiadb.org/giardiadb/) [10], and identified putative homologues of giardial phosphatidylglycerolphosphate synthase (gpgps) and phosphatidylserine decarboxylase (gpsd) genes. In bacterial and yeast cells, phosphatidylglycerolphosphate synthase (PGPS) catalyzes the formation of PG-phosphate from CDP-diacylglycerol and sn-glycerol 3-phosphate. PG is then formed from PG-3-phosphate by PG-3-phosphate phosphatase (also referred to as PG synthase, or PGS) [41]. Phosphatidylethanolamine (PE) is synthesized from phosphatidylserine (PS) by the action of PSD by base-exchange reactions. Since the quantitative reverse transcription polymerase chain reaction (qRT-PCR) results (Fig. 4) indicate that both gpgs and gpsd genes are present and transcribe in vegetative trophozoites, encysting cells and cysts. It is possible that gpgps and gpsd are housekeeping genes and they are required for metabolic functions in all stages of the life cycle.
It appears that both PG and PE in Giardia are generated by base-exchange reactions rather than the de novo (CDP-DAG) synthesis. This postulation can further be supported by the fact that when live trophozoites were metabolically labeled with radioactive glucose, threonine, glycerol, and acetate no radioactivity could be detected in lipid fractions [6]. Conversely, we found that radiolabeled bases, fatty acids, and other preformed lipids can be assembled to larger lipid molecules [2]. In a separate experiment, it was noted that [14C]-glycerol and [14C]-ethanolamine are incorporated into PG and PE, which suggests that Giardia might synthesize enzymes that allows these bases (i.e., glycerol and ethanolamine) to incorporate them into respective phospholipids. Intriguingly, we could not detect PS in Giardia, bile, or serum. This could be due to the reasons that PS is a minor PL that exist in few molecular species. It is possible that during our analyses, PS was broken down to serine and diacylglycerol (DAG). In fact, Table 2 shows the presence of DAG when phospholipid composition of bile and serum was determined by MS analysis. Nonetheless, when live trophozoites are cultured in the presence of [14C]-serine the majority of serine is assimilated into PE, which indicates the presence of strong PSD activity and may explain why we failed to detect PS in Giardia (Yichoy et al., unpublished).
As has previously been demonstrated [7-9] and also confirmed by our MS analysis (Table 1, Supplementary Fig. 1), PC is also abundant in Giardia and is the only major PL available in the growth medium. In light of the fact that the medium contains only PC and lyso-PC, the number of phospholipid species (i.e., PG, PE, and PI) identified in trophozoite and cyst supports the idea that base-exchange enzymes are present in this organism [33]. However, except for gpsd, no genes for base-exchange enzymes have so far been revealed from the genome project, and therefore detailed biochemical analyses are required for further characterization of these enzymes.
In silico analyses have revealed that the giardial PGPS and PSD resemble bacterial and Plasmodium enzymes, respectively (Table 4), and that therefore PL metabolism in this parasite could be similar to that of bacterial and lower eukaryotic cells. Both enzymes can be found in bacteria; homologs of gpgps are present in Eubacteria and Archaea, while gpsd is only found in Eubacteria. Because of the occurrence of these enzymes in prokaryotes and the localization of their activity in the mitochondrial membranes, it is possible that they are originally of endosymbiotic origin in eukaryotes, with their current position in the nuclear genome being a secondary transfer from the mitochondrial genome. It can be speculated that there have been at least two gpgps duplication events and a single gpsd duplication, as evidenced by two paralogs of gpsd and as many as three of gpgps. A fairly consistent pattern is seen from the BLAST search results the presence of two paralogs of gpsd and gpgps among higher eukaryotes. The two enzymes appear to have co-evolved, since many of the multi-cellular eukaryotes with two paralogs of gpsd also have two or even three copies of gpgps.
The phylogenetic distribution of the PGPS paralogs has a number of functional and evolutionary consequences. The “primitive” form, annotated as EC 2.7.8.5, is responsible for the conversion of PGP to PG, a pathway found throughout bacteria and in “lower” eukaryotes. Not surprisingly, it is present in bacteria and as a plesiomorphy in Giardia and Trichomonas (though the question of whether giardial EC 2.7.8.5 was originally of nuclear origin or originally part of the degenerate mitosome remains unclear). It is absent in higher eukaryotes, with the anomalous exception of plants. The latter may be due to horizontal gene transfer from prokaryotes, which is a more parsimonious explanation than postulating multiple independent losses in other “higher” eukaryotes. Regardless, the presence of a bacterial-like paralog in Giardia may have important implications for drug targeting. Specifically, inhibition of EC 2.7.8.5 activity in Giardia will not necessarily entail inhibition of phospholipid synthesis function in the mammalian host, which uses a different paralog and pathway. In the case of PSD, there are two eukaryotic forms: PSD-1, which is shared with prokaryotes, and PSD-2, which appears to be restricted to plants and fungi. Because plants and fungi are not closely related—fungi are more closely related to the Metazoa than to plants [42] —this distribution is unusual and requires further investigation. The form of the enzyme found in Giardia is the “primitive” PSD-1, which is shared with both Eubacteria and most Eukaryotes. The giardial PSD-1 sequences nest within the Eukaryotic subclades.
Our GC-MS analysis of total fatty acid content reveals that Giardia contains few additional fatty acids that are not present in the medium. Fatty acids like 21:1, 23:0, 24:0, and 24:1 are only present in Giardia, which suggests that these are newly generated fatty acids produced by elongation and desaturation reactions (Fig. 2, Table 3). The presence of a fatty acid desaturase enzyme has already been reported by Ellis et al. [9], and the Giardia Genome Database has identified the presence of a fatty acid elongase gene; the two could be responsible for the exchange and elongation of acyl chains, respectively. As to the presence of odd-carbon fatty acids (OCFAs), in our GC-MS analysis conditions we could not determine whether they were linear or methyl-branched structures.
In summary, our lipidomic and genomic data indicate that because of its limited ability to synthesize fatty acids and more complex lipids (e.g., phospholipids and neutral lipids), Giardia scavenges lipids from its environment and changes accordingly by fatty acid remodeling and/or base-exchange reactions. In fact, PG and PE, two major phospholipids, are likely to be generated via base-exchange reactions. Although the conversions of PS→PE or PE→PS are well characterized in yeast and mammalian cells, the synthesis of PG by base-exchange reaction is yet to be described. The post-transcriptional silencing and over-expression of both genes will be helpful in characterizing these genes/gene products and their respective functions in the giardial life cycle. Many of these experiments are already in progress in our laboratory.
Supplementary Material
Suppl. Fig.1. Lipid analysis by MS of G. lamblia. Total phospholipids were fractionated by silica-gel 60 and analyzed by ESI-QTOF-MS. (A and B) Positive-ion mode MS-MS spectra of C18:1/18:1-PC (m/z 792.7) C16:0/d16:1-SM at m/z 709.9, respectively. (C and D) Negative-ion mode MS-MS spectra of C18:1/C16:0-PE (m/z 716.7) and C16:0/C16:0-PI (m/z 809.7), respectively. The number at the top right corner of each spectrum indicates signal strength, measured as ion intensity at 100% relative abundance. m/z, mass to charge ratio.
Suppl. Fig. 2. Maximum parsimony tree for PGPS and its paralogs. The support values are shown at each node for 100 bootstrap replicates. The taxon name is followed by the paralog designation, either E.C. 2.7.8.5, 2.7.8.11, or CLS (cardiolipin synthase). The taxon abbreviations are the same as in Fig. 3A, with the additional organisms: Asper (Aspergillus fumigatus), Methan (Methanococcoides burtonii), Para (Paramecium tetraurelia), Pyro (Pyrococcus furiosus), and Trich (Trichomonas vaginalis).
Suppl. Fig 3. ESI-QTOF-MS spectra of relative quantitative analysis of giardial phospholipids. Total lipids were extracted and fractionated as described in Materials and Methods. Cell numbers were adjusted to 5000 cells/μL. (A and B) Positive- and negative-ion mode analysis, respectively. For positive-ion (ESI+) mode MS analysis, giardial lipid samples were spiked with 2.5 μM 11:0/11:0-PC (m/z 600.4) ([M + Li]+), used as internal standard (IS). For negative-ion (ESI-) mode MS analysis, 5 μM 12:0/12:0-PE (m/z 578.4) ([M - H]−) was used as the IS. m/z, mass to charge ratio.
ACKNOWLEDGEMENTS
We are grateful to Drs. Suparna Ray, Yunuen Hernandez and Sukla Roychowdhury for helpful discussion in the course of this investigation. We also thank Dr. Gary Olsen (University of Illinois at Urbana-Champaign) for his helpful advice on the nomenclature and evolution of PGPS enzymes and Roger Romero for his assistance with the ESI-QTOF-MS. ESN was supported in part by the Georges A. Krutilek Memorial Scholarship from UT-El Paso. This work was supported by the grant S06 GM 008012 (SD) from the National Institutes of Health. ICA is supported by NIH grants S06 GM 008012 and R01AI070655. Mass spectrometric and qRT-PCR analyses were performed in the Biomolecule Analysis Core Facility at BBRC/UTEP supported by 5G12RR008124 grant from NIH/NCRR/RCMI.
Abbreviations
- AA
arachidonic acid
- CDP-DAG
citidyldiphosphate-diacylglycerol
- CLS
cardiolipin synthase
- DAG
diacylglycerol
- DCM
dichloromethane
- ESI-QTOF-MS
electrospray ionization-quadrupole time-of-flight-mass spectrometry
- FA
fatty acid
- GC-MS
gas chromatography-mass spectrometry
- gPGPS
Giardia phosphatidylglycerolphosphate synthase
- MS
mass spectrometry
- OCFA
odd-carbon number fatty acid
- PGP
phosphatidylglycerolphosphate
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PL
phospholipid
- SM
sphingomyelin
REFERENCES
- 1.Vial HJ, Eldin P, Tielens AG, et al. Phospholipids in parasitic protozoa. Mol Biochem Parasitol. 2003;126:143–54. doi: 10.1016/s0166-6851(02)00281-5. [DOI] [PubMed] [Google Scholar]
- 2.Das S, Stevens T, Castillo C, et al. Lipid metabolism in mucous-dwelling amitochondriate protozoa. Int J Parasitol. 2002;32:655–75. doi: 10.1016/s0020-7519(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 3.Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–75. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–64. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez Y, Shpak M, Duarte TT, et al. Novel role of sphingolipid synthesis genes in regulating giardial encystation. Infect Immun. 2008;76:2939–49. doi: 10.1128/IAI.00116-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarroll EL, Muller PJ, Meyer EA, et al. Lipid and carbohydrate metabolism in Giardia lamblia. Mol Biochem Parasitol. 1981;2:187–96. doi: 10.1016/0166-6851(81)90099-2. [DOI] [PubMed] [Google Scholar]
- 7.Mohareb EW, Rogers EJ, Weiner EJ, et al. Giardia lamblia: phospholipid analysis of human isolates. Ann Trop Med Parasitol. 1991;85:591–7. doi: 10.1080/00034983.1991.11812614. [DOI] [PubMed] [Google Scholar]
- 8.Kaneda Y, Goutsu T. Lipid analysis of Giardia lamblia and its culture medium. Ann Trop Med Parasitol. 1988;82:83–90. doi: 10.1080/00034983.1988.11812213. [DOI] [PubMed] [Google Scholar]
- 9.Ellis JE, Wyder MA, Jarroll E, et al. Changes in lipid composition during in vitro encystation and fatty acid desaturase activity of Giardia lamblia. Mol Biochem Parasitol. 1996;81:13–25. doi: 10.1016/0166-6851(96)02677-1. [DOI] [PubMed] [Google Scholar]
- 10.Morrison HG, McArthur AG, Gillin FD, et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–6. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- 11.Keister D. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–8. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 12.Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–2. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 13.Gillin FD, Boucher SE, Rossi SS, et al. Giardia lamblia: the roles of bile, lactic acid and pH in the completion of the life cycle in vitro. Exp Parasitol. 1989;69:164–74. doi: 10.1016/0014-4894(89)90185-9. [DOI] [PubMed] [Google Scholar]
- 14.Kane A, Ward HD, Keusch GT, Pereira MEA. In vitro encystation of Giardia lamblia: large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. J Parasitol. 1991;77:974–81. [PubMed] [Google Scholar]
- 15.Almeida IC, Camargo MM, Procopio DO, et al. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 2000;19:1476–85. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pernet F, Pelletier C J, Milley J. Comparison of three solid-phase extraction methods for fatty acid analysis of lipid fractions in tissues of marine bivalves. J Chromatogr. 2006;1137:127–37. doi: 10.1016/j.chroma.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 17.Fridberg A, Olson CL, Nakayasu ES, et al. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J Cell Sci. 2008;121:522–35. doi: 10.1242/jcs.016741. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado RA, Kuniyoshi RK, Linss JG, et al. Trypanosoma cruzi oleate desaturase: molecular characterization and comparative analysis in other trypanosomatids. J Parasitol. 2006;92:1064–74. doi: 10.1645/GE-845R.1. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez Y, Zamora G, Ray S, et al. Transcriptional analysis of three major putative phosphatidylinositol kinase genes in a parasitic protozoan, Giardia lamblia. J Euk Microbiol. 2007;54:29–32. doi: 10.1111/j.1550-7408.2006.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altschul SF, Madden TL, Schaeffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acid Res. 1997;25:3389–409. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson J, Gibson TJ, Plewniak F, et al. Clustal X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acid Res. 1997;24:4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felsenstein J. PHYLIP - Phylogeny Inference Package (Version 3.2). Cladistics. 1989;5:164–6. [Google Scholar]
- 23.Woese C, Kanderl O, Wheelis M. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA. 1990;87:4576–9. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuccon A, Zuccon D. Department of Vertebrate Zoology and Molecular Systematics Laboratory. Swedish Museum of Natural History; Stockholm, Sweden: 2006. MrEnt v1.2, distributed by the authors. [Google Scholar]
- 25.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 26.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 27.Das S, Schteingart CD, Hofmann AF, et al. Giardia lamblia: Evidence for carrier - mediated uptake and release of conjugated bile acids. Exp. Parasitol. 1997;87:133–41. doi: 10.1006/expr.1997.4197. [DOI] [PubMed] [Google Scholar]
- 28.Das S, Castillo C, Stevens TL. Phospholipid remodeling/generation in Giardia: the role of the Lands cycle. Trends Parasitol. 2001;17:316–9. doi: 10.1016/s1471-4922(01)01901-8. [DOI] [PubMed] [Google Scholar]
- 29.Gillin FD, Reiner DS, Gault MJ, et al. Encystation and expression of cyst antigens by Giardia lamblia in vitro. Science. 1987;235:1040–43. doi: 10.1126/science.3547646. [DOI] [PubMed] [Google Scholar]
- 30.Rayan P, Stenzel D, McDonnell PA. The effects of saturated fatty acids on Giardia duodenalis trophozoite in vitro. Parasitol. Res. 2005;97:191–200. doi: 10.1007/s00436-005-1432-5. [DOI] [PubMed] [Google Scholar]
- 31.Gasteiger EA, Gattiker C, Hoogland I, et al. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;1:3784–8. doi: 10.1093/nar/gkg563. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sogin ML. Early evolution and the origin of eukaryotes. Current Opinion in Genetics and Development. 1991;1:457–63. doi: 10.1016/s0959-437x(05)80192-3. 1991. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian A, Navarro S, Carrasco R, et al. Role of exogenous inositol and phosphatidylinositol in glycosylphosphatidylinositol synthesis of GP49 by Giardia lamblia. Biochim. Biophys. Acta. 2000;1483:69–80. doi: 10.1016/s1388-1981(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 34.Gibson GR, Ramirez D, Maier J, et al. Giardia lamblia: incorporation of free and conjugated fatty acids into glycerol-based phospholipids. Exp. Parasitol. 1999;92:1–11. doi: 10.1006/expr.1999.4389. [DOI] [PubMed] [Google Scholar]
- 35.Besteiro S, Bertrand-Michel J, Lebrun M, Vial H, Dubremetz JF. Lipidomic analysis of Toxoplasma gondii tachyzoites rhoptries: further insights into the role of cholesterol. Biochem J. 2008;415:87–96. doi: 10.1042/BJ20080795. [DOI] [PubMed] [Google Scholar]
- 36.Zhao W, Róg T, Gurtovenko AA, et al. Role of phosphatidylglycerol in the stability of bacterial membranes. Biochimie. 2008;90:930–8. doi: 10.1016/j.biochi.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 37.Garidel P, Blume A. Miscibility of phosphatidylethanolamine-phosphatidylglycerol mixtures as a function of pH and acyl chain length. Eur. Biophys. J. 2000;28:629–38. doi: 10.1007/s002490050003. [DOI] [PubMed] [Google Scholar]
- 38.Tovar J, León-Avila G, Sánchez LB, et al. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature. 2003;426:172–6. doi: 10.1038/nature01945. [DOI] [PubMed] [Google Scholar]
- 39.van der Giezen M, Tovar J. Degenerate mitochondria. EMBO Reports. 2005;6:525–30. doi: 10.1038/sj.embor.7400440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Andrade Rosa I, Einicker-Lamas M, Bernardo RR, et al. Cardiolipin in hydrogenosome: evidence of symbiotic origin. Euk. Cell. 2006;5:784–7. doi: 10.1128/EC.5.4.784-787.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu FY, Kelly SL, Hatch GM, et al. N-Acetylsphingosine stimulates phosphatidylglycerolphosphate synthase activity in H9c2 cardiac cells. Biochem J. 1999;337:483–90. [PMC free article] [PubMed] [Google Scholar]
- 42.Wainwright PO, Hinkle G, Sogin ML, et al. Monophyletic origins of the metazoan: an evolutionary link with fungi. Science. 1993;260:340–42. doi: 10.1126/science.8469985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig.1. Lipid analysis by MS of G. lamblia. Total phospholipids were fractionated by silica-gel 60 and analyzed by ESI-QTOF-MS. (A and B) Positive-ion mode MS-MS spectra of C18:1/18:1-PC (m/z 792.7) C16:0/d16:1-SM at m/z 709.9, respectively. (C and D) Negative-ion mode MS-MS spectra of C18:1/C16:0-PE (m/z 716.7) and C16:0/C16:0-PI (m/z 809.7), respectively. The number at the top right corner of each spectrum indicates signal strength, measured as ion intensity at 100% relative abundance. m/z, mass to charge ratio.
Suppl. Fig. 2. Maximum parsimony tree for PGPS and its paralogs. The support values are shown at each node for 100 bootstrap replicates. The taxon name is followed by the paralog designation, either E.C. 2.7.8.5, 2.7.8.11, or CLS (cardiolipin synthase). The taxon abbreviations are the same as in Fig. 3A, with the additional organisms: Asper (Aspergillus fumigatus), Methan (Methanococcoides burtonii), Para (Paramecium tetraurelia), Pyro (Pyrococcus furiosus), and Trich (Trichomonas vaginalis).
Suppl. Fig 3. ESI-QTOF-MS spectra of relative quantitative analysis of giardial phospholipids. Total lipids were extracted and fractionated as described in Materials and Methods. Cell numbers were adjusted to 5000 cells/μL. (A and B) Positive- and negative-ion mode analysis, respectively. For positive-ion (ESI+) mode MS analysis, giardial lipid samples were spiked with 2.5 μM 11:0/11:0-PC (m/z 600.4) ([M + Li]+), used as internal standard (IS). For negative-ion (ESI-) mode MS analysis, 5 μM 12:0/12:0-PE (m/z 578.4) ([M - H]−) was used as the IS. m/z, mass to charge ratio.