Abstract
Resistance to tamoxifen treatment occurs in approximately 50% of the estrogen receptor (ER)α-positive breast cancer patients. Resistant patients would benefit from treatment with other available antiestrogens. Arzoxifene is an effective growth inhibitor of ERα-positive breast cancer cells, including tamoxifen-resistant tumors. In this study, we show that overexpression of a regular component of the ERα transcription factor complex, cyclin D1, which occurs in approximately 40% of breast cancer patients, renders cells resistant to the new promising antiestrogen, arzoxifene. Overexpression of cyclin D1 alters the conformation of ERα in the presence of arzoxifene. In this altered conformation, ERα still recruits RNA polymerase II to an estrogen response element-containing promoter, inducing transcription of an ERα-dependent reporter gene and of endogenous pS2, and promoting arzoxifene-stimulated growth of MCF-7 cells. Arzoxifene is then converted from an ERα antagonist into an agonist. This can be explained by a stabilization of the ERα/steroid receptor coactivator-1 complex in the presence of arzoxifene, only when cyclin D1 is overexpressed. These results indicate that subtle changes in the conformation of ERα upon binding to antiestrogen are at the basis of resistance to antiestrogens.
Overexpression of cyclin D1 alters the conformation of ERα in the presence of arzoxifene, leading to its conversion from an antagonist to an agonist.
Estrogen receptor α (ERα)-positive breast cancer patients are commonly treated with either aromatase inhibitors or antiestrogens. The clinically most frequently applied antiestrogen, tamoxifen, leads in an adjuvant setting to an approximately 50% reduction in recurrence during 10 yr of follow-up of ER-positive patients, and to a decrease in mortality by one third. Tamoxifen resistance, however, is still a major clinical problem in the treatment of breast cancer. Various mechanisms may account for insensitivity to tamoxifen, including activation of the MAPK, protein kinase A (PKA) and p21-activated kinase-1 (PAK1) signaling pathways that show enhanced activity in tamoxifen-resistant breast tumors (1,2,3). All of these kinase activities directly phosphorylate ERα, resulting in antiestrogen resistance. In the case of PKA- and MAPK-mediated resistance, tamoxifen binds, but then fails to induce, the inactive conformation of ERα (1,4). For PKA, we found that its activity alters the orientation between ERα and steroid receptor coactivator-1 (SRC-1) in the presence of tamoxifen, which ultimately leads to RNA polymerase II recruitment and subsequently, to ERα-dependent transactivation instead (5).
Next to phosphorylation of the ERα, expression levels and/or phosphorylation of cofactors such as SRC-1 (6) and SRC-3 (7,8) are reported to be associated with antiestrogen resistance or tamoxifen nonresponsiveness. Moreover, amplification of 11q13, a gene-rich region on chromosome 11, which is found in approximately 15% of the primary human breast cancers (9,10,11), is associated with tamoxifen resistance. Among the genes present in this region are PAK1 and cyclin D1. PAK1 overexpression is correlated with tamoxifen resistance in patients, whereas cyclin D1 overexpression by itself fails to do so (3,12); in addition, it failed to enhance cell growth in tissue culture experiments under tamoxifen conditions (13). Cyclin D1 enhances ligand-independent activation of ERα activity (14,15). The cyclin D1 protein is overexpressed in approximately 40% of human breast cancers, yet it does not indicate a poor prognosis in ERα-positive cases (16,17).
New antiestrogens are continuously generated and tested for clinical application in the treatment of breast cancer patients. These antiestrogens can be subdivided into selective estrogen receptor modulators (SERMs), such as tamoxifen, and selective estrogen receptor down-regulators (SERDs), such as ICI-182,780 (Fulvestrant), whereas antiestrogen GW5638 has mixed SERM/SERD properties (18). New compounds may then fall in one of these classes. Outcome to treatment and resistance to antiestrogens are as yet unpredictable parameters. Many factors may affect clinical response to new antiestrogens, including overexpression of cyclin D1 and/or SRC-1 in possible conjunction with activation of PKA and or MAPK.
In the present study, we found that SRC-1 overexpression did not influence efficacy of antiestrogens to inhibit ERα. Overexpression of cyclin D1, however, resulted in a conformational arrest of ERα, which indicated an activated ERα, but now in the presence of GW5638 and arzoxifene, two novel antiestrogens that are at present in phase III clinical testing. In this conformation, the ERα was capable of recruiting RNA polymerase II and inducing chromatin remodeling required for initiation of transcription. Whereas these events eventually enhanced ERα-driven transcription and tumor cell growth by arzoxifene, the breakdown of ERα by GW5638 prevented these long-term effects. Arzoxifene resistance resulted from stabilization of the cyclin D1-ERα/SRC-1 complex. Cyclin D1 overexpression did not affect the capacity of the other antiestrogens tested to inhibit ERα. Other modifications of ERα were required for induction of resistance to these antiestrogens. These findings illustrate that ER degradation is a dominant factor over a resistance-associated conformation. Moreover, they define the frequently overexpressed cofactor cyclin D1 as a causal factor in resistance to particular antiestrogens and show that subtle changes in the conformation of ERα can be the basis of resistance to antiestrogens.
Results
Overexpression of cyclin D1/SRC-1 induces resistance to antiestrogens as measured by fluorescence resonance energy transfer (FRET)
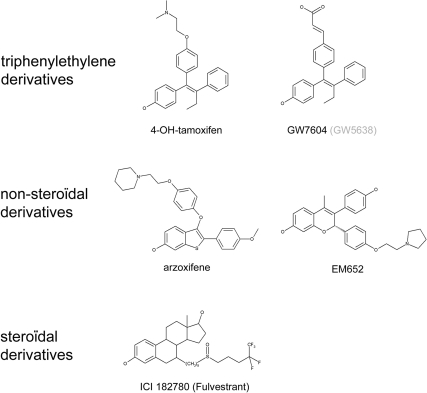
We determined the effect of overexpression of cyclin D1 and/or SRC-1 on the ability of a subset of antiestrogens to inhibit transactivation of ERα. The antiestrogens used in this study and their structure are depicted in Fig. 1. They differ widely in biological effects in vitro and in vivo (19,20). These compounds were tested in U2OS osteosarcoma cells, a highly suitable cell line for testing activation status of ERα and ERβ (1,21). U2OS cells do not express endogenous ER and did not show apparent overexpression of any of the p160 coactivators when related to other cell lines (22,23,24,25). As a first indication for the capacity of the tested antiestrogens to inactivate ERα, we applied a FRET assay. FRET is the energy transfer from a donor fluorophore to a suitable acceptor. When the two fluorophores come in close enough proximity, typically less than 80 Å, energy can be transferred from the cyan fluorescent protein (CFP) to the yellow fluorescent protein (YFP) fluorophore. This depends on the orientation and distance between the two fluorophores and intramolecular FRET (with the donor and acceptor fluorophore coupled to the termini of the ER) thus detects conformational changes. In our setup, an YFP fluorophore was fused to the N terminus and a CFP was fused to the C terminus of ERα, as illustrated in Fig. 2A (left panel). An inactive conformation of ERα is typically associated with an increase in FRET between the CFP and YFP fluorophores, as we reported previously (1,4). This enabled us to monitor the capacity of antiestrogens to inactivate ERα and their sensitivity to overexpression of cyclin D1 and SRC-1.
Figure 1.
Structure of antiestrogens used in this study.
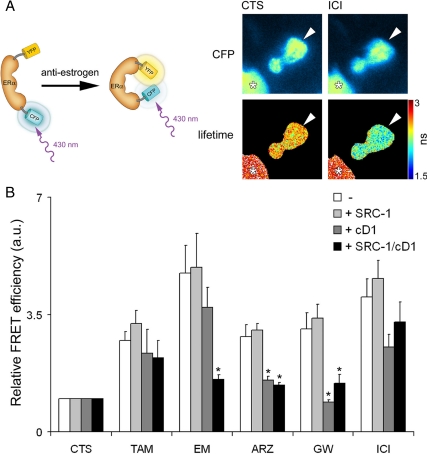
Figure 2.
Inactivation of ERα by antiestrogens measured by FLIM. A (left panel), Principle of FRET. Exciting CFP at 430 nm results in an emission from CFP unless energy is transferred to YFP, resulting in yellow light. An increased YFP emission at the expense of CFP emission can occur as the result of a conformational change of ERα affecting the orientation of the two fluorophores. A (right panel), A representative image from a FLIM measurement before and after ICI-182,780 addition. FLIM measurements were performed in U2OS cells, transfected with YFP-ERα-CFP (arrowhead). As an internal reference, these cells were cocultured with MelJuSo cells only expressing CFP (asterisk). FLIM images were generated from cells cultured in hormone-depleted (CTS) medium only or after treatment with 1 μm ICI-182,780 (ICI) before data acquisition and visualized with a lookup table with the corresponding lifetimes indicated in the bar. ns, Nanoseconds. B, FRET values as determined by FLIM in YFP-ERα-CFP expressing U2OS cells after addition of 1 μm of the indicated antiestrogen. Donor FRET efficiency (ED) was calculated as ED = 1 − (lifetime cell of interest/ lifetime reference cell). Pairwise analysis was performed, where the ED under CTS conditions is set to 1 for each experiment (relative ED); bars indicate mean and sem from n >20 cells per condition. Student’s t test was performed; *, statistically significant relative ED reduction as compared with cells transfected only with YFP-ERα-CFP and treated with the same antiestrogen, P < 0.05. ARZ, Arzoxifene; EM, EM-652; TAM, tamoxifen; GW, GW5638; ICI, ICI-182,780; CD1, cyclin D1.
To perform these experiments in a semi-high throughput manner, we applied fluorescence lifetime imaging microscopy (FLIM), a highly quantitative method for measuring FRET. In FLIM, the lifetime of the donor fluorophore emission is measured, which for the donor CFP is typically 2.7 nsec (26). This lifetime is reduced when the energy of the donor is transferred to the acceptor fluorophore in the case of FRET (27). In our assays, cells expressing YFP-ERα-CFP were cocultured with cells only expressing CFP to provide an internal control for the FLIM measurements (Fig. 2A, right panel). After addition of antiestrogen ICI-182,780, the lifetime of CFP in the YFP-ERα-CFP-expressing cells was reduced from 2.5 nsec to 2.1 nsec after treatment, indicating a donor FRET efficiency of 7% before treatment and 22% after treatment with ICI-182,780. The same cell was monitored before and after treatment with the ligand, where the FRET efficiency after treatment was related to the initial FRET efficiency, which was set at 1 (Fig. 2B). The FRET efficiency before treatment was unaltered by coexpression of cyclin D1 or SRC-1 (data not shown). A significant increase in relative FRET efficiency was found for all antiestrogens tested (Fig. 2B). However, when SRC-1 and cyclin D1 were cotransfected, the relative FRET efficiency after EM-652, arzoxifene and GW5638 addition was significantly reduced. These data were verified using an alternative ratio-based FRET approach (supplemental Fig. S1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), where synergy between cyclin D1/SRC-1 overexpression and PKA activity, which had also been illustrated previously (1), could be observed. When FRET was measured in cells overexpressing SRC-1 alone, no effect of SRC-1 overexpression was found on the relative FRET efficiency for any of the tested antiestrogens. Cyclin D1 overexpression, however, did result in reduced FRET efficiency for arzoxifene and GW5638, indicating that an elevated cyclin D1 level is sufficient to induce resistance against these two compounds as determined by this biophysical approach.
Overexpression of cyclin D1 enhances recruitment of RNA polymerase II to the ERα promoter in the presence of antiestrogens
Our FRET and FLIM results suggested that resistance to antiestrogens GW5638 and arzoxifene may be due to overexpression of cyclin D1. To determine whether this altered conformation of ERα by cyclin D1 also induces transcription in the presence of these antiestrogens, we assayed their effects on RNA polymerase II binding to an artificial prolactin promoter/enhancer (PRL) array in HeLa cells. These cells contain a DNA array, consisting of approximately 200 copies of stably transfected modified PRL (28). The PRL array allowed visualization of a defined DNA structure in the nucleus where ERα and cofactors are recruited to regulate reporter gene mRNA synthesis. Resistance to particular antiestrogens by overexpression of cyclin D1 may result in antiestrogen-mediated transactivation of ERα and consequently to recruitment of RNA polymerase II to the PRL array. To ensure cyclin D1 coexpression in ERα-expressing cells, 5 times more cyclin D1 expression vector was transfected than of ERα. This was verified in a separate staining experiment (see supplemental Fig. S2), where overexpressed cyclin D1 was recruited to the PRL array structure, colocalizing with ERα. Cells expressing ERα-CFP showed colocalization with RNA polymerase II at the DNA array when treated for 2 h under conditions of charcoal-treated serum (CTS; deprived of hormone) and estradiol (E2), as quantified in Fig. 3 and shown in supplemental Fig. S3. The average DNA array size was 1.9 μm2 under CTS conditions, which increased to 2.8 μm2 when E2 was added to the cells. This widening of the DNA array structure was previously reported to be associated with increased transcriptional activity (28,29). Overexpression of cyclin D1 did not significantly alter the array size or RNA polymerase II recruitment under CTS conditions. The effect of an unliganded ERα on the PRL array is apparently different from an arzoxifene-bound ER, because the size of the PRL array is larger under CTS conditions than under antiestrogen conditions. Moreover, RNA polymerase II was recruited to the PRL array in the absence of hormone, but not in the presence of antiestrogens (supplemental Fig. S3).
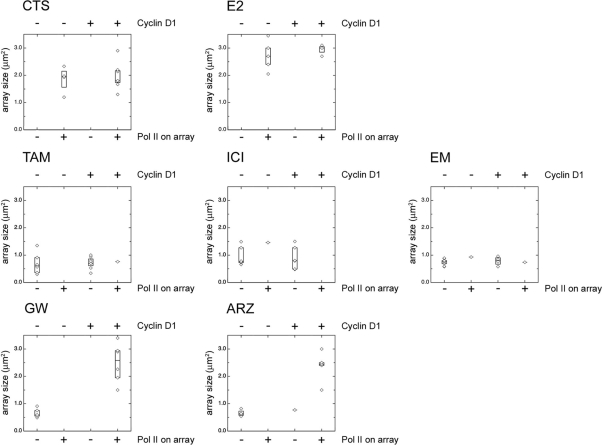
Figure 3.
Cyclin D1 overexpression induces RNA polymerase II recruitment on the PRL array and increased array size in the presence of GW5638 and arzoxifene. Staining for RNA polymerase II signals on the PRL array. PRL array containing HeLa cells were transfected with ERα-CFP alone or cotransfected with cyclin D1. Cells were cultured in hormone-depleted (CTS) medium and treated with 1 μm E2 or with 1 μm of the indicated antiestrogen for 2 h and then fixed and stained for RNA polymerase II. The data are subdivided in the presence or absence of RNA polymerase II on the array and presented in a box plot with the horizontal bar indicating the median array size (determined on RNA polymerase II signal) in μm2. The box size is determined by the upper and lower quartiles, the median value of the upper and lower half of the data points (for each experiment, n > 10), respectively. Microscopic images of the cells are shown in supplemental Fig. S3. ARZ, Arzoxifene; EM, EM-652; TAM, tamoxifen; GW, GW5638; ICI, ICI-182,780; E2, estradiol; Pol II, RNA polymerase II.
Addition of any of the tested antiestrogens resulted in a loss of RNA polymerase II from the PRL array structure, and in a marked condensation of the array size (Fig. 3 and supplemental Fig. S3), which is indicative of an inactive ERα (28). Cyclin D1 overexpression did not affect RNA polymerase II recruitment for cells treated with tamoxifen, ICI-182,780, and EM-652, and the PRL array appeared condensed. However, when cyclin D1 was coexpressed, RNA polymerase II recruitment was restored for cells treated with GW5638 and arzoxifene. In addition, the array size for both GW5638 and arzoxifene was increased from 0.7 to 2.4 μm2 in the presence of exogenous cyclin D1. Our findings demonstrated that cyclin D1 overexpression prevented an increase in the FRET signal for arzoxifene and GW5638 and resulted in a recruitment of RNA polymerase II and in a subsequent widening of the array structure. All of these features indicate that resistance to GW5638 and arzoxifene can be induced by overexpression of cyclin D1.
Overexpression of cyclin D1 enhances ERα-mediated transcription and subsequent growth of MCF-7 cells in the presence of arzoxifene
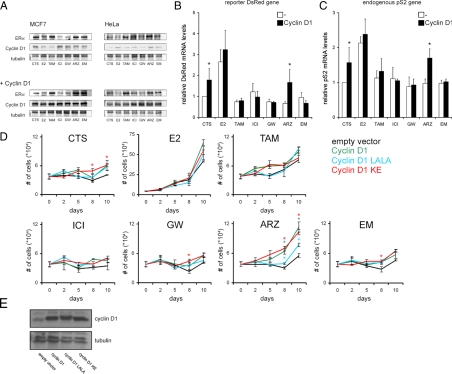
To study the direct consequences of GW5638 and arzoxifene-driven, ERα-mediated RNA polymerase II recruitment under conditions of cyclin D1 overexpression as a means of drug resistance, we measured transcription of an integrated DsRed2 reporter gene that is under control of the ER-responsive elements on the PRL array (28) by quantitative RT-PCR (Fig. 4B). These cells were transfected with ERα-CFP and treated overnight with the ligand, after which RNA was isolated for RT-PCR analysis, as described in Materials and Methods. Only cells treated with E2 showed an increase in DsRed2 mRNA levels of 2.7-fold; this in contrast to any of the antiestrogens tested. DsRed2 mRNA levels increased under hormone-deprived conditions 1.8-fold over control CTS values when cyclin D1 was overexpressed, which is consistent with the hormone-independent activation of ERα in cyclin D1-overexpressing cells (15). When cyclin D1 was overexpressed, only cells treated with arzoxifene showed increased DsRed2 mRNA levels (from 0.7 times related to control without exogenous cyclin D1 to 1.7 times over control in the presence of exogenous cyclin D1). No enhanced transcription was observed in GW5638-treated cells, for reasons described below. Not only activation of an exogenous reporter gene, but also transcription of an estrogen response element-dependent endogenous gene, was tested, namely pS2 in MCF-7 breast cancer cells. pS2 mRNA levels were enhanced by arzoxifene when cyclin D1 was overexpressed, whereas none of the other antiestrogens tested did so (Fig. 4C). The RT-PCR data for the levels of endogenous pS2 mRNA in MCF-7 cells showed similar results as was observed for the DsRed2 mRNA reporter signals in HeLa cells (Fig. 4B). These data indicated that overexpression of cyclin D1 induced resistance to arzoxifene by enhancing expression of endogenous and of exogenously transfected, ER-dependent genes. Because transfection efficiency of cyclin D1 and its mutants was approximately 40–50% in MCF7 and Hela cells (data not shown), the results obtained in Fig. 4 represented underestimates of the effects of these cyclin D1 constructs. Although overexpression of cyclin D1 also showed resistance to GW5638, as indicated by FRET and RNA polymerase II recruitment analysis, this did not result in elevated transcription of ER-dependent genes in the presence of GW5638. GW5638, however, is known to possess SERD capacities, which will lead to degradation of ERα. The discrepancy between the (short term) FRET and RNA polymerase II recruitment assays which indicated resistance, and the (long term) absence of DsRed2 and pS2 mRNA induction could be explained by GW5638-associated degradation of ERα that will then prevent specific transcription on the long term. Western blot analysis of ERα-transfected PRL-HeLa cells and MCF-7 cells expressing only endogenous ERα, which were treated with the different antiestrogens with or without additional cyclin D1 overexpression, confirmed the SERD nature of GW5638 (Fig. 4A). The ERα protein levels in both ERα-transfected HeLa and MCF-7 cells corresponded in a comparable way to the treatment with (anti-)estrogens, where E2 lowered and tamoxifen stabilized the protein level of ERα, as compared with untreated cells. The ERα protein levels for cells treated with GW5638 or ICI-182,780, a widely accepted SERD, were similar and showed that treatment with these compounds induced degradation of ERα. Treatment with arzoxifene and EM-562, however, stabilized the protein level of ERα similarly as for tamoxifen. Cyclin D1 overexpression had no effect on the protein level of ERα in the cells treated with the various antiestrogens.
Figure 4.
Cyclin D1 overexpression enhances ERα-mediated transcription and cell growth of MCF-7 cells in the presence arzoxifene. A, ERα protein stability in MCF-7 and Hela cells, with expression of either endogenous ERα or exogenous ERα-CFP, respectively. Where indicated, cells were cotransfected with cyclin D1. Cells were treated overnight with the indicated antiestrogens, analyzed by Western blot, and stained for ERα and cyclin D1, using tubulin as a loading control. B, QPCR of the DsRed reporter gene was performed on Hela PRL array cells, which were transfected with ERα and cyclin D1 with empty vector as a control and treated overnight with E2 or antiestrogen or left untreated. mRNA levels were measured as described in Materials and Methods, and related to values under CTS conditions, which were set to 1. Bars indicate sd from three independent experiments. *, Statistically significant difference in DsRed mRNA levels between the cyclin D1-transfected cells and the empty vector-transfected cells treated with the same antiestrogen; P < 0.05. C, QPCR of the endogenous pS2 gene was performed on MCF-7 cells, transfected with cyclin D1 or with empty vector as a control. Cells were treated with E2 or antiestrogen for 5 h or left untreated. mRNA levels were measured as described in Materials and Methods and related to CTS values that were set to 1. Bars indicate sd from two independent experiments in duplicate. *, Statistically significant difference in pS2 mRNA levels between the cyclin D1-transfected cells and the empty vector-transfected cells treated with the same antiestrogen; P < 0.05. D, Cell growth of MCF-7 cells transfected with cyclin D1, cyclin D1 LALA, cyclin D1 KE, or empty vector. Cells were seeded in 24-well plates and cultured in the presence of the indicated antiestrogens for 10 d, where samples were taken directly after seeding and after 2, 5, 8, and 10 d of ligand exposure. Shown are mean and sd from triplicate samples. *, Statistically significant difference in cell growth between the cyclin D1-transfected cells and the empty vector-transfected cells (black lines) treated with the same antiestrogen, within the same timepoint; P < 0.05 (green asterisk for cyclin D1 wild type, blue asterisk for cyclin D1 LALA, and red asterisk for cyclin D1 KE). E, Overexpression of cyclin D1 wild type and cyclin D1 mutants in MCF-7 cells. Western blot analysis of MCF-7 cells, transfected by electroporation with an empty vector, cyclin D1 wild type, cyclin D1 LALA, or cyclin D1 KE. Blots were stained with antibodies detecting cyclin D1 and tubulin, verifying equal overexpression of the applied cyclin D1 variants. ARZ, Arzoxifene; EM, EM-652; GW, GW5638; ICI, ICI-182,780; E2, estradiol.
To show a biological effect of cyclin D1 overexpression on resistance to arzoxifene, we performed a cell growth assay using the MCF-7 breast tumor cell line (Fig. 4D), of which the proliferation is dependent on an activated ERα. The MCF-7 cells were therefore transfected with various mutants of cyclin D1 or with an empty pcDNA3-YFP vector as a negative control. In comparison with wild-type cyclin D1, a cyclin D1 LALA mutant that fails to stabilize ERα/SRC-1 interaction, and a cyclin D1 KE mutant that is defective in activating cdk (cyclin-dependent kinase) (30) were used. The cyclin D1 KE mutant stimulates ERα/SRC-1-dependent cell growth, whereas the cyclin D1 LALA mutant might enhance proliferation, independently of ERα activity (29). The cells were cultured for 10 d in the presence of the antiestrogens, and samples were taken every few days for cell counting. For each ligand, except for arzoxifene, minor effects were observed on the cell growth when cyclin D1 variants were expressed. Cells treated with arzoxifene, however, significantly increased in number when cyclin D1 was transfected. The cells transfected with the cyclin D1 KE mutant, stimulating ERα/SRC-1-dependent proliferation, showed a comparable growth response. This was not the case in cells transfected with cyclin D1 LALA, in which the cell growth was increased, but not to the extent of the cyclin D1 variants that are capable of stabilizing ERα/SRC-1 interactions. These data showed that the cyclin D1-dependent cell growth of MCF-7 cells was stimulated in the presence of arzoxifene, indicating genuine antiestrogen resistance to this compound by overexpression of cyclin D1. The effect of cyclin D1 on proliferation is not observed in MCF-7 cells under CTS conditions, indicating that this effect is specific for converting the antiestrogen arzoxifene from an antagonistic into an agonistic compound. Whether, in that case, specific gene targets are involved that induce proliferation by arzoxifene-bound ER but not by unliganded ER when cyclin D1 is overexpressed remains to be studied.
The effects of the cyclin D1 KE mutant on arzoxifene-mediated proliferation were not mediated by enhanced cdk2 activity through sequestration of the cdk inhibitors p21 and p27, as illustrated by coimmunoprecipitation experiments (supplemental Fig. S4).
Cyclin D1 stabilizes ERα/SRC-1 interactions in the presence of arzoxifene
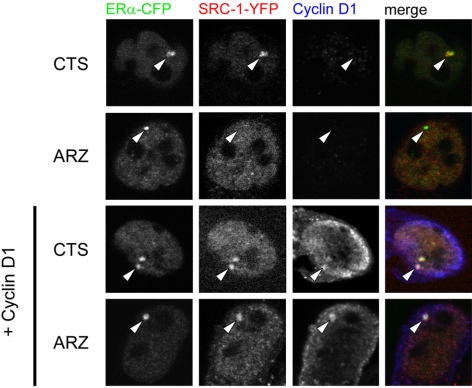
Overexpression of cyclin D1 apparently facilitates transcription and cell growth in the presence of arzoxifene, but the molecular mechanism for this effect is unclear. To investigate the mechanism of the arzoxifene-specific transcription under conditions of cyclin D1 overexpression, we investigated ERα/SRC-1 complex formation in the PRL array containing HeLa cells, with or without cyclin D1 overexpression (Fig. 5). In nontreated cells, colocalization between ERα and SRC-1 occurred in the absence and presence of exogenous cyclin D1. In cells treated with arzoxifene, however, SRC-1 recruitment to the array was lost. When cyclin D1 was cotransfected, however, both SRC-1 and cyclin D1 were recruited to the ERα-containing array structure. These data imply that the arzoxifene-induced dissociation of the ER/SRC-1 complex was prevented by cyclin D1 overexpression. Identical results were obtained by using a fluorescence recovery after photobleaching approach (presented in supplemental Fig. S5).
Figure 5.
Cyclin D1 stabilizes the complex between ERα and SRC-1 under arzoxifene conditions. Cyclin D1 overexpression stabilizes SRC-1 recruitment by ERα on the PRL array. PRL array containing HeLa cells were transfected with ERα-CFP and SRC-1-YFP and either with cyclin D1 or with empty vector. Cells were cultured in hormone-depleted (CTS) medium and treated with 1 μm arzoxifene for 2 h or left untreated and then fixed and stained for cyclin D1. Arrowheads indicate the PRL array. A representative image of n > 15 was chosen for each condition. No endogenous level of cyclin D1 could be detected in HeLa cells with the antibody applied. ARZ, Arzoxifene.
These results indicated that overexpression of cyclin D1 induced resistance to arzoxifene by stabilizing the ERα/SRC-1 complex.
Discussion
Ligands of ERα induce specific conformations of the receptor, with alterations beyond the ligand-binding domain of ERα. These altered conformations include distinctive orientations of the receptor’s carboxyl-terminal transactivation helix (31) and are recognized by particular peptides that bind to distinct ERα-ligand conformations (32). A glimpse of these different conformations is revealed in x-ray crystallography studies of ERα-ligand interactions that are restricted to the activation function 2 moiety of the receptor (33). We have demonstrated that these distinct ERα-ligand conformations have far reaching consequences, because they influence resistance to a particular antiestrogen (1,4). Responses to each antiestrogen can be characterized by a specific set of phosphorylation sites in ERα that, when being phosphorylated by PKA and/or MAPK, are responsible for resistance to that particular antiestrogen. These phosphorylations are responsible for an altered conformation of ERα as detected by intramolecular FRET. In the present study, we showed that overexpression of cyclin D1, here a specific cofactor of ERα, renders ERα resistant to new third generation antiestrogens, arzoxifene and GW5638. Arzoxifene is an effective growth inhibitor of ERα-positive human breast cancer cells, including tamoxifen-resistant cells (34,35), and would be an alternative SERM in the clinic next to the broadly applied tamoxifen. We demonstrated that overexpression of cyclin D1, however, alters the conformation of ERα in the presence of arzoxifene (by FRET and FLIM), and showed that ERα in this altered conformation still forms a complex with SRC-1 and recruits RNA polymerase II to an estrogen response element-containing promoter (Figs. 2, 3, and 5). This then promotes transcription of either endogenous or exogenously transfected, ER-dependent genes and stimulates growth of MCF-7 cells in an arzoxifene-dependent manner (Fig. 4). Overexpression of cyclin D1 thus converts arzoxifene from an antagonist into an agonist. This feature, among others, may have been causally involved in a significantly shorter progression-free survival and time to treatment failure of arzoxifene-treated patients as compared with tamoxifen in the treatment of locally advanced and metastatic breast cancer (36). However, no material of the breast tumors of these patients was available to study this.
In previous studies, we have demonstrated that FRET results in MCF-7 cells are similar to those performed in U2OS cells, excluding any cell-specific FRET effect (1).
Overexpression of cyclin D1 is also responsible for the two short-term markers of antiestrogen resistance (a conformational change of the receptor and a recruitment of RNA polymerase II) when the SERM/SERD compound GW5638 was used but failed to stimulate GW5638-mediated gene expression and growth of MCF-7 cells. The SERD nature of the mixed antagonist GW5638 induced degradation of ERα, even in the presence of overexpressed cyclin D1 and prevented the late features of antiestrogen resistance to occur (Fig. 4, A–D). This is comparable to the resistance found for Fulvestrant, ICI-182,780, where ERα becomes resistant to Fulvestrant when it is phosphorylated by both PKA at Serine 305 and by MAPK at Serine 118 (4), or by phosphorylation of Serine 305 by PKA in conjunction with overexpression of cyclin D1 and SRC-1 (1). This resistance coincides with an altered ERα conformation but is not sustained in enhanced Fulvestrant-dependent reporter read-out or proliferation. The two short-term features of antiestrogen resistance (conformational alteration of ERα and recruitment of cofactors and RNA polymerase II) are immediate effects upon the ERα-SERD binding, whereas the two long-term features (transcription and cell growth) take place during, or after, a SERD-mediated degradation of ERα. Apparently, degradation of ERα is not prevented by the modifications that render ERα resistant to SERMs.
Cyclin D1 forms a specific complex with the ER that facilitates ligand-independent recruitment of coactivators to the receptor complex (15,37). It is noteworthy that cyclin D1 overexpression does not lead to resistance to tamoxifen under experimental conditions (13) and does not predict response to tamoxifen treatment in breast cancer patients (12,38). Our results confirmed these findings by the absence of a FRET change (Fig. 2), RNA polymerase II recruitment (Fig. 3), nor tamoxifen-dependent gene activation and cell growth (Fig. 4) under tamoxifen conditions when cyclin D1 is overexpressed.
This suggests that each antiestrogen induces different conformations of ERα with different downstream effects. This is further complicated by different modifications of ERα that affect the various antiestrogens differently with unpredictable effects on their antagonistic or (sometimes) agonistic action. For arzoxifene and GW5638, we show here that overexpression of cyclin D1 renders ERα resistant to these compounds. Cyclin D1 protein binds to the 338–379 amino acid (aa) region of ERα (39), just adjacent to the hinge region, in which Serine 305, when phosphorylated by PKA, determines resistance to tamoxifen.
Our findings of antiestrogen-specific modifications of ERα that are responsible for resistance to particular antiestrogens may be of clinical relevance, because they indicate resistance to a particular antiestrogen in the treatment of breast cancer (40) and could provide guidelines for the use and development of new antiestrogens. Both of these may aid in developing personalized endocrine treatment in breast cancer, in which different antiestrogens are subsequently applied in a rationalized manner, instead of in an empirical order as is now common practice (41).
Materials and Methods
Cell culture, transfection, constructs, and antibodies
Human osteosarcoma U2OS, MCF-7, and HeLa cells were cultured in DMEM in the presence of 10% fetal calf serum and standard antibiotics. Cells containing ERα were cultured in phenol red-free DMEM containing 5% charcoal-treated serum (CTS; Hyclone Laboratories, Logan, UT). For the FRET and FLIM experiments, cells were cultured overnight on 2-cm round glass coverslips. Cells were transfected 24 h before analysis with pcDNA3-YFP-ERα-CFP or cotransfected with cyclin D1 and/or SRC-1, where indicated, using polyethylenimine (molecular mass, 25 kDa; Polysciences, Warrington, PA) (42). 4-OH-tamoxifen (Sigma, St. Louis, MO), EM-652 (kindly provided by Dr. C. Labrie, University of Quebec, Canada), arzoxifene, GW7604, the active form of GW5638 (43) (kindly provided by GlaxoSmithKline, Research Triangle Park, NC), or ICI-182,780 (Tocris, Ellisville, MO) were added at a final concentration of 1 μm, to ensure saturating ligand concentrations for overexpressed ERα. Forskolin (Sigma) was added 15 min before measurements at a final concentration of 10 μm.
YFP-ERα-CFP, ERα-CFP, SRC-1, SRC-1(aa 623–711)-YFP, SRC-1(aa 1051–1240)-YFP, and cyclin D1 constructs were generated as described previously (1,5,30). All used constructs and point mutants were sequence verified. Antibodies applied in this study were raised against ERα (Novocastra Laboratories, Newcastle, UK), tubulin (Sigma), cyclin D1 (Santa Cruz Biotechnology, Santa Cruz, CA), RNA polymerase II (8WG16; Covance, Laboratories, Inc., Madison, WI), and GFP (44).
FLIM
Before FLIM experiments, cells on coverslips were mounted in bicarbonate-buffered saline in a heated tissue culture chamber at 37 C under 5% CO2. FLIM experiments were performed on a Leica inverted DM-IRE2 microscope (Leica, Inc., Deerfield, IL) equipped with a Lambert Instruments frequency domain lifetime attachment (Leutingewolde, The Netherlands), controlled by the vendor’s LI FLIM software. CFP was excited at 430 nm with approximately 4 mW power using a LED modulated at 40 MHz. Emission was collected at 450–490 nm using an intensified charge-coupled device camera. FLIM measurements were performed in U2OS cells, transfected with YFP-ERα-CFP only, or cotransfected with cyclin D1 and/or SRC-1 constructs. Calculated CFP lifetimes were referenced to a 1 μm solution of Rhodamine-G6 in medium that was set at 4.11 nsec lifetime and internally calibrated using cocultured CFP containing MelJuSo reference cells for which the lifetime was set to 2.7 nsec (26). Donor FRET efficiency (ED) was calculated as ED = 1 − (lifetime cell of interest/lifetime reference cell). Pair-wise analysis was performed for each cell before and after addition of antiestrogen, where the ED under CTS conditions is set to 1 for each experiment (relative ED).
Confocal laser scanning microscopy analysis
For confocal laser scanning microscopy analysis, prolactin promoter/enhancer (PRL) array containing HeLa cells (28) was cultured in DMEM containing CTS and was transfected where indicated with ERα-CFP, SRC-1-YFP and/or cyclin D1 by electroporation and subsequently seeded in DMEM without phenol red supplemented with 5% CTS. Cells were treated for 2 h with 1 μm E2, 1 μm of the indicated antiestrogen, or left untreated, to ensure saturating ligand concentrations for overexpressed ERα. Thereafter, cells were fixed with 3.7% formaldehyde in PBS and subsequently stained with antibodies detecting RNA polymerase II (8WG16; Covance, Inc.) or anticyclin D1 (Santa Cruz) and secondary antibodies conjugated to Alexa 647 (Molecular Probes, Leiden, The Netherlands). Images were taken with a Leica TCS SP2 System equipped with a ×63 oil emersion objective. CFP was excited at 458 nm, and emission was measured at 460–500 nm. Alexa 647 was excited at 633 nm; emission was measured at 645–720 nm. Array size quantification was performed using Leica software and plotted using proFit (QuantumSoft).
Quantitative RT-PCR
Prolactin promoter/enhancer (PRL-) array containing HeLa cells (28) was transfected with ERα-CFP only or cotransfected with cyclin D1 by electroporation and subsequently cultured in phenol-red free DMEM containing CTS. Immediately after seeding, the cells were treated with 10 nm E2 or 100 nm antiestrogen for 16 h or the cells were left untreated. For quantitative RT-PCR in MCF-7 cells, cells were cultured in phenol red-free DMEM containing 5% charcoal-treated serum for 72 h before transfection. Subsequently, cells were transfected with cyclin D1 or pcDNA3 empty vector and cultured overnight, after which cells were treated with 10 nm E2 or 100 nm antiestrogen for 5 h or the cells were left untreated. After exposure to hormones, cells were lysed and RNA was extracted using Trizol (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. RNA was reverse transcribed using SuperScript III Reverse Transcriptase (Invitrogen), on which quantitative PCR (QPCR) was performed using CYBR Green (Applied Biosystems, Foster City, CA), according to the manufacturer’s protocols. The DsRed2 cDNA was amplified with the forward primer 5′-CCAGTTCCAGTACGGCTCCA and the reverse primer 5′-GCCGTCCTCGAAGTTCATCA. pS2 cDNA was amplified with the forward primer 5′-CATCGACGTCCCTCCAGAAGA and the reverse primer 5′-CTCTGGGACTAATCACCGTGCT. As a control for equal loading, the observed signals were related to β-actin RNA levels, using a forward primer 5′-CCTGGCACCCAGCACAAT and reverse primer 5′-GGGCCGGACTCGTCATACT.
Cell growth assay
MCF-7 cells, grown on 5% CTS serum containing phenol-red free DMEM for 7 d, were electroporated with cyclin D1 wild type, cyclin D1-L244A/L245A (LALA), cyclin D1-K112E (KE), or pcDNA3-YFP empty vector as control. Immediately after electroporation, 40,000 cells were seeded in per well (24-well plate). Cells were treated with 10 nm E2, 100 nm antiestrogen or left untreated, and ligand-supplemented medium was replaced every 2 d. At 10 h after seeding, cells were detached from the plate using trypsin and fixed in 3.7% formaldehyde in PBS in triplicate. Similar samples were taken at 2, 5, 8, and 10 d after transfection. Total number of cells obtained from each well was determined using a Casy model TT cell counter (Schärfe-System GmbH, Reutlingen, Germany).
Supplementary Material
Acknowledgments
We thank Lennert Janssen and Cristiane Bentin-Toaldo for assistance in generating the chimeric mutants of ERα and SRC-1 and Coenraad Kuijl for help with fluorescence recovery after photobleaching analysis (all from The Netherlands Cancer Institute).
Footnotes
This work was supported by Grant NKI 2005-3388 of the Dutch Cancer Society and was in part performed within the framework of Top Institute Pharma Project No. T3-107.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 28, 2009
Abbreviations: aa, Amino acid; CFP, cyan fluorescent protein; CTS, charcoal-treated serum; E2, estradiol; ER, estrogen receptor; FLIM, fluorescence lifetime imaging microscopy; FRET, fluorescence resonance energy transfer; PAK1, p21-activated kinase-1; PKA, protein kinase A; PRL, prolactin promoter/enhancer array; SERD, selective ER down-regulator; SERM, selective ER modulator; SRC, steroid receptor coactivator; YFP, yellow fluorescent protein.
References
- Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, Floore A, Velds A, van't Veer L, Neefjes J 2004 Tamoxifen resistance by a conformational arrest of the estrogen receptor α after PKA activation in breast cancer. Cancer Cell 5:597–605 [DOI] [PubMed] [Google Scholar]
- Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Dowsett M 2005 Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol 23:2469–2476 [DOI] [PubMed] [Google Scholar]
- Holm C, Rayala S, Jirström K, Stål O, Kumar R, Landberg G 2006 Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst 98:671–680 [DOI] [PubMed] [Google Scholar]
- Zwart W, Griekspoor A, Rondaij M, Verwoerd D, Neefjes J, Michalides R 2007 Classification of anti-estrogens according to intramolecular FRET effects on phospho-mutants of estrogen receptor α. Mol Cancer Ther 6:1526–1533 [DOI] [PubMed] [Google Scholar]
- Zwart W, Griekspoor A, Berno V, Lakeman K, Jalink K, Mancini M, Neefjes J, Michalides R 2007 PKA-induced resistance to tamoxifen is associated with an altered orientation of ERα towards co-activator SRC-1. EMBO J 26:3534–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Brown M 2002 Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Tsai SY, O'Malley BW 2004 Selective estrogen receptor modulators 4-hydroxytamoxifen and raloxifene impact the stability and function of SRC-1 and SRC-3 coactivator proteins. Mol Cell Biol 24:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R 2003 Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361 [DOI] [PubMed] [Google Scholar]
- Karlseder J, Zeillinger R, Schneeberger C, Czerwenka K, Speiser P, Kubista E, Birnbaum D, Gaudray P, Theillet C 1994 Patterns of DNA amplification at band q13 of chromosome 11 in human breast cancer. Genes Chromosomes Cancer 9:42–48 [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL 2003 Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res Treat 78:323–335 [DOI] [PubMed] [Google Scholar]
- Schuuring E, Verhoeven E, Mooi WJ, Michalides RJ 1992 Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene 7:355–361 [PubMed] [Google Scholar]
- Bostner J, Ahnström Waltersson M, Fornander T, Skoog L, Nordenskjöld B, Stål O 2007 Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene 26:6997–7005 [DOI] [PubMed] [Google Scholar]
- Pacilio C, Germano D, Addeo R, Altucci L, Petrizzi VB, Cancemi M, Cicatiello L, Salzano S, Lallemand F, Michalides RJ, Bresciani F, Weisz A 1998 Constitutive overexpression of cyclin D1 does not prevent inhibition of hormone-responsive human breast cancer cell growth by antiestrogens. Cancer Res 58:871–876 [PubMed] [Google Scholar]
- Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ 1997 CDK-independent activation of estrogen receptor by cyclin D1. Cell 88:405–415 [DOI] [PubMed] [Google Scholar]
- Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R 1998 Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev 12:3488–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R, Hageman P, van Tinteren H, Houben L, Wientjens E, Klompmaker R, Peterse J 1996 A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer 73:728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diest PJ, Michalides RJ, Jannink L, van der Valk P, Peterse HL, de Jong JS, Meijer CJ, Baak JP 1997 Cyclin D1 expression in invasive breast cancer. Correlations and prognostic value. Am J Pathol 150:705–711 [PMC free article] [PubMed] [Google Scholar]
- Wu YL, Yang X, Ren Z, McDonnell DP, Norris JD, Willson TM, Greene GL 2005 Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell 18:413–424 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Zhao H, Fuqua SA 2000 Selective estrogen receptor modulators: structure, function, and clinical use. J Clin Oncol 18:3172–3186 [DOI] [PubMed] [Google Scholar]
- Robertson JF 2004 Selective oestrogen receptor modulators/new antioestrogens: a clinical perspective. Cancer Treat Rev 30:695–706 [DOI] [PubMed] [Google Scholar]
- Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS 2004 Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology 145:3473–3486 [DOI] [PubMed] [Google Scholar]
- Levy N, Zhao X, Tang H, Jaffe RB, Speed TP, Leitman DC 2007 Multiple transcription factor elements collaborate with estrogen receptor α to activate an inducible estrogen response element in the NKG2E gene. Endocrinology 148:3449–3458 [DOI] [PubMed] [Google Scholar]
- Monroe DG, Johnsen SA, Subramaniam M, Getz BJ, Khosla S, Riggs BL, Spelsberg TC 2003 Mutual antagonism of estrogen receptors α and β and their preferred interactions with steroid receptor coactivators in human osteoblastic cell lines. J Endocrinol 176:349–357 [DOI] [PubMed] [Google Scholar]
- Rogatsky I, Zarember KA, Yamamoto KR 2001 Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J 20:6071–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LJ, Levy N, Zhao X, Griffin C, Tagliaferri M, Cohen I, Ricke WA, Speed TP, Firestone GL, Leitman DC 2009 Cell type- and estrogen receptor-subtype specific regulation of selective estrogen receptor modulator regulatory elements. Mol Cell Endocrinol 299:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer JE, Van Munster EB, Vischer NO, Gadella Jr TW 2004 Probing plasma membrane microdomains in cowpea protoplasts using lipidated GFP-fusion proteins and multimode FRET microscopy. J Microsc 214:190–200. [DOI] [PubMed] [Google Scholar]
- Bastiaens PI, Squire A 1999 Fluorescence lifetime imaging microscopy: spatial resolution of biochemical processes in the cell. Trends Cell Biol 9:48–52 [DOI] [PubMed] [Google Scholar]
- Sharp ZD, Mancini MG, Hinojos CA, Dai F, Berno V, Szafran AT, Smith KP, Lele TP, Lele TT, Ingber DE, Mancini MA 2006 Estrogen-receptor-α exchange and chromatin dynamics are ligand- and domain-dependent. J Cell Sci 119:4101–4116 [DOI] [PubMed] [Google Scholar]
- Hatzis P, Talianidis I 2002 Dynamics of enhancer-promoter communication during differentiation-induced gene activation. Mol Cell 10:1467–1477 [DOI] [PubMed] [Google Scholar]
- Bindels EM, Lallemand F, Balkenende A, Verwoerd D, Michalides R 2002 Involvement of G1/S cyclins in estrogen-independent proliferation of estrogen receptor-positive breast cancer cells. Oncogene 21:8158–8165 [DOI] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engström O, Ohman L, Greene GL, Gustafsson JA, Carlquist M 1997 Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758 [DOI] [PubMed] [Google Scholar]
- Wijayaratne AL, Nagel SC, Paige LA, Christensen DJ, Norris JD, Fowlkes DM, McDonnell DP 1999 Comparative analyses of mechanistic differences among antiestrogens. Endocrinology 140:5828–5840 [DOI] [PubMed] [Google Scholar]
- Pike AC 2006 Lessons learnt from structural studies of the oestrogen receptor. Best Pract Res Clin Endocrinol Metab 20:1–14 [DOI] [PubMed] [Google Scholar]
- Buzdar A, O'Shaughnessy JA, Booser DJ, Pippen Jr JE, Jones SE, Munster PN, Peterson P, Melemed AS, Winer E, Hudis C 2003 Phase II, randomized, double-blind study of two dose levels of arzoxifene in patients with locally advanced or metastatic breast cancer. J Clin Oncol 21:1007–1014 [DOI] [PubMed] [Google Scholar]
- Freddie CT, Larsen SS, Bartholomaeussen M, Lykkesfeldt AE 2004 The effect of the new SERM arzoxifene on growth and gene expression in MCF-7 breast cancer cells. Mol Cell Endocrinol 219:27–36 [DOI] [PubMed] [Google Scholar]
- Deshmane V, Krishnamurthy S, Melemed AS, Peterson P, Buzdar AU 2007 Phase III double-blind trial of arzoxifene compared with tamoxifen for locally advanced or metastatic breast cancer. J Clin Oncol 25:4967–4973 [DOI] [PubMed] [Google Scholar]
- Neuman E, Ladha MH, Lin N, Upton TM, Miller SJ, DiRenzo J, Pestell RG, Hinds PW, Dowdy SF, Brown M, Ewen ME 1997 Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol 17:5338–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R, van Tinteren H, Balkenende A, Vermorken JB, Benraadt J, Huldij J, van Diest P 2002 Cyclin A is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br J Cancer 86:402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Fan S, Li Z, Fu M, Rao M, Ma Y, Lisanti MP, Albanese C, Katzenellenbogen BS, Kushner PJ, Weber B, Rosen EM, Pestell RG 2005 Cyclin D1 antagonizes BRCA1 repression of estrogen receptor α activity. Cancer Res 65:6557–6567 [DOI] [PubMed] [Google Scholar]
- Holm C, Kok M, Michalides R, Fles R, Koornstra RH, Wesseling J, Hauptmann M, Neefjes J, Peterse JL, Stål O, Landberg G, Linn SC 2009 Phosphorylation of the oestrogen receptor α at serine 305 and prediction of tamoxifen resistance in breast cancer. J Pathol 217:372–379 [DOI] [PubMed] [Google Scholar]
- Jordan VC, O'Malley BW 2007 Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol 25:5815–5824 [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP 1995 A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92:7297–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardes RC, O'Regan RM, Gajdos C, Robinson SP, Bentrem D, De Los Reyes A, Jordan VC 2002 Effects of a new clinically relevant antiestrogen (GW5638) related to tamoxifen on breast and endometrial cancer growth in vivo. Clin Cancer Res 8:1995–2001 [PubMed] [Google Scholar]
- van Ham SM, Tjin EP, Lillemeier BF, Grüneberg U, van Meijgaarden KE, Pastoors L, Verwoerd D, Tulp A, Canas B, Rahman D, Ottenhoff TH, Pappin DJ, Trowsdale J, Neefjes J 1997 HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr Biol 7:950–957 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.