Abstract
Accumulating evidence highlights the importance of the endocannabinoid anandamide (AEA) as a key mediator in reproductive physiology. Current data suggest potential roles for AEA in gametogenesis, fertilization, and parturition. AEA exerts its actions through two G protein-coupled receptors, termed cannabinoid receptor 1 (CB1), and 2 (CB2), and the ligand-gated transient receptor potential vanilloid receptor type 1 (TRPV1) ion channel. At present, the cellular mechanism(s) and consequences of AEA signaling in reproductive tissues, especially the myometrium, are poorly understood. Here, we examine the expression of CB1, CB2, and TRPV1 in the human myometrial smooth muscle cell-line (ULTR) and characterize intracellular signaling after stimulation with AEA. Radioligand binding analysis revealed a total CB receptor expression of 76 ± 24 fmol/mg protein, with both quantitative PCR and competition binding studies indicating a negligible CB2 component. AEA caused Gαi/o-dependent inhibition of adenylate cyclase to reduce intracellular cAMP levels. In addition, AEA caused a 2.5- to 3.5-fold increase in ERK activation, which was ablated by inhibition of Gαi/o, phosphoinositide-3-kinase and Src-kinase activities, but not by inhibition of Ca2+/calmodulin-dependent protein kinase or protein kinase C activities. TRPV1 channel activation with capsaicin failed to activate ERK. Consistent with these findings, the selective agonists, arachidonyl-2-chloroethylamide (CB1) and L759656 (CB2), and selective antagonists AM251 (CB1) and JTE907 (CB2), provided pharmacological evidence that the ERK signaling pathway is activated through endogenously expressed CB1. These findings provide an insight into myometrial AEA signaling, highlighting a potential role for endocannabinoids in the regulation of gene expression in myometrial smooth muscle cells.
The endocannabinoid anandamide activates endogenous cannabinoid type-1 receptors in myometrial cells to inhibit adenylate cyclase and activate ERK through a Gαi/o-, phosphoinositide-3-kinase- and Src-kinase-dependent pathway.
Marijuana is now the most widely used recreational drug worldwide. The major active component of marijuana, Δ9-tetrahydrocannabinol, binds to two G protein-coupled receptors (GPCRs) termed cannabinoid receptor 1 (CB1) (1) and CB2 (2). These receptors have been localized to various tissues, including the reproductive tract. Marijuana use in pregnancy is associated with adverse outcomes (3,4), including spontaneous and preterm labor (5,6,7), fetal growth restriction (8,9,10), and miscarriage (11). These data, and the presence of cannabinoid receptors in the human reproductive tract, suggest that cannabinoids may play a modulatory role in human reproduction. Endogenous ligands (termed “endocannabinoids”) for both CB1 and CB2 receptors have been isolated and characterized. The most studied endocannabinoids are N-arachidonylethanolamine [anandamide (AEA) (12)], and 2-arachidonylglycerol (13,14,15), which, like Δ9-tetrahydrocannabinol, signal via CB receptors (16).
AEA has been shown to have specific regulatory roles in pre- and periimplantation events (17), gestation (18), early pregnancy loss (19,20), uterine smooth muscle relaxation (21), and postnatal development (22). The plasma levels of AEA fluctuate through the menstrual cycle [higher in the follicular than luteal phase (23)] and are higher in reproductive age women compared with postmenopausal age women. Furthermore, levels fluctuate during pregnancy, falling in the late first and early second trimester, and increasing 4-fold before labor (24). These observations have kindled new research investigating the clinical potential of AEA as a predictor of fertility and pregnancy outcomes (25,26).
Cannabinoid receptors are expressed in reproductive tissues. For example, CB1 receptors have been detected in the oviduct, uterus (27), and placental membranes (28). Expression of cannabinoid receptors is high during embryogenesis, with CB1 and CB2 expression (at least in rodents) observed from the early four- to eight-cell embryo stage through to the preimplantation blastocyst (27). CB1 and CB2 mRNAs have also been localized to myometrial layers of the human uterus (21), suggesting that endocannabinoid signaling may play an important role in modulating myometrial function.
CB1 and CB2 receptors preferentially couple to inhibitory Gαi/o proteins to inhibit adenylate cyclase activity, and hence reduce intracellular cAMP levels (16). CB receptor activation also affects numerous other signaling events, including the regulation of ion channels and nitric oxide generation (29,30). Both CB receptor subtypes are also known to increase the activity of MAPKs, which mediate cell surface-to-nucleus signaling and can influence apoptosis, oncogenesis, cell differentiation, and progression through the cell cycle (e.g Refs. 31 and 32). ERK 1 and 2 are prototypic MAPKs, the activation of which is regulated by various cell-surface receptors through often complex signaling networks (33). Despite this complexity, different classes of receptor often exploit common pathways leading to ERK activation (34).
Currently, little is known about either the expression of cannabinoid receptors or the mechanisms and consequences of endocannabinoid receptor signaling in uterine tissues. Interestingly, AEA-induced myometrial relaxation has been reported (21) as an acute response mediated via the CB1 receptor. However, a full characterization of this, or indeed other, AEA-mediated cell signaling events within the myometrium, has yet to be undertaken. In this study we sought to characterize AEA-mediated signaling events in the ULTR myometrial cell-line, which has been shown to be a useful model of normal myometrial cells (35), with the objective of identifying potentially important signaling pathways through which AEA can modulate myometrial function.
Results
Expression of CB1 and CB2 in ULTR cells: quantitative PCR (qPCR)
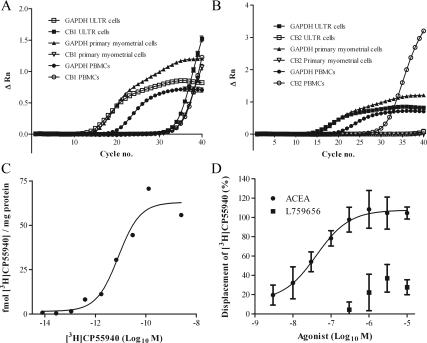
To determine the relative mRNA expression of CB1 and CB2, qPCR techniques using primer sets specifically targeting CB1 or CB2 demonstrated CB1 expression in all RNA extractions. RNA was assayed from three separate subcultures of ULTR cells in triplicate. Typical growth curves for CB1 and CB2, as well as the house keeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are shown in Fig. 1, A and B. As a positive control and in agreement with previous data (36), CB1 and CB2 were detected in peripheral blood mononuclear cells (PBMC; Fig. 1, A and B; and Table 1). CB1 signals were also detected in ULTR and primary myometrial cells with similar mean cycle threshold values (Table 1) similar to those in PBMCs. CB2 was only detected in two of the nine replicate PCR runs (n = 3, sampled in triplicate; mean cycle threshold value for these two runs, 39.1; see Table 1). CB2 was not detected in primary myometrial cells. Levels of CB2 message therefore appear to be very low, if present at all, in ULTR and primary myometrial cells.
Figure 1.
Expression of CB1 and CB2. qPCR assessment of CB1 and CB2 receptor expression. qPCR data show amplification plots for a single RNA extraction experiment from ULTR, primary myometrial, and PBMCs. Data are representative of three separate extractions, sampled in triplicate. A, cDNA from ULTR (▪), primary myometrial (▿), and PBMC ○) cell lysates were amplified with specific commercially verified TaqMan primer/probe sets for CB1. Data are also shown for GAPDH in ULTR (□), primary myometrial (▴), and PBMCs (•). B, cDNA from ULTR (▪), primary myometrial (▿), and PBMC (○) cell lysates were amplified with specific commercially verified TaqMan primer/probe sets for CB2. Again GAPDH data are supplied for ULTR (□), primary myometrial (▴), and PBMC (•). All data are related to ΔRn; the change in the normalized reporter fluorescence, relative to basal. C and D, Radioligand binding: 100 μg of fresh ULTR cell membrane was incubated with [3H]CP55940 and various CB receptor agonists and antagonists for 1 h at 30 C, before filtration through 0.5% polyethylenimine-presoaked Whatman GF/B filters. Associated 3H was determined by standard liquid scintillation counting methods. C, Saturation analysis: ULTR membranes were incubated with varying concentrations of [3H]CP55940 ranging from 1–5000 pm either in the presence (nonspecific binding) or absence (total binding) of 100 μm AEA. Nonspecific binding values were subtracted from total binding and plotted graphically in relation to protein. Data shown are representative of four separate membrane preparations. The Bmax and pKi values obtained were 76.36 ± 24.1 fmol/mg, and 9.59 ± 0.22 (257 pm), respectively. Data are means ± sem (n = 3). D, Displacement of [3H]-CP55940 using CB1 and CB2 specific agonists. ULTR membranes were incubated with saturating concentrations of [3H]CP55940 (1200–1700 pm) in the presence of either 100 μm AEA, or varying concentrations of ACEA (CB1 agonist), or L759656 (CB2 agonist) ranging from 10 nm to 10 μm. Specific binding values obtained for each concentration of ACEA and L759656 are expressed as a percentage of those obtained with 100 μm AEA. pKi value obtained for ACEA, after Cheng-Prusoff conversion, was 7.89 ± 0.45 (13 nm). Data are means ± sem (n = 4).
Table 1.
qPCR analysis of CB1 and CB2 expression
| Cell type | CB1
|
CB2
|
||||
|---|---|---|---|---|---|---|
| Ct-CB1 | Ct-GAPDH | ΔCT | Ct-CB2 | Ct-GAPDH | ΔCT | |
| ULTR | ||||||
| Sample 1 | 35.1 | 15.1 | 20 | |||
| Sample 2 | 35.7 | 15.7 | 20 | |||
| Sample 3 | 35.7 | 15.7 | 20.1 | |||
| Primary myometrial | ||||||
| Donor 1 | 37.6 | 15.9 | 21.7 | |||
| Donor 2 | 35.6 | 16.2 | 19.4 | |||
| Donor 3 | 36.7 | 16.8 | 19.9 | |||
| PBMC | ||||||
| Donor 1 | 36.9 | 18.5 | 18.5 | 32.1 | 18.5 | 13.6 |
| Donor 2 | 35.7 | 17.9 | 17.8 | 29.9 | 17.8 | 12.0 |
| Donor 3 | 35.0 | 20.0 | 15.0 | 30.7 | 20.0 | 10.7 |
Cycle threshold (Ct) values for CB1 and GAPDH in ULTR and primary myometrial cells, and CB1, CB2, and GAPDH in PBMCs. Samples from ULTR were assayed in triplicate from three separate extractions and for primary myometrial and PBMCs in duplicate from three separate donors. Representative growth curves are seen in Fig. 1A. CB2 mRNA transcripts were only detected in two of nine replicates from three separate ULTR samples, giving a mean cycle threshold value 39.1 for CB2 (from the two positive values).
Expression of CB1 and CB2 in ULTR cells: radioligand binding
CP55940 is a CB receptor agonist that binds with equal affinity to CB1 and CB2 (37). The binding of [3H]CP55940 to membranes prepared from ULTR cells was saturable. Specific binding represented approximately 17% of the total binding at a KD concentration of [3H]CP55940 (data not shown). Analysis of saturation binding curves indicated a Bmax value of 76 ± 24 fmol mg/ protein, and a pKD value of 9.59 ± 0.22 (∼257 pm) (Fig. 1B). Data are mean ± sem (n = 3).
To determine the relative expression of CB1 and CB2 receptors, we used CB receptor subtype-selective agonists and antagonists to displace [3H]CP55940 binding. Inclusion of AM251 (CB1 antagonist) and JTE907 (CB2 antagonist) increased the binding of [3H]CP55940 (data not shown), perhaps indicative of an allosteric regulation of CB receptors as reported previously (38). The CB1-selective agonist arachidonyl-2-chloroethylamide (ACEA) completely displaced specific [3H]CP55940 binding. Full concentration analysis for ACEA revealed a pIC50 value of 7.615 ± 0.52 (IC50 24.2 nm) and following Cheng Prusoff (39) correction, a pKi of 7.89 ± 0.45 (13 nm) (data are mean ± sem; n = 4).
In contrast, at high concentrations the CB2-selective agonist L759656 displaced only approximately 30% of specific [3H]CP55940 binding (Fig. 1C). A previous study has reported Ki values of 4.9 μm and 12 nm for L759656 at CB1 and CB2 receptors, respectively (40), indicating a 428-fold selectivity toward CB2. Only at L759656 concentrations above 1 μm was displacement observed, suggesting that this is due to cross-reactivity with CB1. These data concur with those observed using quantitative mRNA methods and are strongly suggestive of CB1 being the major cannabinoid receptor present in ULTR cells.
Inhibition of forskolin-stimulated cAMP levels by AEA
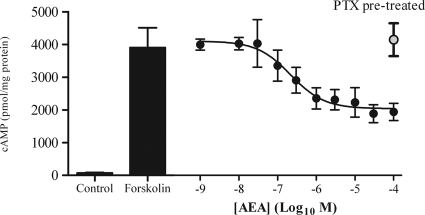
To investigate cannabinoid receptor signaling through Gαi/o proteins, we assessed the ability of AEA to reduce cAMP accumulation stimulated by forskolin (10 μm) in the presence of isobutyl-methylxanthine (a phosphodiesterase inhibitor). In the absence of AEA, the basal cAMP level in ULTR cells was approximately 50 pmol mg/protein, whereas forskolin-stimulated cAMP accumulation was approximately 3800 pmol mg/protein. This was reduced by greater than 55% in the presence of a maximal concentration of AEA (10 or 30 μm; Fig. 2) with a pIC50 value of 6.64 ± 0.28 (216 nm) (data are means ± sem; n = 3). The AEA-mediated inhibition of cAMP accumulation was completely prevented by pretreatment of cells with pertussis toxin (PTX; 100 ng/ml, 20 h) (Fig. 2).
Figure 2.
AEA-mediated inhibition of cAMP. Cultured ULTR cells were seeded into 24-well plates and washed with KHB. In the presence of isobutylmethylxanthine, AEA was added for 10 min, before addition of 10 μm forskolin. The assay proceeded for a further 10 min before termination with ice-cold 0.5 m trichloroacetic acid. Cellular cAMP was extracted, determined by radioreceptor assay, and related to cell protein content as determined by the Bradford method (77). The bar graphs to the left indicate basal and forskolin-elevated levels of cAMP; to the right (•) is the concentration dependency of AEA-mediated inhibition of forskolin-elevated cAMP. pIC50 values obtained were 6.64 ± 0.28 (IC50, 216 nm), data are mean ± sem (n = 3). After a 20-h, 100 ng/ml PTX treatment, AEA failed to inhibit forskolin-elevated cAMP accumulation (○); means ± sem (n = 3).
Characterization of AEA-stimulated ERK1/2 activation in ULTR cells
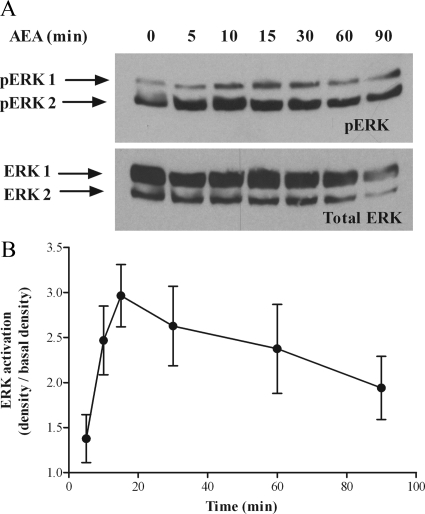
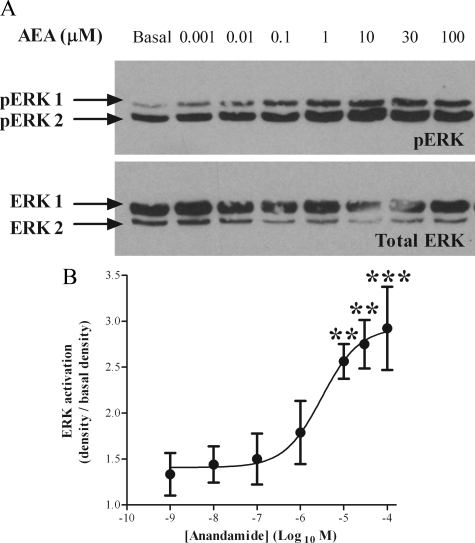
To examine the effects of AEA on MAPK-signaling pathways, we specifically observed the activation of ERKs 1 and 2. Western blotting methods were used to detect the active, phosphorylated ERK 1 and 2 isoforms (pERK1 and pERK2), which were subsequently averaged (pERK). Addition of AEA to ULTR cells resulted in time- and concentration-dependent increases in levels of pERK (Fig. 3 and Fig. 4). ERK activation was maximal 10–15 min after addition of AEA (30 μm) and slowly declined over the subsequent 90 min of the experiment (Fig. 3B). To assess the consistency of protein loading, Western blot membranes were stripped and reprobed for total ERK expression. Detection of total ERK was consistent throughout the study (see Figs. 3–8). The increase in pERK relative to basal (unstimulated) levels was 2.88 ± 0.88 fold (n =4, Fig. 3B). The levels of pERK increased in a concentration-dependent manner for AEA concentrations up to 30 μm (Fig. 4B). Quantification confirmed maximal pERK levels at approximately 30 μm AEA, and revealed a pEC50 value of 6.18 ± 0.59 (mean ± sem (n = 3); Fig. 4B) (EC50 2.63 μm).
Figure 3.
Time course of AEA-mediated ERK activation in ULTR cells. ULTR cells were cultured in six-well plates and stimulated with AEA (30 μm) for up to 90 min. Samples were subjected to standard Western blotting techniques, and levels of phosphospecific ERK proteins, (pERK 44 and pERK 42) were determined (A, upper panel). Antibodies were stripped from the membrane, and blots were reprobed for levels of total ERK as a protein loading control (A, lower panel). Data are representative of four separate experiments. The density of pERK after stimulation with AEA was related to the corresponding basal in the presence of vehicle. pERK density was calculated using the Syngene Genegenius Bioimaging System (panel B). Data are expressed as the percentage increase over basal. Data are means ± sem (n = 4 separate experiments).
Figure 4.
Concentration-response relationship for AEA-mediated ERK activation in ULTR cells. ULTR cells were cultured in six-well plates and stimulated for 15 min with varying concentrations of AEA ranging from 1 nm to 100 μm. Samples were subjected to standard Western blotting techniques, and the levels of pERK were determined (A, upper panel). Antibodies were stripped from the membrane, and blots were reprobed for levels of total ERK as a protein loading control (A, lower panel). Data are representative of four separate experiments. The density of pERK after stimulation with AEA was then related to the corresponding basal (15 min stimulation with vehicle) pERK density using the Syngene Genegenius Bioimaging System (B). Data are expressed as the percentage increase over basal. Data are means ± sem (n = 4 separate experiments). Significant increases over basal were determined by one-way ANOVA and Bonferroni’s post hoc test to give P < 0.05 (*) and P < 0.01 (**).
Figure 5.
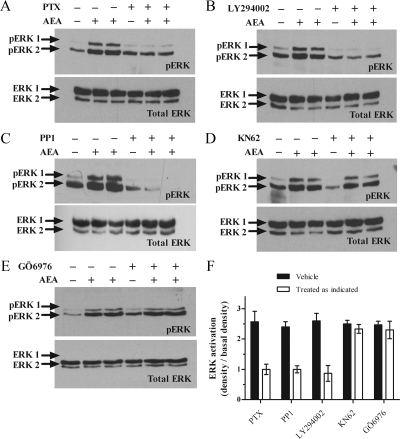
Identifying the signaling pathway mediating AEA activation of ERK signaling. To identify the signaling pathway AEA uses to stimulate ERK phosphorylation, ULTR cells were treated with either A) PTX (100 ng/ml, 20 h, Gαi G-protein inhibitor); B) LY294002 (100 nm, 30 min, PI3K inhibitor); C) PP1 (5 μm, 30 min, Src-kinase inhibitor); D) KN62 (5 μm for 15 min, calmodulin inhibitor); or E) Gö6976 (1 μm for 15 min, PKC inhibitor). After addition of the above inhibitors, cells were challenged with AEA (30 μm) for 15 min. All upper panels show representative pERK blots, and the lower panels show total ERK, after antibody stripping and reprobing (as described in Materials and Methods). F, Cumulative densitometric analysis of AEA-stimulated ERK activation assessed using the Syngene Genegenius Bioimaging System. Data are expressed as mean pERK absorbance increase over basal ± sem (n = 4 separate experiments). Control data are shown in solid bars, whereas data including inhibitors is shown in open bars. **, Significant (P < 0.01, by one-way ANOVA and Bonferroni’s post hoc test) inhibition of ERK phosphorylation.
Figure 6.
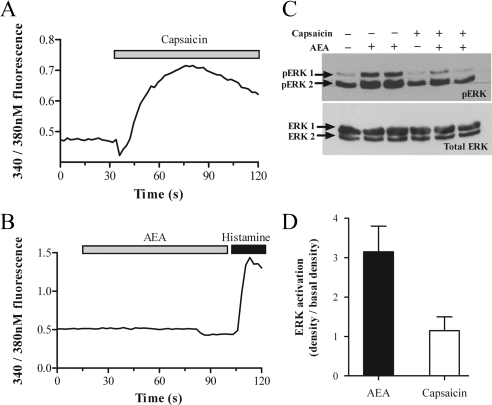
TRPV1 activation increases [Ca2+]i concentration but does not mediate ERK activation. ULTR cells were loaded with the Ca2+-sensitive dye fura-2 (3 μm) for 1 h. Cells were excited at alternating 340 and 380 nm, and changes in cytosolic fluorescence were measured using fluorescence microscopy as described in Materials and Methods. Cells were challenged with either 1 μm capsaicin (A) or 30 μm AEA (B) at t = 30 sec. At t = 100 sec, cells were challenged with 1 μm histamine as a positive control (B only). The traces show average data from six to 10 cells from the field of view and are representative of two further experiments. C, ULTR cells were stimulated with either 30 μm AEA or 1 μm capsaicin as indicated for 15 min, before lysis and processing for pERK detection (C, upper panel). All blots were stripped and reprobed for total ERK as protein loading controls (C, lower panel). Data shown are representative of four separate experiments. D, Cumulative densitometric analysis of AEA-stimulated ERK activation assessed using the Syngene Genegenius Bioimaging System. Data are expressed as mean pERK absorbance increase over basal ± sem (n = 4 separate experiments). **, Significant (P < 0.01, by one-way ANOVA and Bonferroni’s post hoc test) difference between capsaicin- and AEA-mediated ERK phosphorylation. s, Seconds.
Figure 7.
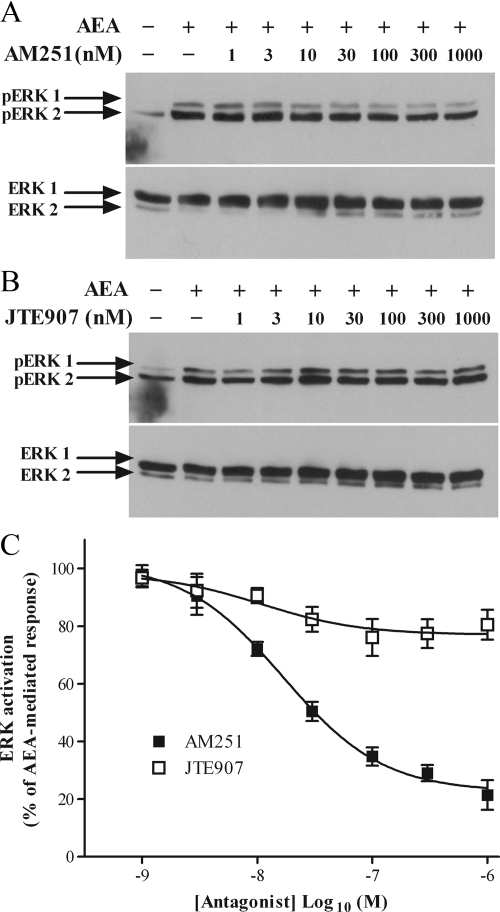
Receptor antagonism of AEA-mediated ERK activation. Representative blots showing inhibition of AEA-stimulated ERK phosphorylation by CB1 antagonist AM251 (A, upper panel) and CB2 antagonist JTE907 (B, upper panel). ULTR cells were incubated with varying concentrations of either the CB1 antagonist AM251 or CB2 antagonist JTE907 for 15 min before challenge with AEA (30 μm) for 15 min. Samples were processed and levels of pERK were determined as described previously. Antibodies were stripped from the membrane, and blots were reprobed for levels of total ERK as a protein loading control (A, lower panel) and (B, lower panel). Data are representative of three separate experiments. C, Cumulative densitometric analysis of AM251 and JTE907 mediated inhibition of AEA-stimulated ERK phosphorylation. Data show the effects of AM251 (▪) and JTE907 (□) on pERK plotted as a percentage of the density of AEA-mediated ERK phosphorylation. Data are means ± sem (n = 3).
Figure 8.
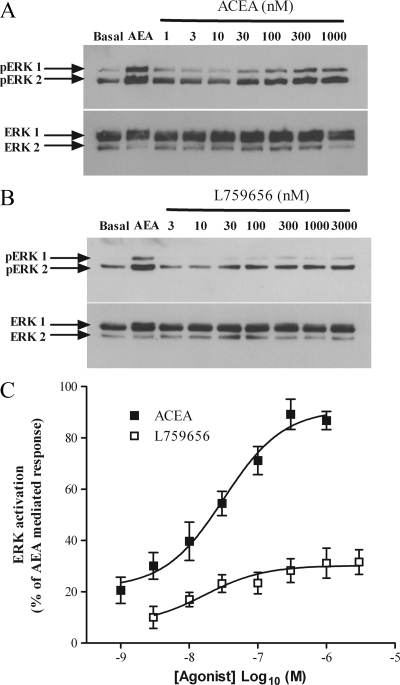
ERK phosphorylation after activation of either CB1 or CB2. ULTR cells were subject to pERK detection as per Fig. 7 after challenge with either AEA (30 μm) or with varying concentrations of either the CB1 agonist ACEA (A, upper panel) or the CB2 agonist L759656 (B, upper panel). Protein loading controls are shown (A, lower panel, and B, lower panel). Data are representative of three separate experiments. C, Cumulative densitometric analysis of pERK in the presence of ACEA (▪) or L759656 (□). Data were plotted as a percentage of the absorbance of AEA-mediated ERK phosphorylation. Data are means ± sem (n = 3).
Delineating the signaling pathway linking AEA to ERK1/2 activation in ULTR cells
To characterize the signaling events linking AEA to the activation of ERK, the effects of a series of specific inhibitors, for proteins and enzymes known to be involved in GPCR-mediated ERK-activation were investigated. Consistent with the cAMP data, pertussis toxin pretreatment (PTX; 100 ng/ml, 20 h) abolished AEA-stimulated phosphorylation of ERK (Fig. 5, A and F). Similarly, pretreatment of ULTR cells with LY294002 [a phosphoinositide-3-kinase (PI3K)] inhibitor (100 nm, 30 min) also abolished AEA-stimulated ERK phosphorylation (Fig. 5, B and F). Similar data were obtained after pretreatment with an alternative PI3K inhibitor, wortmannin (1 μm, 15 min; data not shown). Inhibition of the nonreceptor tyrosine kinase Src with pyrazolo pyrimidine type 1 (PP1) (5 μm, 30 min) also abolished ERK activation (Fig. 5, C and F). In contrast, inhibition of Ca2+/calmodulin-dependent protein kinase with KN62 (5 μm, 15 min) (Fig. 5, D and F) or protein kinase C activity with GÖ6976 (1 μm, 15 min) (Fig. 5, E and F) had no effect on the ability of AEA to stimulate the phosphorylation of ERK1/2.
Is AEA-stimulated TRPV1 channel activation important in ERK1/2 activation?
In addition to being an agonist at both CB1 and CB2 receptors, AEA is also an agonist at the nonspecific cation TRPV1 channel (41). The presence of functional TRPV1 channels was confirmed in ULTR cells by Ca2+ imaging. Cells were loaded with the Ca2+-sensitive dye Fura-2 and challenged with the TRPV1 agonist capsaicin. Capsaicin (1 μm) produced a rapid and sustained 1.5- to 3-fold increase in intracellular Ca2+ ([Ca2+]i) levels (Fig. 6A), whereas addition of vehicle (dimethyl sulfoxide) had no effect (data not shown). In contrast, addition of AEA (30 μm) to Fura-2-loaded ULTR cells failed to elevate [Ca2+]i (Fig. 6B), suggesting little or no AEA-mediated activation of the TRPV1 channel. After AEA addition, cells were challenged with histamine (1 μm), and robust [Ca2+]i elevations were observed, indicating that AEA nonresponsive cells were viable (Fig. 6B) and Ca2+ signaling pathways were intact. In addition, capsaicin treatment (1 μm, 15 min) failed to stimulate ERK phosphorylation (Fig. 6, C and D). Collectively, these data indicate that TRPV1 channel activation is not involved in the pERK response to AEA in ULTR cells.
Determination of the relative roles of CB1 and CB2 receptors in ERK1/2 activation
The roles of CB1 and CB2 receptors in mediating AEA-stimulated ERK1/2 phosphorylation in ULTR cells was investigated using subtype-selective antagonists (Fig. 7) and agonists (Fig. 8). Pretreatment of ULTR cells with AM251, a potent CB1 antagonist (42), for 15 min before challenge with AEA (30 μm) resulted in a concentration-dependent reduction in ERK activation. Maximal reductions of 83 ± 6% compared with AEA alone were achieved after addition of 1 μm AM251 (Fig. 7, A and C). JTE907, a CB2 antagonist (43), also reduced AEA-mediated ERK1/2 activation in a concentration-dependent manner, but in this case the maximum inhibition was only 21 ± 7% (Fig. 7, B and C). These data suggest that AEA-stimulated ERK1/2 activation is predominantly mediated by CB1 receptors. pIC50 values for AM251 and JTE907 were 7.7 ± 0.1 (IC50 17 nm) and 7.9 ± 0.5 (IC50 11 nm), respectively (n = 3; Fig. 7C).
Similar observations were obtained using CB subtype-selective agonists. Activation of CB1 with ACEA (44) resulted in a concentration-dependent activation of ERK (pEC50 value, 7.5 ± 0.16; EC50 ∼28 nm) that at the maximal concentration (300 nm) was 82 ± 6% of that caused by AEA (Fig. 8, A and C). In contrast, the CB2 agonist L759656 (40) increased ERK phosphorylation by 20 ± 9% (pEC50 value, 7.7 ± 0.4; EC50 ∼17 nm) compared with AEA (Fig. 8, B and C), supporting our previous observations with the subtype-selective antagonists.
AEA reduces ULTR cell viability
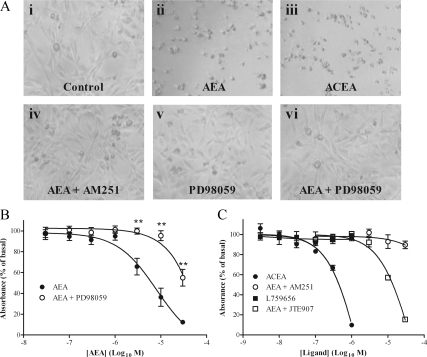
Because ERK signaling is known to regulate cell growth, we assessed whether N-arachidonylethanolamine-stimulated ERK signaling affected ULTR growth. Visual examination of arachidonylethanolamine (AEA)-treated cells after 24 h showed that high agonist concentrations produced rounded cell morphology and reduced cell numbers, suggestive of cell death (Fig. 9A). The CB1-specific agonist ACEA (1 μm) produced similar effects to AEA (Fig. 9A), whereas the CB2 agonist L759656 (data not shown) had no effect. Inclusion of the CB1 antagonist AM251 (1 μm) also prevented AEA-mediated cell death (Fig. 9A), whereas the CB2 antagonist JTE907 (1 μm) was ineffective (data not shown). Interestingly, addition of PD98059 (20 μm), an established MAPK kinase inhibitor (45), at a concentration that completely inhibits ERK phosphorylation in ULTR cells (data not shown) also prevented AEA-mediated cell death (Fig. 9A).
Figure 9.
AEA reduces ULTR cell viability. ULTR cells were seeded into six-well plates and imaged via light microscopy (A). Representative images showing ULTR cells treated with either vehicle (i) or AEA (30 μm) (ii) for 24 h before imaging. To examine the role of CB1, ULTR cells were treated with ACEA (1 μm, CB1 agonist) (iii) or AEA (30 μm) in the presence of AM251 (1 μm, CB1 antagonist) (iv) for 24 h. Representative images showing the effects of 24 h ERK inhibition (PD98059, 20 μm) treatment alone (v) or with (vi) addition of AEA (30 μm) on cell number. Data are representative of three separate experiments. B and C, ULTR cells were seeded into 96-well plates and treated with various CB receptor agonists for 24 h before analysis of cell proliferation via XTT methods as per manufacturers’ instructions. Absorbance was expressed as a percentage of control (vehicle-treated) cells. B, Proliferation was examined after 24 h challenge of cells with selective agonists for CB1 (ACEA, 1 μm; •) or CB2 (L759656, 1 μm; ▪), or with varying concentrations of AEA in the presence of a CB1 antagonist (AM251, 1 μm; ○) or CB2 antagonist (JTE907, 1 μm; □). C, Proliferation was examined after 24 h treatment with varying concentrations of AEA in the presence (○) or absence (•) of the ERK inhibitor PD98059 (20 μm). Data are shown as means ± sem (n = 3 separate experiments). AEA-mediated reduced ULTR cell viability was significantly reversed in the presence of PD98059 (**, P < 0.01 according to one-way ANOVA and Bonferroni’s post hoc test).
The 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) assay (46) was used to quantify the effects of AEA on ULTR cell viability. AEA (24 h) treatment produced a concentration-dependent reduction in XTT fluorescence (Fig. 9B), indicative of reduced cell viability and reduced cell number (46). In agreement with our initial observations, inhibition of ERK activity by PD98059 (20 μm) reversed the effects of AEA (Fig. 9B) on cell viability. The effects of AEA on ULTR cell viability appear to be mediated through CB1 because the CB1 agonist ACEA reduced cell viability, and the CB1 antagonist AM251 blocked the effects of AEA (Fig. 9C). Furthermore, the CB2 agonist L759656 did not affect ULTR cell viability, and CB2 blockade with JTE907 (1 μm) did not prevent AEA reducing cell viability (Fig. 9C). Collectively these data suggest that AEA reduces ULTR cell viability in a CB1- and ERK-dependent manner.
Discussion
Despite evidence for the presence of cannabinoid receptors in tissues of the reproductive system, there is a deficit in information regarding the cellular signaling pathways generated by cannabinoid stimulation within these tissues. Characterization of the cellular pharmacology of these receptors is fundamental to our understanding because it allows us to develop a relationship between cellular, tissue, and physiological processes involved in human reproduction. Our understanding of the effects of AEA on human uterine smooth muscle cells is at present limited to a single study (21). To address the paucity of data we have used a well-characterized immortalized human myometrial smooth muscle cell line (ULTR cells) to explore some aspects of cannabinoid receptor signaling and demonstrate characteristics that are both similar and distinct to those reported for other tissues and cells.
The ULTR cells maintain a smooth-muscle cell phenotype in culture (47), displaying many of the properties of primary isolated myometrial cells (48,49,50,51). Indeed, we have extended these studies to identify similar receptor (steroid and GPCR) expression patterns and agonist response profiles between ULTR and primary myometrial cells (Taylor, A. H., and J. M. Willets, unpublished observations and Ref. 35). ULTR cells are therefore an excellent cell model of human myometrial cells in which to study aspects of receptor pharmacology and signal transduction.
Both CB1 and CB2 receptors have the potential to inhibit adenylate cyclase and activate ERK (52,53). In addition, CB1 and CB2 mRNA transcripts have previously been identified in pregnant myometrial tissues (21). Here we have used qPCR, [3H]CP55940 binding and receptor subtype-selective agonists and antagonists to assess the presence or absence of CB1 and CB2 receptors. In contrast to previous findings (21), we have detected CB1, but essentially no CB2 mRNA transcripts. Moreover, [3H]CP55940 and antagonist competition binding data confirmed these findings by identifying a predominant CB1 receptor population. Indeed, in myometrial membranes the CB2 ligand L759656 displaced [3H]CP55940 only at concentrations above 1 μm, reflecting its affinity for CB1 (KD at CB1 = 4888 nm) rather than CB2 (KD at CB2 = 11.8 nm) (40). It is not therefore surprising, that at high L759656 concentrations (1–10 μm) inhibition of CB1 receptor activity is observed. These findings are also mirrored in our functional ERK1/2 activation studies in which CB2 agonist or antagonists are effective only at micromolar concentrations, correlating with their ability to bind to CB1 receptors. Our data suggest that in ULTR cells AEA signaling is mediated through CB1 receptors with a minimal contribution from CB2 receptors. The fact that CB2 mRNA transcripts are found in pregnant myometrium (21) suggests a possibility that CB2 receptors are up-regulated in myometrium during pregnancy. However, it should be noted that the only previously reported effect of AEA on myometrial physiology is mediated by CB1 receptors alone (21).
The presence of functional CB1 receptors in myometrial cells inevitably leads to speculation about their physiological purpose and indeed the role of AEA signaling in the myometrium. To the best of our knowledge, very few studies have addressed this issue. Dennedy et al. (21) showed that AEA (acting through CB1) had an immediate relaxant effect on contracting ex vivo human uterine tissue preparations (21). This finding suggests that AEA signaling may be beneficial during pregnancy, although the mechanism governing this AEA-mediated myometrial relaxation has not been fully investigated. Under certain conditions, increased cAMP levels following CB1 activation have been observed (54,55,56), implying possible coupling to Gαs. Similar observations, however, were not reported for CB2 (57,58).
Several mechanisms dictate smooth muscle tone; for example, elevation of cAMP levels leads to smooth muscle relaxation. However, our data demonstrate that AEA signaling inhibits adenylate cyclase, thereby reducing cAMP levels. Clearly, an AEA-mediated reduction in cAMP levels does not result, as one may expect, in myometrial contraction (21), implying that alternative mechanisms control AEA-stimulated myometrial relaxation. Smooth muscle tone is also regulated by membrane potential, a property regulated by the activity of various ion channels. Indeed, our data identify the presence of functional TRPV1 channels in ULTR cells, activation of which leads to elevated intracellular Ca2+ concentrations. However, in contrast to the previous findings reported by others (59,60), AEA was unable to gate Ca2+ influx, which would suggest that AEA is unlikely to induce Ca2+-dependent myometrial contractions after TRPV1 activation. Interestingly, CB receptor signaling is also known to induce hyperpolarization caused by indirect activation of K+ channels, or by inhibition of L-type Ca2+ channels, and inhibition of intracellular Ca2+ store release in muscle cells leading to relaxation (61,62). Myometrial cells express many K+ channels and also L-type Ca2+ channels, and a similar interaction between CB receptors and channels may explain the ability of AEA to induce relaxation.
This study is the first to demonstrate that AEA activates ERK1/2 in human myometrial cells. ERK1/2 proteins are members of the MAPK family, which can provide a link between extracellular stimuli and transcription factors to regulate gene expression. Consistent with previous reports (31), activation of ERK1/2 was sensitive to PTX, indicating that the effects were mediated directly through CB receptor-Gαi/o coupling. The same conclusion can be made from the findings of antagonism of CB receptors (31,63) and reduction in ERK1/2 activation in the presence of specific antagonists demonstrated here. In the presence of inhibitors of PI3K and Src, we observed a complete inhibition of AEA-mediated ERK1/2 activation. In contrast, inhibition of Ca2+/calmodulin-dependent protein kinase and protein kinase C had no inhibitory effect, suggesting that the activation of ERK1/2 occurs independently of these proteins. Gαi/o-coupled GPCRs are known to activate ERK through PI3K-dependent mechanisms, often as a result of Gβγ-mediated actions on PI3K, and involve members of the Src nonreceptor tyrosine kinase family (64); however, signaling to ERK1/2 downstream of Gαi/o does not exclusively follow this pathway. These data are both consistent and contradictory to previous reports of CB1-ERK1/2 signaling in various tissues. For example, within the rodent hippocampus, cannabinoid-mediated activation of ERK1/2 was independent of both PI3K and Src (65), whereas in human astrocytoma cells, ERK responses were insensitive to the inhibition of Src but dependent on PI3K (66). Although the ability of cannabinoid receptors to facilitate ERK1/2 phosphorylation is well documented, it would appear that the exact nature of the signal transduction mechanism is heavily dependent on cell background.
Activation of ERK signaling pathways are known to regulate a multitude of cell functions such as apoptosis, oncogenesis, cell differentiation, and progression through the cell cycle (33,67). Our findings suggest that AEA may regulate gene expression in myometrial cells after CB receptor activation, potentially through ERK1/2 activation. Indeed, longer term AEA exposure (48 h, 10 μm) suppresses calponin and smoothlin expression (A.H. Taylor, unpublished observations) in ULTR cells. Taken together these data suggest that AEA may further confer a relaxatory phenotype on the myometrial cells.
Recent studies from our laboratory highlighted the fluctuations of plasma AEA concentrations throughout both the menstrual cycle and pregnancy (24). Strikingly, small increases in plasma AEA levels occur before labor but are followed by a 4-fold increase during active labor. At present, it is not known whether this is a consequence of, or coincidental, with active labor, but because labor is a painful process and AEA has a well-documented role in pain transmission (68,69), the rise in plasma AEA may well be a byproduct of the labor process. However, more recent data from our group have confirmed that elevated plasma AEA concentrations occur when a women converts to the active labor state (20), suggesting that AEA may play an important role in labor. It is interesting to speculate that the long-term effects of AEA during pregnancy may be related to alterations in myometrial gene expression, a process mediated potentially through ERK1/2 signaling. Indeed, we show here that AEA reduced ULTR cell viability in a CB1- and ERK-dependent manner. The antiproliferative, proapoptotic effects of AEA have been well documented (70) and have been proposed to be ERK dependent (71). CB1 receptor signaling also inhibits human endometrial stromal cell decidualization and promotes apoptosis (72). It is therefore tempting to speculate that elevated AEA levels during labor may play a role in promoting myometrial cell death and uterine remodeling after birth.
The data presented here add to the growing evidence of multiple roles for AEA and cannabinoid receptors in human reproduction and myometrial physiology. We have demonstrated a time- and concentration-dependent ERK1/2 activation involving Gαi/o, Src, and PI3K, but not phospholipase C, protein kinase C, Ca2+/calmodulin-dependent protein kinase, Ca2+, or the TRPV1 channel. We have also observed that in ULTR the AEA-mediated pERK1/2 signal is generated downstream of CB1, with negligible contribution from CB2. Considering the potential of pERK1/2 to affect a multitude of cellular functions through regulation of transcription and protein expression, the next step would clearly be to investigate changes in the levels of mRNA and protein after receptor activation and link these to the pathways characterized here.
Materials and Methods
Materials
ULTR cells were a kind gift from Dr. James McDougall (Fred Hutchinson Cancer Center, Seattle, WA) and cultured in reagents supplied by Invitrogen (Paisley, UK) and cell culture plastic ware from NUNC (Roskilde, Denmark). AEA and capsaicin were supplied by Sigma-Aldrich (Poole, UK). KN62, LY294002, wortmannin, GÖ6976, and PP1 were all supplied by Tocris (Bristol, UK), as were the selective CB1 and CB2 agonists and antagonists AM251, JTE907, L759656, and ACEA. Acrylamide gels were formed using acrylamide supplied by Flowgen (Nottingham, UK) and run using Protean-II Western blotting equipment (Bio-Rad, Hemel Hemstead, UK) with running buffer from the same supplier. Enhanced chemiluminescence reagent and autoradiography film were obtained from GE Healthcare (Uppsala, Sweden), and nitrocellulose membrane (Protran) was supplied by Schleicher & Schuell (Keene, NH). The antibody for pERK1/2 was supplied by Promega (Southampton, UK), and the antibody for total ERK1/2 was supplied by Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Horseradish peroxidase-conjugated mouse antirabbit antibody was supplied by Sigma-Aldrich. cDNA Reverse Transcription kits, TaqMan gene expression assays for CB1 (Hs01038522_s1), CB2 2 (Hs00275635_m1) and the housekeeper GAPDH and Turbo DNA-free were obtained from Applied Biosystems (Foster City, CA). Whatman GF/B filters were supplied by Brandell (Gaithersburg, MD). [3H]CP55940 (139.6 Ci/mmol) was obtained from PerkinElmer Life Sciences (Cambridge, UK), and [3H]cAMP was from GE Healthcare (Uppsala, Sweden). All other reagents were supplied by either Sigma Chemical (Poole, Dorset, UK) or, Fisher Scientific (Loughborough, UK).
Cell culture
ULTR cells were maintained in DMEM + Glutamax-1, supplemented with 10% fetal calf serum, penicillin (100 IU/ml), streptomycin (100 μg/ml), and fungizone (2.5 μg/ml). Cells were routinely maintained in 75-cm2 flasks at 37 C in a 95%/5% air/CO2-humidified environment and subcultured at 80% confluency.
Quantitative PCR analysis of CB1 and CB2 expression
RNA extraction
Total RNA was extracted from cultured ULTR cells and primary myometrial cells (p1–3) using Tri-reagent. After extraction RNA pellets were washed in alcohol and dissolved in PCR-grade H2O, the mass of RNA determined using an Eppendorf Biophotometer. RNA purity was assessed from the 260:280 nm ratio which was greater than 1.7 in for all samples.
Total RNA was subjected to DNA clean-up using a proprietary kit, Turbo DNA-free, according to the manufacturer’s instructions. To facilitate standardization of further procedures, 10 μg RNA was added to a 50 μl clean-up reaction. Cleaned samples were reverse transcribed using a high-capacity cDNA reverse transcription Kit (Applied Biosystems), and the resulting cDNA was stored at −20 C. Cannabinoid receptor subtype mRNA was assessed by quantitative PCR using commercially available TaqMan gene expression assays from Applied Biosystems for the human cannabinoid receptor 1 (Hs01038522_s1), human cannabinoid receptor 2 (Hs00275635_m1), and the housekeeper GAPDH. The thermal profile for qPCRs in the StepOne instrument (Applied Biosystems) was 2 min at 50 C, 10 min at 95 C, 50 cycles of 15 sec at 95 C, and 1 min at 60 C. Nontemplate controls were included for all samples. Baseline-corrected normalized reporter (ΔRn); the magnitude of normalized fluorescence signal generated by the reporter at each cycle during the PCR. Delta Rn is calculated at each cycle as: ΔRn = Rn (cycle) − baseline, where Rn = fluorescence signal from the reporter dye normalized to the passive reference.
PBMCs were isolated from two healthy volunteers within the Division of Anesthesia, Critical Care, and Pain Management as described previously (73). Because CB2 receptors were essentially undetectable in ULTR cell cultures, PBMC samples were used as a positive control for both CB1 and CB2 TaqMan probes.
[3H]CP55940 binding
Membrane preparation
Confluent ULTR cell monolayers were dissociated with harvesting buffer (composition: HEPES, 10 mm; NaCl, 0.9%, EDTA, 0.2%; wt/vol), collected by centrifugation (400 rpm, 2 min, 4 C), and resuspended in homogenization buffer (composition: 50 mm Tris-HCl; 2.5 mm EDTA; 5 mm MgSO4, pH 7.4; with KOH). Cells were homogenized and centrifuged (15,000 rpm; 4 C, 15 min) before pellets were resuspended in homogenization buffer, and protein was measured as per the Lowry method (74). Membranes were used on the day of harvest and were not frozen.
Saturation binding
Experiments were performed in assay buffer (composition; as for homogenization buffer with the inclusion of 1 mg/ml BSA) in 500 μl volumes containing 100 μg of fresh ULTR cell membrane and [3H]CP55940 at concentrations ranging from 1–5000 pm. Nonspecific binding was determined using 100 μm AEA. After 1 h at 30 C, membranes were harvested by addition of ice-cold assay buffer and filtration through 0.5% polyethylenimine presoaked Whatman GF/B filters. Recovered radiation was determined by standard liquid scintillation counting. Specific binding was determined as total binding less nonspecific binding.
Displacement binding
Experiments were performed as above, with the exception that nonspecific binding was determined by inclusion of either the CB1 agonist ACEA or CB2 agonist L759656, at varying concentrations. The specific binding value obtained was compared with that of the inclusion of 100 μm AEA.
Inhibition of cAMP accumulation
ULTR cell monolayers were cultured to confluency in 24-well plates. For dose-response analysis, cell monolayers were washed with 1 ml Krebs-Henseleit buffer (KHB) (HEPES, 10 mm; NaHCO3, 1.3 mm; d-glucose, 11.7 mm; MgSO4 · 7 H2O, 1.2 mm; KH2PO4 , 1.2 mm; KCl, 4.7 mm; NaCl, 118 mm; CaCl2 · 2 H2O, 1.3 mm; pH 7.4) and incubated at 37 C for 10 min in 1 ml KHB. Cells were pretreated with 300 μm isobutylmethylxanthine for 10 min before challenge with varying concentrations of AEA for 10 min. cAMP was then elevated by a 10 min treatment with 10 μm forskolin. Extraction methods were identical to that described previously (75) and cAMP content was determined using a radioreceptor assay with binding protein purified from calf adrenal glands (76) and related to cellular protein levels as determined by the Bradford method (77).
Western blotting
Methods for the detection of ERK using Western blotting techniques followed methods described previously (78). Briefly, cells were grown to confluency in six-well plates and serum starved for an additional 18–24 h before assay. Cells were washed in KHB (composition: HEPES, 10 mm; NaHCO3, 1.3 mm; d-glucose, 11.7 mm; MgSO4, 1.2 mm; KH2PO4, 1.2 mm; KCl, 4.7 mm; NaCl, 118 mm; CaCl2, 1.3 mm, pH 7.4). At this stage, cells were either treated with inhibitors or antagonists (see details below) or stimulated with 30 μm AEA or other test agents at 37 C. Cells were lysed with lysis buffer [Tris-HCl (pH 7.4), 20 mm; 1% (vol/vol); Triton X-100, 10% (vol/vol); glycerol, NaCl, 137 mm; EDTA, 2 mm; β-glycerophosphate, 25 mm; sodium orthovanidate; 1 mm; phenylmethanesulfonylfluoride, 500 μm; leupeptin, 0.1 mg/ml; benzamidine, 0.2 mg/ml; pepstatin, 0.1 mg/ml]. Cell lysates were centrigued at 10,000 rpm for 10 min at 4 C, and 150 μl of the supernatant was added to an equal volume of 2× sample buffer [250 mm Tris-HCl, pH 6.8; 0.01% bromophenol blue (wt/vol); 2% sodium dodecyl sulfate (wt/vol); 40% (vol/vol) glycerol; and 50 mm dithiothreitol]. Samples were then boiled for 5 min before separation on 10% SDS-PAGE gels, transferred to nitrocellulose, and blocked using standard Western blotting techniques. To detect pERK1/2, membranes were probed with anti-pERK1/2 antibody (1:5000 dilution) in TBS-T [50 mm Tris-base, 150 mm NaCl, 0.1% Tween-20 (vol/vol), pH 7.5] with 0.01% (wt/vol) BSA and incubated overnight at 4 C. Visualization of immunoreactive bands was achieved using horseradish peroxidase-conjugated secondary antirabbit antibodies (1:1000 dilution in TBS-T with 5% milk, 1 h, room temperature) followed by chemiluminescence detection, and exposed to autoradiography film.
Loading controls
To ensure all gels were equally loaded for protein, membranes were subsequently stripped and reprobed for total ERK. Briefly, membranes were incubated for 30 min in stripping buffer [Tris-HCl; 62.5 mm, 2-mercaptoethanol; 100 mm, sodium dodecyl sulfate; 2% (wt/vol), pH 6.7] at 50 C with occasional agitation. Membranes were thoroughly washed in TBS-T before being blocked in 5% milk as described above. Total ERK1/2 was detected using a specific anti-ERK1/2 antibody (Santa Cruz Biotechnology; 1:1000 in TBS-T/5% milk, 4 C overnight) before the addition of secondary antibody and chemiluminescence detection. It should be noted that the detection of total ERK was undertaken using an antibody raised against ERK1, and thus ERK1 staining is heavier than ERK2 and does not indicate that ULTR cells express more ERK1 than ERK2.
Ca2+ imaging
Cells were seeded onto glass uncoated coverslips and cultured until 80% confluent. Cells were washed with KHB and loaded with fura-2 (5 μm, 1 h) at room temperature in KHB before being mounted onto the stage of an Olympus 1X70-S1F inverted microscope (Olympus Corp., Lake Success, NY). Temperatures were maintained at 37 C by continuous perfusion of KHB (5 ml/ min) through a Peltier unit. Cells were excited using a monochromator at 340 and 380 nm at alternate 1-sec intervals by light from a xenon lamp (PerkinElmer Life Sciences, Cambridge, UK). Fluorescent images were captured above 510 nm by a charge-coupled device camera at a rate of 0.5 frames/sec. The ratio of changes in fluorescence between 340 and 380 nm were used as an indication of changes in [Ca2+]i concentration and measured using purpose-written UltraView software (PerkinElmer Life Sciences). For quantitation, data were averaged from six to 10 cells from the field of view.
XTT cell viability assay
ULTR cells were seeded into 96-well plates and grown to approximately 50% confluency. Cells were then serum starved overnight before being treated with AEA alone or in the presence of AM251 (1 μm; CB1 antagonist), JTE907 (1 μm; CB2 antagonist), or PD98059 (20 μm) for 24 h. ULTR cells were also exposed to the CB1 agonist ACEA or CB2 agonist L759656. Analysis of cell proliferation via the XTT method was conducted as per manufacturer’s instructions (Roche, Indianapolis, IN). Briefly, XTT (0.3 mg/ml) was added to cells and incubated for 4 h at 37 C before fluorescence readings at 450 nm and 620 nm.
Data analysis
Densitometric analysis of the autoradiographs was achieved using a Syngene GeneGnome Bio Imaging System using Genesnap software (Syngene, Cambridge, UK). The densities of the p42 and p44 ERK proteins (ERK 1 and 2) were averaged and normalized to basal (unstimulated) level. Concentration-response curves were fitted using Prism version 5.0 (GraphPad Software, Inc., San Diego, CA). All data shown are expressed as the mean of at least three experiments (unless otherwise stated) ± sem. For representative data, experiments were also performed to an n = 3 or more. Data were analyzed using one-way ANOVA, followed by Bonferroni’s post hoc test (Excel 5.0; Microsoft, Redmond, WA). Significance was accepted when P < 0.05.
Saturation radioligand binding data were fitted using Prism version 5.0 and the Bmax, and PKD values were obtained from these graphs. For displacement analysis, graphs were again fitted using Prism 5.0, and IC50 values were obtained. The pKi value was yielded from these data according to the Cheng-Prusoff equation (39) where Ki = IC50/(1 + [3H]CP55940/KD).
Acknowledgments
We thank Dr. Robert Hirst for providing invaluable advice during our radioligand binding studies.
Footnotes
Disclosure Summary: The authors have nothing to declare.
First Published Online May 28, 2009
Abbreviations: ACEA, Arachidonyl-2-chloroethylamide; AEA, anandamide; [Ca2+]i, intracellular calcium concentration; CB1, type 1 cannabinoid receptor; CB2, type 2 cannabinoid receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GPCR, G protein-coupled receptor; pERK, threonine- and tyrosine-phosphorylated ERK; KHB, Krebs-Henseleit buffer; PBMCs, peripheral blood mononuclear cells; PI3K, phosphoinositide-3-kinase; PP1, pyrazolo pyrimidine type 1; PTX, pertussis toxin; qPCR, quantitative PCR; TBS-T, Tris-NaCl-Tween 20; TRPV1, transient receptor potential vanilloid receptor type 1; XTT, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide.
References
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI 1990 Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564 [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M 1993 Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65 [DOI] [PubMed] [Google Scholar]
- Wengler T, Croix D, West M, Tramma G 1988 Marijuana and pregnancy. In: Dohler K, Pawlikowsky M, eds. Progress in neuropeptide research. Basel: Birkhauser Verlage Basel; 111–119 [Google Scholar]
- Hall W, Solowij N 1998 Adverse effects of cannabis. Lancet 352:1611–1616 [DOI] [PubMed] [Google Scholar]
- Tennes K 1984 Effects of marijuana on pregnancy and fetal development in the human. NIDA Res Monogr 44:115–123 [PubMed] [Google Scholar]
- Greenland S, Richwald GA, Honda GD 1983 The effects of marijuana use during pregnancy. II. A study in a low-risk home-delivery population. Drug Alcohol Depend 11:359–366 [DOI] [PubMed] [Google Scholar]
- Greenland S, Staisch KJ, Brown N, Gross SJ 1982 Effects of marijuana on human pregnancy, labor, and delivery. Neurobehav Toxicol Teratol 4:447–450 [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Willan A 1984 Marijuana use during pregnancy and decreased length of gestation. Am J Obstet Gynecol 150:23–27 [DOI] [PubMed] [Google Scholar]
- Abel EL 1980 Prenatal exposure to cannabis: a critical review of effects on growth, development, and behavior. Behav Neural Biol 29:137–156 [DOI] [PubMed] [Google Scholar]
- Fried PA 1993 Prenatal exposure to tobacco and marijuana: effects during pregnancy, infancy, and early childhood. Clin Obstet Gynecol 36:319–337 [DOI] [PubMed] [Google Scholar]
- Fried PA, Buckingham M, Von Kulmiz P 1983 Marijuana use during pregnancy and perinatal risk factors. Am J Obstet Gynecol 146:992–994 [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R 1992 Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949 [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R 2001 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA 98:3662–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Baywitch M, Barg J, Vogel Z 1995 Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K 1995 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97 [DOI] [PubMed] [Google Scholar]
- Pertwee RG 1999 Pharmacology of cannabinoid receptor ligands. Curr Med Chem 6:635–664 [PubMed] [Google Scholar]
- Wang H, Xie H, Dey SK 2006 Endocannabinoid signaling directs periimplantation events. AAPS J 8:E425–E432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger T, Fragkakis G, Giannikou P, Probonas K, Yiannikakis N 1997 Effects of anandamide on gestation in pregnant rats. Life Sci 60:2361–2371 [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A 2000 Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet 355:1326–1329 [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Finney M, Evans MD, Konje JC 2008 Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA 299:1135–1136 [DOI] [PubMed] [Google Scholar]
- Dennedy MC, Friel AM, Houlihan DD, Broderick VM, Smith T, Morrison JJ 2004 Cannabinoids and the human uterus during pregnancy. Am J Obstet Gynecol 190:2–9; discussion, 3A [DOI] [PubMed] [Google Scholar]
- Wenger T, Fragkakis G, Giannikou P, Yiannikakis N 1997 The effects of prenatally administered endogenous cannabinoid on rat offspring. Pharmacol Biochem Behav 58:537–544 [DOI] [PubMed] [Google Scholar]
- El-Talatini MR, Taylor AH, Konje JC 5 February 2009 The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril 10.1016/j.fertnstert.2008.12.033 [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Evans MD, Cooke MS, Taylor DJ, Bell SC, Konje JC 2004 Plasma levels of the endocannabinoid anandamide in women—a potential role in pregnancy maintenance and labor? J Clin Endocrinol Metab 89:5482–5487 [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Bell SC, Konje JC 2002 Endogenous cannabinoids: metabolism and their role in reproduction. Life Sci 70:1963–1977 [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ang C, Bell SC, Konje JC 2007 The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum Reprod Update 13:501–513 [DOI] [PubMed] [Google Scholar]
- Paria BC, Das SK, Dey SK 1995 The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci USA 92:9460–9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B, Gibbons HM, Mitchell MD, Glass M 2003 Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta 24:990–995 [DOI] [PubMed] [Google Scholar]
- Pertwee RG 1997 Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74:129–180 [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG 2002 International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202 [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P 1995 Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J 312:637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda D, Galve-Roperh I, Haro A, Guzmán M 2000 The CB1 cannabinoid receptor is coupled to the activation of c-Jun N-terminal kinase. Mol Pharmacol 58:814–820 [DOI] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH 2001 MAP kinases. Chem Rev 101:2449–2476 [DOI] [PubMed] [Google Scholar]
- Garrington TP, Johnson GL 1999 Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 11:211–218 [DOI] [PubMed] [Google Scholar]
- Willets JM, Taylor AH, Shaw H, Konje JC, Challiss RA 2008 Selective regulation of H1 histamine receptor signaling by G protein-coupled receptor kinase 2 in uterine smooth muscle cells. Mol Endocrinol 22:1893–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong L, Newton C, Cheng Q, Friedman H, Roth MD, Klein TW 2002 Altered cannabinoid receptor mRNA expression in peripheral blood mononuclear cells from marijuana smokers. J Neuroimmunol 127:169–176 [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME 1996 Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther 278:989–999 [PubMed] [Google Scholar]
- Pertwee RG 2005 Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76:1307–1324 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH 1973 Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition I50 of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, Pertwee RG 1999 Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol 126:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB 2000 The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol 129:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A 1999 Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem 42:769–776 [DOI] [PubMed] [Google Scholar]
- Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T 2001 In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J Pharmacol Exp Ther 296:420–425 [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB 1999 Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J Pharmacol Exp Ther 289:1427–1433 [PubMed] [Google Scholar]
- Schauwienold D, Plum C, Helbing T, Voigt P, Bobbert T, Hoffmann D, Paul M, Reusch HP 2003 ERK1/2-dependent contractile protein expression in vascular smooth muscle cells. Hypertension 41:546–552 [DOI] [PubMed] [Google Scholar]
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR 1988 Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 48:4827–4833 [PubMed] [Google Scholar]
- Perez-Reyes N, Halbert CL, Smith PP, Benditt EP, McDougall JK 1992 Immortalization of primary human smooth muscle cells. Proc Natl Acad Sci USA 89:1224–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball A, Wang JW, Wong S, Zielnik B, Mitchell J, Wang N, Stemerman MB, Mitchell BF 2006 Phorbol ester treatment of human myometrial cells suppresses expression of oxytocin receptor through a mechanism that does not involve activator protein-1. Am J Physiol Endocrinol Metab 291:E922–E928 [DOI] [PubMed] [Google Scholar]
- Miggin SM, Kinsella BT 2001 Thromboxane A2 receptor mediated activation of the mitogen activated protein kinase cascades in human uterine smooth muscle cells. Biochim Biophys Acta 1539:147–162 [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Molnar M, Romero R 2002 Interferon γ antagonizes interleukin-1β-induced cyclooxygenase-2 expression and prostaglandin E2 production in human myometrial cells. J Soc Gynecol Invest 9:215–219 [PubMed] [Google Scholar]
- Zaragoza DB, Wilson RR, Mitchell BF, Olson DM 2006 The interleukin 1β-induced expression of human prostaglandin F2α receptor messenger RNA in human myometrial-derived ULTR cells requires the transcription factor, NFκB. Biol Reprod 75:697–704 [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Bourrié B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P 1995 Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. J Biol Chem 270:13973–13980 [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrié B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P 1996 Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem 237:704–711 [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Brotchie JM 1997 Paradoxical action of the cannabinoid WIN 55,212-2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. Br J Pharmacol 120:1397–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch L, Sterin-Borda L, Borda E 2004 Expression and biological effects of CB1 cannabinoid receptor in rat parotid gland. Biochem Pharmacol 68:1767–1774 [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Chang LK, Kwan J, Martin GR 1998 Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther 287:884–888 [PubMed] [Google Scholar]
- Glass M, Felder CC 1997 Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci 17:5327–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra B, Portier M, Kernéis A, Delpech M, Carillon C, Le Fur G, Ferrara P, Shire D 1999 Dual intracellular signaling pathways mediated by the human cannabinoid CB1 receptor. Eur J Pharmacol 374:445–455 [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED 1999 Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457 [DOI] [PubMed] [Google Scholar]
- Lam PM, Hainsworth AH, Smith GD, Owen DE, Davies J, Lambert DG 2007 Activation of recombinant human TRPV1 receptors expressed in SH-SY5Y human neuroblastoma cells increases [Ca2+]i, initiates neurotransmitter release and promotes delayed cell death. J Neurochem 102:801–811 [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR 1999 Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol 276:H2085–H2093 [DOI] [PubMed] [Google Scholar]
- Högestätt ED, Zygmunt PM 2002 Cardiovascular pharmacology of anandamide. Prostaglandins Leukot Essent Fatty Acids 66:343–351 [DOI] [PubMed] [Google Scholar]
- Rubino T, Forlani G, Viganò D, Zippel R, Parolaro D 2004 Modulation of extracellular signal-regulated kinases cascade by chronic Δ9-tetrahydrocannabinol treatment. Mol Cell Neurosci 25:355–362 [DOI] [PubMed] [Google Scholar]
- Werry TD, Sexton PM, Christopoulos A 2005 “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol Metab 16:26–33 [DOI] [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA 2003 Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci 23:2371–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-Roperh I, Rueda D, Gómez del Pulgar T, Velasco G, Guzmán M 2002 Mechanism of extracellular signal-regulated kinase activation by the CB1 cannabinoid receptor. Mol Pharmacol 62:1385–1392 [DOI] [PubMed] [Google Scholar]
- Friday BB, Adjei AA 2008 Advances in targeting the Ras/Raf/MEK/Erkmitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res 14:342–346 [DOI] [PubMed] [Google Scholar]
- Grotenhermen F 2005 Cannabinoids. Curr Drug Targets CNS Neurol Disord 4:507–530 [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita 2nd RL 2006 Endocannabinoid mechanisms of pain modulation. Aaps J 8:E693–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agró A 2003 The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ 10:946–955 [DOI] [PubMed] [Google Scholar]
- Melck D, Rueda D, Galve-Roperh I, De Petrocellis L, Guzmán M, Di Marzo V 1999 Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett 463:235–240 [DOI] [PubMed] [Google Scholar]
- Moghadam KK, Kessler CA, Schroeder JK, Buckley AR, Brar AK, Handwerger S 2005 Cannabinoid receptor I activation markedly inhibits human decidualization. Mol Cell Endocrinol 229:65–74 [DOI] [PubMed] [Google Scholar]
- Williams JP, Thompson JP, McDonald J, Barnes TA, Cote T, Rowbotham DJ, Lambert DG 2007 Human peripheral blood mononuclear cells express nociceptin/orphanin FQ, but not μ, δ, or κ opioid receptors. Anesth Analg 105:998–1005 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Brown BL, Albano JD, Ekins RP, Sgherzi AM 1971 A simple and sensitive saturation assay method for the measurement of adenosine 3′:5′-cyclic monophosphate. Biochem J 121:561–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton PJ, Szekeres PG, Wise A, Willars GB 2004 Signaling and ligand binding by recombinant neuromedin U receptors: evidence for dual coupling to Gαq/11 and Gαi and an irreversible ligand-receptor interaction. Mol Pharmacol 66:1544–1556 [DOI] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Thandi S, Blank JL, Challiss RA 2002 Group-I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to extracellular signal-regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. J Neurochem 83:1139–1153 [DOI] [PubMed] [Google Scholar]