Abstract
Activation of the G protein-coupled TRH receptor leads to its phosphorylation and internalization. These studies addressed the fundamental question of whether phosphorylation regulates receptor trafficking or endosomal localization regulates the phosphorylation state of the receptor. Trafficking of phosphorylated and dephosphorylated TRH receptors was characterized using phosphosite-specific antibody after labeling surface receptors with antibody to an extracellular epitope tag. Rab5 and phosphoreceptor did not colocalize at the plasma membrane immediately after TRH addition but overlapped extensively by 15 min. Dominant-negative Rab5-S34N inhibited receptor internalization. Later, phosphoreceptor was in endosomes containing Rab5 and Rab4. Dephosphorylated receptor colocalized with Rab4 but not with Rab5. Dominant-negative Rab4, -5, or -11 did not affect receptor phosphorylation or dephosphorylation, showing that phosphorylation determines localization in Rab4+/Rab5− vesicles and not vice versa. No receptor colocalized with Rab7; a small amount of phosphoreceptor colocalized with Rab11. To characterize recycling, surface receptors were tagged with antibody, or surface receptors containing an N-terminal biotin ligase acceptor sequence were labeled with biotin. Most recycling receptors did not return to the plasma membrane for more than 2 h after TRH was removed, whereas the total cell surface receptor density was largely restored in less than 1 h, indicating that recruited receptors contribute heavily to early repopulation of the plasma membrane.
Dephosphorylated TRH receptor recycles slowly via Rab4 while plasma membrane receptors are more rapidly repopulated from an intracellular pool.
The TRH receptor is a Gαq/11-coupled seven-transmembrane receptor expressed in the anterior pituitary and various areas of the central nervous system. TRH causes a transient increase in inositol 1,4,5-triphosphate production and intracellular calcium and activation of protein kinase C. Cells expressing TRH receptor remain refractory to further stimulation with TRH (termed “desensitization”) until agonist is removed and the signaling pathway allowed to recover (i.e. resensitization). Whereas regulation of downstream effectors is important for controlling G protein-coupled receptor (GPCR) signaling, desensitization and resensitization are primarily achieved at the level of the receptor.
The TRH receptor is rapidly phosphorylated (t1/2∼15 sec) after binding agonist (1). The phosphoreceptor recruits arrestin to the plasma membrane (2,3) and internalizes with arrestin in a clathrin- and dynamin-dependent manner (4). GPCR internalization has long been seen as a means for cells to control responsiveness to extracellular signals, and the extent of resensitization depends on how much of internalized receptor is degraded and how much recycles (5,6,7). Desensitized inositol triphosphate and Ca2+ responses are restored gradually after TRH is removed (4,8). A simple explanation is that internalized receptor recycles to the plasma membrane, but this has never been directly tested.
Arrestin is required for TRH receptor internalization (2,9,10,11,12). When TRH receptors are stimulated with agonist, receptor is rapidly and extensively sequestered, and when agonist is removed by washout, the plasma membrane is gradually repopulated with TRH receptors (t1/2∼30 min) (4,13,14,15,16). The half-time of receptor dephosphorylation after agonist is removed is quite rapid (t1/2 < 5 min) (1). It is not known how the internalized TRH receptor is trafficked, when it dissociates from arrestin, or how it returns to the plasma membrane. Although many GPCRs activate MAPK proteins through arrestin, and for some receptors this requires internalization of the arrestin-receptor complex (17), the TRH receptor does not rely on arrestin to activate MAPK (18).
Rab guanosine triphosphatases (GTPases) are ubiquitously expressed small G proteins (reviewed in Refs. 19,20,21). Rabs associate with specific endosomes, act as molecular scaffolds by recruiting various effectors, and ensure proper membrane trafficking (22,23,24,25). Rab5 regulates clathrin-dependent endocytosis and the formation of early endosomes. Recycling to the plasma membrane occurs relatively rapidly via Rab4. Recycling through Rab11 is generally much slower, and GPCRs trafficked via Rab11 pass through a perinuclear compartment (22,26,27,28,29).
Despite important advances in understanding GPCR trafficking via Rab GTPases (6,7), it is still unclear how receptor phosphorylation affects, or is affected by, receptor trafficking. Is phosphorylation an address label that directs the receptor to stay in or go to a specific vesicle, possibly through direct interaction with a specific Rab, or does subcellular localization affect dephosphorylation, perhaps by regulating access to the cognate receptor phosphatase? After endocytosis, do phosphorylated receptors traffic differently than dephosphorylated receptors? To answer such questions, we used a phosphosite-specific antibody to distinguish between phosphorylated and nonphosphorylated TRH receptor and colocalized the receptor with Rab GTPases and arrestin. We show that after TRH receptors have internalized, they appear to be dephosphosphorylated before they move to a specific Rab4 vesicle population and recycle. We also show that once TRH is removed, an intracellular pool of receptors is recruited to the plasma membrane before dephosphorylated receptors have recycled extensively.
Results
Subcellular localization of TRH receptor
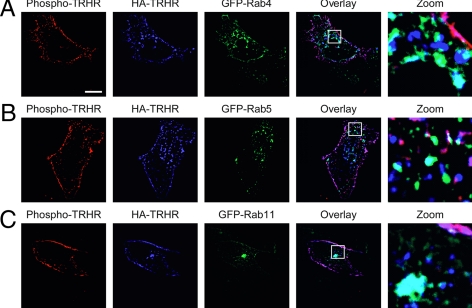
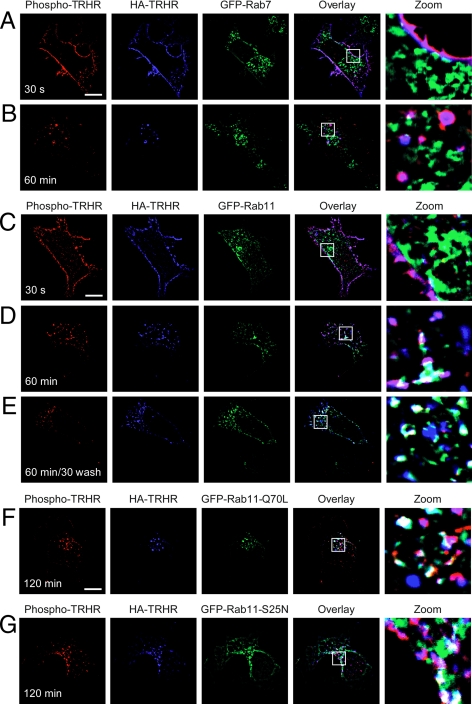
To study TRH receptor localization, we transfected human embryonic kidney (HEK)293 cells with receptors tagged with hemagglutinin (HA) at the extracellular N terminus and green fluorescent protein (GFP)-Rab GTPases. Before fixation, we incubated the cells with TRH for 30 sec to cause phosphorylation of receptors on the cell surface. Most of the TRH receptor in naïve cells (data not shown) or cells briefly exposed to TRH (Fig. 1) was localized to the plasma membrane, but some was contained in intracellular vesicles that contained Rab4, Rab5, or Rab11 (blue HA-TRH receptor and green GFP-Rabs are cyan in overlay). Phospho-TRH receptor was detected with an affinity-purified rabbit polyclonal antibody (Ab6959) raised against a peptide representing residues 351–370 on the receptor cytoplasmic C-tail with all four Ser/Thr residues phosphorylated. This antibody shows no reactivity with unphosphorylated receptor (1). Receptor on the plasma membrane was stained with phosphosite-specific antibody (red phospho-TRH receptor and blue HA-TRH receptor are pink in overlay). Nearly all of the receptor at the cell surface is phosphorylated after treatment with TRH, based on SDS-PAGE mobility shift and immunodepletion with phosphoreceptor antibody (1,11).
Figure 1.
TRH receptor in permeabilized cells. Cells expressing 2HA-AP-TRH receptor and GFP-Rab4, -5, or -11 were treated with 100 nm TRH for 30 sec, fixed, permeabilized, and stained with anti-HA and anti-phospho-TRH receptor antibodies. Overlap of blue HA-TRH receptor and green GFP-Rabs appears cyan in overlay. Bar, 10 μm. TRHR, TRH receptor.
TRH receptor endocytosis
We used the following strategy to monitor trafficking of only receptor at the plasma membrane at the start of the experiment. We incubated intact cells expressing HA-tagged TRH receptor with mouse anti-HA antibody such that only receptors on the surface of the cell were labeled; HA antibody-labeled cells were subsequently fixed, permeabilized, and probed with phosphosite-specific antibody against phospho-TRH receptor. By labeling TRH receptor simultaneously with two different antibodies, we were able to distinguish between phosphorylated (both antibodies bound) and dephosphorylated (only HA antibody bound) receptor.
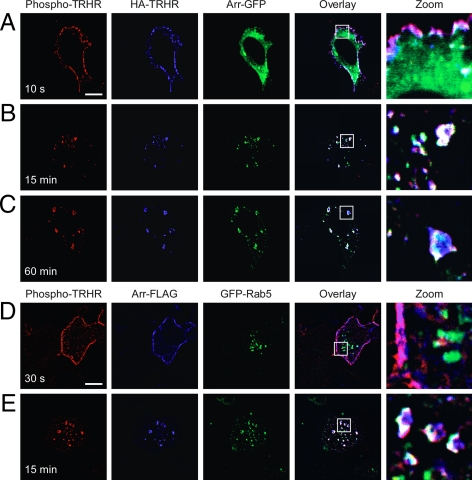
We followed the intracellular localization of arrestin and phosphorylated and dephosphorylated TRH receptors in cells expressing TRH receptor and arrestin-GFP. After addition of TRH for 10 sec, only a small amount of arrestin could be seen at the plasma membrane even though surface receptor was phosphorylated (Fig. 2A; pink shows overlay of blue HA-receptor and red phosphoreceptor). Arrestin colocalized with phosphorylated receptor on the plasma membrane by 1 min (see below), and by 15 min colocalized with phosphorylated receptor on large vesicles where it remained for up to 60 min (Fig. 2, B and C; white shows overlay of all three color channels).
Figure 2.
Phospho-TRH receptor traffics with arrestin. A–C, Cells expressing 2HA-AP-TRH receptor and arrestin-GFP (green) were incubated with mouse anti-HA antibody to label surface receptors and then stimulated with 100 nm TRH for the indicated times. After fixation, cells were stained with antimouse Alexa Fluor 647 antibody to identify anti-HA antibody-labeled receptors (blue) or rabbit anti-phospho-TRH receptor antibody and antirabbit Alexa Fluor 555 antibody to identify phospho-TRH receptors (red). Pink shows overlay of red and blue channels; white shows overlay of all three channels. D and E, Cells expressing 2HA-AP-TRH receptor, arrestin-FLAG, and GFP-Rab5 were stimulated with 100 nm TRH for the indicated times and fixed and stained. Mouse anti-FLAG antibody followed by antimouse Alexa Fluor 647 antibody was used to label arrestin-FLAG (blue). Bar, 10 μm. Arr-, Arrestin; s, seconds; TRHR, TRH receptor.
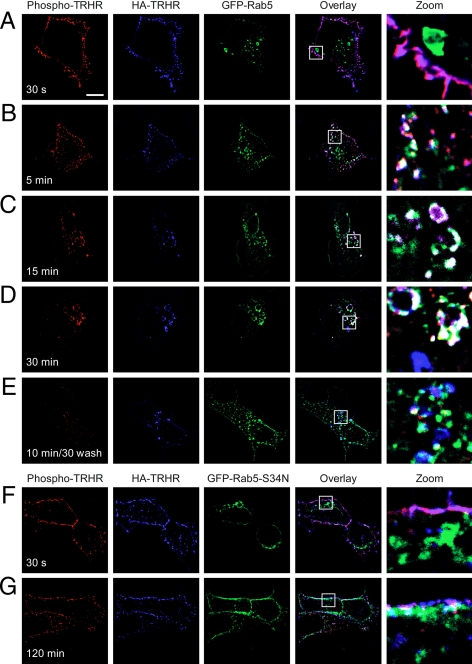
Rab5 contributes to the formation and function of clathrin-coated pits (30). We asked when the phospho-TRH receptor colocalizes with Rab5 in cells treated with 100 nm TRH. Rab5 and phosphoreceptor did not colocalize at the plasma membrane at very early time points, but they were together by 5 min after agonist treatment and overlapped extensively by 15 min in structures that appeared to be early endosomes (Fig. 3, A–C) and stained positive for EEA1 (data not shown). A quantitative analysis of receptor and Rab5 colocalization is shown in Fig. 6. Overexpression of dominant-negative Rab5-S34N inhibited TRH receptor internalization from early times to as long as 2 h after hormone addition (Fig. 3 and supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals online web site at http://mend.endojournals.org).
Figure 3.
TRH receptor internalizes via Rab5-positive endosomes. Cells expressing 2HA-AP-TRH receptor and (A–E) GFP-Rab5 or (F and G) GFP-Rab5-S34N were incubated with anti-HA antibody, stimulated with 100 nm TRH for the indicated times, and fixed and stained. Some cells were washed to remove TRH and allowed to recover for 30 min before fixation. The localization of GFP-Rab5-S34N was quite variable in different cells (e.g. panels F and G and supplemental Fig. 1). Bar, 10 μm. s, Seconds; TRHR, TRH receptor.
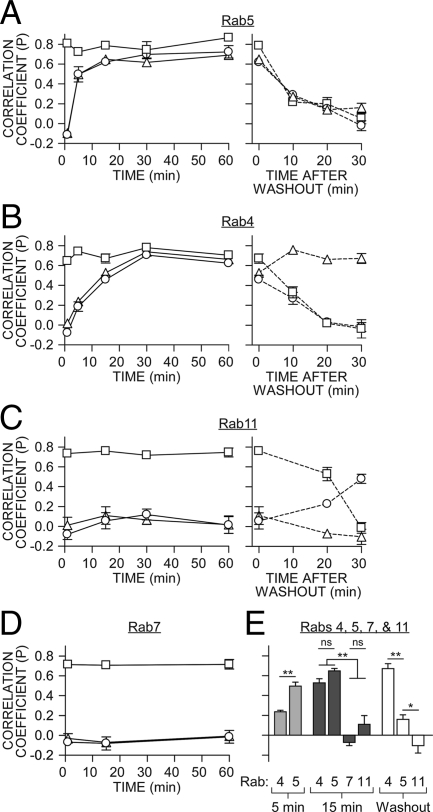
Figure 6.
Colocalization of Rabs with TRH receptor. Pearson’s correlation coefficients for: 2HA-AP-TRH receptor with phospho-TRH receptor (squares), 2HA-AP-TRH receptor with GFP-Rabs (triangles) or phospho-TRH receptor with GFP-Rabs (circles) are shown as mean ± se (n = 3–12). A–D: Left panel, values from cells stimulated with TRH; A–C, right panel, values after agonist washout. E, Comparison of selected coefficients from panels A–D for colocalization of 2HA-AP-TRH receptor and particular Rab GTPases 5 or 15 min after stimulation with TRH or 30 min after agonist washout. **, P < 0.001; *, P < 0.05; nonsignificant (ns), P > 0.05 by one-way ANOVA.
We monitored Rab5, arrestin, and phosphoreceptor at intervals after stimulating cells with TRH. Arrestin moved to the plasma membrane within 30 sec, when Rab5 was still cytosolic (Fig. 2D). By 15 min, phosphoreceptor, Rab5, and arrestin colocalized in large vesicles, where they remained for as long as 60 min (Fig. 2E).
Because essentially all of the HA-labeled TRH receptor on the surface was phosphorylated after addition of TRH, HA-labeled receptor that did not stain with phosphosite-specific antibody represented dephosphorylated receptor (blue in overlay). Importantly, dephosphorylated receptors did not colocalize with Rab5; this was true for receptors that became dephosphorylated in the presence of TRH or after agonist was removed (Fig. 3, D and E, respectively; blue in overlay; quantitative depiction in Fig. 6). Thus, phospho-TRH receptor and arrestin were both found in Rab5-positive vesicles, whereas dephosphorylated receptor was confined to Rab5-negative endosomes.
Rab7 and lysosomal degradation
After internalization, GPCRs recycle to the plasma membrane or undergo lysosomal degradation (5,7,31). Lysosomal sorting of receptors is dependent on Rab7 (32) and often on receptor ubiquitination (33,34). The TRH receptor is neither ubiquitinated nor degraded in the first few hours after TRH stimulation (35,36). TRH receptor internalized but did not colocalize with Rab7, even after 1 h of TRH exposure (Fig. 4, A and B). Live-cell imaging of GFP-tagged TRH receptor and the red lysosomal marker LysoTracker Red also showed no localization of receptor in lysosomes for up to 4 h after addition of TRH (data not shown).
Figure 4.
TRH receptor does not colocalize with Rab7 and minimally with Rab11. Cells expressing 2HA-AP-TRH receptor and either GFP-Rab7 (A and B) or GFP-Rab11 (C and D) were incubated with anti-HA antibody and stimulated with 100 nm TRH for the indicated times. Cells in panel E were washed to remove TRH and allowed to recover for 30 min before fixation and staining. Cells expressing 2HA-AP-TRH receptor and constitutively active GFP-Rab11-Q70L (panel F) or dominant-negative GFP-Rab11-S25N (panel G). Bar, 10 μm. s, Seconds; TRHR, TRH receptor.
Slow recycling endosomes
Rab11 mediates the slow recycling of cargo proteins and some GPCRs to the plasma membrane through a perinuclear region (6,21,22,29,37). We asked whether Rab11 mediates TRH receptor trafficking during TRH exposure or after TRH removal. TRH receptor colocalization with Rab11 was first noticeable 30–60 min after addition of agonist (Fig. 4D), but this association never involved more than a small fraction of total receptor or Rab11 (see Fig. 6). Dephosphorylated receptor was never found in Rab11-positive endosomes (Fig. 4D, blue in overlay). When Rab11 activity was maximized by overexpression of a constitutively active form of Rab11, Rab11-Q70L, there was very little overlap with TRH receptor (Fig. 4F). Likewise, coexpression of dominant-negative Rab11-S25N did not change receptor trafficking (Fig. 4G).
Rapid recycling endosomes
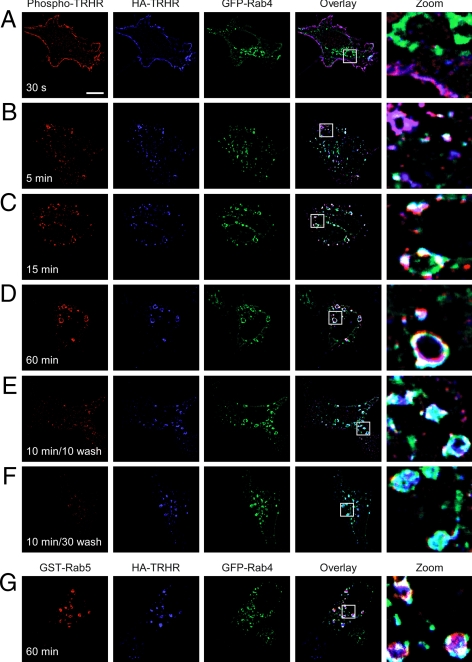
Rab4 colocalizes with Rab5 on early endosomes and is responsible for the rapid recycling of the β2-adrenergic receptor to the plasma membrane (24). Because only a small amount of TRH receptor trafficked with Rab11 or Rab7, we asked whether Rab4 organized the major pathway following after Rab5. Phospho-TRH receptor began to colocalize with Rab4 by 15 min after stimulation with TRH (Figs. 5, A–C, and 6). Colocalization with Rab4 thus began considerably later than the first detectable overlap with Rab5, which occurred by 5 min after binding TRH (Fig. 3B). As with Rab5, phosphoreceptor colocalization with Rab4 increased at later time points and was widespread by 60 min (Fig. 5D). Although individual Rab GTPases have distinct functions and distributions, there is some overlap, with different Rabs occupying the same endosome (25). Coexpression of GFP-tagged Rab4 and GST-tagged Rab5 confirmed that internalized phospho-TRH receptor resides on common endosomes containing both Rab4 and Rab5 (Fig. 5G).
Figure 5.
Dephosphorylated TRH receptor traffics via Rab4. A–F, Cells expressing 2HA-AP-TRH receptor and GFP-Rab4 were incubated with anti-HA antibody, stimulated with 100 nm TRH for the indicated times, and fixed and stained. Some cells were washed to remove TRH and allowed to recover for the indicated times before fixation. G, Cells expressing 2HA-AP-TRH receptor, GFP-Rab4, and GST-Rab5 were treated as in panel D except that rabbit anti-GST antibody was used to label GST-Rab5. Bar, 10 μm. s, Seconds; TRHR, TRH receptor.
A major difference between Rab4 and Rab5, however, was seen in their colocalization with dephosphorylated (blue in Fig. 5, E and F) vs. phosphorylated (red in Fig. 5, A–D) TRH receptor: dephosphorylated receptor colocalized extensively with Rab4 (Fig. 5, E and F, cyan in overlay) but not with Rab5 (Fig. 3E). Taken together, the data show that phosphorylated TRH receptor is found in Rab5-positive, Rab4-positive vesicles, whereas dephosphorylated receptor is confined to Rab5-negative, Rab4-positive endosomes.
To quantitatively compare trafficking with various Rabs, we calculated Pearson’s correlation coefficient, which reflects a pixel-by-pixel comparison of two-color channels (38), from images from multiple independent experiments (Fig. 6). Values can range from 1 (indicating perfect correlation) to −1 (exclusion), with 0 indicating no correlation. Phosphorylated receptor colocalized with Rab5 earlier than Rab4 but was never appreciably colocalized with Rabs 7 or 11 (Fig. 6E). After TRH washout, the small amount of receptor that remained phosphorylated colocalized with Rab11, as indicated by an increased Pearson’s coefficient (Fig. 6C). Agonist washout abolished 2HA-AP-TRH receptor colocalization with Rab5, whereas correlation with Rab4 actually increased (Fig. 6B, right panel). This analysis emphasizes that dephosphorylated receptors are confined to Rab5-negative, Rab4-positive vesicles.
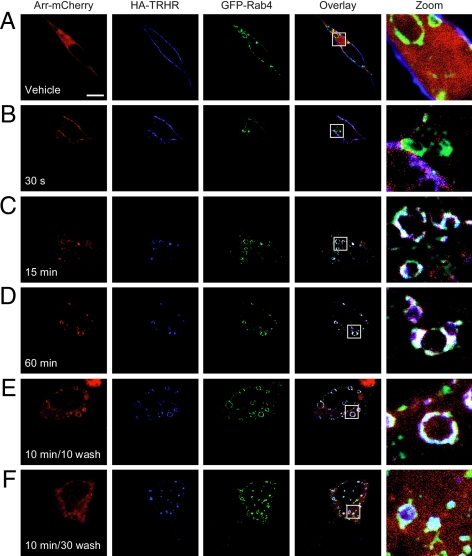
Arrestin is an important regulator of receptor internalization, and recent reports indicate that it continues to play a role in endosomal sorting (39,40,41,42). We coexpressed receptor, GFP-tagged Rab4, and arrestin-mCherry in human embryonic kidney (HEK)293 cells and asked whether the receptor and arrestin colocalized in Rab4-positive vesicles. We used arrestin-mCherry in these experiments because its emission wavelength does not overlap with GFP. Before stimulation with TRH, arrestin was diffuse in the cytosol, Rab4 was concentrated on endosomes, and HA-TRH receptor was on the plasma membrane, i.e. there was no overlap among any of the expressed proteins (Fig. 7A). Upon stimulation with TRH, arrestin moved rapidly to the membrane and overlapped with receptor (Fig. 7B, arrestin in red and HA-receptor in blue are pink in overlay). From 15–60 min, receptor in Rab4-positive endosomes was also colocalized with arrestin (Fig. 7, C and D, white in overlay). Thirty minutes after TRH removal, when nearly all of the receptor was dephosphorylated (Fig. 5F), much of the arrestin had returned to the cytosol, whereas receptor remained with Rab4 (Fig. 7F).
Figure 7.
Arrestin-receptor complex colocalizes with Rab4. Cells expressing 2HA-AP-TRH receptor, GFP-Rab4, and arrestin-mCherry were incubated with anti-HA antibody, stimulated with 100 nm TRH for the indicated times, fixed, and stained. Some cells were washed to remove TRH and allowed to recover for the indicated times before fixation. Bar, 10 μm. Arr-, Arrestin; s, seconds.
Repopulating the plasma membrane with TRH receptor
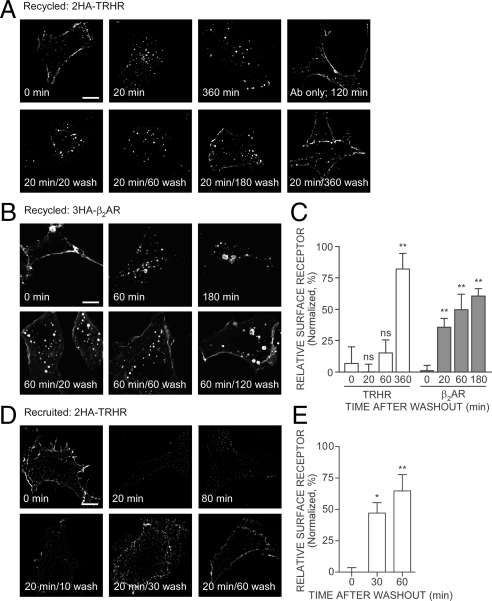
Proteins that traffic via Rab4 are generally regarded as being part of a rapid recycling pathway. We failed to see HA-antibody-labeled, internalized TRH receptor return to the plasma membrane up to 30 min after removal of agonist (Figs. 3E, 4E, 5F, and 7F), even though receptor was dephosphorylated and in Rab4 vesicles. Receptor was visible on the plasma membrane 3 h after agonist was removed, but some receptor still remained inside the cell (Fig. 8A).
Figure 8.
TRH and β2-adrenergic receptor trafficking. To measure recycling, cells expressing (A) 2HA-AP-TRH receptor or (B) HA-β 2-adrenergic receptor were incubated with anti-HA antibody, stimulated with 100 nm TRH or 1 μm isoproterenol, respectively, for the indicated times, fixed, and stained. Some cells were washed to remove agonist and allowed to recover for the indicated times before fixation; 1 μm propranolol was included in the recovery buffer for cells expressing HA-β2-adrenergic receptor. C, Quantification by line scan of relative surface receptor from cells treated as in panels A and B. D, To measure recruitment, cells expressing 2HA-AP-TRH receptor were treated as indicated; anti-HA antibody was then added to intact cells to identify surface receptors selectively. mCherry was coexpressed to allow positive identification of transfected cells (data not shown). E, Quantification by line scan of relative surface receptor from cells treated as in panel D. Shown are mean ± se of three to five cells from independent experiments, except the 60-min washout in panel E, which is the mean ± range from two determinations. **, P < 0.01; *, P < 0.05; nonsignificant (ns), P > 0.05 by one-way ANOVA compared with 0 min after washout. Bar, 10 μm. β2-AR, β2-adrenergic receptor; TRHR, TRH receptor.
We compared the behavior of the TRH receptor with the trafficking of another GPCR, the β2-adrenergic receptor, which internalizes relatively slowly, recycles rapidly to the plasma membrane via Rab4, and also traffics slowly to lysosomes via Rab7 (6). When we labeled HA-tagged β2-adrenergic receptor with anti-HA antibody, added and then removed agonist, we saw robust recycling as early as 20 min after agonist was washed out (Fig. 8B). The rates of recycling were analyzed by two methods. In one (Fig. 8, C and E, and Fig. 9C), line scans were performed and the ratios of fluorescence intensity on the plasma membrane relative to the cytoplasm were compared. In the other (supplemental Fig. 2), the fraction of cells with moderate or strong fluorescence on the cell surface was estimated visually. The results of both approaches, which are compared in supplemental Fig. 2, are in good agreement and confirm that recycling of antibody-labeled TRH receptor is slower than recycling of antibody-labeled β2-adrenergic receptor.
Figure 9.
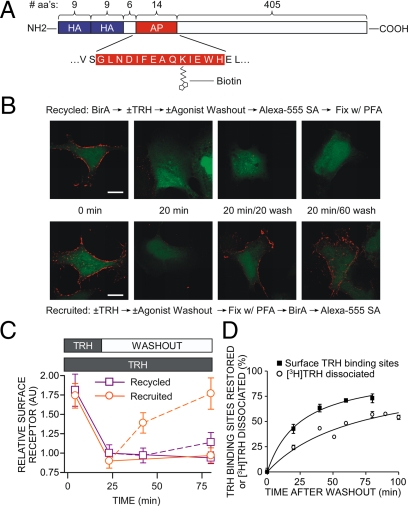
Recycling of biotinylated TRH receptor. A, Model of 2HA-AP-TRH receptor. B and C, Cells expressing 2HA-AP-TRH receptor were incubated with or without 100 nm TRH with or without washout, as shown in the middle of panel B. Cells were fixed with paraformaldehyde (PFA), biotinylated with BirA, and labeled with Alexa Fluor 555 streptavidin in the order shown above (recycled) or below (recruited) the micrographs. Control experiments established that staining of naïve cells was comparable when fixation preceded incubation with BirA or when the order was reversed. Bar, 10 μm. C, Quantification by line scan of relative surface receptor. Shown are mean ± se of four to seven cells from four independent experiments. Solid lines show results of cells exposed to TRH throughout, and dashed lines show cells after TRH washout. D, To measure restoration of TRH binding sites after internalization, cells stably expressing 2HA-TRH receptors were incubated with or without 100 nm TRH for 20 min to cause receptor internalization, washed, and incubated for various times. Dishes were placed on ice, and the number of TRH-binding sites on the cell surface was titrated by incubating cells with 10 nm [3H]MeTRH on ice as described in Materials and Methods. To measure receptor cycling, cells were incubated with [3H]TRH for 20 min, washed, and incubated for different times before determination of the fraction of [3H]TRH released as described in Materials and Methods. Points show the mean ± se from five to nine experiments, each performed in duplicate. Error bars were within symbol size where not visible. aa, Amino acids.
The experiments described above tracked the behavior of receptors after endocytosis but could not identify receptors that were inside the cell at the start of the experiment. To ask whether such intracellular receptors traffic to the membrane, i.e. are recruited, we incubated cells with TRH, withdrew hormone for various times, and then added anti-HA antibody to label all receptors on the plasma membrane, i.e. recycled plus recruited. TRH receptors were easily detected at the plasma membrane before treatment with TRH, but rapidly disappeared from the cell surface within 20 min after addition of agonist (Fig. 8, D and E, and supplemental Fig. 2). The plasma membrane remained essentially free of receptors for up to 80 min while TRH was present. After removal of TRH, TRH receptors were again detectable on the plasma membrane within 10 min. Replenishment of TRH receptors is not affected by protein synthesis inhibitors (14,43). The results in Fig. 8 suggest that after TRH is removed, the plasma membrane is repopulated by receptors recruited from an intracellular pool distinct from receptors that had recently internalized.
Trafficking and recycling of a biotinylated receptor
To confirm that TRH receptor recycling was slow relative to receptor recruitment, we used an alternate labeling strategy that uses the Escherichia coli enzyme biotin ligase (BirA) to biotinylate a 15-amino acid acceptor peptide (AP) (44) genetically inserted near the TRH receptor N terminus (Fig. 9A). BirA is highly specific, recognizing lysine only in the context of the AP tag, and because it does not cross the plasma membrane, the enzyme only biotinylates proteins with an extracellular AP tag.
To distinguish between receptors that internalized and then returned to the plasma membrane (recycled) and receptors that trafficked to the plasma membrane from an intracellular store (recruited), we biotinylated receptors on the surface of the cell before or after, respectively, addition of TRH and agonist washout. In the first protocol, intact cells were incubated with BirA to biotinylate surface receptors, stimulated with TRH, washed to remove agonist, and then labeled with Alexa Fluor 555 streptavidin to detect recycled receptors. Biotinylated receptors were clearly visible on cells that were not treated with TRH but had largely disappeared from the plasma membrane after 20 min of exposure to agonist (Fig. 9B). Internalized receptors recycled to the plasma membrane very slowly after TRH was removed. In the second protocol, cells were stimulated with TRH to cause internalization of unlabeled receptors on the surface, washed to remove agonist and allow receptor recycling and/or recruitment, and fixed with paraformaldehyde. Fixed cells were incubated with BirA to biotinylate all receptors on the surface and labeled with Alexa Fluor 555 streptavidin. Receptors were detected on vehicle-treated, but not on TRH-treated, cells (Fig. 9B). Agonist washout brought receptors to the plasma membrane within 20 min, where they were accessible to BirA, biotinylated, and labeled with streptavidin. Quantitative analyses by two methods again indicate that internalized receptors recycle slowly whereas receptors from an internal pool move to the plasma membrane quickly after agonist washout (Fig. 9C and supplemental Fig. 2).
A third strategy was used to distinguish between receptor recruitment and recycling. Cells were incubated with 100 nm TRH for 20 min, and hormone was then withdrawn, as in the experiments described above. In this case, the density of receptors on the plasma membrane was measured after different times of washout by incubating cells with membrane-impermeant [3H]MeTRH at 0 C, where further cycling could not take place. The half-time for restoration of surface receptor concentration was 25 min (Fig. 9D), close to the estimates of 35 min based on microscopic methods. To measure the rate of receptor recycling, cells were labeled with [3H]TRH for 20 min and washed, and the release of radioactivity into the medium was determined. [3H]TRH dissociation (t1/2 ∼70 min) was again slower than the rate of receptor replenishment.
Phosphorylation and dephosphorylation kinetics
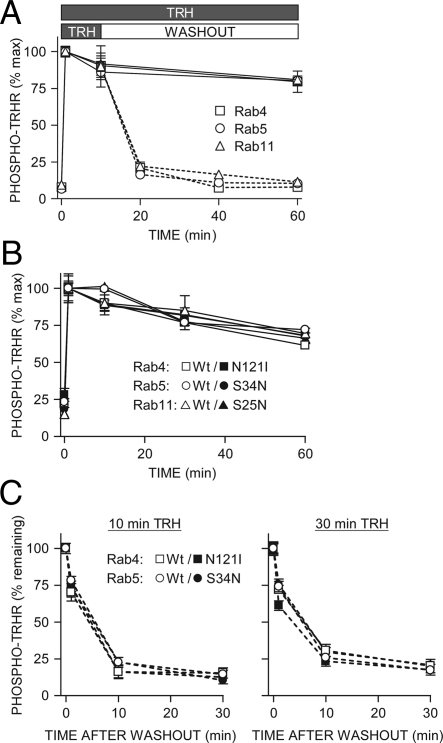
We used a previously validated ELISA for phosphoreceptor to measure the kinetics of TRH receptor phosphorylation and dephosphorylation after overexpression of wild-type or dominant-negative Rab GTPases (1). The initial rate of phosphorylation and the amount of phosphorylation over time for 60 min and longer were identical when receptor was coexpressed with Rab4, -5, -7, or -11 (Fig. 10A). Overexpression of dominant-negative forms of Rab4, -5, or -11 likewise had no effect on receptor phosphorylation (Fig. 10B). The rate of dephosphorylation after hormone withdrawal was also the same, whether the initial TRH challenge was 10, 30, or 60 min (Fig. 10, A and C). The total concentration of TRH receptor was unchanged under conditions in which dephosphorylation was extensive as measured by Western blot, radioligand binding, and ELISA (Refs. 35 and 36) and data not shown). Examination of the intensity of total receptor staining in Figs. 3–5, 7, and 8 also suggests that receptor levels remain the same after TRH washout. The rate and extent of dephosphorylation were comparable to those reported in nontransfected rat pituitary cells that endogenously express TRH receptor (1).
Figure 10.
Effect of Rab GTPase overexpression on TRH receptor phosphorylation and dephosphorylation. Cells expressing 2HA-AP-TRH receptor and (A–C) wild-type or (B and C) dominant-negative GFP-Rab4, -5, or -11 were stimulated with 100 nm TRH for the indicated times. Some cells were washed to remove TRH and incubated for the indicated times. Phosphorylated receptor was quantified by ELISA; shown are mean ± se of pooled replicates from at least three independent experiments. Solid lines show results of cells exposed to TRH throughout, and dashed lines show cells after TRH washout. GFP fluorescence was examined in all experiments. All of the GFP-rabs were well expressed and detectable in essentially all cells expressing receptors. Differences were not significant by one-way ANOVA. TRHR, TRH receptor; Wt, wild type.
Discussion
The experiments described here provide a detailed characterization of the subcellular localization and trafficking of a GPCR that distinguishes between a phosphorylated and nonphosphorylated receptor. The TRH receptor is phosphorylated predominantly by GPCR kinase 2 (1). Phosphorylated receptor recruits arrestin to the plasma membrane and then internalizes with it onto Rab5-postive endosomes (Figs. 2 and 3). Shortly after TRH is removed, receptors are dephosphorylated, dissociated from arrestin, and trafficked exclusively via Rab4 vesicles that no longer contain Rab5 (Figs. 3 and 5–7). This observation prompts a fundamental question: does dephosphorylation occur immediately once the TRH receptor reaches Rab4-positive/Rab5-negative endosomes, or is the receptor sorted into these vesicles only after it has been dephosphorylated? Because the rate of TRH receptor dephosphorylation was unaffected by overexpression of dominant-negative Rabs 4, 5, or 11, it appears that the phosphorylation state of the TRH receptor determines receptor localization and not vice versa. Dominant-negative Rab5, for example, blocks internalization but not dephosphorylation, showing that the phosphoreceptor does not need to advance to any particular endosomal compartment to undergo dephosphorylation. Furthermore, TRH receptor dephosphorylation is not inhibited when internalization is prevented by hypertonic sucrose or absence of arrestin (1,11), which also prevent receptor from reaching Rab4 vesicles. The μ-opioid receptor also traffics by different pathways depending on its phosphorylation state (45).
Dephosphorylation of the TRH receptor is markedly different than that of the β2-adrenergic receptor, even though both receptors traffic to early endosomes via Rab5 and recycle to the plasma membrane via Rab4 after being dephosphorylated (6). Dephosphorylation of protein kinase A sites on the β2-adrenergic receptor requires receptor entry into early endosomes that have a low pH and are enriched in a protein phosphatase-2A-like phosphatase (24,46), whereas dephosphorylation of GPCR kinase sites can occur at the plasma membrane (47). The TRH receptor is readily dephosphorylated at all sites whether it is on the plasma membrane or in endosomes, and pharmacological inhibition of endosomal acidification has no effect on dephosphorylation (1). Dominant-negative Rab5-S34N blocks β2-adrenergic receptor (24) but not TRH receptor dephosphorylation (Fig. 10). Thus, whereas one can conclude that β2-adrenergic receptor dephosphorylation is dependent on its localization, the inverse is true for the TRH receptor.
As long as the TRH receptor is phosphorylated and associated with arrestin, it appears to remain in Rab5- or, to a limited extent, Rab11-containing endosomes. It is not clear what factors cause retention of phosphoreceptors in these vesicles. Two GPCRs have been shown to directly bind to Rabs: the angiotensin II type 1a receptor binds Rab5 (48), and the thromboxane A2 receptor binds Rab11 (49). We were unable to detect direct binding of TRH receptor to any of the Rabs described in this report through coimmunoprecipitation with or without chemical cross-linking. Once dephosphorylated, TRH receptor moves into Rab4-positive vesicles, although it is not clear whether it is actively sorted into Rab4-positive endosomes or enters that pathway as passive cargo. An active sorting model is supported by the fact that dephosphorylated receptor traffics exclusively with Rab4 but not with Rab11 or Rab7, which also form pathways extending from the Rab5-rich early endosome.
Dephosphorylation of internalized TRH receptors began immediately when TRH was withdrawn and was much faster (t1/2 ∼ 5 min) (Figs. 3E, 4E, 5, E and F, and 10) than replenishment of receptors at the plasma membrane (Figs. 8 and 9). Biochemical measurements of receptor dephosphorylation and cycling give similar results (1,4,13,14,15,50). Thus, it is puzzling that intracellular receptors dephosphorylate so quickly in response to the removal of extracellular agonist, because this implies that the intra- and extracellular receptor pools are somehow connected. It is unlikely that G protein-coupled signaling is involved because phospholipase C and protein kinase C inhibitors have no effect on receptor phosphorylation, and the kinetics of phosphorylation and dephosphorylation are normal when receptors are expressed in fibroblasts from mice lacking Gαq and Gα11 (1,11,51). Further study is required to explain how internalized receptor detects the presence of extracellular TRH.
GPCRs are typically divided into two classes: class A receptors bind arrestin but internalize without it and typically recycle quickly (t1/2 ∼10 min), whereas class B receptors remain complexed with arrestin after endocytosis and recycle slowly (t1/2 > 1 h) or are degraded (52,53). The TRH receptor has been classified as a class B receptor (53) but is atypical in not entering Rab7 vesicles or trafficking to lysosomes (Figs. 4 and 6). Information within the C-tail sequences of GPCRs determines whether internalized receptor recycles or is degraded, because swapping the tails of two receptors changes receptor fate (54,55). The TRH receptor tail is sufficient to convert the noninternalizing mammalian GnRH receptor to a class B receptor, but the recycling properties of the chimeric receptor have not been characterized thoroughly (56).
Recycling to the plasma membrane typically follows dephosphorylation for GPCRs that are not degraded (31,52). The TRH receptor has been reported to recycle after removal of agonist based on the reappearance of TRH binding sites at the plasma membrane, the return of plasma membrane FLAG antibody staining in cells expressing N-terminal FLAG-tagged receptors, the redistribution of GFP-tagged TRH receptors to the plasma membrane, and restoration of TRH signaling (4,13,14,15). Analysis by all of these methods indicates that the plasma membrane is at least 50% repopulated with TRH receptors 30 min after TRH withdrawal (reminiscent of class A receptors), but the methods used do not distinguish between recycled and recruited receptors. Two avenues of investigation previously suggested that recycling receptors might not fully account for replenishment of the surface receptor population. If recycling from endosomes provides most of the receptors that repopulate the membrane, one might expect that the time to replenish surface receptors would depend on the time of agonist exposure, which dictates where the receptors reside when agonist is withdrawn (Rab5, Rab5/Rab4, or Rab11 vesicles), yet Drmota and Milligan (15) found that the duration of the initial pulse of TRH did not correlate with the rate of replenishment of surface binding sites. In addition, when we studied a TRH receptor fused to a DsRed fluorescent protein that changes from green to red fluorescence over time, we found that the plasma membrane was repopulated preferentially by younger (green) receptors (14).
Our data raise the questions of where intracellular TRH receptors reside, what triggers their movement to the plasma membrane, and what pathway they follow. The intracellular pool of TRH receptors is initially localized in part in Rab11-positive endosomes (Fig. 1). Recycling of the thromboxane A2 receptor TPβ, for example, proceeds through Rab11 vesicles after constitutive endocytosis (49). The unactivated thrombin receptor cycles constitutively, and the intracellular pool replenishes the plasma membrane after irreversible cleavage and activation of surface receptors (57). Constitutive cycling of the TRH receptor has been reported (43) but must proceed quite slowly, because neither antibody- nor biotin-labeled surface receptors underwent significant internalization over 3 h in the absence of hormone (Figs. 8 and 9). Additional approaches will be required to determine whether recruitment is a constitutive process or one involving signaling.
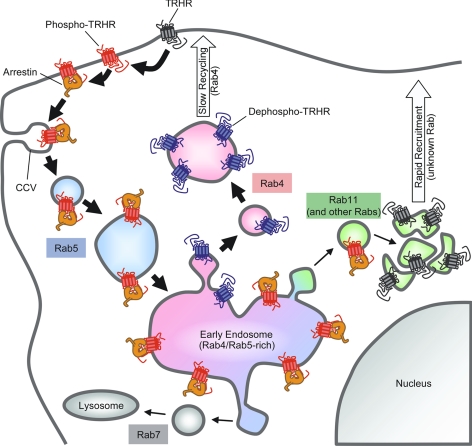
We used three independent methods to assess the contributions of recycled vs. recruited receptors. When we prelabeled surface receptors selectively with anti-HA antibody and followed the return of these receptors to the surface after agonist-mediated endocytosis and different recovery periods, we did not detect recycled receptors at the plasma membrane until 3 h after TRH was removed (Fig. 8A). In contrast, when we identified the total cell surface receptor population by applying anti-HA antibody to intact cells after TRH-mediated endocytosis and various times of recovery, we found that surface receptors had largely reappeared within 30 min after TRH removal (Fig. 8, D and E), consistent with previous reports (13). These findings suggest that recruited, rather than recycled, receptors repopulate the membrane at early times, but they could be misleading if labeling the N terminus of receptors with antibody retards normal trafficking of receptors. We corroborated our findings by studying recycling of biotinylated TRH receptors. Addition of a biotin residue is unlikely to alter trafficking, and there was no possibility that the covalently attached biotin could fall off. We again found that recycling of internalized receptors proceeded very slowly relative to the rate of replenishment of the surface receptor pool, measured by biotinylating receptors after internalization and recycling (Fig. 9). We also demonstrated that after receptor internalization, surface binding sites appeared faster than internalized [3H]TRH dissociated. Taken together, the data indicate that internalized TRH receptors are neither degraded nor immediately recycled to the cell surface but, rather, retained in a Rab4-positive endosomal compartment for prolonged periods while the plasma membrane is repopulated by a different pool of intracellular receptors (Fig. 11).
Figure 11.
TRH receptor trafficking. Primary routes of receptor trafficking are marked with thick arrows; minor or unused pathways have thin arrows. TRH receptors on the cell surface (gray) are rapidly phosphorylated (red) in response to TRH and recruit arrestin to the plasma membrane. Receptors can be dephosphorylated while still on the plasma membrane after agonist is removed. Arrestin escorts phospho-TRH receptor to clathrin-coated vesicles (CCV), where the arrestin-receptor complex is internalized on Rab5-positive endosomes (shaded blue). The arrestin-receptor complex is delivered to early endosomes rich in Rab5 and Rab4. Some phosphoreceptor bound to arrestin moves into Rab11-positive endosomes (shaded green). Once dephosphorylated, TRH receptor (blue) moves rapidly to exclusively Rab4-positive vesicles (shaded pink) and is slowly recycled to the plasma membrane. Before treatment with TRH, an intracellular pool of reserve TRH receptors (gray) colocalizes in part with Rab11 in a perinuclear compartment. TRHR, TRH receptor.
Characterization of the trafficking of phosphorylated and dephosphorylated TRH receptors improves our understanding of TRH receptor signaling and highlights the variety that exists in GPCR trafficking. Like most GPCRs, once phosphorylated, the TRH receptor is sequestered with arrestin into Rab5-positive endosomes that later include Rab4. Unlike most class B GPCRs, however, the TRH receptor does not recycle via the slow Rab11-dependent pathway but, instead, undergoes dephosphorylation and then recycles via Rab4. Recruitment from an intracellular pool of receptors contributes to rapid replenishment of the surface receptor population, whereas recycling contributes to replenishment at later times. Future studies will determine the role of specific phosphosites on receptor trafficking as well as the identity and role of the relevant phosphatase.
Materials and Methods
Plasmids
mCherry from Dr. Roger Tsien (University of California San Diego, San Diego, CA) was cloned into pcDNA3 (Invitrogen, Carlsbad, CA), and arrestin 3 from Dr. Vsevolod Gurevich (Vanderbilt University, Nashville, TN) without a STOP codon was cloned in front of mCherry. Biotin ligase affinity peptide (AP) sequence was inserted in the N-terminal region of a rat TRH receptor with double N-terminal HA epitopes using QuikChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The resulting protein, 2HA-AP-TRH receptor, expresses, binds TRH, becomes phosphorylated, and traffics the same as receptor with no AP tag. Sequences were verified by direct sequencing. Plasmid encoding BirA enzyme was a gift from Dr. Alice Ting (Massachusetts Institute of Technology, Cambridge, MA) (44). Rab-encoding plasmids were very generous gifts from Dr. Stephen Ferguson (Robarts Research Institute, London, Ontario, Canada); Rab5 constructs are canine and all others are human. Arrestin 3-GFP was from Dr. Marc Caron (Duke University, Durham, NC). Primer sequences are available on request.
Cell culture and transfection
HEK293 cells were maintained in DMEM/F-12 with 5% fetal bovine serum and passaged with trypsin/EDTA. Cells in 25-cm2 flasks were transfected at 50–75% confluence using 4 μg of plasmid DNA and 9–11 μl of FuGene HD (Roche Diagnostics, Indianapolis, IN) and reseeded 1:5 48 h later on ECL cell attachment matrix-coated (Millipore Corp., Bedford, MA) coverslips for microscopy or poly-l-lysine-coated (Sigma Chemical Co., St. Louis, MO) 12-well dishes for ELISA. Experiments were performed 48 h after reseeding, when receptor density was comparable to that in rat pituitary cells (≤1 pmol/mg protein). Cells stably expressing 2HA-AP-TRH receptor were selected with 500 μg/ml G418. The distribution of TRH receptors was not detectably different in transiently and stably transfected cells.
Confocal microscopy
GFP was detected by excitation with a 488-nm argon laser, 543-nm bandpass emission filter, mCherry and Alexa Fluor 555 with a 543-nm He-Ne laser, 585-nm bandpass emission filter, and Alexa Fluor 647 with a 633-nm He-Ne laser, 670-nm bandpass emission filter on a Nikon C1 visible light laser scanning confocal microscope (Nikon, Inc., Melville, NY) with a ×60 (1.4 NA) oil immersion objective. Fields were randomly selected, but cells with aberrant morphology or very bright signals were ignored. All images within each experiment were scaled and deconvoluted identically for each color channel using Metamorph Imaging Software (Molecular Devices, Sunnyvale, CA), with the exception of color channels showing fluorescent protein-tagged arrestins, which were not deconvoluted. Fluorescent signal crossover was eliminated by scanning channels individually, and background was determined by imaging cells not expressing individual proteins or not stained with individual fluorophores. At least five cells per coverslip were imaged, and all images shown are representative of three or more independent experiments.
Antibody labeling of surface receptors
To selectively label surface receptors, cells were rinsed once with Hanks’ balanced salt solution (HBSS) or Dulbecco’s PBS (2.67 mm KCl, 1.5 mm KH2PO4, 138 mm NaCl, 8.1 mm Na2HPO4), pH 7.4, incubated for 5 min with 1:250 anti-HA antibody (mHA-ll; Covance Laboratories, Inc., Madison, WI) in HBSS, and rinsed twice with HBSS. Alternatively, cells were treated with agonist followed by washout for various times and then labeled with anti-HA antibody in HBSS. No antibody bound to nontransfected cells, and incubation with antibody alone for 30 min did not change [3H]MeTRH binding.
To label receptors on the plasma membrane after agonist treatment and washout, we incubated cells in 1:500 anti-HA antibody for 5 min, rinsed to remove excess antibody, and fixed with 3% paraformaldehyde for 10 min.
Subcellular localization of phosphoreceptor complexes
To induce TRH receptor phosphorylation and endocytosis, cells labeled with HA-antibody or not were stimulated 96 h after transfection with 100 nm TRH in HBSS for various times at 37 C. TRH was removed by washing dishes twice in HBSS and then incubating for various times in HBSS. Cells were fixed with 3.7% paraformaldehyde in HBSS, at room temperature, for 20 min.
To label phosphorylated TRH receptors, fixed cells were rinsed once with PBS, incubated 90 min with 1:100 affinity-purified phosphosite-specific polyclonal antibody in RIPA/milk buffer (150 mm NaCl; 50 mm Tris; 1 mm EDTA; 1% Triton X-100; 0.1% sodium dodecyl sulfate; 0.5% Na deoxycholate, pH 8.0; 5% nonfat dried milk), washed three times with PBS, incubated 45 min with 1:250 goat antirabbit Alexa Fluor 555 secondary antibody (Molecular Probes, Sunnyvale, CA) in RIPA/milk, washed three times with PBS, and mounted using ProLong Gold plus 4′,6-diamidino-2-phenylindole (Molecular Probes). Arrestin-FLAG was detected the same way, using 1:250 M2 anti-FLAG antibody (Sigma) and 1:500 goat antimouse Alexa Fluor 647 secondary antibody (Molecular Probes). GST-Rab5 was detected using 1:500 rabbit polyclonal anti-GST antibody ab9085 (Abcam, Inc., Cambridge, MA) and 1:500 antirabbit Alexa Fluor 555. HA antibody was detected using 1:500 goat antimouse Alexa Fluor 647 secondary antibody for triple-color experiments, or goat antimouse Alexa Fluor 488 for single- or dual-color experiments.
BirA purification and biotinylation of surface receptors
His6-tagged BirA was purified as described (44). 2HA-AP-TRH receptors in HEK293 cells plated on glass coverslips were biotinylated as follows. To fluorescently label only recycled receptors, coverslips were rinsed once with Dulbecco’s PBS (DPBS), placed on parafilm, and incubated at room temperature for 20 min with 4 μm BirA in 100 μl biotinylation buffer [5 mm MgCl2, 1 mm ATP, 1 or 10 μm d-biotin (pH 7.4) in DPBS]. Cells were rinsed to remove enzyme, treated with 100 nm TRH in DPBS, washed to remove agonist, and incubated to allow recycling. Dishes were placed on ice to stop trafficking, and biotinylated receptors on the plasma membrane were labeled with Alexa Fluor 555 streptavidin (Molecular Probes) for 20 min on ice. Cells were rinsed to remove excess streptavidin and fixed with 3% paraformaldehyde in DPBS for 10 min, and coverslips were mounted with ProLong Gold plus 4′,6-diamidino-2-phenylindole. To fluorescently label all receptors on the plasma membrane at the end of the experiment (i.e. recycled plus recruited receptors), cells were treated with 100 nm TRH, washed, incubated as described, and fixed for 10 min with 3% paraformaldehyde. Receptors on the cell surface were then biotinylated with BirA, and biotinylated receptors were labeled with Alexa Fluor 555 streptavidin. No fluorescence was detected in nontransfected cells or cells that were incubated without BirA.
Quantification of recycling and recruitment rates by microscopy
TRH receptors on the plasma membrane were labeled with antibody or biotin as noted. Quantification of relative surface receptor by line scan was performed as follows. Average intensity of fluorescence at the plasma membrane or inside the cell was measured by line scan using ImageJ software. Each cell was analyzed by drawing 10 lines approximately one third of the way into the cell from random points along the plasma membrane. Relative surface receptor is expressed as the ratio of plasma membrane to intracellular fluorescence. In Fig. 8 and supplemental Fig. 2 this value was normalized to a range from 0–100% to allow comparison between different receptors (Fig. 8) or quantification methods (supplemental Fig. 2).
Recycling or recruitment rates were also determined by counting the number of cells with moderate to high staining of receptor on the plasma membrane. This method may overestimate trafficking rates, but any bias affects both rate measurements equally, permitting reliable comparison. Slides from the experiments shown in Figs. 8 and 9 were pooled, and counts were made by an observer unaware of the experimental conditions of each sample.
Quantification of recycling and recruitment rates with radiolabeled hormone
[3H]TRH and [3H]N3-Me-His2-TRH (MeTRH) were obtained from NEN/Dupont (Boston, MA). HEK293 cells stably expressing HA-tagged TRH receptor were grown on 12-well dishes and equilibrated in HBSS at 37 C for 20 min. To measure recycling, cells were incubated with 2–5 nm [3H]TRH for 20 min, washed three times with ice-cold HBSS, and incubated further in HBSS at 37 C. At intervals, medium was removed and cells were lysed in 0.1% sodium dodecyl sulfate. Radioactivity in medium and cells was counted. To measure receptor recruitment, cells were incubated for 20 min with or without 100 nm TRH to drive internalization and then washed twice with HBSS and incubated at 0 C with 10 nm [3H]MeTRH for 1.5 to 2 h to occupy surface receptors. Dishes were washed three times with cold saline, and cells were lysed and counted. Nonspecific binding in both experiments was determined in wells containing 1 μm unlabeled TRH. Receptor density in cells never exposed to unlabeled TRH was taken as 100%, and receptor density before washout was taken as 0% binding restored. The percent [3H]TRH dissociated was (specifically bound [3H]TRH in the medium)/(total in cells plus medium).
Other methods
Phosphorylated TRH receptor was quantified by a modified ELISA (1). Experiments were performed at least three times, and error bars show mean ± se of triplicate determinations, unless noted. Where not visible, error bars fell within symbol size. Differences were determined by two- or one-way ANOVA and post hoc Tukey’s test or Student’s unpaired t test, as appropriate. Pearson’s correlation coefficients (38) were calculated using Imaris software (Bitplane AG, Zurich, Switzerland) on three to 12 images per point.
Supplementary Material
Acknowledgments
The authors are grateful to the researchers who generously provided reagents.
Footnotes
This work was supported by Grant DK19974 from the National Institutes of Health (to P.M.H.) and a National Institutes of Health Cardiovascular Research Training Grant and a Pharmaceutical Manufacturers’ Association Predoctoral Fellowship (to B.W.J.).
Disclosure Summary: None of the authors have anything to declare.
First Published Online June 18, 2009
Abbreviations: AP, Acceptor peptide; BirA, biotin ligase; DPBS, Dulbecco’s PBS; GFP, green fluorescent protein; GPCR, G protein-coupled receptor; GST, glutathione-S-transferase; GTPase, guanosine triphosphatase; HA, hemagglutinin; HEK, human embryonic kidney; HBSS, Hanks’ balanced salt solution.
References
- Jones BW, Song GJ, Greuber EK, Hinkle PM 2007 Phosphorylation of the endogenous thyrotropin-releasing hormone receptor in pituitary GH3 cells and pituitary tissue revealed by phosphosite-specific antibodies. J Biol Chem 282:12893–12906 [DOI] [PubMed] [Google Scholar]
- Groarke DA, Wilson S, Krasel C, Milligan G 1999 Visualization of agonist-induced association and trafficking of green fluorescent protein-tagged forms of both β-arrestin-1 and the thyrotropin-releasing hormone receptor-1. J Biol Chem 274:23263–23269 [DOI] [PubMed] [Google Scholar]
- Vrecl M, Anderson L, Hanyaloglu A, McGregor AM, Groarke AD, Milligan G, Taylor PL, Eidne KA 1998 Agonist-induced endocytosis and recycling of the gonadotropin-releasing hormone receptor: effect of β-arrestin on internalization kinetics. Mol Endocrinol 12:1818–1829 [DOI] [PubMed] [Google Scholar]
- Yu R, Hinkle PM 1998 Signal transduction, desensitization, and recovery of responses to thyrotropin-releasing hormone after inhibition of receptor internalization. Mol Endocrinol 12:737–749 [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL 2007 Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69:451–482 [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Ferguson SS 2003 Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74:225–235 [DOI] [PubMed] [Google Scholar]
- Sorkin A, Von Zastrow M 2002 Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol 3:600–614 [DOI] [PubMed] [Google Scholar]
- Yu R, Hinkle PM 1997 Desensitization of thyrotropin-releasing hormone receptor-mediated responses involves multiple steps. J Biol Chem 272:28301–28307 [DOI] [PubMed] [Google Scholar]
- Groarke DA, Drmota T, Bahia DS, Evans NA, Wilson S, Milligan G 2001 Analysis of the C-terminal tail of the rat thyrotropin-releasing hormone receptor-1 in interactions and cointernalization with β-arrestin 1-green fluorescent protein. Mol Pharmacol 59:375–385 [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, Seeber RM, Kohout TA, Lefkowitz RJ, Eidne KA 2002 Homo- and hetero-oligomerization of thyrotropin-releasing hormone (TRH) receptor subtypes. Differential regulation of β-arrestins 1 and 2. J Biol Chem 277:50422–50430 [DOI] [PubMed] [Google Scholar]
- Jones BW, Hinkle PM 2005 β-Arrestin mediates desensitization and internalization but does not affect dephosphorylation of the thyrotropin-releasing hormone receptor. J Biol Chem 280:38346–38354 [DOI] [PubMed] [Google Scholar]
- Scott MG, Benmerah A, Muntaner O, Marullo S 2002 Recruitment of activated G protein-coupled receptors to pre-existing clathrin-coated pits in living cells. J Biol Chem 277:3552–3559 [DOI] [PubMed] [Google Scholar]
- Ashworth R, Yu R, Nelson EJ, Dermer S, Gershengorn MC, Hinkle PM 1995 Visualization of the thyrotropin-releasing hormone receptor and its ligand during endocytosis and recycling. Proc Natl Acad Sci USA 92:512–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LB, Hinkle PM 2004 Fate of internalized thyrotropin-releasing hormone receptors monitored with a timer fusion protein. Endocrinology 145:3095–3100 [DOI] [PubMed] [Google Scholar]
- Drmota T, Milligan G 2000 Kinetic analysis of the internalization and recycling of [3H]TRH and C-terminal truncations of the long isoform of the rat thyrotropin-releasing hormone receptor-1. Biochem J 346:711–718 [PMC free article] [PubMed] [Google Scholar]
- Hinkle PM, Shanshala 2nd ED 1989 Pituitary thyrotropin-releasing hormone (TRH) receptors: effects of TRH, drugs mimicking TRH action, and chlordiazepoxide. Mol Endocrinol 3:1337–1344 [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK 2007 β-Arrestins and cell signaling. Annu Rev Physiol 69:483–510 [DOI] [PubMed] [Google Scholar]
- Jones BW, Hinkle P 2008 Arrestin binds to different phosphorylated regions of the TRH receptor with distinct functional consequences. Mol Pharmacol 74:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneka M, Neeft M, van der Sluijs P 2003 Regulation of membrane transport by rab GTPases. Crit Rev Biochem Mol Biol 38:121–142 [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P 2006 Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103:11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A 2007 Rab GTPases at a glance. J Cell Sci 120:3905–3910 [DOI] [PubMed] [Google Scholar]
- Fan GH, Lapierre LA, Goldenring JR, Richmond A 2003 Differential regulation of CXCR2 trafficking by Rab GTPases. Blood 101:2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin F, Dery O, DeFea KO, Slice L, Patierno S, Sternini C, Grady EF, Bunnett NW 2001 Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. J Biol Chem 276:25427–25437 [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Anborgh PH, Ferguson SS 2000 β2-Adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem 275:27221–27228 [DOI] [PubMed] [Google Scholar]
- Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M 2000 Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149:901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday ND, Holst B, Rodionova EA, Schwartz TW, Cox HM 2007 Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol Endocrinol 21:3100–3112 [DOI] [PubMed] [Google Scholar]
- Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, Lodge R, Catt KJ, Balla T 2002 Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol 157:1211–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorati G, Le Gouill C, Balamotis M, Birnbaumer M 2001 The long and the short cycle. Alternative intracellular routes for trafficking of G-protein-coupled receptors. J Biol Chem 276:13096–13103 [DOI] [PubMed] [Google Scholar]
- Volpicelli LA, Lah JJ, Fang G, Goldenring JR, Levey AI 2002 Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J Neurosci 22:9776–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E 1998 A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol 8:34–45 [DOI] [PubMed] [Google Scholar]
- Ferguson SS 2001 Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24 [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B 2000 Rab7: a key to lysosome biogenesis. Mol Biol Cell 11:467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK 2007 Seven-transmembrane receptors and ubiquitination. Circ Res 100:1142–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub O, Rotin D 2006 Role of ubiquitylation in cellular membrane transport. Physiol Rev 86:669–707 [DOI] [PubMed] [Google Scholar]
- Cook LB, Hinkle PM 2004 Agonist-dependent up-regulation of thyrotrophin-releasing hormone receptor protein. Biochem J 380:815–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LB, Zhu CC, Hinkle PM 2003 Thyrotropin-releasing hormone receptor processing: role of ubiquitination and proteasomal degradation. Mol Endocrinol 17:1777–1791 [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn SC 2006 Recycling endosomes. J Cell Sci 119:1679–1681 [DOI] [PubMed] [Google Scholar]
- Bolte S, Cordelières FP 2006 A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232 [DOI] [PubMed] [Google Scholar]
- Pan L, Gurevich EV, Gurevich VV 2003 The nature of the arrestin x receptor complex determines the ultimate fate of the internalized receptor. J Biol Chem 278:11623–11632 [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ 2003 Trafficking patterns of β-arrestin and G protein-coupled receptors determined by the kinetics of β-arrestin deubiquitination. J Biol Chem 278:14498–14506 [DOI] [PubMed] [Google Scholar]
- Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Höllt V, Schulz S 2004 Differential β-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem 279:21374–21382 [DOI] [PubMed] [Google Scholar]
- Vines CM, Revankar CM, Maestas DC, LaRusch LL, Cimino DF, Kohout TA, Lefkowitz RJ, Prossnitz ER 2003 N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J Biol Chem 278:41581–41584 [DOI] [PubMed] [Google Scholar]
- Petrou C, Chen L, Tashjian Jr AH 1997 A receptor-G protein coupling-independent step in the internalization of the thyrotropin-releasing hormone receptor. J Biol Chem 272:2326–2333 [DOI] [PubMed] [Google Scholar]
- Chen I, Howarth M, Lin W, Ting AY 2005 Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat Methods 2:99–104 [DOI] [PubMed] [Google Scholar]
- Wang F, Chen X, Zhang X, Ma L 2008 Phosphorylation state of μ-opioid receptor determines the alternative recycling of receptor via Rab4 or Rab11 pathway. Mol Endocrinol 22:1881–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ 1997 The role of sequestration in G protein-coupled receptor resensitization. Regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem 272:5–8 [DOI] [PubMed] [Google Scholar]
- Iyer V, Tran TM, Foster E, Dai W, Clark RB, Knoll BJ 2006 Differential phosphorylation and dephosphorylation of β2-adrenoceptor sites Ser262 and Ser355,356. Br J Pharmacol 147:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seachrist JL, Laporte SA, Dale LB, Babwah AV, Caron MG, Anborgh PH, Ferguson SS 2002 Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem 277:679–685 [DOI] [PubMed] [Google Scholar]
- Hamelin E, Thériault C, Laroche G, Parent JL 2005 The intracellular trafficking of the G protein-coupled receptor TPβ depends on a direct interaction with Rab11. J Biol Chem 280:36195–36205 [DOI] [PubMed] [Google Scholar]
- Drmota T, Novotny J, Kim GD, Eidne KA, Milligan G, Svoboda P 1998 Agonist-induced internalization of the G protein G11α and thyrotropin-releasing hormone receptors proceed on different time scales. J Biol Chem 273:21699–21707 [DOI] [PubMed] [Google Scholar]
- Yu R, Hinkle PM 1999 Signal transduction and hormone-dependent internalization of the thyrotropin-releasing hormone receptor in cells lacking Gq and G11. J Biol Chem 274:15745–15750 [DOI] [PubMed] [Google Scholar]
- Drake MT, Shenoy SK, Lefkowitz RJ 2006 Trafficking of G protein-coupled receptors. Circ Res 99:570–582 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS 2000 Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17201–17210 [DOI] [PubMed] [Google Scholar]
- Liang W, Austin S, Hoang Q, Fishman PH 2003 Resistance of the human β1-adrenergic receptor to agonist-mediated down-regulation. Role of the C terminus in determining β-subtype degradation. J Biol Chem 278:39773–39781 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG 1999 Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 274:32248–32257 [DOI] [PubMed] [Google Scholar]
- Heding A, Vrecl M, Bogerd J, McGregor A, Sellar R, Taylor PL, Eidne KA 1998 Gonadotropin-releasing hormone receptors with intracellular carboxyl-terminal tails undergo acute desensitization of total inositol phosphate production and exhibit accelerated internalization kinetics. J Biol Chem 273:11472–11477 [DOI] [PubMed] [Google Scholar]
- Shapiro MJ, Trejo J, Zeng D, Coughlin SR 1996 Role of the thrombin receptor’s cytoplasmic tail in intracellular trafficking. Distinct determinants for agonist-triggered versus tonic internalization and intracellular localization. J Biol Chem 271:32874–32880 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.