Abstract
The mineralocorticoid receptor (MR) plays a central role in electrolyte homeostasis and in cardiovascular disease. We have previously reported a ligand-dependent N/C-interaction in the MR. In the present study we sought to fully characterize the MR N/C-interaction. By using a range of natural and synthetic MR ligands in a mammalian two-hybrid assay we demonstrate that in contrast to aldosterone, which strongly induces the interaction, the physiological ligands deoxycorticosterone and cortisol weakly promote the interaction but predominantly inhibit the aldosterone-mediated N/C-interaction. Similarly, progesterone and dexamethasone antagonize the interaction. In contrast, the synthetic agonist 9α-fludrocortisol robustly induces the interaction. The ability of the N/C interaction to discriminate between MR agonists suggests a subtle conformational difference in the ligand-binding domain induced by these agonists. We also demonstrate that the N/C interaction is not cell specific, consistent with the evidence from a glutathione-S-transferase pull-down assay, of a direct protein-protein interaction between the N- and C-terminal domains of the MR. Examination of a panel of deletions in the N terminus suggests that several regions may be critical to the N/C-interaction. These studies have identified functional differences between physiological MR ligands, which suggest that the ligand-specific dependence of the N/C-interaction may contribute to the differential activation of the MR that has been reported in vivo.
The ligand-specific dependence of the MR N/C-interaction may contribute to its differential activation in vivo.
The aldosterone-mineralocorticoid receptor (MR) signaling pathway plays an important role in the regulation of electrolyte balance in epithelial cells, primarily in the distal collecting tubules of the kidney and in the colon (1,2). MR is also expressed in various nonepithelial cells, such as cardiomyocytes (3,4,5) and neurons (6). The MR plays a critical role in the pathophysiology of hypertension and cardiac dysfunction as well as in neurophysiological dysfunction in humans (6,7). Two recent clinical trials, the Randomized Aldactone Evaluation Study (RALES) and Eplerenone Post-acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) underscore the efficacy of antagonizing MR with spironolactone or eplerenone, respectively, to reduce the morbidity and mortality of patients with congestive heart failure (8). Despite the clinical significance of the MR, the molecular mechanisms underlying signal transduction by the MR are not clearly understood.
Of the steroid hormone receptors, the MR is unique in being a physiologically important receptor for two different classes of steroid hormones: 1) the mineralocorticoids, aldosterone and deoxycorticosterone (DOC) and 2) the glucocorticoids, cortisol and corticosterone (in rodents). In addition, progesterone is an antagonist of MR although the physiological significance of this is not understood. The MR mediates the action of aldosterone and cortisol, in a complex, tissue-specific manner (5). However, the molecular basis of the tissue-specific dichotomy remains unexplained.
The MR (NR3C2) is a ligand-dependent transcription factor that belongs to the nuclear receptor superfamily (9). MR shares a similar modular structure with other members of the nuclear receptor superfamily with an amino-terminal region that encodes a ligand-independent activation function-1 (AF-1), followed by the highly conserved DNA-binding domain (DBD), the hinge or linker region, and the C-terminal ligand-binding domain (LBD), which includes a ligand-dependent transcriptional domain, denoted as activation function-2 (AF-2) (10). We have previously demonstrated an interaction between the N-terminal domain (NTD) and the LBD of the MR that occurs in response to aldosterone but not cortisol (11).
An N/C-interaction in the steroid receptors is best characterized for the androgen receptor (AR) (12,13). In the AR, the interaction is mediated by the binding of the AF-2 region of the LBD to specific FxxLF and WxxLF sequences in the NTD (14). These motifs in the N terminus bind to the groove formed by helices 3, 4, and 12 in the LBD (14,15). The MR NTD lacks both these motifs and the LxxLL motifs that mediate coactivator binding to the AF-2 region of steroid receptors. Rogerson and Fuller (11) found that mutation of the AF-2 region (E962A) decreased but did not eliminate the interaction of the MR LBD with the N terminus, in contrast to the interaction with the coactivators such as steroid receptor coactivator-1 (SRC-1) and peroxisome proliferator γ coactivator 1-α, which are AF-2 dependent (our unpublished observation). This substitution in the MR may be analogous to the AF-2 charge clamp mutation, K720A in the AR, which abrogates both the N/C-interaction and coactivator binding (13). Rogerson and Fuller (11) also reported that the AR NTD did not interact with the MR LBD, again pointing to fundamental differences in the structural determinants of the N/C-interaction in the AR and in the MR.
In this study we have further characterized the MR N/C-interaction by utilizing a range of MR ligands to contrast their ability to induce or antagonize the N/C-interaction in a mammalian two-hybrid (M-2-H) assay. We demonstrate that the MR ligand DOC weakly promotes the interaction but predominantly inhibits the aldosterone-mediated N/C-interaction. Progesterone and dexamethasone also antagonize the aldosterone-mediated interaction. In contrast, the synthetic agonist 9α-fludrocortisol robustly induces the interaction. To examine the response in a more physiological context we have also examined the N/C-interaction in different cell lines. The M-2-H studies using various cell lines indicate that the cellular milieu does not alter the interaction, which is consistent with the evidence of a direct protein-protein interaction in a glutathione-S-transferase (GST) pull-down assay between the N- and C-terminal domains of MR. The regions involved in mediating the N/C-interaction in the NTD have also been examined.
Results
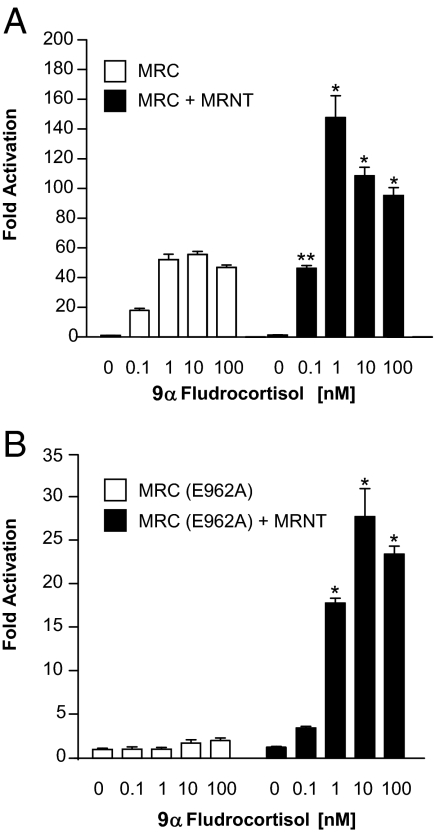
9α-Fludrocortisol induces the MR N/C-interaction
It has been previously demonstrated that an interaction between the MR NTD and the LBD occurs in response to its physiological ligand aldosterone. In contrast, cortisol is a weak agonist that predominantly antagonizes the aldosterone-mediated N/C-interaction (11). We have used the M-2-H assay to further characterize the ligand-dependent MR N/C-interaction. Figure 1 shows the effect of 9α-fludrocortisol, a potent synthetic MR ligand, on the interaction between the MR NTD (MRNT) and the MR C-terminal domain (MRC). Both the wild-type GAL4-MRC (Fig. 1A) and GAL4-MRC containing E962A mutation in the AF-2 region (Fig. 1B) constructs were coexpressed in COS-1 cells with either VP16 or VP16-MRNT. GAL4-MRC and GAL4-MRC (E962A), when expressed with VP16 alone (open bars, Figs. 1A and 1B, respectively), induce luciferase activity in the presence of 9α-fludrocortisol. The ligand-dependent activity of the GAL4-MRC is essentially a one-hybrid assay that reflects the potency of the ligand-dependent transactivation mediated by the AF-2 region in the MR (11). In the original report of the MR N/C-interaction, we inactivated this function (the E962A mutation) to reduce this background activity to enable clear demonstration of the N/C-interaction in the M-2-H assay (11). Significantly greater activity is observed when GAL4-MRC or GAL4-MRC (E962A) is expressed with VP16-MRNT (closed bars; Fig. 1, A and B, respectively), indicating a ligand-dependent interaction between the MR N-terminal and C-terminal domains.
Figure 1.
9α-Fludrocortisol dose response for the MR N/C-interaction. COS-1 cells were transiently transfected with expression vectors and the GAL4-responsive luciferase reporter vector g5-LUC. The cells were treated 14–16 h after transfection with various concentrations of 9α-fludrocortisol as indicated. Luciferase activity was measured after a 24-h incubation and is represented as fold activation relative to luciferase activity in the absence of ligand. A, 9α-Fludrocortisol dose response with wild-type MRC. The open bars correspond to GAL4-MRC + pVP16, and the solid bars correspond to GAL4-MRC + pVP16-MRNT. B, 9α-Fludrocortisol dose response with MRC(E962A). The open bars correspond to GAL4-MRC(E962A) + pVP16, and the solid bars correspond to GAL4-MRC(E962A) + pVP16-MRNT. Each data point represents the mean ± sem derived from three independent experiments; *, P < 0.001; **, P < 0.01 denotes significance when compared with the same concentration of 9α-fludrocortisol in the absence of MRNT.
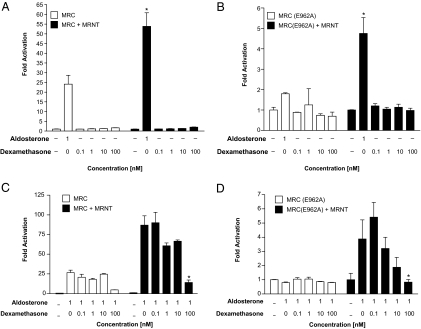
Dexamethasone antagonizes the aldosterone-mediated N/C-interaction
Previous studies have demonstrated that the potent synthetic glucocorticoid, dexamethasone, binds to MR and shows a clear, albeit weak, agonistic activity via the human MR (16). We have examined, therefore, the effect of dexamethasone on the MR N/C-interaction. A dose-response curve with dexamethasone was generated and compared with the N/C-interaction in the presence of 1 nm of aldosterone. However, there was no significant response observed on the N/C-interaction between the GAL4-MRC and/or GAL4 (E962A) with VP16-MRNT in the presence of increasing concentrations of dexamethasone (Fig. 2, A and B, respectively). In contrast, dexamethasone suppressed the aldosterone-mediated N/C-interaction in a dose-dependent manner (Fig. 2, C and D).
Figure 2.
Dexamethasone dose response for the MR N/C-interaction, alone and in the presence of aldosterone. COS-1 cells were transiently transfected for the M-2-H assay as described in Fig. 1. The cells were treated with various concentrations of dexamethasone alone (A and B) or in the presence of 1 nm aldosterone (C and D) to examine the effect on the MR N/C-interaction with wild-type MRC (A and C) and MRC(E962A) (B and D). Luciferase activity is represented as fold activation relative to luciferase activity in the absence of ligand. Each data point represents the mean ± sem derived from three independent experiments; *, P < 0.001 denotes significance when compared with 1 nm aldosterone in the absence of MRNT (A and B) or when compared with aldosterone alone in the presence of MRNT (C and D).
DOC attenuates the aldosterone-mediated N/C-interaction
Given that DOC binds and activates the MR with an equivalent efficiency to aldosterone and indeed has been viewed as the primordial mineralocorticoid (17), we examined the effect of DOC on the MR N/C-interaction. As with dexamethasone, DOC only weakly induced the N/C-interaction between GAL4-MRC and VP16-MRNT (Fig. 3A); however, no response was observed with GAL4-MRC (E962A) and VP16-MRNT in response to DOC (Fig. 3B). In Fig. 3, C and D, we have examined the effect of DOC on the aldosterone-mediated N/C-interaction. We observed that DOC unexpectedly acts as an antagonist and suppresses the aldosterone-mediated N/C-interaction.
Figure 3.
DOC dose response for the MR N/C-interaction, alone and in the presence of aldosterone. COS-1 cells were transiently transfected for the M-2-H assay as described in Fig. 1. The cells were treated with increasing concentrations of DOC in the absence (A and B) and presence (C and D) of aldosterone (1 nm) to examine the effect on the MR N/C-interaction with wild type MRC (A and C) and MRC(E962A) (B and D). Luciferase activity is represented as fold activation relative to luciferase activity in the absence of ligand. Each data point represents the mean ± sem derived from three independent experiments; *, P < 0.001 denotes significance when compared with 1 nm aldosterone in the absence of MRNT (A and B) or when compared with aldosterone alone in the presence of MRNT (C and D).
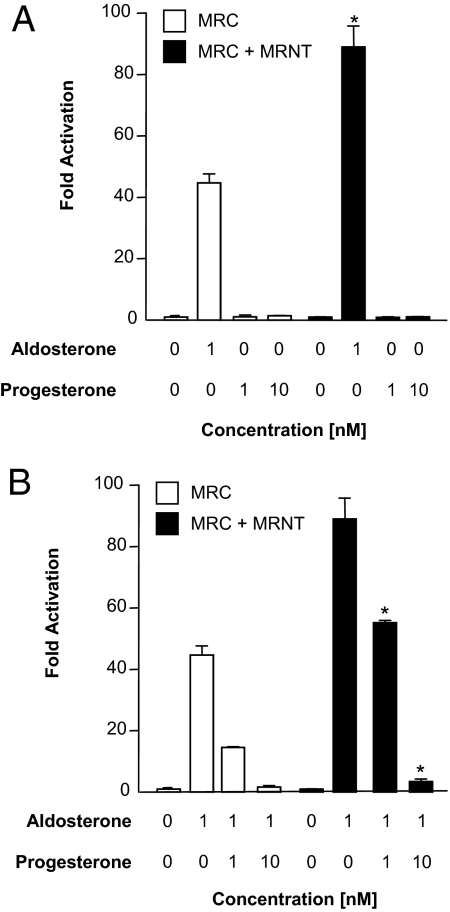
Progesterone antagonizes the aldosterone-mediated N/C-interaction
Transactivation studies have shown that progesterone acts as a weak partial agonist but predominant antagonist for hMR (18). We therefore assessed the ability of progesterone to induce or antagonize the aldosterone-mediated N/C-interaction. We observe that progesterone, by itself, does not induce the interactions between GAL4-MRC and VP16-MRNT (Fig. 4). However, as expected, progesterone clearly attenuates the aldosterone-mediated N/C-interaction, as has been previously reported for the antagonists spironolactone (11) and eplerenone (our unpublished data). Similar observation was also made with GAL4-MRC (E962A) (data not shown).
Figure 4.
Effect of progesterone on the aldosterone-mediated MR N/C-interaction. COS-1 cells were transiently cotransfected with GAL4-MRC as described in Fig. 1. The cells were treated with aldosterone (1 nm) and progesterone (1 and 10 nm) alone (A) or cotreated with aldosterone (1 nm) and progesterone (1 and 10 nm) as indicated. B, Luciferase activity is represented as fold activation relative to luciferase activity in the absence of ligand. Each data point represents the mean ± sem derived from three independent experiments; *, P < 0.001 denotes significance when compared with 1 nm aldosterone in the absence of MRNT (A) or when compared with aldosterone alone in the presence of MRNT (B).
The MR N/C-interaction is not cell specific
To examine the influence of the cellular milieu on the N/C-interaction, we performed the M-2-H assay in several different cell types. The N/C-interaction was examined in the porcine renal epithelial LLC-PK1 (Fig. 5A), rat cardiomyocyte H9c2 (Fig. 5B), human granulosa COV434 (Fig. 5C), and human embryonic kidney HEK293 (Fig. 5D) cell lines. We observed an aldosterone-mediated N/C-interaction in the cell lines with GAL4-MRC. However, in contrast to COS-1 cells, we did not observe an aldosterone-dependent N/C-interaction with GAL4-MRC (E962A) with the four cell lines (data not shown).
Figure 5.
The MR N/C-interaction in LLC-PK1 (A), H9C2 (B), COV-434 (C), and HEK293 (D) cells. The cells were transiently cotransfected with GAL4-MRC with or without VP16-MRNT in the presence or absence of aldosterone (10 nm). Luciferase activity is represented as fold activation relative to luciferase activity in the absence of ligand. Each data point represents the mean ± sem derived from three independent experiments; *, P < 0.001; and ***, P < 0.05 denote significance when compared with aldosterone in the absence of MRNT.
Structural determinants of the N/C-interaction
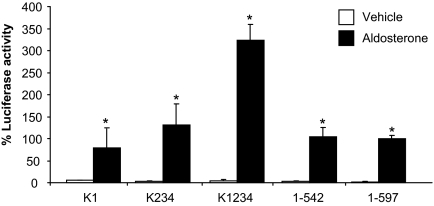
The NTD of the MR is unstructured; it contains four sumoylation sites as well as several putative phosphorylation, acetylation, and ubiquitinylation sites (19,20), but it lacks motifs that might be predicted to mediate or interact with the LBD. The only well-characterized motifs in the NTD are the four sumoylation sites at positions 89, 399, 428, and 494. To assess the role of these sites and/or sumoylation, the lysine residues in these sites were sequentially mutated to arginine as described previously by Pascual-Le Tallec et al. (21) and more recently by Tirard et al. (19). Elimination of all four sumoylation sites did not block the N/C-interaction (Fig. 6). We also sought to explore the consequences of increasing sumoylation by cotransfecting PIAS1 [protein inhibitor of activated STAT (signal transducer and activator of transcription)]. PIAS1 has previously been shown to interact with the NTD of the MR and to increase sumoylation (21). Unfortunately PIAS-induced luciferase activity in the absence of ligand with either the NTD or the NTD with the four sumoylation sites mutated, indicating a nonspecific effect, unrelated to interactions with, or sumoylation of, the NTD (data not shown).
Figure 6.
Analysis of the role of sumoylation in the MR N/C-interaction. COS-1 cells were transiently cotransfected with GAL4-MRC(E962A) with or without VP16-MRNT (wild type; 1–597) and VP16-MRNT in which the four sumoylation sites have been mutated. Where K1 represents the lysine mutation at position 89, K234 at 399, 428, and 494 and K1234 incorporates the lysine mutations at all four positions. The cells were treated with vehicle (open bars) or aldosterone (10 nm) (solid bars). Luciferase activity is represented as a percentage of the maximal response. Each data point represents the mean ± sem derived from three independent experiments; *, P < 0.001 denotes significance when compared with vehicle.
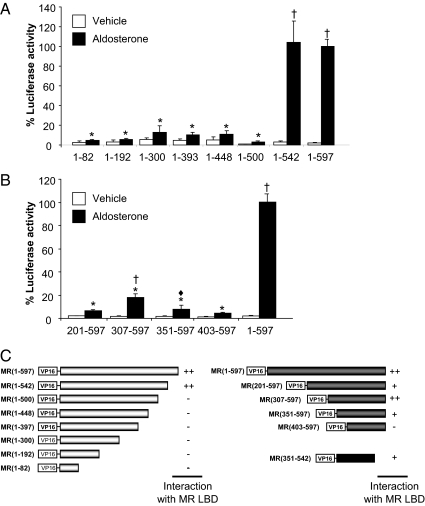
To identify regions and/or sequences in the N terminus mediating the interaction, we created deletions from both ends of the 597-amino acid N terminus (Fig. 7). Expression of the constructs was confirmed by Western blot analyses (data not shown), and the interaction was examined in the presence of 1, 10, and 100 nm aldosterone. There was no difference observed in the dose-response curves so only the maximal response (10 nm) is shown (Fig. 7). The analysis was conducted in the context of MRC (E962A) to reduce the impact of the high background observed with the intact AF2 region (MRC). The interaction was lost when 97 or more amino acids were truncated from the C-terminal end of the NTD [VP-MR (1–500)] (Fig. 7A). Conversely, the interaction was lost when 402 amino acids were deleted from the N-terminal of the NTD [VP16-MR (403–597)] (Fig. 7B). As indicated in Fig. 7C, although the constructs VP16-MR (201–597) and VP16-MR (351–597) also exhibit a lower response, it is significantly less than the full-length NTD. On the basis of these results, when the region MR (amino acids 351–542) was examined, an interaction was observed although of a lower magnitude than that observed for the full-length NTD (data not shown).
Figure 7.
Structural determinants of the MR N/C-interaction in the NTD. COS-1 cells were transiently cotransfected with GAL4-MRC(E962A) with or without VP16-MRNT and with a series of deletions of VP16-MRNT as indicated in panels A and B. The cells were treated with vehicle (open bars) or aldosterone (10 nm) (solid bars). Each data point represents the mean ± sem derived from three independent experiments; *, P < 0.001 denotes significance when compared with wild type [VP16-MRNT (1–597) + MRC(E962A)] in the presence of aldosterone; ♦, P < 0.01; and †, P < 0.001 denote significance when compared with vehicle alone. C, Schematic representation of N/C-interaction between fragments of MRNT and MRC(E962A).
To more precisely define the amino acids within the segment 351–542 of the NTD necessary for the N/C-interaction, amino acids spanning MR 351–542 were deleted from the NTD. All of these internal deletions, Δ328–381, Δ382–510, Δ511–534, and Δ535–561, retain the ability to interact in a ligand-dependent manner with the LBD in the M-2-H assay (Fig. 8). In view of this, we examined the deletion Δ351–542; in contrast to the prediction based on the truncation study (Fig. 7), this construct was able to interact with the MRC in the M-2-H assay. In all cases the magnitude of the N/C-interaction significantly differed from that of the full-length NTD. A decrease was particularly prominent for Δ382–510 and other deletions that encompass that region. In contrast, a significantly greater interaction is observed with Δ328–381, suggesting the presence of sequences that inhibit the N/C-interaction.
Figure 8.
Deletion of amino acids 351–542 from the NTD. A, COS-1 cells were transiently cotransfected with GAL4-MRC(E962A) with or without VP16-MRNT and with deletions of VP16-MRNT. The cells were treated with vehicle (open bars) or aldosterone (10 nm) (solid bars). Each data point represents the mean ± sem derived from three independent experiments; *, P < 0.001 denotes significance when compared with wild type [VP16-MRNT (1–597) + MRC(E962A)] in the presence of aldosterone; Δ, P < 0.05; and †, P < 0.001 denote significance when compared with vehicle alone. B, Schematic representation of series of internal deletions in the MR NT that retain N/C-interaction with MRC(E962A).
The results of both the deletion and truncation experiments led us to conclude that more than one region in the NTD is involved in the N/C-interaction.
The MR N/C-interaction is a direct protein-protein interaction
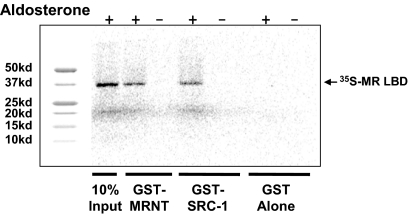
It has been demonstrated for the progesterone receptor (PR) (22) and estrogen receptor-α (23) that the N/C- interaction can be mediated by coactivator recruitment to the LBD. We therefore sought to establish whether the N/C-interaction in MR involved a direct contact between both domains or if it was mediated by an intermediate protein present in the M-2-H assay. A GST pull-down assay with the MR LBD and a fragment of SRC-1 has been described previously (24). This fragment of SRC-1 was used as the positive control. The GST-N terminus fusion protein expressed in vitro was able to pull down the 35S-labeled MR LBD in the presence of aldosterone as was the GST-SRC-1 fusion protein whereas GST alone did not demonstrate an interaction (Fig. 9). We also examined the region of the N terminus 351–542 and observed a ligand-dependent interaction (data not shown). The data clearly demonstrates that the N/C-interaction in the MR is a direct protein-protein interaction. This is consistent with our findings that the N/C-interaction, determined using the M-2-H assay, was present in all the cell lines examined (Fig. 5).
Figure 9.
GST pull-down assay with the N and C termini. The MR NTD expressed as GST fusion protein, which previously had been coupled to Sepharose glutathione beads, was incubated with [35S]methionine-labeled MR-LBD in the presence (lanes 2) or absence (lane 3) of 1 μm aldosterone. As controls, incubation with SRC-1 (570–780) (positive; lanes 4 and 5), GST alone (lanes 6 and 7) and one tenth of the receptor input (lane 1) are shown. kd, Kilodaltons.
Discussion
Several studies provide clear evidence that cross talk between individual domains of the steroid receptors occurs in a ligand- and cell-specific manner. The most important of these is an interaction between the NTD and the LBD. This interdomain or N/C-interaction is best characterized in the AR (13) and has been used as a platform for the development of selective AR modulators (25). The N/C-interactions have also been described for the PR (22) and the estrogen receptor-α (23). The functional significance of the N/C-interaction is well established in the AR where mutations in the AR that compromise the N/C-interaction cause the androgen insensitivity syndrome (13,26,27,28,29,30). The N/C-interaction is mediated by the binding of specific FxxLF and WxxLF sequences in the AR NTD (14,32) to the groove formed by helices 3, 4, and 12 in the LBD (14,15). The FxxLF motif interacts with the AF-2 region such that it may serve to modulate the interactions of the LxxLL motifs found in steroid receptor coactivators (13).
In our previous studies, we identified a ligand-specific N/C-interaction using the M-2-H assay in the MR. Interestingly, the interaction occurs in the presence of aldosterone but only very weakly in the presence of cortisol. In fact, cortisol antagonizes aldosterone-mediated N/C- interaction (12). In this study we used a number of MR ligands to further characterize the N/C-interaction. As expected, dexamethasone antagonized the aldosterone-mediated N/C-interaction in a dose-dependent manner. This is consistent with the ability of dexamethasone to compete for [3H] aldosterone binding albeit at relatively high concentrations (16). Similarly, the N/C-interaction is also inhibited by the MR antagonists progesterone, eplerenone, and spironolactone. Interestingly, in contrast to aldosterone, which strongly induces the interaction, the high affinity MR physiological ligand and agonist, DOC, weakly induces the interaction but predominantly inhibits the aldosterone-mediated N/C-interaction. In contrast, the synthetic agonist, 9α-fludrocortisol, robustly induces the interaction, which is consistent with a ligand-dependent interaction between the NTD and LBD. These studies suggest that the difference in the ability of the physiological MR ligands to induce the N/C-interaction may be a key determinant of the tissue-specific agonist/antagonist activity of these ligands.
In epithelial tissues, the ability of cortisol/corticosterone to access and activate the MR is controlled by 11β hydroxysteroid dehydrogenase 2 (33); when glucocorticoid receptor occupancy is precluded and 11β hydroxysteroid dehydrogenase 2 blocked, corticosterone produces almost identical antinatriuretic effects to aldosterone (5). In some nonepithelial cells, such as cardiomyocytes, glucocorticoids bind to the MR and antagonize the effects of aldosterone (34). In the rat, aldosterone acts in the brain to raise blood pressure, an effect antagonized by corticosterone but not by a specific glucocorticoid receptor agonist (6). Nuclear hormone receptors act by interaction with a range of coregulatory proteins: coactivators, which enhance transcription, and corepressors, which repress transcription. A likely explanation for the tissue-specific actions of cortisol/corticosterone at the MR is differential interactions with coregulators, perhaps through the N/C-interaction. Pascual-Le Tallec et al. (20) have reported that the elongation factor, ELL, is a selective coregulator of the MR that interacts with the NTD of the hMR. It however enhances both aldosterone and cortisol-induced MR transactivation. Similarly a SUMO-1 E3 ligase (PIAS1) and a SUMO-1-conjugation enzyme (Ubc9) coactivate transactivation of the MR through the NTD but without evidence of ligand specificity (21,35). A coactivator complex that interacts with the MR AF-1 region was purified from HeLa cells and found to contain RNA helicase A (RHA) (36). Importantly, this complex interacts with the receptor via RHA in the presence of aldosterone but not cortisol. Given that the RHA complex interacts with the MR NTD, but in a ligand-dependent manner, the N/C-interaction may mediate the interaction.
The transcriptional activity and specificity of steroid receptors are strongly influenced by the synergistic interactions between multiple receptor dimers (16,37). Studies from the laboratory of David Pearce (37) examining the factors that are responsible for synergy by the MR at multiple glucocorticoid response elements (GREs) indicate a contribution from both N and C termini, suggesting that an N/C-interaction is important in this context. Synergism depends on whether a promoter containing multiple GREs or a single GRE is used. In this context, we investigated the MR N/C-interaction using the M-2-H assay in the presence of a reporter gene containing one and three GAL4 response elements, in addition to the reporter containing five GAL4 response elements that we and others routinely use in the M-2-H assay. Unfortunately, the reporter containing three GAL4 response elements, and more particularly, the crucial reporter containing one GAL4 response element, lacked adequate sensitivity, even with the positive controls in the assay kit, to enable a meaningful analysis.
The mechanism of the MR N/C-interaction differs from that of the AR in that mutation of the AF-2 domain neither abrogates the N/C-interaction, nor does the MR contain FxxLF and WxxLF sequences in its N terminus (14). The NTD of the steroid receptors exhibit little or no conservation of sequence between receptors and of these, MR has the largest NTD. Pascual-Le Tallec and Lombes (38) have recently reviewed the function of the MR NTD, although they identify potential sites of posttranslational modification, including phosphorylation, acetylation, ubiquitinylation, and sumoylation (38), many of which are conserved among species; motifs that might mediate an interaction with the AF-2 domain of the LBD were not identified.
The four sumoylation sites stand out as potential sites of interaction and three of the four are absolutely conserved across the species of MR (17); sumoylation sites are also found in the AR, GR, and PR. The consensus motif ΨKxD/E contains an acceptor lysine. Sumoylation has variously been reported to decrease or increase transactivation. These motifs were originally identified as synergy control sequences where their deletion in the context of multiple response elements was shown to increase transactivation. Our analysis strongly suggests that the sumoylation sites per se are not the point of interaction with the LBD; however, it may be that sumoylation at these sites may modify the N/C-interaction; the increased activity observed with all the four lysine residues converted to arginine is consistent with this, although other explanations including protein stability may be relevant.
Although, the MR NTD is reported to contain an AF-1, its physical location differs between studies (24,39,40). Secondary structure analysis suggests that the MR NTD is primarily disordered, consistent with the concept that its structure will be defined by the interacting partner. The deletion experiments suggest that the region between amino acids 351–542, which encompasses the AF-1 function identified by Fuse et al. (39), is critical for the interactions with the sequences 351–403 and 500–542 being somehow critical. Although, a comparison across species of the published MR N-terminal amino acid sequences from human to rainbow trout (17) suggests significant conservation of sequences within this region, the responsible sequence is not apparent. A caveat in considering this comparison is that an MR N/C-interaction for species other than the human has not yet been published. The segment 351–542 does indeed mediate the interaction in the M-2-H assay, and on this basis, with a view to identifying the critical residues, a series of deletions were made across this region. All retained the interaction, and, indeed, deletion from the MR NTD of amino acids 351–542 did not abolish the N/C-interaction in the M-2-H assay. These results clearly demonstrate that sequences outside of the 351–542 segment, presumably at least two regions, N-terminal and C-terminal to this segment, also contribute to the interaction. Given that the interaction may involve interreceptor interactions between more than one other LBD, the possibility of multiple interacting domains within the NTD is plausible. In the case of the AR, Buchanan et al. (41) have demonstrated that the distance between the two interacting motifs influences the N/C-interaction; it is therefore possible that such an effect may also confound the interpretation of the present studies. In view of the unstructured nature of the MR NTD, it is also possible that there are multiple interacting regions, defined more by composition than sequence, with a hierarchy of affinity determined by both content and spatial factors.
A variety of coregulatory proteins serve as partners for nuclear receptors orchestrating the molecular events required for receptor-dependent transcriptional regulation. Tissue specificity is likely to be mediated, at least partly, by the pattern of coregulator expression. Neither ligand- nor tissue-specific MR coactivators have yet been identified. We therefore wished to determine whether the N/C-interaction involved a direct interaction between the two domains or whether an intermediate protein may mediate the interaction. We have demonstrated a direct, aldosterone-dependent, interaction in vitro (Fig. 9) using a GST pull-down assay with the MR N terminus. As might be predicted from the GST pull-down result, the cellular milieu does not alter the interaction in that the interaction was also observed in a range of cell lines. It is however important to note that the cell type independence of the interaction does not preclude a cell-dependent consequence of the N/C-interaction. It is apparent from these studies that the MR N/C-interaction exhibits similarity to the N/C-interaction in the AR. However, these studies clearly demonstrate two significant differences; first, the structural determinants of the MR N/C-interaction differ from those of the AR and second, the ability of the N/C-interaction to discriminate between MR ligands suggests a conformational difference in the LBD induced by these ligands.
Although the functional significance of the N/C-interaction is well established for other steroid receptors, particularly the AR, it remains to be determined for the MR. The ligand discrimination is an intriguing finding; with the exception of an interaction with RHA (36) molecular interactions within the receptor that discriminate physiological ligands have not been described. In contrast, in vivo and ex vivo studies have identified differences between the consequences of aldosterone and cortisol signaling at the MR. It is tempting to speculate that the N/C-interaction may contribute to this tissue-specific discrimination, which in turn would suggest that as with the AR N/C-interaction (25), it might prove to be a useful assay for the development of tissue-specific ligands. Tissue-specific MR agonists and antagonists may be of potential therapeutic benefit in neurological and cardiovascular diseases, respectively.
Materials and Methods
Plasmid constructs
The M-2-H assay was performed using the Mammalian MATCHMAKER Two-Hybrid Assay Kit (CLONTECH Laboratories, Inc., Palo Alto, CA), except that the reporter vector was g5-LUC, which contains the luciferase gene driven by a GAL4-responsive promoter. In this kit the plasmid pM encodes the GAL4 DBD (amino acids 1–147) upstream of a multiple cloning site for insertion of the test sequence, and the plasmid pVP16 encodes the VP16 activation domain (amino acids 411–456) upstream of a multiple cloning site for insertion of the test sequence.
The sequence encoding amino acids 672–984, encompassing the MR hinge and LBD, was amplified by PCR from the pRShMR expression vector and cloned into the pM vector to create the GAL4-MRC. The MRC E962A mutant was created with primer resulting in an A→C mutation. The sequence encoding amino acids 1–597, encompassing the MR NTD, was amplified by PCR from the pRShMR expression vector and cloned into pVP16 to create the VP16-MRNT construct (11).
The various fragments of the human MR N terminus used in this study were generated by PCR using the previously described 5′- and 3′-primers incorporating a SalI site and a HindIII restriction site, respectively, for cloning into pVP16 (11). These primers were used with internal reverse and forward primers (supplemental Table 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), respectively, containing the appropriate SalI site or HindIII restriction site to amplify in-frame truncations of the NTD. Internal deletions of the NTD were created using the original NTD flanking primers with internal primers (supplemental Table 1) to create PCR products for subsequent overlap extension PCR using the flanking primers. All constructs were created by overlap extension PCR using pRShMR as the template.
Point mutations were made sequentially into pVP16-MRNTD to convert the lysine in each of the four sumoylation motifs to an arginine. These mutations were created using the QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), as per manufacturer’s instructions. The primers used for PCR are presented in supplemental Table 2.
All constructs were fully sequenced to ensure there were no PCR-derived errors using cycle sequencing with BigDye Terminator Cycle Sequencing Ready Reaction Kit, version 3.1 (PerkinElmer Applied Biosystems, Foster City, CA) according to the manufacturer’s protocols. Samples were run on an Applied Biosystems 3130xl Genetic Analyzer. Data were then collected using Data Collection version 3.0 and analyzed using Sequence Analysis version 5.2 software in The Gandel Charitable Trust Sequencing Centre, Monash Medical Centre, Melbourne.
Cell culture and M-2-H assays
The M-2-H assays were performed in monkey kidney COS-1, human epithelial HEK293, porcine kidney epithelial LLC-PK1, rat heart cardiomyocyte H9c2, and human granulosa tumor COV434 cells. The cells were cultured in DMEM supplemented with 10% fetal bovine serum, 1 mm glutamine, nonessential amino acids, and penicillin (10 U/liter)-streptomycin (10 μg/liter) in 5% CO2. The cells were trypsinized and seeded at a density of 8 × 104 cells per well in 12-well plates in DMEM supplemented with 10% fetal bovine serum. Cells were incubated overnight before transfection. Transfections were performed using Fugene 6 (Roche Molecular Biochemicals, Indianapolis, IN) in triplicate for each data point as per manufacturer’s protocol, and the medium was changed to DMEM supplemented with 5% charcoal-stripped serum.
The cells were transfected with the expression vectors for GAL4-DBD fused with wild-type or mutated LBDs of MR (0.1 μg; pM-MRC constructs), a pVP16-MRNTD (0.5 μg), and pG5-Luc (0.5 μg) reporter vector. Kit controls included in the assays were pG5-Luc alone (negative), pM3-VP16 (positive), pM-53 + pVP16-T (positive), and pM-53 + pVP16-CP (negative). After transfection the cells were incubated for 14–16 h. Transfected cells were then treated with steroids and incubated for a further 24 h and harvested for luciferase analysis.
Luciferase assay
Luciferase activity was assayed using the Luclite kit (PerkinElmer Applied Biosystems) according to the manufacturer’s instructions. Briefly, cells were washed once in PBS supplemented with 0.5 mm MgCl2 and 0.5 mm CaCl2 and resuspended in 100 μl of supplemented PBS with 100 μl of Luclite substrate buffer. Cell lysates were transferred to a 96-well plate, and relative light units were measured for 1 sec in a PerkinElmer 2103 Lumi count luminometer.
GST pull-down assays
The NTD (amino acids 1–597) was PCR amplified and ligated into pGEX-4T-1 (Pharmacia, Uppsala, Sweden) using the SalI and NotI sites. The MR NT-pGEX-4T-1 clone was transformed into BL21 pLysS bacterial cells for expression induced by isopropyl-β-d-thiogalactopyranoside. The cells were lysed, and the crude lysates were examined for expression of GST-MR NT. Coomassie Blue staining showed the appearance of a band of the correct size in isopropyl-β-d-thiogalactopyranoside-induced cells, and Western blotting using an anti-GST antibody confirmed high-level expression of the GST-MRNT. The pGEX2Tk-SRC1 (amino acids 570–780) plasmid, which contains a fragment of SRC-1, was used as a positive control. GST and GST fusion proteins were purified using glutathione-agarose affinity chromatography as previously described (24). The GST fusion proteins were analyzed on 10% SDS-PAGE gels for integrity and to normalize the amount of each protein.
For the MR LBD construct, a Kozak sequence and an ATG were incorporated into the PCR primer upstream of the receptor sequence. The PCR product was ligated into pcDNA3.1 (+) (Invitrogen, Carlsbad, CA) using the HindIII and BamHI sites. The Promega TNT-T7 quick coupled transcription/translation system was used to produce the [35S]methionine-labeled MR LBD. Aldosterone (1 μm) was then added before binding assay. In vitro binding assays were performed with glutathione-Sepharose beads (20 μl) coated with approximately 500 ng of GST fusion protein and 2–10 μl of [35S]methionine-labeled protein in 200 μl of binding buffer containing 100 mm NaCl, 20 mm Tris-HCl (pH 8.0), 1 mm EDTA, 0.5% Nonidet P-40, 5 μg of ethidium bromide, and 100 μg of BSA. The beads were resuspended in 20 μl of SDS-PAGE sample buffer and boiled for 5 min. The eluted proteins were fractionated by SDS-PAGE, and the gel was dried at 80 C and autoradiographed.
Western blot analysis
The expression of the VP16 activation domain fusion proteins in COS-1 cells was assessed by Western blotting. COS-1 cells (2 × 106 cells per well in six-well plates) were transfected with 2 μg of expression plasmid using Fugene 6. At 48 h after transfection, cells were washed with PBS and lysed using 200 μl of gel loading buffer [10 mm Tris (pH 6.8), containing 2% sodium dodecyl sulfate, 10% glycerol, 4% β-mercaptoethanol, and 1% bromophenol blue]. The lysates were collected and boiled for 5 min at 95 C. Lysate (10 μl) was run on a 10% polyacrylamide gel under denaturing conditions. The protein was then electroblotted onto Hybond P membrane (Amersham-Pharmacia Biotech AB, Uppsala, Sweden).
The expression of the VP16 activation domain fusion proteins was assessed using anti-MR antibody (antibody rMR1-18 6G1) (29) kindly provided by Professor Celso Gomez-Sanchez, University of Mississippi Medical Center (Jackson, MS). Nonspecific binding was blocked by incubating the membranes for 1 h at room temperature in 20 mm Tris (pH 7.6), 0.14 m NaCl and 0.1% Tween 20 (TBS-T), containing 5% skim milk powder. The membranes were incubated with primary antibody for 2 h at room temperature. The membrane was then incubated with horseradish peroxidase-linked antimouse secondary antibody (1:1000) (DAKO Corp., Carpinteria, CA) for 45 min at room temperature. Antibody binding was visualized by chemiluminescence using the ECL Plus kit (Amersham-Pharmacia Biotech).
Statistical analyses
Statistical analyses were conducted using Graph Pad Prism (version 4; Graph Pad Software Inc., CA). Each data point represents the mean ± sem derived from three independent experiments. Means were compared using either a Student’s t test or one-way ANOVA followed by Tukey’s or Bonferroni’s post hoc test. Means were considered significantly different if P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Marie-Edith Rafestin-Oblin for the gift of the pGEX2Tk-SRC1 (570–780) plasmid and Marc Lombés for pcDNA3.1-PIAS1. We also thank Dr. David Pearce for helpful discussion during the course of this study; and Claudette Thiedeman and Sue Panckridge for help in preparation of the manuscript.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia through Career Fellowship 122200 (to P.J.F.), by Prince Henry’s Institute of Medical Research through the Fred Boylan and Bill Burke Fellowship (to J.B.P.), and by a Bridge Grant from The Endocrine Society.
Current address for F.M.R.: Royal Children’s Hospital, Parkville 3050, Victoria, Australia.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 18, 2009
Abbreviations: AF, Activation function; AR, androgen receptor; DBD, DNA-binding domain; DOC, deoxycorticosterone; GRE, glucocorticoid response element; GST, glutathione-S-transferase; HEK, human embryonic kidney; LBD, ligand-binding domain; M-2-H, mammalian two-hybrid; MR, mineralocorticoid receptor; MRC, MR C-terminal domain; MRNT, MR NTD; NTD, N-terminal domain; PIAS, protein inhibitor of activated STAT (signal transducer and activator of transcription); PR, progesterone receptor; RHA, RNA helicase A; SRC, steroid receptor coactivator.
References
- Rogerson FM, Fuller PJ 2000 Mineralocorticoid action. Steroids 65:61–73 [DOI] [PubMed] [Google Scholar]
- Farman N, Rafestin-Oblin ME 2001 Multiple aspects of mineralocorticoid selectivity. Am J Physiol Renal Physiol 280:F181–F192 [DOI] [PubMed] [Google Scholar]
- Cachofeiro V, Miana M, de Las Heras N, Martín-Fernández B, Ballesteros S, Fernández-Tresguerres J, Lahera V 2008 Aldosterone and the vascular system. J Steroid Biochem Mol Biol 109:331–335 [DOI] [PubMed] [Google Scholar]
- Struthers AD 2004 Aldosterone in heart failure: pathophysiology and treatment. Curr Heart Fail Rep 1:171–175 [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Young MJ 2005 Mechanisms of mineralocorticoid action. Hypertension 46:1227–1235 [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, DeRijk R, de Kloet ER 2008 The coming out of the brain mineralocorticoid receptor. Trends Neurosci 31:1–7 [DOI] [PubMed] [Google Scholar]
- Connell JM, MacKenzie SM, Freel EM, Fraser R, Davies E 2008 A lifetime of aldosterone excess: long-term consequences of altered regulation of aldosterone production for cardiovascular function. Endocr Rev 29:133–154 [DOI] [PubMed] [Google Scholar]
- Pitt B 2004 Effect of aldosterone blockade in patients with systolic left ventricular dysfunction: implications of the RALES and EPHESUS studies. Mol Cell Endocrinol 217:53–58 [DOI] [PubMed] [Google Scholar]
- Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL, Wilson EM, McDonnell DP, Cidlowski JA 2006 International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev 58:782–797 [DOI] [PubMed] [Google Scholar]
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM 1987 Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268–275 [DOI] [PubMed] [Google Scholar]
- Rogerson FM, Fuller PJ 2003 Interdomain interactions in the mineralocorticoid receptor. Mol Cell Endocrinol 200:45–55 [DOI] [PubMed] [Google Scholar]
- Langley E, Zhou ZX, Wilson EM 1995 Evidence for an anti-parallel orientation of the ligand-activated human androgen receptor dimer. J Biol Chem 270:29983–29990 [DOI] [PubMed] [Google Scholar]
- He B, Kemppainen JA, Voegel JJ, Gronemeyer H, Wilson EM 1999 Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH2-terminal domain. J Biol Chem 274:37219–37225 [DOI] [PubMed] [Google Scholar]
- He B, Kemppainen JA, Wilson EM 2000 FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J Biol Chem 275:22986–22994 [DOI] [PubMed] [Google Scholar]
- Estébanez-Perpiñá E, Moore JM, Mar E, Delgado-Rodrigues E, Nguyen P, Baxter JD, Buehrer BM, Webb P, Fletterick RJ, Guy RK 2005 The molecular mechanisms of coactivator utilization in ligand-dependent transactivation by the androgen receptor. J Biol Chem 280:8060–8068 [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Arriza JL, Spengler D, Reul JM, Evans RM, Holsboer F, Damm K 1993 Transactivation and synergistic properties of the mineralocorticoid receptor: relationship to the glucocorticoid receptor. Mol Endocrinol 7:597–603 [DOI] [PubMed] [Google Scholar]
- Sturm A, Bury N, Dengreville L, Fagart J, Flouriot G, Rafestin-Oblin ME, Prunet P 2005 11-Deoxycorticosterone is a potent agonist of the rainbow trout (Oncorhynchus mykiss) mineralocorticoid receptor. Endocrinology 146:47–55 [DOI] [PubMed] [Google Scholar]
- Oelkers W 2004 Drospirenone, a progestogen with antimineralocorticoid properties: a short review. Mol Cell Endocrinol 217:255–261 [DOI] [PubMed] [Google Scholar]
- Tirard M, Almeida OF, Hutzler P, Melchior F, Michaelidis TM 2007 Sumoylation and proteasomal activity determine the transactivation properties of the mineralocorticoid receptor. Mol Cell Endocrinol 268:20–29 [DOI] [PubMed] [Google Scholar]
- Pascual-Le Tallec L, Simone F, Viengchareun S, Meduri G, Thirman MJ, and Lombès M 2005 The elongation factor ELL (eleven-nineteen lysine-rich leukemia) is a selective coregulator for steroid receptor functions. Mol Endocrinol 19:1158–1169 [DOI] [PubMed] [Google Scholar]
- Pascual-Le Tallec L, Kirsh O, Lecomte MC, Viengchareun S, Zennaro MC, Dejean A, Lombès M 2003 Protein inhibitor of activated signal transducer and activator of transcription 1 interacts with the N-terminal domain of mineralocorticoid receptor and represses its transcriptional activity: implication of small ubiquitin-related modifier 1 modification. Mol Endocrinol 17:2529–2542 [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP 1999 Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol Endocrinol 13:910–924 [DOI] [PubMed] [Google Scholar]
- Métivier R, Stark A, Flouriot G, Hübner MR, Brand H, Penot G, Manu D, Denger S, Reid G, Kos M, Russell RB, Kah O, Pakdel F, Gannon F 2002 A dynamic structural model for estrogen receptor-α activation by ligands, emphasizing the role of interactions between distant A and E domains. Mol Cell 10:1019–1032 [DOI] [PubMed] [Google Scholar]
- Hellal-Levy C, Fagart J, Souque A, Wurtz JM, Moras D, Rafestin-Oblin ME 2000 Crucial role of the H11–H12 loop in stabilizing the active conformation of the human mineralocorticoid receptor. Mol Endocrinol 14:1210–1221 [DOI] [PubMed] [Google Scholar]
- Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, López FJ, Marschke KB, Rosen J, Schrader W, Turner R, van Oeveren A, Viveros H, Zhi L, Negro-Vilar A 2007 An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology 148:363–373 [DOI] [PubMed] [Google Scholar]
- McPhaul MJ, Marcelli M, Tilley WD, Griffin JE, Isidro-Gutierrez RF, Wilson JD 1991 Molecular basis of androgen resistance in a family with a qualitative abnormality of the androgen receptor and responsive to high-dose androgen therapy. J Clin Invest 87:1413–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi S, Wilson CM, Harbison MD, Griffin JE, Wilson JD, McPhaul MJ, Marcelli M 1993 Complete testicular feminization caused by an amino-terminal truncation of the androgen receptor with downstream initiation. J Clin Invest 91:1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley E, Kemppainen JA, Wilson EM 1998 Intermolecular NH2-/carboxyl-terminal interactions in androgen receptor dimerization revealed by mutations that cause androgen insensitivity. J Biol Chem 273:92–101 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP 2006 Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147:1343–1348 [DOI] [PubMed] [Google Scholar]
- Quigley CA, Tan JA, He B, Zhou ZX, Mebarki F, Morel Y, Forest MG, Chatelain P, Ritzén EM, French FS, Wilson EM 2004 Partial androgen insensitivity with phenotypic variation caused by androgen receptor mutations that disrupt activation function 2 and the NH(2)- and carboxyl-terminal interaction. Mech Ageing Dev 125:683–695 [DOI] [PubMed] [Google Scholar]
- Thompson J, Saatcioglu F, Jänne OA, Palvimo JJ 2001 Disrupted amino- and carboxyl-terminal interactions of the androgen receptor are linked to androgen insensitivity. Mol Endocrinol 15:923–935 [DOI] [PubMed] [Google Scholar]
- He B, Gampe Jr RT, Kole AJ, Hnat AT, Stanley TB, An G, Stewart EL, Kalman RI, Minges JT, Wilson EM 2004 Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol Cell 16:425–438 [DOI] [PubMed] [Google Scholar]
- White PC, Mune T, Agarwal AK 1997 11β -Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 18:135–156 [DOI] [PubMed] [Google Scholar]
- Odermatt A, Atanasov AG 2009 Mineralocorticoid receptors: Emerging complexity and functional diversity. Steroids 74:163–171 [DOI] [PubMed] [Google Scholar]
- Yokota K, Shibata H, Kurihara I, Kobayashi S, Suda N, Murai-Takeda A, Saito I, Kitagawa H, Kato S, Saruta T, Itoh H 2007 Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J Biol Chem 282:1998–2010 [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Yanagisawa J, Fuse H, Ogawa S, Yogiashi Y, Okuno A, Nagasawa H, Nakajima T, Matsumoto T, Kato S 2002 Ligand-selective potentiation of rat mineralocorticoid receptor activation function 1 by a CBP-containing histone acetyltransferase complex. Mol Cell Biol 22:3698–3706 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu W, Wang J, Yu G, Pearce D 1996 Steroid receptor transcriptional synergy is potentiated by disruption of the DNA-binding domain dimer interface. Mol Endocrinol 10:1399–1406 [DOI] [PubMed] [Google Scholar]
- Pascual-Le Tallec L, Lombès M 2005 The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol 19:2211–2221 [DOI] [PubMed] [Google Scholar]
- Fuse H, Kitagawa H, Kato S 2000 Characterization of transactivational property and coactivator mediation of rat mineralocorticoid receptor activation function-1 (AF-1). Mol Endocrinol 14:889–899 [DOI] [PubMed] [Google Scholar]
- Lavery DN, McEwan IJ 2005 Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem J 391:449–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan G, Yang M, Cheong A, Harris JM, Irvine RA, Lambert PF, Moore NL, Raynor M, Neufing PJ, Coetzee GA, Tilley WD 2004 Structural and functional consequences of glutamine tract variation in the androgen receptor. Hum Mol Genet 13:1677–1692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.