Abstract
Nuclear hormone receptors (NRs) are transcription factors responsible for mediating the biological effects of hormones during development, metabolism, and homeostasis. Induction of NR target genes is accomplished through the assembly of hormone-bound NR complexes at target promoters and coincides with changes in histone modifications that promote transcription. Some coactivators and corepressors of NR can enhance or inhibit NR function by covalently modifying histones. One such modification is methylation, which plays important roles in transcriptional regulation. Histone methylation is catalyzed by histone methyltransferases and reversed by histone demethylases. Recent studies have uncovered the importance of these enzymes in the regulation of NR target genes. In addition to histones, these enzymes have nonhistone substrates and can methylate and demethylate NRs and coregulatory proteins in order to modulate their function. This review discusses recent progress in our understanding of the role of methylation and demethylation of histones, NRs, and their coregulators in NR-mediated transcription.
This review discusses recent advances regarding the role of protein methylation and demethylation in regulating NR mediated transcription.
Nuclear hormone receptors (NRs) are a group of structurally related transcription factors that are responsible for mediating the biological effects of hormones. There are approximately 50 mammalian NRs that include receptors for thyroid hormones, steroid hormones, retinoic acid, and vitamin D, as well as orphan receptors in which the ligand is unknown (1,2,3).
Transcriptional activation by NR is a multistep process and involves NRs and primary and secondary coactivators (4). Binding of hormones to their cognate NRs can induce allosteric changes that permit the NR to recognize specific DNA elements at promoter regions. These conformational alterations allow for the hormone-bound receptor to recruit coactivators, some of which possess intrinsic enzymatic activities that modify chromatin. The concerted action of these proteins usually results in changes in local chromatin structure, which facilitates recruitment of the RNA polymerase II transcriptional machinery to the promoter (5).
In recent years, much attention has been focused on chromatin modifications due to their critical role in regulating gene expression. The fundamental unit of chromatin is the nucleosome, which consists of 146 bp of DNA wrapped around a histone octamer made up of two copies each of the four core histones, H2A, H2B, H3, and H4 (6). The N-terminal histone tails extend from the globular domain and are subject to a variety of posttranslational modifications that include acetylation, methylation, phosphorylation, and ubiquitination. The presence of these modifications in different combinations can have profound effects on local chromatin structure, allowing the cell to fine tune its transcriptional output (7).
Many coactivators and corepressors of NRs possess intrinsic histone-modifying activities. Whereas the ability to alter local chromatin structure is crucial for transcriptional regulation, some of the histone-modifying enzymes can also target nonhistone substrates including NRs themselves as well as their coregulators. Recent studies have uncovered the importance of several posttranslational modifications, including acetylation, phosphorylation, and methylation in regulating NR function. In this review, we focus on the role of protein methylation and demethylation in regulating NR-mediated transcription.
Transcriptional Regulation by Histone Methylation and Demethylation
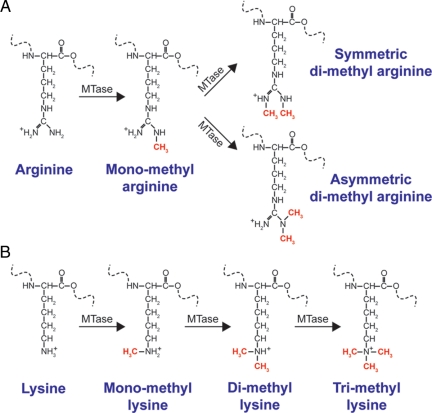
Histone methylation, which can occur on lysine and arginine residues, has been implicated in many biological processes including transcriptional regulation, heterochromatin formation, X inactivation, and genomic imprinting (8,9). Lysine residues can accept up to three methyl groups whereas arginine residues can be monomethylated, symmetrically dimethylated, or asymmetrically dimethylated (Fig. 1). Thus far, there are at least five arginine residues (H3R2, H3R8, H3R17, H3R26, and H4R3) and six lysine residues (H3K4, H3K9, H3K27, H3K36, H3K79, and H4K20) on histones H3 and H4 that can be methylated (10). Depending on the site of methylation, histone methylation can have both positive and negative effects on transcription. Unlike acetylation and phosphorylation, methylation does not affect the overall charge of the residue. However, in some cases, methylation can serve as a binding site for the recruitment of additional regulatory proteins (11,12). Nevertheless, the precise mechanism by which histone methylation regulates transcription is poorly understood.
Figure 1.
Chemical structures of the various methylated arginine and lysine residues catalyzed by methyltransferases (MTase). A, Arginines can be monomethylated, symmetrically dimethylated, or asymmetrically dimethylated. B, Lysines can be monomethylated, dimethylated, or trimethylated.
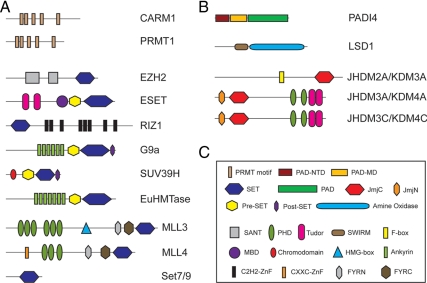
Histone methyltransferases (HMTs) catalyze the transfer of methyl groups from the methyl donor S-adenosyl-l-methionine to either the ω- or the δ-NH2 group of arginine residues or the ε-NH2 group of lysine residues that are present in histones. HMTs can be divided into three families based on their catalytic domains (8). The three families include: the protein arginine methyltransferase (PRMT) family, the Su(var)3–9, enhancer of zeste, trithorax (SET)-domain-containing family, and the non-SET domain enzymes (Dot1/Dot1L). With regard to NR-mediated transcriptional regulation, much of the literature has pointed to the participation of PRMTs and the SET-domain-containing HMTs in this biological process (Fig. 2A). The 10 mammalian PRMTs (PRMT1–PRMT10) are categorized into four groups based on their reaction mechanism and end product (13). The evolutionarily conserved SET domain is responsible for methylation of lysine residues. Although the SET domain is present in about 60 human proteins, only a fraction of these proteins have been shown to possess the ability to methylate histones.
Figure 2.
Domain architecture of HMTs (panel A) and demethylases (panel B) involved in nuclear hormone signaling. The names of the various functional domains in A and B are illustrated in panel C. MLL, Mixed-lineage leukemia; MBD, methyl-CpG-binding domain; SANT, Swi3 (switching-defective protein 3), Ada2 (adaptor 2), N-CoR (nuclear receptor corepressor), TFIIIB (transcription factor IIIB).
Unlike histone acetylation and phosphorylation, the reversibility of histone methylation was hotly debated for many years due to the low turnover rate of methylated histones (14). However, recent studies have demonstrated that histone methylation can also be removed enzymatically by histone demethylases that include PADI4 (peptidyl arginine deiminase, type 4), LSD1 (lysine specific demethylase 1), and the JmjC (Jumonji C)-domain containing proteins (10). Accumulating evidence suggests that these enzymes participate in diverse biological processes including NR signaling (Fig. 2B).
Regulation of Hormone-Responsive Genes by Histone Methylation and Demethylation
Activation of NR target genes requires that the local chromatin environment be transcriptionally competent. This can be accomplished, in part, by specific chromatin-modifying enzymes that promote accessibility of the transcriptional machinery to target promoters. Consequently, many NRs can directly or indirectly recruit chromatin-modifying enzymes such as HMTs and demethylases to facilitate NR target gene activation. Below we summarize recent studies regarding histone methylation and demethylation with respect to various NRs.
Estrogen receptor (ER)
Coactivator-associated arginine methyltransferase 1 (CARM1) is one of the best characterized examples of HMTs that plays an important role in mediating NR function. Also known as PRMT4, CARM1 is a type I PRMT that produces asymmetric dimethylarginine in addition to monomethylarginine. Methylation of arginines 2, 17, and 26 of histone H3 are mediated by CARM1 and are associated with transcriptional activation (15,16,17). The catalytic domain resides within a highly conserved set of four sequence motifs that is also found in members of the PRMT family.
Initially identified as an interaction partner of the p160 coactivators (16), CARM1 is a secondary coactivator of ER (as well as other NRs) functioning only when p160 coactivators are present. The importance of CARM1 in ER-dependent transcription is exemplified by the observation that CARM1 null fibroblasts and embryos exhibit aberrant expression of estrogen-responsive genes (18). Not surprisingly, CARM1 is overexpressed in breast tumors (19). In the breast cancer cell line MCF-7, addition of E2 (estradiol) to the culture leads to activation of the ER target gene pS2, which coincides with CARM1 recruitment and increased H3R17 methylation at the gene promoter (15). It has also been demonstrated that estrogen-stimulated cell proliferation depends on CARM1-mediated methylation of the E2F1 promoter (20). CARM1, itself, is also regulated by phosphorylation that inhibits its HMT activity and results in decreased activation of ER-mediated transcription (21).
In addition to CARM1, other PRMTs have also been implicated in ER signaling. PRMT1 is an H4R3 methyltransferase (22,23) that can be recruited by ER complexes as well as other NR complexes. Although the role of H4R3 methylation in gene activation is poorly understood, in vitro studies have indicated that this modification facilitates acetylation of H4 through the recruitment of the histone acetyltransferase, p300 (23). Furthermore, PRMT1 appears to act synergistically with CARM1 to enhance NR function (24). However, it is not entirely clear whether the coactivator function of PRMT1 is dependent on its enzymatic activity. Whereas this is true in the case of the androgen receptor (AR) (23), this has not been addressed in the context of ER-mediated transcription.
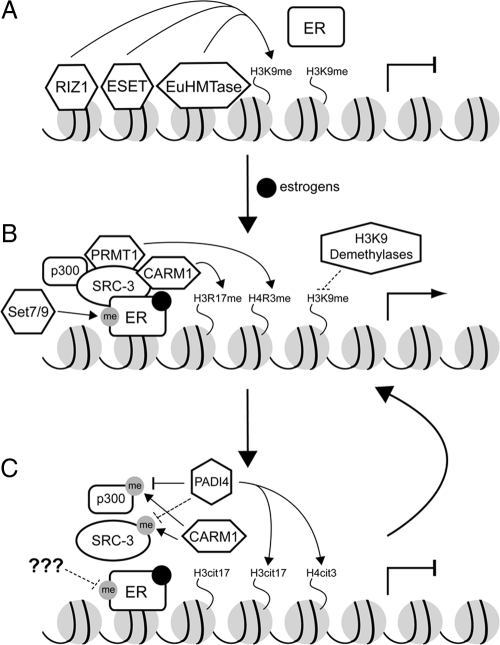
With regard to the role of HMTs in ER function, much of the work has been focused on CARM1 and PRMT1. Although other HMTs have recently been implicated in ER-dependent transcription, their precise function remains unclear. In general, very little has been done in elucidating the role of lysine methylation in regulating ER-dependent transcription. Methylation on H3K9 has been linked to gene repression, and its role in ER-dependent transcription may become apparent in the near future. Recently, several H3K9 HMTs, including ERG-associated protein with SET domain (ESET), retinoblastoma-interacting zinc factor (RIZ1), and EuHMTase, have been implicated in repressing ER targets pS2 and GREB1 in the absence of ligand E2 (25). These data point to a model in which ER target genes are repressed by H3K9 HMTs in the absence of estrogens (Fig. 3A) and activated by CARM1 and PRMT1 in the presence of estrogens (Fig. 3B).
Figure 3.
Schematic representation of the HMTs and demethylases involved in ER signaling during the absence of hormones (panel A), presence of hormones (panel B), and coactivator complex disassembly (panel C). Dashed lines represent unknown events.
In addition to H3K9 methylation, the involvement of H3K4 and H3K27 methylation in ER function is of particular interest due to their correlation with gene activation and repression, respectively. Recent studies have begun to reveal the potential function of enhancer of zeste 2 (EZH2), the catalytic subunit of the H3K27 methyltransferase (26), in ER signaling. As a Polycomb group protein, EZH2 has been implicated in gene silencing during development and differentiation through methylation on H3K27 (27). Consistent with its known function, EZH2 was reported to act as a corepressor by interacting with repressor of ER activity (28). However, EZH2 has also been reported to act as a coactivator for ER target genes, c-Myc and cyclin D1 (29). Although it is unclear how this discrepancy is reconciled, we note that different breast cancer cell line were used in these studies. It is possible that EZH2 might perform different functions depending on the cell lines and the specific promoter context. Additionally, it is important to note that the coactivator function of EZH2 is independent of its HMT activity (29). To clarify the role of H3K27 methylation in the silencing of ER target genes, it will be interesting to determine whether components of the EZH2 complex co-occupy ER target promoters because EZH2, SUZ12, and EED are all required for the H3K27 methyltransferase activity (30). Alternatively, it is possible that EZH2 may have functions outside the context of the complex.
Given that the steady state of histone methylation is controlled by a balance between the action of two groups of opposing enzymes, HMTs and histone demethylases, it is expected that enzymes that remove the methyl mark can also have effects on NR-dependent transcription. For example, the peptidyl deiminase PADI4 can convert methylated arginines to citrulline at positions 2, 17, and 26 of histone H3 and position 3 of histone H4 (31,32). Thus, PADI4 functions by antagonizing the effects of CARM1 and PRMT1 on expression of ER target genes (Fig. 3C). Interestingly, PADI4 itself is also a target of ER (33), which is consistent with the notion that ER-mediated transcription can be quickly attenuated after the initial induction.
AR
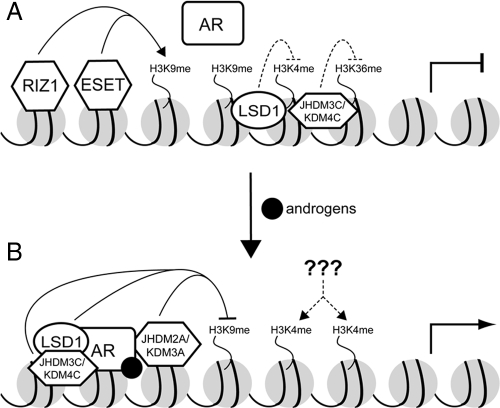
Recently, histone demethylases have emerged as key players in the regulation of AR-mediated transcription. In particular, current literature has placed much emphasis on demethylation of H3K9. In the absence of androgens, target genes are turned off due to the presence of H3K9 methylation. In the presence of androgens, H3K9 methylation is removed, which leads to the derepression of AR target genes. This model is supported by data from several recent studies. For example, LSD1, an amine oxidase initially identified as an H3K4 demethylase (34), was reported to also demethylate H3K9 in the presence of the AR agonist R1881 (35). Importantly, LSD1 can interact with AR and induce demethylation of H3K9me1 and H3K9me2 at the prostate-specific antigen (PSA) promoter region (35).
Interestingly, under the same conditions, decreased levels of H3K9me3 were also observed at the PSA promoters. However, this could not be attributed to LSD1 because LSD1 is not capable of catalyzing trimethyl demethylation (34). Subsequent work from Schüle and colleagues (36) identified JHDM3C/KDM4C as the demethylase responsible for the H3K9me3 demethylation. However, both LSD1 and JHDM3C/KDM4C appear to be present at the PSA promoter constitutively even in the absence of AR agonist. It is unclear why these two enzymes are present at a time when AR is not activated. We note that in addition to H3K9, LSD1 and JHDM3/KDM4 possess capability to demethylate H3K4 and H3K36, respectively (34,37,38). It is tempting to speculate that LSD1 and JHDM3C/KDM4C may also play a role in maintaining repression by demethylating these active marks when ligands are absent. If this is indeed the case, it would of great interest to investigate whether the presence of agonists is sufficient to switch the substrate specificities of these two enzymes.
In addition to LSD1 and JHDM3C/KDM4C, the H3K9me2-specific demethylase JHDM2A/KDM3A also participates in AR activation. Unlike LSD1 and JHDM3C/KDM4C, JHDM2A/KDM3A interacts with AR and is recruited to the PSA and NKX3.1 promoters in a hormone-dependent manner (39). Knockdown followed by chromatin immunoprecipitation (ChIP) demonstrates that this demethylase is only responsible for the removal of the H3K9me2 mark at the PSA and the NKX3.1 promoters (39). JHDM3A/KDM4A and JHDM3D/KDM4D are also AR coactivators that interact with AR only in the presence of the hormone, mibolerone (40). Interestingly, knockdown of JHDM3A/KDM4A in the absence of miberolone resulted in reduced levels of PSA mRNA, indicating that JHDM3A/KDM4A is important for maintaining basal levels of PSA. Because JDHM3A/KDM4A is capable demethylating H3K9me3 (37,38,41), further work is required to determine whether this activity is important for maintaining basal levels of target gene expression.
Conversely, if H3K9 demethylation is important for AR-dependent transcription, one might expect that H3K9 methyltransferases would promote repression at these loci. Consistent with this notion, both ESET and RIZ1 contribute to repression of AR target genes in the absence of hormone because knockdown of these HMTs results in derepression of these genes (25). However, because only partial derepression was observed when both enzymes were knocked down, it is evident that other H3K9 methyltransferases must participate in this process. Although the H3K9me2-specific HMT G9a was demonstrated to act as a coactivator for AR as well as ER (42), this function does not appear to be dependent on its HMT activity. Whether other H3K9 HMTs, such as SUV39H and EuHMTase, have a role in maintaining the repressed state of AR target genes in the absence of androgens remains to be determined.
It is evident that multiple members of the HMTs and demethylases appear to play significant roles in AR-mediated transcription (Fig. 4). Consequently, many of these enzymes are dysregulated in prostate cancer cell lines and tumors (41,43,44). Because many HMTs and demethylases are involved in this process, it is possible that precise transcriptional regulation of specific AR target genes may require specific combinations of HMTs and demethylases depending on cell type and promoter context.
Figure 4.
Schematic representation of the HMTs and demethylases involved in AR signaling during the absence of hormones (panel A) and presence of hormones (panel B). Dashed lines represent unknown events.
Other NRs
In addition to participating in ER- and AR-dependent transcription, histone methylation and demethylation have also been implicated in the function of other NRs. For example, both CARM1 and PRMT1 have been shown to be recruited to the thyroid response element by ligand-bound thyroid receptor (TR) resulting in increases in arginine methylation and transcription (45,46). CARM1 also functions as a coactivator for AR (47) and PPARγ (peroxisome proliferator-activated receptor γ) (48).
Similar to AR, H3K9 methylation also contributes to the transcriptional regulation of other NR target genes. Repression of TR-dependent transcription in the absence of hormone T3 is attributed to methylation of H3K9 by SUV39H1 (49). As expected, activation of TR results in decreases in H3K9me3 methylation (50), which could result from histone demethylation or histone replacement. However, based on what is known about H3K9 demethylation and NR-mediated transcription, it is likely that the JHDM3/KDM4 family of histone demethylases may be involved because this family has the capacity to remove H3K9me3. Interestingly, knockdown of CARM1 abolishes the decrease in H3K9me3 (50), suggesting that there may be a cross talk between arginine methylation and H3K9 demethylation.
In addition to its involvement in AR-mediated transcription, recent studies of the Jhdm2a knockout mice have revealed the function of JHDM2A/KDM3A in regulating the expression of metabolic genes (51). Loss of Jhdm2a function results in increased H3K9me2 levels at peroxisome proliferator response elements as well as reduced recruitment of NRs PPARγ and retinoid X receptor α, and their coactivators Pgc1α, p300, and Src1 (51). These results suggest that JHDM2A can serve as a coactivator of PPARγ. Furthermore, the H3K9 demethylase, JHDM3C/KDM4C, was reported to function as a coactivator of the glucocorticoid receptor (GR) and the progesterone receptor (PR) (36). It is likely that H3K9 demethylation also plays a role in transcriptional activation of the NR target genes.
In contrast to the established role of arginine methylation and H3K9 methylation in regulating NR function, very little is known about the role of H3K4 methylation, a mark strongly correlated with transcriptional activation. However, one recent study has implicated MLL3 and MLL4, HMTs that target H3K4, in the transcriptional activation of liver X receptor target genes (52). Recruitment of these HMTs to target promoters leads to induction of H3K4me3. It is likely that other H3K4 methyltransferases may also play critical roles in the transcriptional activation of other NR target genes.
Regulation of NRs by Methylation
In many cases, NRs achieve their function by recruiting coactivators and corepressors, some of which possess intrinsic enzymatic activities toward histones. Given that proteins other than histones can also be modified, it is possible that the proteins that associate with histone-modifying enzymes can also serve as substrates. Consistent with this notion, some of these histone-modifying enzymes can also modify the NRs themselves. Although much of the attention has been given to phosphorylation and acetylation of NRs, it is becoming apparent that methylation of NRs is also an important regulatory mechanism. Below, we summarize some of the recent findings in this area.
ER
It has been known for some time that ER is subject to a number of posttranslational modifications that include acetylation (53,54), phosphorylation (55,56), ubiquitination (57), and sumoylation (58). To add to the growing list of modifications, recent work has revealed that ER can also be methylated by Set7/9 (59). ER methylation leads to its stabilization and efficient recruitment to target genes (59). It remains to be investigated whether a demethylase can antagonize Set7/9 function in the absence of estrogens.
Methylation also regulates nongenomic functions of ER. The classical mechanism of ER action involves recruitment of the ligand-bound receptor to estrogen response elements within the genome. On the other hand, nongenomic functions involve cytoplasmic pools of ER that participate in multiprotein complexes in the presence of estrogens (60). Proteins that interact with cytoplasmic, ligand-bound ER include growth-dependent kinases and adaptor molecules. The formation of these complexes ultimately leads to the activation of many downstream signaling pathways including MAPK and Akt (61,62). Through this mechanism, ER is able to regulate a broader set of genes compared with the classical mechanism alone. The formation of cytoplasmic ER complexes is not only regulated by the presence of estrogens but also by methylation of ER. PRMT1, which resides in both the nucleus and the cytoplasm (63), can methylate the ER DNA-binding domain at R260. This modification is essential for E2-induced formation of the ERα/Src/p85 complex as well as activation of Akt (64).
Other NRs
In addition to ER, methylation can also occur in other NRs. The orphan NR, hepatocyte nuclear factor 4, is involved in regulation of genes controlling the metabolism of glucose and lipids and is another example of a NR that is regulated by methylation. PRMT1 methylates hepatocyte nuclear factor 4 within its DNA-binding domain, which enhances its ability to bind to target genes (65). In addition, a mass spectrometric approach revealed that mouse RARα (retinoic acid receptor α) is trimethylated on lysine 347, which leads to enhanced interactions with coactivators p300/CBP [cAMP response element binding protein (CREB)-binding protein] and RIP140 (receptor interacting protein 1) as well as its heterodimeric partner retinoid X receptor (66). This is the first demonstration that NRs can be lysine methylated although the methyltransferase is still currently unknown. Because more attention is now being given to nonhistone substrates of methyltransferases, it is likely that additional examples of NRs as methylation substrates will be identified in the near future.
Regulation of NR Coregulators by Methylation
Coregulators (coactivators and corepressors) of NRs play important roles in the regulation of transcriptional activity by ligand-bound NRs. This is accomplished through interactions between NRs and their coregulators to form multiprotein NR complexes that are crucial for efficient activation of target genes. Many of the coregulators discussed below do not act on a specific NR but, instead, broadly regulate several NRs. Due to their close proximity to other proteins with intrinsic enzymatic activities, coactivators and corepressors are also subject to posttranslational modifications including methylation.
Steroid receptor coactivator (SRC)/p160 coactivators
One of the best characterized group of coactivators is the SRC/p160 family proteins, which consist of three evolutionarily related members that directly interact with hormone-bound NRs. 1 SRC-1 was the founding member of this family and was shown to be a coactivator for PR (67). Subsequent characterization of SRC-2 [GR-interacting protein 1 (GRIP1), TIF2] and SRC-3 (p/CIP, Rac3, ACTR, AIB1, TRAM1) led to the understanding that these coactivators can enhance the function of multiple NRs (68). Through interaction with hormone-bound receptors, the SRC/p160 family of coactivators function as crucial scaffolds allowing for the assembly of coregulator complexes that enhance NR-mediated transcriptional activation.
For example, ER-mediated transcription involves multiple rounds of coregulator recruitment, assembly, and disassembly at target promoters (69,70). This process is both ordered and cyclical and may also be indicative of how other NRs and their coregulators function to induce and attenuate transcription. Although the mechanism of coregulator assembly is relatively well understood, very little is known regarding how coregulator complexes are disassembled.
Recent studies have suggested that methylation of SRC-3 may play a role in coregulator complex disassembly by reducing the affinity of SRC-3 for other coactivators. CARM1-mediated methylation of SRC-3 within its glutamine-rich region results in its dissociation from CARM1 (71). It was reported that methylation-deficient mutant CARM1 exhibited an enhanced coactivator function as well as higher affinity for the coactivators p300 (71) and CBP (72), indicating that methylation of SRC-3 affects the stability of the interactions and leads to the disassembly of the coactivator complex (Fig. 3C). In addition to affecting the stability of the coactivator complex, SRC-3 methylation also appears to regulate SRC-3 stability (72), which may provide an additional mechanism by which ER-mediated transcription can be rapidly attenuated.
In addition to the methylation site located in the glutamine-rich region, mass spectrometric analysis has revealed additional sites located between the NR interaction domain and the activation domain 1 of SRC-3 (72). Interestingly, these methylation sites are in close proximity to phosphorylation sites required for the coactivation of ER and AR (73), suggesting potential cross talk between these two modifications. Indeed, the presence of phosphorylation at these sites dramatically inhibited methylation within this region (72). However, the functional relevance of these newly identified sites remains to be determined. If methylation of these sites also diminishes the coactivating function of SRC-3, then cross talk between phosphorylation and methylation would add another layer of complexity to the mechanism by which the function of NRs can be switched on and off.
CBP/p300
Secondary coactivators such as CBP and p300 play important roles in enhancing transcription in response to hormone treatment. CBP and its paralogue p300 possess intrinsic histone acetyltransferase (HAT) activity. Acetylation of lysine resides of histones is thought to neutralize the positive charge of the residue, thus opening up the local chromatin structure and making it more accessible for transcriptional machinery. Furthermore, CBP and p300 can interact with RNA polymerase II (74,75,76,77), basal transcription factors (78,79), and other coactivators (80,81) to facilitate assembly of transcription initiation complexes. Interactions between CBP/p300 and the transcription machinery are subject to regulation by posttranslational modification, such as methylation (82). In fact, CBP/p300 was among the first coregulators demonstrated to be regulated by methylation (83).
Methylation within the KIX domain of CBP/p300 interferes with its interaction with CREB, resulting in inhibition of CREB-dependent recruitment and subsequent activation (83). In addition, CBP was also found to be methylated at R742 near the KIX domain, which is important for hormone-dependent interaction with GRIP-1 (84). The C-terminal GRIP-binding domain of p300 is also subject to methylation, which inhibits its interaction with GRIP (85). Interestingly, methylation of this site can be reversed by PADI4. Because p300-GRIP interaction is important for the recruitment of the coactivator complex, methylation of CBP/p300 may be a mechanism by which complex assembly and disassembly can be dynamically regulated.
PGC-1α
Peroxisome proliferator-activated receptor γ coactivator 1 α (PGC-1α) regulates energy homeostasis by functioning as a transcriptional coactivator for several NRs including PPARγ, ER, TR, GR, and RAR (86). Methylation of PGC-1α by PRMT1 appears to be crucial for its coactivator function (87). In transient transfection reporter assays, coactivation is abolished in the absence of PRMT1 or when PGC-1α methylation sites are mutated. Although PGC-1α can be methylated by PRMT1, but not CARM1, in vitro, it is unclear whether PGC-1α methylation occurs in vivo. Furthermore, how methylation of PGC-1α leads to a positive effect on transcription is unknown.
RIP140
Also known as NRIP1 (NR-interacting protein 1), RIP140 is a general coregulator for many NRs (88,89,90) whose precise function is complex. It appears that RIP140 interacts with agonist-bound receptors but can function as either a coactivator or a corepressor, depending on the cellular and promoter context (91,92). Nevertheless, its role in gene silencing has been characterized and appears to be linked with its capability to interact with histone deacetylases HDAC1 and HDAC3 (93,94) as well as the corepressor CtBP (95).
Posttranslational modifications appear to be an important mechanism by which the biological function of RIP140 can be regulated. In addition to phosphorylation (96,97) and acetylation (98), methylation of RIP140 can modulate its repressive activity. Accumulating evidence indicates that PRMT1-mediated arginine methylation can suppress RIP140-repressive function by at least two mechanisms: 1) RIP140 methylation decreases the recruitment of HDAC3 to the RARβ2 promoter; and 2) increases its interaction with exportin-1 resulting in the nuclear export of RIP140 (99). Interestingly, this methylation event depends on protein kinase C ε activation, RIP140 phosphorylation, and subsequent recruitment of 14-3-3 and PRMT1 (100). In addition, mass spectrometric analyses indicate that RIP140 itself is also lysine methylated (101). However, the functional relevance of RIP140 methylation and the responsible methyltransferase remains unknown.
Concluding Remarks
Recent identification and characterization of numerous HMTs and demethylases has led to great efforts in understanding their biological function. As a result, accumulating evidence suggests that methylation and demethylation play an important role in regulating nuclear hormone signaling. In addition to histones, these methyltranserases and demethylases can also modify NRs and their coregulators to contribute to the regulation of NR-mediated transcription (Table 1).
Table 1.
Substrates of HMTs and demethylases involved in nuclear hormone signaling
| Enzyme | Effector of | Substrates
|
||
|---|---|---|---|---|
| Histone | NR | Coregulator | ||
| HMTs | ||||
| Arginine | ||||
| CARM1 (PRMT4) | ER, AR, TR, PPARγ | H3R2, H3R17, H3R26 | SRC-3, CBP/p300 | |
| PRMT1 | ER, AR, TR, HNF | H4R3 | ER, HNF4 | PGC-1α, RIP140 |
| Lysine | ||||
| EZH2 | ER | H3K27 | ||
| ESET | ER, AR | H3K9 | ||
| RIZ1 | ER, AR | H3K9 | ||
| G9a | ER | H3K9 | ||
| SUV39H | TR | H3K9 | ||
| EuHMTase | ER | H3K9 | ||
| MLL3 | LXR | H3K4 | ||
| MLL4 | LXR | H3K4 | ||
| Set7/9 | H3K4 | ER | ||
| HDMTs | ||||
| Arginine | ||||
| PADI4 | ER | H3R2, H3R17, H3R26 | CBP/p300 | |
| Lysine | ||||
| LSD1 | ER, AR | H3K4, H3K9 | ||
| JHDM2A/KDM3A | AR, PPARγ, RXRα | H3K9 | ||
| JHDM3A/KDM4A | AR | H3K9, H3K36 | ||
| JHDM3C/KDM3C | AR, GR, PR | H3K9, H3K36 | ||
HNF4, Hepatocyte nuclear factor 4; LXR, Liver X receptor; PR, progesterone receptor; RXR, retinoid X receptor.
With regard to histone methylation and its effect on NR-mediated transcription, much attention has been focused on arginine methylation and H3K9 methylation as discussed above. Although many of the relevant HMTs and demethylases have been demonstrated to be NR coregulators, several key questions remain. 1) Evidence supports a model in which NR target genes are repressed in the absence of hormones due to the recruitment of H3K9 HMTs to target promoters. During transcriptional activation, these HMTs must be evicted from the promoter region. How this is achieved is not clear. Although competition with hormone-bound NRs may be one explanation, it is also possible that posttranslational modifications of HMTs may result in reduced affinity for chromatin. 2) PADI4 antagonizes the effects of CARM1 and PRMT1 by converting methylated arginine to citrulline. Transcription mediated by ER and possibly other NRs is a cyclical process. It is unclear how the presence of citrulline may affect the next round of transcription. It is possible that CARM1 and PRMT1 only enhance the first round of transcription. Alternatively, restoration of the arginine residue could be achieved by histone replacement.
Preliminary studies have begun to reveal the role of other methylation events, including H3K4 and H3K27methylation, in NR-mediated transcription. Genomic regions containing both of these marks are termed “bivalent domains” and are of great interest due to their role in maintaining a poised transcriptional state (102,103). Because genes containing bivalent domains are not considered transcriptionally active, it is possible that NR response elements contain these marks in the absence of a ligand. In response to hormone stimulation, removal of the silencing H3K27 mark would allow rapid activation. Bivalent domains have been reported to be present at retinoic acid response elements (104). However, further characterization of other response elements is required to determine whether bivalent domains play a general role in NR-mediated transcription. Although bivalent domains were initially identified in embryonic stem cells, whether they exist in cell lines relevant to NR signaling remains to be determined.
There is some evidence that NRs may utilize different combinations of histone methyltransferases and demethylases to activate their target genes (25). With the recent development of the ChIP-chip and ChIP-Seq technologies, it is possible to generate genome-wide histone methylation maps in the presence and absence of hormones. Although these techniques have been applied to examine genome-wide localization of NRs (105) as well as changes in histone acetylation (106), there is no report investigating changes in histone methylation before and after hormone stimulation. Although it is clear that levels of certain marks such as arginine methylation and H3K9 methylation dynamically change in the presence of ligands, a genome-wide approach would not only provide a bigger picture of the methylation changes, but could also provide insight into the groups of target genes that are coregulated.
Until recently, protein methylation had not been given much attention compared with other posttranslational modifications such as phosphorylation. However, it is evident that some of the HMTs and demethylases have nonhistone protein substrates. Therefore, it is expected that methylation of some of these proteins should contribute to the regulation of the protein function. This notion has attracted people to begin categorizing methylated proteins. For example, mass spectrometric technologies have been used to identify novel methylated proteins in the human genome. Although some success has been achieved in identifying novel arginine-methylated proteins (107), the result may not represent the complete human arginine methylome because identification was limited to the proteins that bound to methyl-specific antibodies. To overcome this problem, entire cell extracts could be analyzed by mass spectrometry as this has been done previously for phosphoproteins (108,109). Because only proteins that are methylated in high abundance can be identified by this approach, it is likely that improved sensitivity of the mass spectrometer will be necessary to characterize the human methylome. In view of the fact that methylation is a reversible reaction, development of specific demethylase inhibitors may be required for achieving this task.
Given the importance of NR signaling in the maintenance of normal cellular function, great efforts have been devoted to the development of drugs that interfere with NR signaling (110). The discovery that many of the HMTs and demethylases are part of the NR signaling pathway has raised the possibility that these enzymes could be potential drug targets for therapeutic intervention. Available data suggest that HMTs and demethylases play critical roles in NR signaling, not only through their ability to modify histones but also through their ability to directly regulate NRs and their coregulators. Because these enzymes appear to function in multiple ways, understanding their precise contributions to transcriptional activation by NRs will be necessary if therapeutic drugs are to be designed.
Footnotes
This work was supported by Howard Hughes Medical Institute and National Institutes of Health Grant R01-GM068804-07.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 30, 2009
Abbreviations: AR, Androgen receptor; CARM1, coactivator-associated arginine methyltransferase 1; CBP, CREB-binding protein; CREB, cAMP response element-binding protein; ESET, ERG-associated protein with SET domain; E2H2, enhancer of zeste 2; E2, estradiol; ER, estrogen receptor; GR, glucocorticoid receptor; GRIP1, GR-interacting protein 1; HDAC, histone deacetylase; HMT, histone methyltransferase; LSD1, lysine-specific demethylase 1; PADI4, peptidyl arginine deiminase, type 4; PGC-1α, PPARγ coactivator 1α; PPAR, peroxisome proliferator-activated receptor; PRMT, protein arginine methyltransferase; PSA, prostate-specific antigen; RAR, retinoic acid receptor; RIP140, receptor interacting protein 1; RIZ1, retinoblastoma-interacting zinc finger; SET, Su(var)3–, enhancer of zeste, trithorax; SRC, steroid receptor coactivator; TR, thyroid receptor.
References
- Conzen SD 2008 Minireview: nuclear receptors and breast cancer. Mol Endocrinol 22:2215–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B 2008 The year in basic science: nuclear receptors and coregulators. Mol Endocrinol 22:2751–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson TM, Moore JT 2002 Genomics versus orphan nuclear receptors—a half-time report. Mol Endocrinol 16:1135–1144 [DOI] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH 2005 Nuclear hormone receptor coregulator: role in hormone action, metabolism, growth, and development. Endocr Rev 26:583–597 [DOI] [PubMed] [Google Scholar]
- Wolf IM, Heitzer MD, Grubisha M, DeFranco DB 2008 Coactivators and nuclear receptor transactivation. J Cell Biochem 104:1580–1586 [DOI] [PubMed] [Google Scholar]
- Kornberg RD, Lorch Y 1999 Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98:285–294 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD 2000 The language of covalent histone modifications. Nature 403:41–45 [DOI] [PubMed] [Google Scholar]
- Martin C, Zhang Y 2005 The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6:838–849 [DOI] [PubMed] [Google Scholar]
- Bedford MT, Richard S 2005 Arginine methylation an emerging regulator of protein function. Mol Cell 18:263–272 [DOI] [PubMed] [Google Scholar]
- Klose RJ, Zhang Y 2007 Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol 8:307–318 [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T 2001 Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410:120–124 [DOI] [PubMed] [Google Scholar]
- Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T 2001 Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116–120 [DOI] [PubMed] [Google Scholar]
- Bedford MT 2007 Arginine methylation at a glance. J Cell Sci 120:4243–4246 [DOI] [PubMed] [Google Scholar]
- Byvoet P, Shepherd GR, Hardin JM, Noland BJ 1972 The distribution and turnover of labeled methyl groups in histone fractions of cultured mammalian cells. Arch Biochem Biophys 148:558–567 [DOI] [PubMed] [Google Scholar]
- Bauer UM, Daujat S, Nielsen SJ, Nightingale K, Kouzarides T 2002 Methylation at arginine 17 of histone H3 is linked to gene activation. EMBO Rep 3:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR 1999 Regulation of transcription by a protein methyltransferase. Science 284:2174–2177 [DOI] [PubMed] [Google Scholar]
- Ma H, Baumann CT, Li H, Strahl BD, Rice R, Jelinek MA, Aswad DW, Allis CD, Hager GL, Stallcup MR 2001 Hormone-dependent, CARM1-directed, arginine-specific methylation of histone H3 on a steroid-regulated promoter. Curr Biol 11:1981–1985 [DOI] [PubMed] [Google Scholar]
- Yadav N, Lee J, Kim J, Shen J, Hu MC, Aldaz CM, Bedford MT 2003 Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc Natl Acad Sci USA 100:6464–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Messaoudi S, Fabbrizio E, Rodriguez C, Chuchana P, Fauquier L, Cheng D, Theillet C, Vandel L, Bedford MT, Sardet C 2006 Coactivator-associated arginine methyltransferase 1 (CARM1) is a positive regulator of the Cyclin E1 gene. Proc Natl Acad Sci USA 103:13351–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietze S, Lupien M, Silver PA, Brown M 2008 CARM1 regulates estrogen-stimulated breast cancer growth through up-regulation of E2F1. Cancer Res 68:301–306 [DOI] [PubMed] [Google Scholar]
- Higashimoto K, Kuhn P, Desai D, Cheng X, Xu W 2007 Phosphorylation-mediated inactivation of coactivator-associated arginine methyltransferase 1. Proc Natl Acad Sci USA 104:12318–12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Briggs SD, Brame CJ, Caldwell JA, Koh SS, Ma H, Cook RG, Shabanowitz J, Hunt DF, Stallcup MR, Allis CD 2001 Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr Biol 11:996–1000 [DOI] [PubMed] [Google Scholar]
- Wang H, Huang ZQ, Xia L, Feng Q, Erdjument-Bromage H, Strahl BD, Briggs SD, Allis CD, Wong J, Tempst P, Zhang Y 2001 Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science 293:853–857 [DOI] [PubMed] [Google Scholar]
- Koh SS, Chen D, Lee YH, Stallcup MR 2001 Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem 276:1089–1098 [DOI] [PubMed] [Google Scholar]
- Garcia-Bassets I, Kwon YS, Telese F, Prefontaine GG, Hutt KR, Cheng CS, Ju BG, Ohgi KA, Wang J, Escoubet-Lozach L, Rose DW, Glass CK, Fu XD, Rosenfeld MG 2007 Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell 128:505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y 2002 Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298:1039–1043 [DOI] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M 2006 Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 6:846–856 [DOI] [PubMed] [Google Scholar]
- Hwang C, Giri VN, Wilkinson JC, Wright CW, Wilkinson AS, Cooney KA, Duckett CS 2008 EZH2 regulates the transcription of estrogen-responsive genes through association with REA, an estrogen receptor corepressor. Breast Cancer Res Treat 107:235–242 [DOI] [PubMed] [Google Scholar]
- Shi B, Liang J, Yang X, Wang Y, Zhao Y, Wu H, Sun L, Zhang Y, Chen Y, Li R, Zhang Y, Hong M, Shang Y 2007 Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells. Mol Cell Biol 27:5105–5119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Zhang Y 2004 SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell 15:57–67 [DOI] [PubMed] [Google Scholar]
- Cuthbert GL, Daujat S, Snowden AW, Erdjument-Bromage H, Hagiwara T, Yamada M, Schneider R, Gregory PD, Tempst P, Bannister AJ, Kouzarides T 2004 Histone deimination antagonizes arginine methylation. Cell 118:545–553 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S, Stallcup MR, Allis CD, Coonrod SA 2004 Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 306:279–283 [DOI] [PubMed] [Google Scholar]
- Dong S, Zhang Z, Takahara H 2007 Estrogen-enhanced peptidylarginine deiminase type IV gene (PADI4) expression in MCF-7 cells is mediated by estrogen receptor-α-promoted transfactors activator protein-1, nuclear factor-Y, and Sp1. Mol Endocrinol 21:1617–1629 [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y 2004 Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941–953 [DOI] [PubMed] [Google Scholar]
- Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AH, Günther T, Buettner R, Schüle R 2005 LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437:436–439 [DOI] [PubMed] [Google Scholar]
- Wissmann M, Yin N, Müller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Günther T, Buettner R, Metzger E, Schüle R 2007 Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol 9:347–353 [DOI] [PubMed] [Google Scholar]
- Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y 2006 The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature 442:312–316 [DOI] [PubMed] [Google Scholar]
- Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y 2006 Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125:467–481 [DOI] [PubMed] [Google Scholar]
- Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y 2006 JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 125:483–495 [DOI] [PubMed] [Google Scholar]
- Shin S, Janknecht R 2007 Activation of androgen receptor by histone demethylases JMJD2A and JMJD2D. Biochem Biophys Res Commun 359:742–746 [DOI] [PubMed] [Google Scholar]
- Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K 2006 The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature 442:307–311 [DOI] [PubMed] [Google Scholar]
- Lee DY, Northrop JP, Kuo MH, Stallcup MR 2006 Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors. J Biol Chem 281:8476–8485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J, Metzger E, Schüle R, Buettner R 2006 Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res 66:11341–11347 [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Matsubara A, Teishima J, Seki M, Mita K, Usui T, Oue N, Yasui W 2007 DNA methylation of the RIZ1 gene is associated with nuclear accumulation of p53 in prostate cancer. Cancer Sci 98:32–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Hasebe T, Shi YB 2009 Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol Cell Biol 29:745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Shi YB 2007 Contrasting effects of two alternative splicing forms of coactivator-associated arginine methyltransferase 1 on thyroid hormone receptor-mediated transcription in Xenopus laevis. Mol Endocrinol 21:1082–1094 [DOI] [PubMed] [Google Scholar]
- Majumder S, Liu Y, Ford 3rd OH, Mohler JL, Whang YE 2006 Involvement of arginine methyltransferase CARM1 in androgen receptor function and prostate cancer cell viability. Prostate 66:1292–1301 [DOI] [PubMed] [Google Scholar]
- Yadav N, Cheng D, Richard S, Morel M, Iyer VR, Aldaz CM, Bedford MT 2008 CARM1 promotes adipocyte differentiation by coactivating PPARγ. EMBO Rep 9:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lin Q, Yoon HG, Huang ZQ, Strahl BD, Allis CD, Wong J 2002 Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Mol Cell Biol 22:5688–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Choi KC, Oh SY, Kang HB, Lee YH, Haam S, Ahn YH, Kim KS, Kim K, Yoon HG 2007 The functional role of the CARM1-SNF5 complex and its associated HMT activity in transcriptional activation by thyroid hormone receptor. Exp Mol Med 39:544–555 [DOI] [PubMed] [Google Scholar]
- Tateishi K, Okada Y, Kallin EM, Zhang Y 2009 Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 458:757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee J, Lee SK, Lee JW 2008 Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol Endocrinol 22:1312–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL 2006 Acetylation of estrogen receptor α by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol 20:1479–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Fu M, Angeletti RH, Siconolfi-Baez L, Reutens AT, Albanese C, Lisanti MP, Katzenellenbogen BS, Kato S, Hopp T, Fuqua SA, Lopez GN, Kushner PJ, Pestell RG 2001 Direct acetylation of the estrogen receptor α hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem 276:18375–18383 [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA 2004 Phosphorylation of estrogen receptor α blocks its acetylation and regulates estrogen sensitivity. Cancer Res 64:9199–9208 [DOI] [PubMed] [Google Scholar]
- Lannigan DA 2003 Estrogen receptor phosphorylation. Steroids 68:1–9 [DOI] [PubMed] [Google Scholar]
- Wijayaratne AL, McDonnell DP 2001 The human estrogen receptor-α is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem 276:35684–35692 [DOI] [PubMed] [Google Scholar]
- Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L 2005 Sumoylation of the estrogen receptor α hinge region regulates its transcriptional activity. Mol Endocrinol 19:2671–2684 [DOI] [PubMed] [Google Scholar]
- Subramanian K, Jia D, Kapoor-Vazirani P, Powell DR, Collins RE, Sharma D, Peng J, Cheng X, Vertino PM 2008 Regulation of estrogen receptor α by the SET7 lysine methyltransferase. Mol Cell 30:336–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnström L, Sjöberg M 2005 Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RX, Zhang Z, Santen RJ 2005 Estrogen rapid action via protein complex formation involving ERα and Src. Trends Endocrinol Metab 16:347–353 [DOI] [PubMed] [Google Scholar]
- Herrmann F, Lee J, Bedford MT, Fackelmayer FO 2005 Dynamics of human protein arginine methyltransferase 1(PRMT1) in vivo. J Biol Chem 280:38005–38010 [DOI] [PubMed] [Google Scholar]
- Le Romancer M, Treilleux I, Leconte N, Robin-Lespinasse Y, Sentis S, Bouchekioua-Bouzaghou K, Goddard S, Gobert-Gosse S, Corbo L 2008 Regulation of estrogen rapid signaling through arginine methylation by PRMT1. Mol Cell 31:212–221 [DOI] [PubMed] [Google Scholar]
- Barrero MJ, Malik S 2006 Two functional modes of a nuclear receptor-recruited arginine methyltransferase in transcriptional activation. Mol Cell 24:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq MD, Tsai NP, Khan SA, Wei LN 2007 Lysine trimethylation of retinoic acid receptor-α: a novel means to regulate receptor function. Mol Cell Proteomics 6:677–688 [DOI] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW 1995 Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- Xu J, Li Q 2003 Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 17:1681–1692 [DOI] [PubMed] [Google Scholar]
- Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- Feng Q, Yi P, Wong J, O'Malley BW 2006 Signaling within a coactivator complex: methylation of SRC-3/AIB1 is a molecular switch for complex disassembly. Mol Cell Biol 26:7846–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem H, Cheng D, Zhao Q, Underhill C, Tini M, Bedford MT, Torchia J 2007 The activity and stability of the transcriptional coactivator p/CIP/SRC-3 are regulated by CARM1-dependent methylation. Mol Cell Biol 27:120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O'Malley BW 2004 Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol Cell 15:937–949 [DOI] [PubMed] [Google Scholar]
- Cho H, Orphanides G, Sun X, Yang XJ, Ogryzko V, Lees E, Nakatani Y, Reinberg D 1998 A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol 18:5355–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Uchida C, Anderson SF, Lee CG, Hurwitz J, Parvin JD, Montminy M 1997 RNA helicase A mediates association of CBP with RNA polymerase II. Cell 90:1107–1112 [DOI] [PubMed] [Google Scholar]
- Nakajima T, Uchida C, Anderson SF, Parvin JD, Montminy M 1997 Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev 11:738–747 [DOI] [PubMed] [Google Scholar]
- Neish AS, Anderson SF, Schlegel BP, Wei W, Parvin JD 1998 Factors associated with the mammalian RNA polymerase II holoenzyme. Nucleic Acids Res 26:847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok RP, Lundblad JR, Chrivia JC, Richards JP, Bächinger HP, Brennan RG, Roberts SG, Green MR, Goodman RH 1994 Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature 370:223–226 [DOI] [PubMed] [Google Scholar]
- Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A 1996 Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem 271:9009–9013 [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM 1997 Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569–580 [DOI] [PubMed] [Google Scholar]
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y 1996 A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382:319–324 [DOI] [PubMed] [Google Scholar]
- Kalkhoven E 2004 CBP and p300: HATs for different occasions. Biochem Pharmacol 68:1145–1155 [DOI] [PubMed] [Google Scholar]
- Xu W, Chen H, Du K, Asahara H, Tini M, Emerson BM, Montminy M, Evans RM 2001 A transcriptional switch mediated by cofactor methylation. Science 294:2507–2511 [DOI] [PubMed] [Google Scholar]
- Chevillard-Briet M, Trouche D, Vandel L 2002 Control of CBP co-activating activity by arginine methylation. EMBO J 21:5457–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Coonrod SA, Kraus WL, Jelinek MA, Stallcup MR 2005 Regulation of coactivator complex assembly and function by protein arginine methylation and demethylimination. Proc Natl Acad Sci USA 102:3611–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM 2003 Peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1 α): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90 [DOI] [PubMed] [Google Scholar]
- Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR 2005 Activation of nuclear receptor coactivator PGC-1α by arginine methylation. Genes Dev 19:1466–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillès V, Dauvois S, L'Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG 1995 Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J 14:3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Horset F, Dauvois S, Heery DM, Cavaillès V, Parker MG 1996 RIP-140 interacts with multiple nuclear receptors by means of two distinct sites. Mol Cell Biol 16:6029–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Wei LN 1999 Characterization of receptor-interacting protein 140 in retinoid receptor activities. J Biol Chem 274:31320–31326 [DOI] [PubMed] [Google Scholar]
- Subramaniam N, Treuter E, Okret S 1999 Receptor interacting protein RIP140 inhibits both positive and negative gene regulation by glucocorticoids. J Biol Chem 274:18121–18127 [DOI] [PubMed] [Google Scholar]
- Treuter E, Albrektsen T, Johansson L, Leers J, Gustafsson JA 1998 A regulatory role for RIP140 in nuclear receptor activation. Mol Endocrinol 12:864–881 [DOI] [PubMed] [Google Scholar]
- Wei LN, Farooqui M, Hu X 2001 Ligand-dependent formation of retinoid receptors, receptor-interacting protein 140 (RIP140), and histone deacetylase complex is mediated by a novel receptor-interacting motif of RIP140. J Biol Chem 276:16107–16112 [DOI] [PubMed] [Google Scholar]
- Wei LN, Hu X, Chandra D, Seto E, Farooqui M 2000 Receptor-interacting protein 140 directly recruits histone deacetylases for gene silencing. J Biol Chem 275:40782–40787 [DOI] [PubMed] [Google Scholar]
- Vo N, Fjeld C, Goodman RH 2001 Acetylation of nuclear hormone receptor-interacting protein RIP140 regulates binding of the transcriptional corepressor CtBP. Mol Cell Biol 21:6181–6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Huq MD, Khan SA, Tsai NP, Wei LN 2005 Regulation of co-repressive activity of and HDAC recruitment to RIP140 by site-specific phosphorylation. Mol Cell Proteomics 4:1776–1784 [DOI] [PubMed] [Google Scholar]
- Huq MD, Khan SA, Park SW, Wei LN 2005 Mapping of phosphorylation sites of nuclear corepressor receptor interacting protein 140 by liquid chromatography-tandem mass spectroscopy. Proteomics 5:2157–2166 [DOI] [PubMed] [Google Scholar]
- Huq MD, Wei LN 2005 Post-translational modification of nuclear co-repressor receptor-interacting protein 140 by acetylation. Mol Cell Proteomics 4:975–983 [DOI] [PubMed] [Google Scholar]
- Mostaqul Huq MD, Gupta P, Tsai NP, White R, Parker MG, Wei LN 2006 Suppression of receptor interacting protein 140 repressive activity by protein arginine methylation. EMBO J 25:5094–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Ho PC, Huq MD, Khan AA, Tsai NP, Wei LN 2008 PKCε stimulated arginine methylation of RIP140 for its nuclear-cytoplasmic export in adipocyte differentiation. PLoS ONE 3:e2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq MD, Ha SG, Barcelona H, Wei LN 2009 Lysine methylation of nuclear co-repressor receptor interacting protein 140. J Proteome Res 8:1156–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES 2006 A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315–326 [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O'Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE 2007 Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie RF, Gudas LJ 2007 Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARβ(2), and Cyp26A1 in F9 embryonal carcinoma cells. J Mol Biol 372:298–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois G, Giguère V 2008 Nuclear receptor location analyses in mammalian genomes: from gene regulation to regulatory networks. Mol Endocrinol 22:1999–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL 2007 Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert FM, Côté J, Boulanger MC, Richard S 2003 A proteomic analysis of arginine-methylated protein complexes. Mol Cell Proteomics 2:1319–1330 [DOI] [PubMed] [Google Scholar]
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Cohn MA, Cantley LC, Gygi SP 2004 Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci USA 101:12130–12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle K, White FM 2006 Phosphoproteomic approaches to elucidate cellular signaling networks. Curr Opin Biotechnol 17:406–414 [DOI] [PubMed] [Google Scholar]
- Chen T 2008 Nuclear receptor drug discovery. Curr Opin Chem Biol 12:418–426 [DOI] [PubMed] [Google Scholar]