Abstract
Purpose:
Prior cross-sectional studies have shown that cancer patients have sleep-wake activity cycles that show little distinction between daytime and nighttime, a pattern indicative of circadian disruption. This pattern is seen both before and during cancer treatment. Long-term data are needed, however, to assess to what extent circadian rhythm impairments evolve over the course of chemotherapy. The goal of this study was to assess the longitudinal course of sleep-wake activity rhythms before and during chemotherapy for breast cancer.
Patients and Methods:
Ninety-five women scheduled to receive neoadjuvant or adjuvant anthracycline based chemotherapy for a stage I-III breast cancer participated. The participants wore a wrist actigraph for 72 consecutive hours at baseline (pre-chemotherapy), as well as during the weeks 1, 2 and 3 (W1, W2, W3) of cycle 1 and cycle 4 of chemotherapy. Sleep-wake circadian activity variables were computed based on actigraphic data.
Results:
Compared to baseline, with the exception of acrophase, all circadian rhythm variables examined, including amplitude, mesor, up-mesor, down-mesor, and rhythmicity were significantly impaired during the first week of both chemotherapy cycles. Although the circadian variables approached baseline values during W2 and W3 of cycle 1, most remained significantly more impaired during W2 and W3 of cycle 4.
Conclusion:
These data suggest that the first administration of chemotherapy is associated with transient disruption of sleep-wake rhythm, while repeated administration of chemotherapy results in progressively worse and more enduring impairments in sleep-wake activity rhythms.
Citation:
Savard J; Liu L; Natarajan L; Rissling MB; Neikrug AB; He F; Dimsdale JE; Mills PJ; Parker BA; Sadler GR; Ancoli-Israel S. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. SLEEP 2009;32(9):1155-1160.
Keywords: Cancer, circadian rhythms, sleep-wake activity, chemotherapy
SLEEP DISTURBANCES ARE VERY COMMON IN CANCER PATIENTS. PREVIOUS STUDIES HAVE REVEALED THAT 30% TO 50% OF CANCER PATIENTS REPORT insomnia symptoms.1,2 There is also evidence suggesting that breast cancer patients constitute the subgroup of cancer patients most at risk for experiencing sleep difficulties.3,4 In addition to resulting in restless sleep (i.e., increased activity during the night), breast cancer and its treatment may also reduce daytime activity. Although data are rather sparse, there is some evidence to suggest that cancer patients have disturbed circadian rhythms.
Studies using actigraphy and comparing cancer patients to healthy controls have been consistent in showing less contrast between daytime and night time activity in cancer patients, a pattern indicative of circadian disruption.5–9 But few have examined circadian variables before and during treatment in cancer patients. Some data on breast cancer patients suggest that sleep and circadian rhythms may be altered even prior to the initiation of chemotherapy.10,11 These studies found that breast cancer patients slept for about 6 hours, spent about a quarter of the night awake and napped for a total of about one hour a day. While their circadian rhythms were not desynchronized, those with more phase-delayed rhythms experienced more daily dysfunction. Together, these studies are consistent in showing some alterations in the rest-activity circadian rhythms of cancer patients. However, as no longitudinal study has been published, it is unknown to what extent these impairments evolve over time.
As part of a larger study on sleep, fatigue, rhythms and breast cancer, the goal of this study was to assess the evolution of circadian impairments longitudinally, i.e., before and during chemotherapy for breast cancer.
METHODS
Participants
The majority of patients were referred by oncologists from the Rebecca and John Moores University of California San Diego (UCSD) Cancer Center. Patients were also referred from community oncologists in the San Diego, CA, and the Yakima, WA, areas. Patients were eligible for the study if they had recently received a diagnosis of stage I-III breast cancer and were scheduled to receive ≥ 4 cycles of neoadjuvant or adjuvant anthracycline-based chemotherapy as part of their cancer treatment. The study excluded women who were shift workers, pregnant, had metastatic or IIIB (including inflammatory) breast cancer, had significant pre-existing anemia, had received radiation therapy prior to their chemotherapy, were undergoing bone marrow transplant, and those with confounding comorbid medical illnesses or any other physiological or psychological impairments (e.g., major depression) that would have limited their participation.
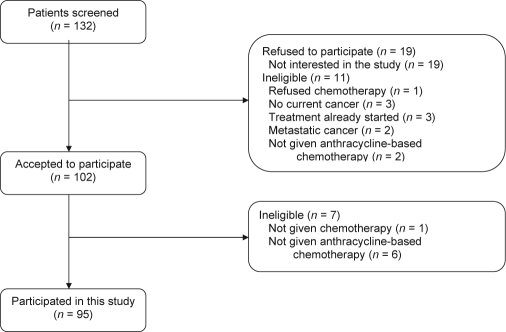
A total of 132 women were screened for the study, of whom 11 did not meet study criteria and 19 refused to participate because of a lack of interest in the study (see Figure 1 for details). Of the 102 patients who consented to participate, 7 additional women were found ineligible, thus leaving a final sample of 95 participants. Of those, 75% were Caucasian, 69% were married, 77% had at least some college education, and 73% reported an annual income of over $30,000 (Table 1).
Figure 1.
Screening and enrollment flowchart
Table 1.
Demographic and Medical Characteristics at Baseline (N = 95)
| Variable | M (SD) | n (%) |
|---|---|---|
| Age (years; n = 94; range: 34-79) | 50.72 (9.66) | |
| Marital Status (n = 94) | ||
| Married | 65 (69.15) | |
| Never married | 10 (10.64) | |
| Divorced | 15 (15.96) | |
| Separated | 3 (3.19) | |
| Widowed | 1 (1.06) | |
| Education (n = 94) | ||
| Some high school or less | 4 (4.26) | |
| Completed high school | 17 (18.09) | |
| Some college | 26 (27.66) | |
| College degree | 47 (50.0) | |
| Annual Family Income (n = 81) | ||
| Less than $30,000 | 13 (16.05) | |
| More than $30,000 | 68 (83.95) | |
| Occupation (n = 93) | ||
| Working | 87 (93.55) | |
| Retired | 6 (6.45) | |
| Menopausal status pre-chemotherapy (n = 87) | ||
| Premenopausal | 37 (42.53) | |
| Perimenopausal | 7 (8.05) | |
| Postmenopausal | 25 (28.74) | |
| Post-hysterectomy | 18 (20.69) | |
| Cancer stage (n = 85) | ||
| Stage I | 25 (29.41) | |
| Stage II | 42 (49.41) | |
| Stage III | 18 (21.18) | |
| Surgery (n = 84) | ||
| Lumpectomy | 34 (40.0) | |
| Mastectomy | 36 (42.35) | |
| Double mastectomy | 5 (5.88) | |
| Pre-op chemotherapy | 9 (10.59) | |
| Chemotherapy regimen (n = 79) | ||
| Exactly 4 cycles of AC | 24 (30.38) | |
| Exactly 4 cycles of AC + docetaxel | 21 (26.58) | |
| Exactly 4 cycles of AC + paclitaxel | 6 (7.59) | |
| More than 4 cycles of AC | 3 (3.80) | |
| Exactly 4 cycles of AC followed by docetaxel | 4 (5.06) | |
| Exactly 4 cycles of AC followed by paclitaxel | 10 (12.66) | |
| 4 or more cycles of AC + 5-fluorouracil | 4 (5.06) | |
| Other regimen | 7 (8.86) | |
| Prior use of hormone replacement therapy (n = 84) | ||
| Yes | 23 (27.38) |
AC = doxorubicin + cyclophosphamide.
MEASURES
Circadian Rhythms
Circadian rhythms were computed from data recorded using an Actillume (Ambulatory Monitoring Inc., Ardsley, NY, USA). The Actillume is a small actigraphy device (1 × 3 × 6 cm) that is worn on the wrist and is similar in size and appearance to a large wrist watch. The actigraph contains a piezoelectric linear accelerometer (sensitive to 0.003 g and above), a microprocessor, 32K RAM memory, and associated circuitry. By calculating the orientation and movement, the Actillume records sleep-awake activity and allows for an objective measure of the duration and disruption of sleep. Sensitivity and effectiveness of sleep-awake inference from wrist activity by the Actillume has previously been validated.12 Measuring wrist activity over time also produces an index of the daily rhythm as well as the circadian activity rhythm over days. The recorded actigraphy data were analyzed using Action 3 (Ambulatory Monitoring Inc.).
Circadian rhythms were analyzed by fitting each participant's actigraphy data to a 5-parameter extended cosine model,13 which resulted in 6 derived outcome variables (Table 2). These measures characterize the rhythmicity of activity levels as well as the timing of the onset and offset of activity.
Table 2.
Circadian Rhythms Variables Derived from Actigraphy
| Variables | Definition | Meaning |
|---|---|---|
| Amplitude | The height of the rhythm. Value = maximum activity – minimum activity | Lower amplitude suggests a dampened circadian rhythm. |
| Acrophase (h) | Time of day of the peak of the curve | A later time suggest more phase delay. |
| Mesor | The mean of the rhythm; Value = Minimum + 1/2 amplitude Half-way between minimum and maximum activity | Mean activity level |
| Up-Mesor (h) | The time of day when the women switched from low activity to high activity, i.e., from below the mesor to above the mesor | Higher value suggests a later starting time of activity; the time the women “got going” in the morning. |
| Down-Mesor (h) | The time of day when the women switched from high activity to low activity, i.e., from above the mesor to below the mesor | Higher value suggests a later time of decline of activity; the time the women “settled down” for the evening. |
| R-Squared | The reduction in squared error from using a model to summarize data (and predict future values) compared to using the mean | Higher value suggests a more rhythmic or robust rhythm. |
Procedure
The detailed procedure is described in Liu et al.14 Briefly, study approval was obtained from the UCSD Committee on Protection of Human Subjects and by the Rebecca and John Moores UCSD Cancer Center's Protocol Review and Monitoring Committee before the study's initiation. After patients were referred by the oncologist, the research coordinator scheduled a meeting with them to discuss their participation in the study. During this meeting, informed consent was obtained, and a release of information form (HIPAA) was signed. Medical records were abstracted for medical history and medication use.
Data were collected at 7 time points: baseline (mean of 7.7 days before the start of chemotherapy), during cycle 1 week 1 (C1W1; week of chemotherapy), cycle 1 week 2 (C1W2; week of lowest blood counts), cycle 1 week 3 (C1W3; recovery week) and during the 3 weeks of cycle 4 (C4W1, C4W2, C4W3). All measures at W1 were begun the day after the administration of chemotherapy and started on the same day of the week at subsequent time points within each cycle.
At each assessment, the participants wore the Actillume recorder for 3 consecutive 24-h periods (i.e., total of 72 h) and completed a sleep log to record their bedtime, wake time, and napping periods. The sleep log information was used as an aid for the actigraphy data editing and scoring.
Statistical Analyses
Descriptive statistics (means, standard deviations, medians, frequencies, ranges) for demographic and clinical characteristics of the study sample were calculated. Preliminary analyses (correlations and t tests) were performed to assess the association between all circadian rhythm variables and several potential confounders including demographic variables (age, body mass index, ethnicity, marital status, education, familial income, occupation) and clinical characteristics (prior use of hormone replacement therapy, menopausal status, tumor size, cancer stage, estrogen-receptor-positive tumors, progesterone-receptor-positive tumors, presence of positive nodes, cancer surgery, and medication use) at baseline, as well as the chemotherapy regimen received during the course of the study. It was decided that demographic and clinical variables that would be significantly associated with at least half of the circadian rhythm variables would be controlled for in the inferential analyses. As none of the variables met this criterion, regression analyses were performed without the inclusion of covariates.
The evolution of circadian rhythms during chemotherapy was modeled using linear mixed-effects models, fitted with restricted maximum likelihood methods.15 A separate model was developed for each of the 6 outcome variables of interest, namely, amplitude, acrophase, mesor, up-mesor, down-mesor, and R-squared. Each model included a random effect at the patient level (random intercept term in the model) to allow for variability in baseline rhythms between individuals. Time of chemotherapy (C1W1, C1W2, C1W3, C4W1, C4W2, C4W3) was included as a categorical fixed effect in the models. Circadian rhythms during each week of the chemotherapy regimen were compared to baseline (pre-treatment) values. A likelihood ratio test was used to test the inclusion of a time (of chemotherapy) effect in the models by comparing the likelihood for the intercept only model to the likelihood of the model with both intercept and time. Residual plots and quantile-quantile plots were used to assess adequacy of fit of the models.
An important advantage of mixed model paradigms is that under a “missing at random” assumption,15 the model allowed for unbalanced data, whereby the number of available measures per individual could vary. Patients could be included in the models even if they did not have outcome information for all time-points. Thus potential biases from a “completers” only analysis are reduced considerably in a mixed-model analysis. In our analyses, data of all patients with actigraphic recordings available for at least one time point were included in the analyses. This yielded a sample size of 86 at baseline; 76 at C1W1; 69 at C1W2; 72 at C1W3; 70 at C4W1; 60 at C4W2; and 65 at C4W3; and a total of 498 observations in each mixed-model analysis. Reasons for not having data available at some time points included technical difficulties with the actigraphic recorder, patients unavailable for testing on particular weeks, and patients dropping out from the study.
RESULTS
Evolution of Circadian Rhythms Variables Over Time
Significant overall time effects were obtained on amplitude, χ2 (6) = 101.01, P < 0.0001; mesor, χ2 (6) = 102.95, P < 0.0001; up-mesor, χ2 (6) = 14.23, P < 0.05; down-mesor, χ2 (6) = 24.09, P < 0.001; and R-squared, χ2 (6) = 120.88, P < 0.0001; suggesting that that the sleep-wake activity became more disrupted during chemotherapy compared to baseline. Only acrophase did not change significantly over the course of the study, χ2 (6) = 5.59, P = 0.47, in spite of the similarity of the pattern of results with that of other variables. Figure 2 shows one participant's raw actigraph data for 3 days during each time point of data collection. In this patient, the sleep-wake rhythm was robust at baseline with a clear contrast between daytime and night time activity. The rhythm then became disrupted at cycle 1 and even more disrupted at cycle 4, as indicated by more activity during the night and less constant bedtime and wake time.
Figure 2.
One participant's raw actigraph data for 3 days during each time point of data collection. Double plot with the first row representing day 1 (midnight to midnight) and day 2 (midnight to midnight), the second row representing day 2 again followed by day 3 and so on. This patient had a robust circadian rhythm at baseline with a clear contrast between daytime and nighttime activity, minimal body movements during the night, and constant bedtime and wake time across the 3 nights. At cycle 1, the rhythm became disrupted with less constant bedtime and wake time and more activity during the night, a pattern that was aggravated at cycle 4 where the contrast between daytime and nighttime is less clear, particularly during week 1. Red bars = missing data (off wrist).
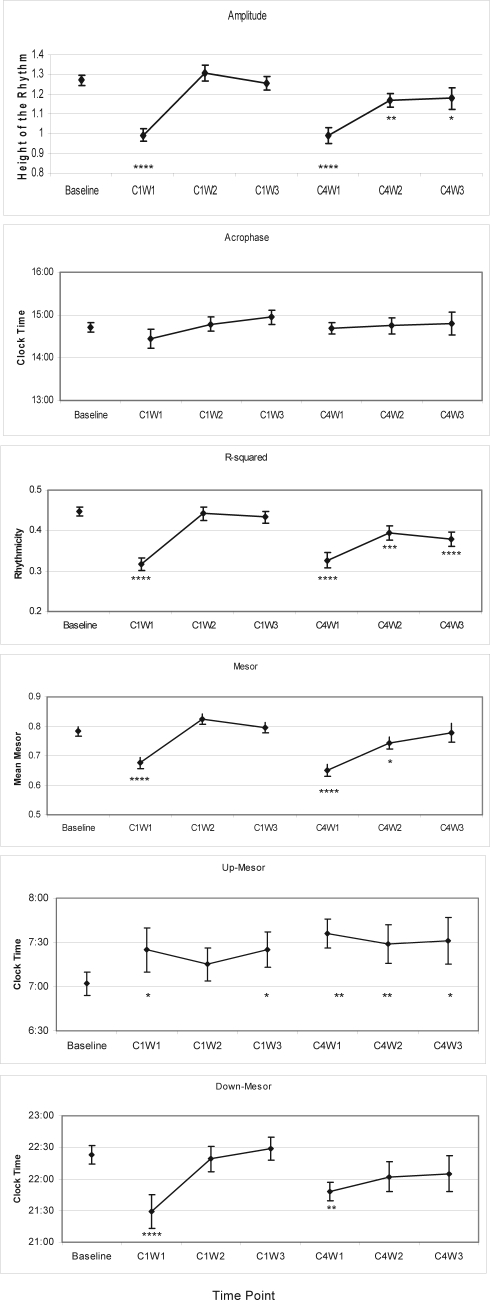
Figure 3a-f illustrates the plots of circadian rhythm variable means (and standard errors) over time. A priori contrasts showed that all circadian rhythm variables (except acrophase) were significantly more impaired at C1W1 than at baseline, i.e., cycles were less rhythmic (lower amplitude, lower R-squared, lower mesor). Moreover, the participants switched from low to high activity later in the day (increased up-mesor of about 30 min) while decreasing their level of activity earlier during the night (reduced down-mesor of about 50 min), suggesting that their days were shorter. Except for up-mesor, there were no significant differences between baseline and C1W2 and C1W3, thus indicating that most variables returned to baseline levels at those time points of cycle 1.
Figure 3.
Means (and standard errors) obtained on each circadian rhythm variable, at each time assessment. *P < 0.05; **P < 0.01; ***P < 0.001; ***P < 0.0001 for comparisons between each time point vs. baseline.
Significant differences were also found between C4W1 and baseline on all circadian rhythms variables, except acrophase. Again, the cycles were less rhythmic (lower amplitude, lower R-squared, lower mesor) and the women increased their level of activity later in the day (increased up-mesor of about 37 min), while switching from high to low activity earlier at night (reduced down-mesor of about 34 min). However, in contrast with data of cycle 1, these impairments were maintained on several variables at C4W2 and C4W3, particularly for differences between baseline and C4W2 (amplitude, mesor, up-mesor, and R-squared) and between baseline and C4W3 (amplitude, up-mesor, and R-squared).
DISCUSSION
The results of this study suggest that the sleep-wake activity cycles of breast cancer patients are impaired during the first week of each chemotherapy cycle (the week of chemotherapy administration), and get progressively worse with each cycle of treatment. During weeks 2 and 3 of cycle 1, the rhythm approached the normal values seen at baseline. However, during weeks 2 and 3 of cycle 4, values remained significantly more impaired than baseline. Together, these findings suggest that a first administration of chemotherapy is associated with transient impairments in circadian rhythms, whereas a repeated administration of chemotherapy is associated with enduring impairments.
To our knowledge, this is the first study to longitudinally assess the evolution of circadian rhythms of cancer patients prior to and during chemotherapy. These study findings are nonetheless consistent with previous cross-sectional studies showing that sleep quality is impaired,/?/3.4/?/ and indicating circadian disruption prior to chemotherapy in breast cancer patients.10,11 This longitudinal study suggests that these pre-treatment impairments are further aggravated with the administration of chemotherapy.
There are several potential negative consequences associated with impaired circadian rhythms. Studies have found that cancer patients with disturbed sleep-wake cycles report higher levels of fatigue and decreased quality of life.16–19 In the specific context of breast cancer, Ancoli-Israel et al.10 and Berger et al.11 both found that women with more phase-delayed rhythms experienced more daily dysfunctions. A study conducted in patients with metastatic colorectal cancer showed that patients with marked activity rhythms (i.e., greater activity out of bed than in bed) not only had a better quality of life and less reported fatigue,18 but also a 5-fold higher survival at 2-year follow-up than those with less synchronized rhythms.20 There is also indirect evidence that disruptions in circadian rhythms may be associated with increased cancer mortality.21 Cancer patients display some abnormalities in the variations of cortisol levels (e.g., flattened cortisol slope) throughout the 24-h cycle which have been associated with reduced immune functioning and increased mortality in metastatic breast cancer patients,22 after controlling for other known predictors.
This study is characterized by several strengths including the utilization of a longitudinal design, of actigraphy recording at each time assessment, and of mixed-model analyses that allow appropriate management of missing data. There are also some limitations. First, because this study did not include measures during the second and third cycle of chemotherapy, it is unclear whether the impairments observed became chronic only at the fourth cycle or gradually worsened with each successive cycle. Another limitation is that in order to decrease patient burden, actigraphs were worn for only 72 h rather than the preferred duration of 5-7 days. Although more days are better, reviews of actigraphy generally recommend a minimum of 3 days for determining circadian rhythm variables.12 Another limitation was the heterogeneity of chemotherapy regimens received by the study participants, although our preliminary analyses did not show significant differences on any of the circadian rhythm variables across them. Finally, although actigraphic data strongly reflect sleep/wake cycles in healthy individuals, and data suggest that actigraphy can reliably estimate sleep-wake in women with breast cancer, more research is needed to understand how reliable actigraphy is in cancer patients.12,10,23,11,18
Further studies are needed to understand the mechanisms through which chemotherapy may contribute to these impairments in sleep-wake activity, a goal that was beyond the scope of this study. Potential mechanisms include psychological (e.g., worries, depression) and behavioral factors (e.g., increased daytime napping) as well as physiological factors such as physical symptoms (e.g., fatigue, nausea), decreased levels of estrogens, impaired cortisol responses and inflammation.2,24 Mills et al. previously reported that anthracycline chemotherapy leads to a generalized and progressive (with cycles of chemotherapy) increase in inflammation that could negatively influence sleep.25 The same mechanism may contribute to changes in circadian rhythms.
Longer follow-ups are also warranted to assess how the circadian rhythm variables evolve with the cessation of chemotherapy and the initiation of other adjuvant treatments such as radiation therapy and hormone therapy. In the meanwhile, it would be important to screen more routinely for sleep and circadian disruptions in breast cancer patients undergoing chemotherapy and to offer appropriate management, such as cognitive-behavioral therapy or light treatment, in order to prevent these disturbances from becoming chronic with their resulting potential negative consequences.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ancoli-Israel has received research support from Sepracor, Takeda, Litebook, Inc.; has consulted for or been on the advisory board of Arena, Acadia, Cephalon, Ferring, Orphagen Pharmaceuticals, Pfizer, Sanofi-Aventis, Sepracor, Somaxin, and Takeda; and has received discounted equipment from Litebook, Inc. and Sepracor. Dr. Dimsdale has received research support from and consulted for Sepracor. Dr. Parker has received research support from GlaxoSmithKline. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Supported by NCI CA112035, NCI CA85264, NIH M01 RR00827, Moores UCSD Cancer Center, the Research Service of the VASDHS and the Fonds de la recherche en santé du Québec. We would like to thank Sherella Johnson, Sue Lawson, and the women who volunteered their time for the study.
Parts of this manuscript were presented at the 6th annual conference of the American Psychosocial Oncology Society, Charlotte, North Carolina, USA, February 2009.
REFERENCES
- 1.Fiorentino L, Ancoli-Israel S. Sleep dysfunction in patients with cancer. Curr Treat Options Neurol. 2007;9:337–46. [PMC free article] [PubMed] [Google Scholar]
- 2.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 3.Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54:1309–21. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 4.Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.21.6333. in press. [DOI] [PubMed] [Google Scholar]
- 5.Chevalier V, Mormont MC, Cure H, Chollet P. Assessment of circadian rhythms by actimetry in healthy subjects and patients with advanced colorectal cancer. Oncol Rep. 2003;10:733–7. [PubMed] [Google Scholar]
- 6.Fernandes R, Stone P, Andrews P, Morgan R, Sharma S. Comparison between fatigue, sleep disturbance, and circadian rhythm in cancer inpatients and healthy volunteers: evaluation of diagnostic criteria for cancer-related fatigue. J Pain Symptom Manage. 2006;32:245–54. doi: 10.1016/j.jpainsymman.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Levin RD, Daehler MA, Grutsch JF, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. 2005;93:1202–8. doi: 10.1038/sj.bjc.6602859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pati AK, Parganiha A, Kar A, Soni R, Roy S, Choudhary V. Alterations of the characteristics of the circadian rest-activity rhythm of cancer in-patients. Chronobiol Int. 2007;24:1179–97. doi: 10.1080/07420520701800868. [DOI] [PubMed] [Google Scholar]
- 9.Parker KP, Bliwise DL, Ribeiro M, et al. Sleep/wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26:2464–72. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Liu L, Marler M, et al. Fatigue, sleep and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–9. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ancoli-Israel S, Cole R, Alessi CA, Chambers M, Moorcroft WH, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 13.Marler MR, Martin JL, Gehrman PR, Ancoli-Israel S. The sigmoidally-transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25:3893–904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Marler M, Parker BA, et al. The relationship between fatigue and light exposure during chemotherapy. Support Care Cancer. 2005;13:1010–7. doi: 10.1007/s00520-005-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford: Clarendon Press, ; 1996. [Google Scholar]
- 16.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- 17.Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 1999;26:1663–71. [PubMed] [Google Scholar]
- 18.Mormont MC, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int. 2002;19:313–23. doi: 10.1081/cbi-120002606. [DOI] [PubMed] [Google Scholar]
- 19.Roscoe JA, Morrow GR, Hickok JT, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10:329–36. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 20.Mormont MC, Waterhouse J, Bleuzen P, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Res. 2000;6:3038–45. [PubMed] [Google Scholar]
- 21.Spiegel D. Losing sleep over cancer. J Clin Oncol. 2008;26:2431–2. doi: 10.1200/JCO.2008.16.2008. [DOI] [PubMed] [Google Scholar]
- 22.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 23.Mormont MC, De Prins J, Levi F. Study of circadian rhythms of activity by actometry: preliminary results in 30 patients with metastatic colorectal cancer. Path Biologie. 1996;44:165–71. [PubMed] [Google Scholar]
- 24.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–82. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills PJ, Parker BA, Jones V, et al. The effects of standard anthracycline-based chemotherapy on soluble icam-1 and VEFG levels in breast cancer. Clin Cancer Res. 2004;10:4998–5003. doi: 10.1158/1078-0432.CCR-0734-04. [DOI] [PubMed] [Google Scholar]