Abstract
Study Objectives:
Obstructive sleep apnea syndrome (OSAS) is associated with cognitive and functional deficits, most of which are corrected after positive airway pressure (PAP) treatment. Previous studies investigating the neural underpinnings of OSAS failed to provide consistent results both on the cerebral substrates underlying cognitive deficits and on the effect of treatment on these anomalies. The aims of the study were a) to investigate whether never-treated OSA patients demonstrated differences in brain activation compared to healthy controls during a cognitive task; and b) to investigate whether any improvements in cognitive functioning found in OSA patients after treatment reflected a change in the underlying cerebral activity.
Design:
OSA patients and healthy controls underwent functional magnetic resonance imaging (fMRI) scanning. They were compared on performance and brain activation during a 2-back working-memory task. Patients were also re-evaluated after 3 months treatment with PAP. Cognitive functions were evaluated using neurocognitive tests. Sleepiness (ESS), mood (Beck Depression Inventory) and, quality-of-life (SF-36) were also assessed.
Setting:
The Sleep Disorders Center and CERMAC at the Vita-Salute San Raffaele University.
Patients or Participants:
17 OSA patients and 15 age- and education-matched healthy controls.
Interventions:
PAP treatment for 3 months.
Measurements and Results:
Compared to controls, never-treated OSA patients showed increased activations in the left frontal cortex, medial precuneus, and hippocampus, and decreased activations in the caudal pons. OSA patients showed decreases in activation with treatment in the left inferior frontal gyrus and anterior cingulate cortex, and bilaterally in the hippocampus. Most neurocognitive domains, impaired at baseline, showed significant improvement after treatment.
Conclusions:
OSA patients showed an overrecruitment of brain regions compared to controls, in the presence of the same level of performance on a working-memory task. Decreases of activation in prefrontal and hippocampal structures were observed after treatment in comparison to baseline. These findings may reflect a neural compensation mechanism in never-treated patients, which is reduced by effective treatment.
Citation:
Castronovo V; Canessa N; Ferini Strambi L; Aloia MS; Consonni M; Marelli S; Iadanza A; BruschiA; Falini A; Cappa SF. Brain activation changes before and after PAP treatment in obstructive sleep apnea. SLEEP 2009;32(9):1161-1172.
Keywords: OSA, fMRI, PAP treatment, working memory, compensatory recruitment
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON CLINICAL SLEEP DISORDER THAT AFFECTS AT LEAST 2% TO 4% OF MIDDLE-AGED WOMEN AND MEN.1 Relatively increased prevalence rates have been reported in older adults2,3 and African Americans.4,5 OSA is clinically characterized by chronically fragmented sleep and intermittent hypoxemia, defined as repeated episodes of oxygen desaturation that alternate with episodes of reoxygenation. OSA is recognized as a significant health problem with neurocognitive and cardiovascular morbidities. The disorder is associated with a range of significant medical and psychological consequences, including obesity, hypertension, increased risk for vascular disease, depression, and excessive daytime sleepiness.6–12 The precise mechanisms responsible for the cognitive and psychological consequences associated with OSA are still unknown. Sleep fragmentation may have detrimental effects on daytime functioning as a result of excessive daytime sleepiness.13 Lanfranchi and Somers14 have suggested that OSA-related hypoxemia results in changes to the structure and function of the brain vessels, which can adversely affect cognitive function and contribute to both morbidity and mortality.8,15
The use of neuroimaging methodologies can contribute to our understanding of brain structure and function in individuals with OSA. Neuroimaging studies may aid in the identification of those OSA individuals at greatest risk for poor outcome by examining relationships between brain integrity and functional response to treatment. These results may subsequently serve as a potent clinical motivator for OSA individuals struggling with treatment adherence.
Two reviews of neuroimaging in OSA have been published to date.15,16 The results are largely inconsistent because of methodological and sample variability. The most consistent findings have come from magnetic resonance spectroscopy (MRS) studies, which have shown changes in the frontal white matter.15 The results of structural studies are also heterogeneous, although hippocampal involvement is frequently reported.17,18
Functional imaging studies utilizing cognitive tasks have resulted either in reduced activation of the dorsolateral prefrontal cortex (DLPFC), or in increased neural response in frontal lobe, cingulate, thalamus, cerebellum, and temporoparietal junction, depending on the cognitive task employed. In particular, Thomas et al.19 compared 16 untreated OSA patients with 16 matched controls. Decreased DLPFC activation was demonstrated in the OSA patients during a working-memory challenge (2-back task) accompanied by a reduction in 2-back performance in reaction times (poorer overall performance on the 2-back task) compared to controls. In the 6 of the patients followed after successful treatment activation increases were observed in posterior parietal areas. Ayalon et al.20 compared untreated OSA patients to matched controls on a verbal learning task, and demonstrated an over-recruitment of brain regions in the OSA group with no differences in performance. The overactivation was attributed to compensatory mechanisms, supporting a relatively unimpaired level of performance.
To date, there are no reported functional MR studies comparing brain activation during a cognitive task in OSA patients and normal controls followed by a posttreatment study of the entire group. The current study was designed to examine the cerebral substrates underlying a working memory task in OSA patients before and after treatment compared to healthy controls in order to fill this gap in the existing literature.
METHODS
Participants
Seventeen male never-treated OSA patients (mean age = 43.93, SD = 7.78, mean education level = 12.57, SD = 2.71) and 15 male age- and education matched healthy controls (mean age = 42.15, SD = 6.64, mean education level = 13.23, SD = 3.09) were recruited. All participants were right-handed21 monolingual native speakers of Italian, and had normal or corrected-to-normal visual acuity. Participants had no evidence of stroke, neurological disorder, dementia, major psychiatric disorder, uncontrolled hypertension (> 100/160), respiratory failure and had no current use of any psychoactive medications. Inclusion criteria for OSA patients were: (a) diagnosis of severe OSA (apnea/hypopnea index [AHI] > 30), and (b) age between 30 and 55 years.
Healthy controls had an AHI < 5. Moreover Restless Legs Syndrome and Periodic Limb Movements were ruled out by a structured sleep interview performed by a sleep specialist; insomnia was excluded by 1-week sleep diary prior to inclusion in the study. Participants were excluded if they demonstrated: (a) symptoms of cognitive deterioration (as indicated by a score at Mini-Mental below 24), or (b) brain structural abnormalities, as shown by evaluation of MR images by an experienced neuroradiologist. All participants including healthy controls reported regular sleep-wake schedules based on daily sleep diaries with an average TST of 6.9 ± 1.1 h in the 4 days prior the study. All participants provided their written informed consent to the experimental procedure, which was previously approved by the local ethics committee. All patients were evaluated at baseline (BL) and after 3 months of treatment (positive airway pressure [PAP] with C-Flex). Full nocturnal polysomnography (PSG), functional magnetic resonance imaging (fMRI), neurocognitive functioning (attention, memory and executive function), sleepiness (ESS), mood (BDI) and quality of life (SF-36) were assessed at both time points. Healthy controls were only evaluated at BL. One OSA patient dropped out (for low adherence to PAP treatment) and 3 participants (2 patients and one control) were excluded from the final analysis due to excessive head movements during scanning (> 4 mm).
Polysomnography
All OSA patients underwent PSG the night before functional scanning. Apnea events were defined, based on PSG, as a ≥ 80% drop of respiratory amplitude, lasting at least 10s. Hypopneas were defined as a 50% drop of respiratory amplitude, lasting ≥ 10s, associated with repeated respiratory effort and arousals or oxygen saturation drops of ≥ 3%. The AHI was defined as an index of the number of apnea and hypopnea events per hour of sleep. Time of oxygen saturation (SpO2) below 90% during total sleep, the lowest nocturnal oxygen saturation (SpO2) value and the mean of the lowest peaks of SpO2 were also recorded.
Neuropsychological Evaluation
Both OSA and control participants underwent a brief neuropsychological evaluation, that included Rey list recall (learning, recall, and recognition memory), Stroop color-word interference test (executive functions: inhibition, selective attention), Paced Auditory Serial Addition Test (PASAT; vigilance and executive functions) (for references, see22). Administration of the neuropsychological test battery lasted approximately 30 min. An alternative equivalent version of the Rey list learning test was employed to avoid learning effects at follow-up.
In addition to the neuropsychological tests, participants were given the self-report Epworth Sleepiness Scale (ESS) to evaluate the subjective daytime somnolence, the Beck Inventory to evaluate mood and Quality of Life (SF-36) to assess overall quality of life. Tests were administered and scored according to the published procedures.22
Positive Airway Pressure (PAP) Treatment
All patients were fully adherent to manual titration night of PAP. They were all sent home with fixed PAP with C-Flex™ for 3 months (Philips Respironics, M series). Adherence at home was objectively reported by Encore software and patients with compliance lower than 4 hours/night and < 80% of days of usage were excluded (n = 1).
Working Memory Task During Functional Scanning
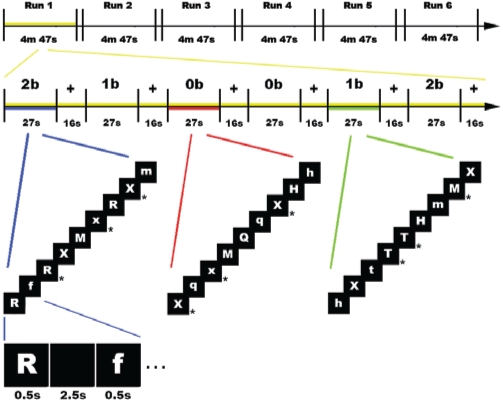
Participants performed a verbal version of the n-back task,23–25 a classical test of working-memory.26,27 In this task, the participant is required to monitor a series of stimuli and to respond whenever a stimulus (henceforth the “target” stimulus) is presented that is the same as the one presented n trials previously, where n is a pre-specified integer, usually 1, 2, or 3. Since the stimuli appear continuously, the task requires to temporarily store each of them in memory for evaluation (based on the contingent task), and to discard it before the appearance of the next one. In the current study, stimuli were pseudo-random sequences of letters, and three conditions were used that varied WM-load incrementally from zero to two items. In the 0-back (0b) condition, participants responded to a single pre-specified target letter (“X”). In the 1-back (1b) condition, the target was any letter identical to the one immediately preceding it (i.e., one trial back). In the 2-back (2b) condition, the target was any letter that was identical to the one presented 2 trials back (Figure 1). Thus, WM load (storage and manipulation demands) increased incrementally from the 0b to the 2b condition.
Figure 1.
Experimental design. From top to bottom, a schematic depiction of the experimental design is shown. The study was composed of 6 functional runs, each lasting 4 m 47 s (top). The first run, in yellow, is enlarged below, to highlight its structure. Every run included 2 blocked repetitions of each task (27 s) in a pseudo-random order, separated by a rest period (fixation of a white cross; 16 s) (middle; the 3 tasks are depicted in blue, red and green colors). The colored lines link highlighted tasks with a graphical description of the sequence of stimuli in each of them (bottom). Each sequence included 9 letters, 3 of which (33%, indicated by an asterisk at the right of the corresponding panel) were “targets.” As shown in the inferior-most part of the figure, each letter within the sequence was shown for 0.5 s, and was separated by the onset of the next one by a black screen lasting 2.5 s.
Experimental Design and Procedure
Every participant underwent one scanning session, composed by 6 functional runs, each lasting 4 min 47 s. A blocked design for the presentation of the stimuli was used, with every run including 2 blocked repetitions of each task (0b, 1b, 2b) in a pseudo-random order (Figure 1). Blocks lasted 27 s, and started with a screen displaying the instructions (2.4 s). Each block included a sequence of 9 letters, of which 3 (33%) were targets. Both stimuli and instructions were depicted in white font on a black background. Stimuli were successively presented in the center of the screen for 0.5 s, and were separated by a 2.5-s black-screen interval. Participants responded to target letters by pressing one button on a keyboard with their right index finger. Successive blocks were separated by a baseline control block (white fixation-cross) lasting 16 s, to allow the cerebral response to return to baseline level. The order of the functional runs was individually randomized for every participant.
Visual stimuli were viewed via a back-projection screen located in front of the scanner and a mirror placed on the head-coil. The software Presentation 11.0 (Neurobehavioral systems, Albany, CA, http://www.neurobs.com) was used for both stimulus presentation and subjects' answers recording. All participants underwent a training session before fMRI scanning to ensure accurate performance of the task.
fMRI Data Acquisition
Anatomical T1-weighted and functional T2*-weighted MR images were acquired with a 3 Tesla Philips Achieva scanner (Philips Medical Systems, Best, NL), using an 8-channel Sense head coil (sense reduction factor = 2). Functional images were acquired using a T2*-weighted gradient-echo, echo-planar (EPI) pulse sequence (30 interleaved slices parallel to the AC-PC line, covering the whole brain, TR = 1700 ms, TE = 30 ms, flip angle = 85 degrees, FOV = 240 mm × 240 mm, no gap, slice thickness = 4 mm, in-plane resolution 2 mm × 2 mm). Each scanning sequence comprised 167 sequential volumes. Immediately after the functional scanning a high-resolution T1-weighted anatomical scan (3D, SPGR sequence, 150 slices, TR = 600 ms, TE = 20 ms, slice thickness = 1 mm, in-plane resolution 1 mm × 1 mm) was acquired for each subject.
fMRI Data Preprocessing and Statistical Analysis
Image preprocessing28 and statistical analysis were performed using SPM5 (Wellcome Department of Cognitive Neurology, http://www.fil.ion.ucl.ac.uk/spm), implemented in Matlab v7.4 (Mathworks, Inc., Sherborn, MA). Preliminary to analyses, the structural MR images from all subjects were inspected by an experienced neuroradiologist. In line with previous imaging studies of working memory,29 we opted for a parametric analysis of the data to identify cerebral regions where activity showed a positive linear relationship (i.e., a linear increase) with increasing WM load. The reason behind this procedure is that parametric analyses allow to overcome some of the well-known limitations of the classical “subtractive” approach.30 The latter, in fact, assumes that the cognitive processes involved in the baseline task (that are subtracted out from those of interest, highlighted by “active” tasks) are performed in the same way, and in the same cerebral regions, in different tasks and/or different participants. In previous studies of working-memory the parametric approach has been implemented to explore cerebral regions where activity was positively and linearly correlated with WM load both in healthy individuals29 and clinical populations.31 In the present study, such an approach was used to investigate the cerebral regions where an increased intensity of brain activation related to WM-load showed significant differences, as well as commonalities, across (a) healthy controls and pretreatment OSA patients, (b) pretreatment and posttreatment OSA patients, and (c) controls and posttreatment OSA patients.
For all participants, the first 5 volumes of every run were discarded to allow for T1 equilibration effects. All volumes were then spatially realigned to the first volume of the first run to correct for between-scan motion32 and unwarped,33 and a mean-image from the realigned volumes was created. This image was spatially normalized to the Montreal Neurological Institute (MNI152) brain template using a 12-parameter affine normalization and 16 nonlinear iterations with 7 × 9 × 7 basis functions.34 The derived spatial transformations were then applied to the realigned-and-unwarped T2*-weighted volumes, that were resampled in 2 × 2 × 2 mm3 voxels after normalization. All functional volumes were then spatially smoothed with an 8-mm full-width half-maximum (FWHM) isotropic Gaussian kernel to compensate for residual between-subject variability after spatial normalization, and globally scaled to 100. The resulting time series across each voxel were then high-pass filtered to 1/128 Hz, and serial autocorrelations were modeled as an Auto Regressive AR(1) process.
Statistical maps were generated using a random-effect model, implemented in a 2-level procedure.35 At the first level, all the 6 blocks within each run were modeled in a single generic regressor (“task”), and one additional regressor modeled a linear parametric modulation of the task-related activity by WM-load (0, 1, 2). The regressors were then convolved with a canonical hemodynamic response function (HRF), and the corresponding parameter estimates were obtained at each voxel by maximum-likelihood estimation. Contrasts of parameter estimates were then calculated to produce a “contrast images” for the parametric regressor of interest for each subject. The inter-blocks cross-fixation periods served as an implicit baseline level.
In order to investigate the cerebral regions where activity showed a linear increase related with task difficulty, at the second (group) level the statistical analyses were performed on the first-level “contrast-images” of the linear parametric modulation by WM-load. These images were first entered into one sample t-tests to highlight WM parametric effects on cerebral activity separately in the single groups of subjects (controls, pretreatment OSA, posttreatment OSA). Conjunction analyses were then performed, using an inclusive masking procedure, to highlight cerebral regions showing common parametric effects of WM-load in a) controls and pretreatment OSA patients, b) pretreatment OSA patients and posttreatment OSA patients, and c) controls and posttreatment OSA patients. The resulting statistical maps were thresholded at P < 0.05 family-wise error [FWE] corrected for multiple comparisons. Finally, 2-sample t-tests and a paired-sample t-test were used to investigate the regions showing significantly different parametric effects of WM-load in a) controls vs. pretreatment OSA patients, b) pretreatment OSA patients vs. posttreatment OSA patients, and c) controls vs. posttreatment OSA patients. In all direct-comparisons analyses, the resulting statistical maps were masked at P < 0.001 uncorrected by those of the conjunction analyses either (a) inclusively, to highlight the regions showing significant differences between the two groups within those commonly activated and (b) exclusively, to highlight the regions showing significant differences between them outside those commonly activated. The statistical maps for direct comparisons were thresholded at P < 0.001 uncorrected for multiple comparisons, and only clusters larger than 4 voxels were reported.
In order to investigate the relationship between the reduction in cerebral activity and the improvement in cognitive functioning following treatment, we performed analyses on specified regions of interest (ROIs). We used the SPM-toolbox Marsbar36 to create ROIs that were defined as 4-mm radius spheres centered on the foci of maximum activation in the regions of significant reduction of activity after treatment. We then extracted from these ROIs the contrast values for the pre- and posttreatment parametric statistical maps of increasing WM-load. For each ROI, the resulting pre- and posttreatment contrast values were subtracted (pre minus post) in order to obtain a measure of the reduction in cerebral activity from pre- to posttreatment in any given region. Therefore, a positive number represents a decrease in activation. A similar procedure was applied to the pre- and posttreatment neuropsychological scores shown in Tables 2 and 7, to obtain an index of behavioral change following PAP. A positive number represents a decrease in the relative neuropsychological score. These 2 measures were then entered in a simple correlation test (Pearson correlation coefficient).
Table 2.
Results of OSA Patients Versus Controls at BL and OSA Pre-Post Treatment Comparisons
| Test | Control BL (n = 14) | Patients BL (n = 14) | Patients at 3-Months (n = 14) | P* |
|---|---|---|---|---|
| Rey's List (learning) | 58.00 ± 7.01 | 48.70 ± 9.67 | 58.19 ± 7.93 | 0.005, 0.001 |
| Rey's List (recall) | 13.00 ± 1.96 | 10.59 ± 2.48 | 13.13 ± 2.25 | 0.003, 0.002 |
| PASAT Error | 5.13 ± 3.58 | 21.53 ± 10.07 | 7.56 ± 7.15 | 0.000, 0.001 |
| Stroop Test | 23.07 ± 8.14 | 39.12 ± 21.88 | 34.73 ± 17.58 | 0.008, n.s. |
| Stroop Test Error | 0.73 ± 1.03 | 5.31 ± 3.57 | 0.87 ± 1.35 | 0.000, 0.001 |
| ESS | 3.00 ± 1.25 | 11.94 ± 5.47 | 2.81 ± 2.79 | 0.000, 0.000 |
| BDI | 1.46 ± 2.16 | 3.76 ± 3.94 | 1.75 ± 2.95 | 0.013, 0.013 |
| SF-36 (total score) | 80.89 ± 9.38 | 68.9 ± 19.72 | 80.36 ± 19.31 | 0.027, 0.005 |
First P-value is for comparison between patients and controls at BL. Second P-value represents the comparison between BL and 3-month in patients only. n.s. = non significant
Table 7.
Results of the Difference Between Pre- and Posttreatment Cognitive Scores, and Correlations of Cognitive Change with Change in Activation after Treatment
| ESS | Rey list learning | Rey recall | PASAT errors | Stroop time | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Mean | 11.94 | 48.70 | 10.59 | 21.53 | 39.12 | ||
| SD | 5.47 | 9.67 | 2.48 | 10.07 | 21.88 | |||
| Post | Mean | 2.81 | 58.19 | 13.13 | 7.56 | 34.73 | ||
| SD | 2.79 | 7.93 | 2.25 | 7.15 | 17.58 | |||
| Region | x | y | z | |||||
| L Hippocampus | −32 | −18 | −16 | −0.08 | −0.25 | 0.03 | 0.70 | 0.10 |
| P-value | 0.79 | 0.39 | 0.93 | 0.01* | 0.74 | |||
| R Hippocampus | 42 | −20 | −24 | −0.32 | 0.16 | −0.12 | 0.23 | 0.26 |
| P-value | 0.27 | 0.59 | 0.68 | 0.42 | 0.36 | |||
| R Hippocampus | 38 | −14 | −30 | 0.08 | 0.40 | −0.26 | 0.29 | −0.30 |
| P-value | 0.79 | 0.16 | 0.38 | 0.31 | 0.30 | |||
| R Hippocampus | 30 | −16 | −34 | −0.42 | 0.33 | −0.32 | 0.61 | −0.14 |
| P-value | 0.13 | 0.24 | 0.27 | 0.02* | 0.64 | |||
| RSMA | 10 | −26 | 50 | 0.02 | −0.26 | 0.09 | −0.16 | 0.53 |
| P-value | 0.96 | 0.37 | 0.77 | 0.59 | 0.05* | |||
| L IFG p. triangularis | −40 | 36 | 4 | 0.12 | −0.36 | −0.45 | 0.00 | 0.56 |
| P-value | 0.68 | 0.20 | 0.11 | 0.99 | 0.04* | |||
| L/R ACC | −2 | 24 | 18 | −0.17 | −0.32 | −0.18 | 0.47 | 0.51 |
| P-value | 0.56 | 0.28 | 0.53 | 0.09 | 0.07 |
Note. Positive correlations reflect a reduction in both measures. SMA = supplementary motor area, ACC = anterior cingulate cortex, IFG p. triangularis = inferior frontal gyrus, pars triangularis. L = left, R = Right, ESS = Epworth Sleepiness Scale, PASAT = Paced Auditory Serial Addition Task.
The location of the activation foci in terms of Brodmann areas (BAs) was determined in the stereotaxic space of Talairach and Tournoux37 after correcting for differences between the latter and the MNI coordinate systems by means of a nonlinear transformation (see http://www.mrccbu.cam.ac.uk/Imaging/Common/mnispace.shtml). Those cerebral regions for which maps were provided were also localized with reference to cytoarchitectonic probabilistic maps of the human brain, using the SPM Anatomy toolbox.38
Statistical analyses on behavioral data during scanning were performed using SPSS 11.0. Nonparametric Mann-Whitney U Tests were employed to evaluate differences between OSA patients and normal controls (BL) and nonparametric Wilcoxon Tests were used to assess treatment effects (BL versus 3 months).
RESULTS
Results of group comparisons (OSA patients versus healthy controls at BL) on key sample characteristics are shown in Table 1, whereas results of within-subjects analyses (OSA patients at BL versus 3 months) on outcome variables are shown in Table 2. The groups were similar on all measures except BMI, sleepiness (OSA patients higher than controls), and all cognitive measures (OSA patients lower than controls). Follow-up demonstrated significant improvement in sleepiness and all cognitive tests (except total time on the Stroop test) with treatment. Also mood (BDI) significantly improved after treatment, even if always in normal ranges (3.76 ± 3.94 vs 1.75 ± 2.95; P = 0.013), as well as quality of life (SF-36) (68.91 ± 19.72 vs 80.36 ± 15.09; P = 0.005).
Table 1.
Demographic and Severity Characteristics of Patients and Controls
| Control BL (n = 14) | Patients BL (n = 14) | P* | |
|---|---|---|---|
| Age (y) | 42.15 ± 6.64 | 43.93 ± 7.78 | n.s. |
| Education (y) | 13.23 ± 3.09 | 12.57 ± 2.71 | n.s. |
| BMI (kg/m2) | 26.10 ± 2.50 | 30.29 ± 4.76 | 0.01 |
| AHI | — | 50.14 ± 24.84 | — |
| Mean SpO2 | — | 89.09 ± 5.62 | — |
| Time SpO2 below 90% (min.) | — | 26.19 ± 23.16 | — |
| PAP use (min/night) | — | 349.36 ± 34.15 | — |
n.s. = non significant
Behavioral Performance During Scanning
Participants' performance on the n-back task during scanning was evaluated by means of 3 different statistical analyses, considering: (a) the number of wrong responses, (b) the number of missed responses, and (c) the response time (Table 3). Due to technical problems with the response recording system, it was not possible to evaluate the behavioral performance of 2 follow-up participants. In all the analyses no significant difference was observed either between controls and pretreatment OSA patients, or between the latter and posttreatment OSA patients, in any of the three experimental conditions (0b, 1b, 2b; Table 3).
Table 3.
Results for Behavioral Performance at the n-back Task During Functional Scanning
| Behavioral Measure | Control (n = 14) | Patients at BL (n = 14) | Patients at 3 months (n = 14) | P* |
|---|---|---|---|---|
| 0-Back | ||||
| Number of Errors (/36) | 0.27 ± 0.46 | 0.64 ± 0.93 | 0.92 ± 1.08 | n.s., n.s. |
| Missed Responses (/36) | 0.20 ± 0.56 | 0.57 ± 1.09 | 0.25 ± 0.62 | n.s., n.s. |
| Response Time | 0.55 ± 0.17 | 0.51 ± 0.12 | 0.51 ± 0.14 | n.s., n.s. |
| 1-Back | ||||
| Number of Errors (/36) | 0.40 ± 0.63 | 0.57 ± 1.40 | 0.50 ± 0.91 | n.s, n.s |
| Missed Responses (/36) | 0.67 ± 1.23 | 1.29 ± 1.73 | 0.00 ± 0.00 | n.s., 0.06 |
| Response Time (s) | 0.57 ± 0.18 | 0.54 ± 0.13 | 0.54 ± 0.15 | n.s., n.s. |
| 2-Back | ||||
| Number of Errors (/36) | 1.73 ± 1.49 | 2.50 ± 2.07 | 2.25 ± 1.66 | n.s., n.s |
| Missed Responses (/36) | 2.60 ± 3.30 | 3.57 ± 3.58 | 1.50 ± 1.44 | n.s., n.s. |
| Response Time | 0.58 ± 0.17 | 0.60 ± 0.18 | 0.52 ± 0.13 | n.s., n.s. |
First P-value is for comparison between patients and controls at BL. Second P-value represents the comparison between BL and 3-month in patients only. n.s. = non significant
Imaging Results
Pretreatment Analyses: Controls vs. Pretreatment OSA Patients
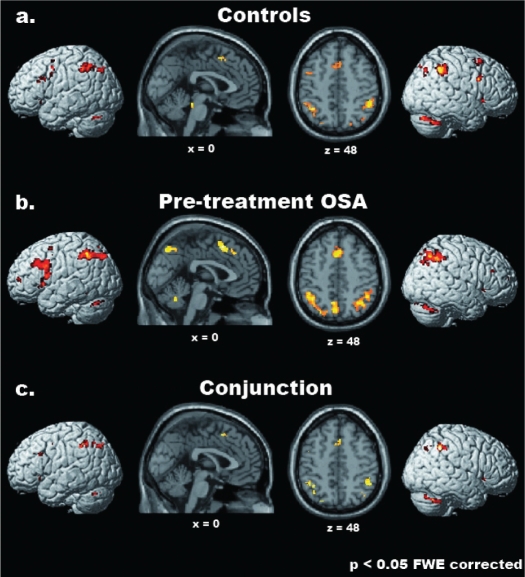
The statistical maps associated with the parametric effects of increasing WM-load in control subjects and pretreatment OSA patients showed a largely overlapping cerebral network in the 2 groups (see Table 4 and Figures 2a and b; see also Tables S1, S2 for the complete list of the activated foci in the 2 groups separately). Such commonalities were further highlighted by the results of the conjunction analysis between the 2 groups (Table 4 and Figure 2c). Commonly activated regions were noted within a widespread frontolateral-frontomedial-parietal network, which included the superior and inferior parietal lobule bilaterally (BAs 7,40), the supplementary motor area (SMA, 6) in the medial wall, as well as the left precentral gyrus (BA 6) and fronto-insular cortex (pars triangularis of the inferior frontal gyrus [IFG; BA 45] and the anterior insula). The latter region, extending into the caudal portion of the pars orbitalis in the IFG (BA 47), was activated also in the right hemisphere. Further common activations were observed bilaterally in the cerebellum, as well as in the left thalamus.
Table 4.
Parametric Effects of WM-load: Conjunction Controls and Pre-Treatment OSA Patients
| H | Anatomical region (BA) Controls and Pre-treatment OSA | ATp | K | Cluster labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| L | Superior parietal lobule (7) | 46 | −24 | −76 | 46 | 5.87 | ||
| Inferior parietal lobule (7) | −32 | −70 | 44 | 5.29 | ||||
| L | Superior parietal lobule (7/39) | 13 | −36 | −62 | 48 | 5.57 | ||
| L | Inferior parietal lobule (40) | 108 | 26.9% in L hIP1 | −34 | −50 | 44 | 6.46 | |
| Inferior parietal lobule (hIP1*) | 50* | 13.8% in L hIP2 | −40 | −48 | 46 | 5.63 | ||
| Inferior parietal lobule (hIP2*) | 60* | −48 | −44 | 44 | 5.24 | |||
| R | Superior occipital gyrus (7) | 9 | 32 | −68 | 42 | 5.42 | ||
| R | Inferior parietal lobule (hIP1*) | 40* | 129 | 19.9% in R hIP1 | 38 | −46 | 40 | 6.44 |
| Inferior parietal lobule (40) | 10.4% in R hIP2 | 50 | −50 | 46 | 6.28 | |||
| L/R | SMA (6*) | 20* | 46 | 41.3% in L area 6 | −2 | 12 | 50 | 5.62 |
| SMA (6/32) | 13.6% in R area 6 | 4 | 20 | 48 | 5.43 | |||
| L | Precentral gyrus (6) | 30 | 4 | −44 | 2 | 46 | 5.19 | |
| L | Middle frontal gyrus (44/45) | 40* | 11 | 34.1% in L area 45 | −46 | 22 | 30 | 5.35 |
| IFG pars triangularis (45*/45) | 29.5% in L area 44 | |||||||
| L | Insula lobe | 48 | −30 | 22 | 0 | 6.43 | ||
| R | Insula lobe | 42 | 34 | 22 | −2 | 5.81 | ||
| IFG pars orbitalis (47) | 32 | 26 | −6 | 5.75 | ||||
| L | Cerebellum (Crus1) | 71 | −28 | −66 | −32 | 6.50 | ||
| L | Cerebellum (Crus 1) | 6 | −16 | −76 | −30 | 5.16 | ||
| R | Cerebellum (Crus 1) | 171 | 32 | −66 | −32 | 6.33 | ||
| R | Cerebellum (Crus 1) | 25 | 12 | −78 | −28 | 5.37 | ||
| Cerebellum (Crus 2) | 6 | −86 | −32 | 5.52 | ||||
| L | Thalamus | 8 | −16 | −28 | 14 | 5.41 | ||
Regions showing a significant linear increase of cerebral activation related to WM load in both controls and pretreatment OSA patients (P < 0.05 FWE corrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann area, ATp = most probable anatomical regions (where available) in the Anatomy toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), hIP = human Intra-Parietal area, SMA = supplementary motor area, IFG = inferior frontal gyrus.
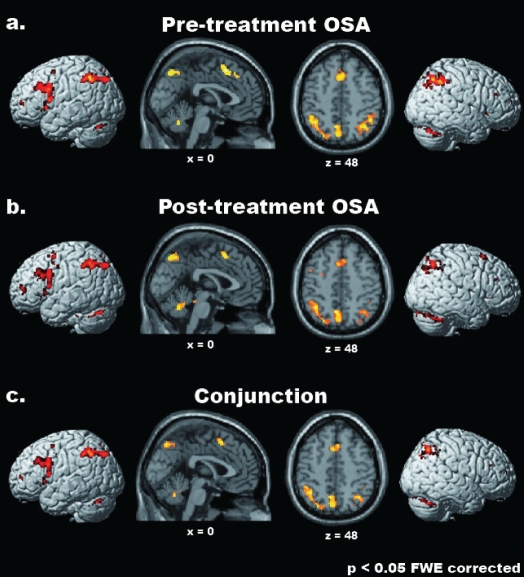
Figure 2.
Common parametric effects of WM load in healthy controls and pretreatment OSA patients. From top to bottom, the regions showing a significant linear increase of cerebral activation related to WM load on cerebral activity in healthy controls (a), pretreatment OSA patients (b), and in both groups (Conjunction analysis; c) are shown (P < 0.05 FWE corrected for multiple comparisons, minimum cluster-size = 4 voxels). Activations were superimposed onto 3D-renderings of the MNI template and representative sagittal (x = 0) and transverse (z = 48) slices from the same brain.
Table S1.
Parametric Effects of WM-Load in Controls
| H | Anatomical region (BA) Controls | ATp | K | Cluster labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| L | Middle occipital gyrus | 235 | −30 | −74 | 40 | 5.51 | ||
| Superior parietal lobule | −24 | −76 | 46 | 5.87 | ||||
| Inferior parietal lobule | −30 | −64 | 40 | 5.41 | ||||
| L | Superior parietal lobule | 590 | 29.8% in L hIP1 | −36 | −62 | 48 | 5.57 | |
| Inferior parietal lobule | 14.6% in L hIP2 | −48 | −50 | 52 | 5.39 | |||
| Inferior parietal lobule (hIP2*) | 40* | −42 | −44 | 44 | 6.01 | |||
| R | Middle occipital gyrus | 95 | 32 | −72 | 36 | 5.23 | ||
| Superior occipital gyrus | 32 | −68 | 42 | 5.42 | ||||
| Angular gyrus | 38 | −70 | 46 | 5.31 | ||||
| R | Superior parietal lobule | 27 | 20 | −78 | 50 | 5.74 | ||
| R | Inferior parietal lobule (hIP1*) | 40 | 669 | 12.3% in R hIP1 | 38 | −46 | 40 | 6.44 |
| Inferior parietal lobule | 4.7% in R hIP2 | 54 | −40 | 52 | 5.99 | |||
| L/R | SMA (6*) | 20* | 175 | 23.6% in L area 6 | −2 | 12 | 50 | 5.73 |
| SMA | 10.5% in R area 6 | 4 | 20 | 48 | 5.43 | |||
| Superior medial gyrus | 4 | 30 | 42 | 5.72 | ||||
| L | Precentral gyrus | 82 | 9.9% in L area 6 | −42 | 2 | 46 | 5.88 | |
| L | Precentral gyrus | 21 | 17% in L area 44 | −50 | 4 | 38 | 5.34 | |
| Precentral gyrus (44*) | 40* | −48 | 6 | 32 | 5.04 | |||
| L | Middle frontal gyrus | 13 | −30 | 2 | 56 | 5.22 | ||
| L | IFG pars triangularis (44*) | 50* | 45 | 60.6% in L area 44 | −40 | 14 | 26 | 5.55 |
| IFG pars triangularis (45*) | 40* | 14.4% in L area 45 | −46 | 22 | 30 | 5.35 | ||
| L | IFG pars triangularis (45*) | 70* | 24 | 100% in L area 45 | −54 | 24 | 26 | 5.71 |
| L | Insula lobe | 112 | −30 | 22 | 0 | 6.43 | ||
| R | Superior frontal gyrus | 69 | 28 | 6 | 58 | 5.35 | ||
| Middle frontal gyrus | 32 | 14 | 58 | 5.61 | ||||
| R | IFG pars opercularis (44*) | 40* | 64 | 51.9% in R area 44 | 52 | 12 | 30 | 5.57 |
| R | Insula lobe | 117 | 36 | 20 | −2 | 6.61 | ||
| L | Cerebellar vermis | 118 | 4 | −36 | −20 | 5.48 | ||
| L | Cerebellum (Crus 1) | 186 | −28 | −66 | −32 | 6.84 | ||
| R | Cerebellum (Crus 1) | 319 | 36 | −64 | −34 | 7.08 | ||
| Cerebellum (Crus 2) | 42 | −48 | −40 | 5.44 | ||||
| R | Cerebellum (Crus 1) | 51 | 12 | −78 | −28 | 5.51 | ||
| Cerebellum (Crus 2) | 6 | −86 | −32 | 6.19 | ||||
| L | Thalamus | 29 | −16 | −28 | 14 | 5.41 | ||
| R | Thalamus | 17 | 14 | −26 | 14 | 5.65 | ||
Regions showing a significant linear increase of cerebral activation related to WM-load in control participants (p < 0.05 FWE corrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann Area, ATp = most probable anatomical regions (where available) in the Anatomy Toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), hIP = human Intra-Parietal area, SMA = Supplementary Motor Area, IFG = Inferior Frontal Gyrus.
Table S2.
Parametric Effects of WM-Load in Pre-Treatment OSA Patients
| H | Anatomical region (BA) Pre-treatment OSA | ATp | K | Cluster labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| L | Superior parietal lobule | 726 | 13.4% of L hIP1 | −34 | −64 | 50 | 6.57 | |
| Inferior parietal lobule | 6.8% of L hIP2 | −30 | −70 | 46 | 5.34 | |||
| Inferior parietal lobule (hIP1*) | 50* | −40 | −48 | 46 | 5.63 | |||
| Inferior parietal lobule (hIP2*) | 60* | −48 | −44 | 44 | 5.24 | |||
| R | Superior occipital gyrus | 27 | 32 | −68 | 42 | 5.55 | ||
| R | Superior parietal lobule | 39 | 28 | −64 | 54 | 5.53 | ||
| R | Angular gyrus | 394 | 16.6% of R hIP2 | 38 | −62 | 48 | 5.58 | |
| Inferior parietal lobule (hIP1*) | 40* | 14.5% of R hIP1 | 38 | −46 | 40 | 6.75 | ||
| Inferior parietal lobule (hIP2*) | 50* | 42 | −44 | 48 | 5.69 | |||
| L/R | Precuneus | 205 | −6 | −70 | 54 | 7.07 | ||
| Precuneus | 4 | −58 | 44 | 5.10 | ||||
| R | Precuneus | 30 | 6 | −70 | 52 | 5.55 | ||
| L/R | SMA (6*) | 40* | 159 | 51.2% in L area 6 | −2 | 14 | 54 | 5.96 |
| SMA (6*) | 40* | 10.8% in R area 6 | 4 | 20 | 50 | 5.86 | ||
| L | Precentral gyrus | 21 | 2.3% in L area 44 | −40 | 8 | 44 | 5.28 | |
| Precentral gyrus | −38 | 6 | 38 | 5.13 | ||||
| L | Middle frontal gyrus | 32 | −38 | 54 | 8 | 5.61 | ||
| L | Middle frontal gyrus | 198 | 23.8% in L area 45 | −44 | 30 | 32 | 6.65 | |
| IFG pars opercularis (44*) | 30* | 13.7% in L area 44 | −50 | 22 | 34 | 5.92 | ||
| IFG pars triangularis (45*) | 40* | −46 | 22 | 30 | 5.95 | |||
| IFG pars triangularis (45) | −44 | 26 | 20 | 5.19 | ||||
| L | IFG pars opercularis (44*) | 40* | 118 | 73.4% in L area 44 | −52 | 8 | 14 | 6.22 |
| L | IFG pars triangularis (44*) | 50* | 10 | 100% in L area 44 | −52 | 14 | 24 | 5.14 |
| L | Middle orbital gyrus | 8 | −36 | 58 | −2 | 5.35 | ||
| L | Insula lobe | 270 | 9.4% in L area 44 | −30 | 22 | 0 | 6.67 | |
| IFG pars triangularis (44) | 3.5% in L area 45 | −48 | 16 | 2 | 6.19 | |||
| R | Middle frontal gyrus | 29 | 42 | 34 | 28 | 5.12 | ||
| R | Middle frontal gyrus | 5 | 38 | 54 | 12 | 5.41 | ||
| R | Insula lobe | 79 | 34 | 22 | −2 | 5.81 | ||
| IFG pars orbitalis | 32 | 26 | −6 | 5.75 | ||||
| R | IFG pars triangularis | 3 | 46 | 32 | 26 | 5.06 | ||
| L | Cerebellum (Crus 1) | 104 | −28 | −66 | −32 | 6.50 | ||
| R | Cerebellum (Crus 1) | 300 | 32 | −66 | −32 | 6.33 | ||
| Cerebellum (Crus 2) | 6 | −86 | −32 | 5.52 | ||||
| L | Thalamus | 106 | −16 | −26 | 16 | 6.19 | ||
| R | Thalamus | 3 | 16 | −18 | 10 | 5.31 | ||
| L | Putamen | 118 | −20 | 2 | 6 | 6.66 | ||
Regions showing a significant linear increase of cerebral activation related to WM-load in pre-treatment OSA patients (p < 0.05 FWE corrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann Area, ATp = most probable anatomical regions (where available) in the Anatomy Toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), hIP = human Intra-Parietal area, SMA = Supplementary Motor Area, IFG = Inferior Frontal Gyrus.
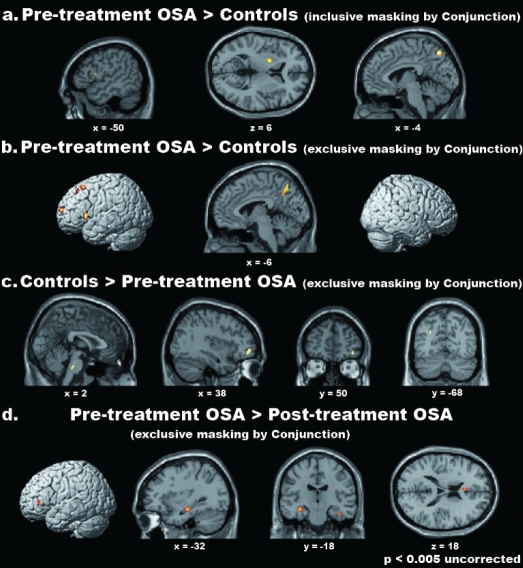
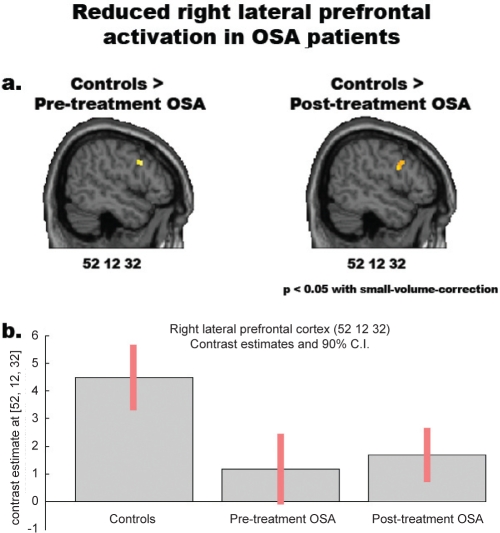
We also observed areas showing significant differences of activation in the two groups of participants (Table 5, Figure 4). Within the regions highlighted by the conjunction analysis (i.e., inclusively masking by the conjunction analysis), stronger activations in OSA patients were observed in the dorsal-most portion of the medial precuneus (BA 7), the pars triangularis of the left IFG (BA 45) and the left putamen (Figure 4a). Outside the regions resulting from the conjunction analysis (i.e., exclusively masking by the conjunction analysis), we observed stronger activations in OSA patients than controls in a portion of the medial precuneus (BA 7), which was more ventral and rostral than the one reported above, and in several regions within the left frontal cortex, namely the pars triangularis of the IFG (BAs 44 and 45), the middle frontal gyrus (44/46), the superior frontal gyrus (8/6) and the fronto-polar cortex (BA 10) (Figure 4b). Enhanced activation in patients was also observed in the medial and dorsal cerebellum, as well as in the left hippocampus. No region was more strongly activated in controls than OSA patients within those that were also commonly activated in the two groups. By contrast, stronger activations outside the regions resulting from the conjunction analysis were observed in control participants in the left middle occipital gyrus (BA 19), the right middle orbital gyrus (47/46) and the caudal pons in the brainstem. The approximate location of the latter activation appears to be in the reticular formation (Figure 4c). Notably, no reduced activation in pretreatment OSA, as compared with controls, was observed in the right lateral prefrontal cortex, as shown by Thomas et al.(2005), at the threshold of P < 0.001 uncorrected. However, an a priori hypothesis motivated by those results justified the use of a small volume correction44 on a specific a priori region observed at a lenient uncorrected threshold of P < 0.005. This procedure highlighted a significantly reduced activation in pretreatment OSA, compared with controls, in the right lateral prefrontal cortex (4-mm radius sphere centered on the coordinates x = 52, y = 12, z = 32; P < 0.05 FWE small volume corrected) (Figure 6).
Table 5.
Differential parametric effects of WM load in controls and pretreatment OSA patients
| H | Anatomical region (BA) | ATp | K | Cluster labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| a. Controls > Pretreatment OSA,Inclusively masked by Conj | ||||||||
| No significant clusters | ||||||||
| b. Controls > Pretreatment OSA, Exclusively masked by Conj | ||||||||
| L/R | Brainstem | 27 | 2 | −28 | −36 | 3.40 | ||
| Brainstem | −4 | −28 | −32 | 3.30 | ||||
| L | Middle occipital gyrus (19) | 19 | −28 | −68 | 30 | 3.30 | ||
| R | Middle orbital gyrus (47/46) | 11 | 38 | 50 | −8 | 3.34 | ||
| c. Pretreatment OSA > Controls, Inclusively masked by Conj | ||||||||
| L | Precuneus (7) | 61 | −4 | −66 | 52 | 4.14 | ||
| L | IFG pars triangularis (45*/44) | 40*/30 | 4 | 37.5% in L area 44 | −50 | 16 | 2 | 3.59 |
| L | Putamen | 19 | −20 | 2 | 6 | 4.03 | ||
| d. Pretreatment OSA > Controls, Exclusively masked by Conj | ||||||||
| L/R | Precuneus (7) | 33 | −6 | −66 | 52 | 3.95 | ||
| L | Superior frontal gyrus (8/6) | 59 | −18 | 22 | 54 | 4.21 | ||
| Middle frontal gyrus (8) | −26 | 22 | 56 | 3.46 | ||||
| L | Middle frontal gyrus (44/46) | 6 | −36 | 20 | 34 | 3.41 | ||
| L | IFG pars triangularis (45/44) | 30/20 | 40 | 25.5% in L area 44 | −48 | 18 | 2 | 3.98 |
| 10.2% in L area 45 | ||||||||
| L | Superior frontal gyrus (10) | 18 | −20 | 62 | 12 | 4.07 | ||
| L | Hippocampus | 40* | 10 | 16.7% in L hippocampus | −38 | −18 | −14 | 3.35 |
| L | Cerebellum (IV-V) | 21 | −8 | −50 | −8 | 3.57 | ||
From top to bottom, cerebral regions showing significantly different parametric effects of WM-load in controls vs. pretreatment OSA within, and outside, the commonly activated regions (a and b, respectively), and in pretreatment OSA vs. controls within, and outside, the commonly activated regions (c and d, respectively) (P < 0.001 uncorrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann area, ATp = most probable anatomical regions (where available) in the Anatomy toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), IFG = inferior frontal gyrus.
Figure 4.
Differential parametric effects of WM load in healthy controls, pretreatment OSA patients and posttreatment OSA patients. From top to bottom, the regions showing significantly stronger parametric effects of WM load on cerebral activity (i.e., increase) in pretreatment OSA patients vs. controls within (a) and outside (b) those commonly activated in the 2 groups; the areas that were more strongly activated in controls than pretreatment OSA patients, outside those highlighted by their Conjunction (c), and those that were more strongly activated in pretreatment than posttreatment OSA patients (d). Only for graphical purposes, activations are shown at an uncorrected threshold of P < 0.005. Activations were superimposed onto 3D-renderings of the MNI template and representative slices from the same brain.
Figure 6.
Reduced right lateral prefrontal activation in both pre- and posttreatment OSA patients compared to healthy controls. Top: significantly reduced activity in the right lateral prefrontal cortex of OSA patients, before and after treatment, compared with healthy controls [P < 0.05 FWE small-volume-corrected, based on an a priori hypothesis from Thomas et al. (2005)]. Bottom: from left to right, mean parameter estimates with 90% confidence-interval in the peak-voxel of the right lateral prefrontal cortex (x = 52, y = 12, z = 32) in controls, pretreatment and posttreatment OSA patients.
Posttreatment analyses: pretreatment vs. Posttreatment OSA patients
Direct comparisons between pre- and posttreatment OSA patients highlighted cerebral regions showing significantly different effects of WM load across time (Table 6, Figures 3 and 4d; see also Tables S2, S3 for the complete list of the activated foci in the two groups separately). No area was more strongly activated after treatment (post- > pretreatment) either within the commonly activated regions in the two groups or outside them (see Table S4 for the list of the common activations in the two groups). Furthermore, no region showed significantly reduced activation after treatment (pre- > posttreatment) within the commonly activated regions. Instead, a significant reduction of activation in posttreatment, as compared with pretreatment, was observed outside the commonly activated regions. Such differences involved the rostral portion of pars triangularis of the left IFG (BA 45), the left anterior cingulate cortex (BA 24), the right SMA (BA 6), as well as several hippocampal clusters in both hemispheres (Table 6 and Figure 4d).
Table 6.
Differential Parametric Effects of WM Load in Pretreatment and Posttreatment OSA Patients
| H | Anatomical region (BA) | ATp | K | Cluster Labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| X | y | z | ||||||
| Pretreatment OSA > Posttreatment OSA, Inclusively masked by Conj | ||||||||
| No significant clusters | ||||||||
| Pretreatment OSA > Posttreatment OSA, Exclusively masked by Conj | ||||||||
| L | Hippocampus | 80* | 42 | 99% in L hippocampus | −32 | −18 | −16 | 3.80 |
| R | Fusiform gyrus/hippocampus | 10 | 35 | 34.4% in R hippocampus | 42 | −20 | −24 | 3.92 |
| R | Fusiform gyrus/hippocampus | 20 | 15 | 25% in R hippocampus | 38 | −14 | −30 | 3.26 |
| Hippocampus | 90* | 52 | 100% in R hippocampus | 30 | −16 | −34 | 3.48 | |
| R | SMA (6) | 20 | 26 | 23.6% in R area 6 | 10 | −26 | 50 | 3.67 |
| L | IFG pars triangularis (45) | 7 | −40 | 36 | 4 | 3.47 | ||
| L | Anterior cingulate cortex (24) | 9 | −2 | 24 | 18 | 3.36 | ||
| Posttreatment OSA > Pretreatment OSA, Inclusively masked by Conj | ||||||||
| No significant clusters | ||||||||
| Posttreatment OSA > Pretreatment OSA, Exclusively masked by Conj | ||||||||
| No significant clusters | ||||||||
From top to bottom, cerebral regions showing significantly different parametric effects of WM-load in pretreatment OSA vs. posttreatment OSA within, and outside, the commonly activated regions (a and b, respectively), and in posttreatment OSA vs. pretreatment OSA within, and outside, the commonly activated regions (c and d, respectively) (P < 0.001 uncorrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann area, ATp = most probable anatomical regions (where available) in the Anatomy toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), SMA = supplementary motor area, IFG = inferior frontal gyrus.
Figure 3.
Common parametric effects of WM load in pretreatment and posttreatment OSA patients. From top to bottom, the regions showing a significant linear increase of cerebral activation related to WM load in pretreatment OSA patients (a), posttreatment OSA patients (b), and in both groups (Conjunction analysis; c) are shown (P < 0.05 FWE corrected for multiple comparisons, minimum cluster-size = 4 voxels). Activations were superimposed onto 3D-renderings of the MNI template and representative sagittal (x = 0) and transverse (z = 48) slices from the same brain.
Table S3.
Parametric Effects of WM-Load in Post-Treatment OSA Patients
| H | Anatomical region (BA) Post-treatment OSA | ATp | K | Cluster labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| L | Middle occipital gyrus | 1971 | 14.9% in L hIP1 | −26 | −66 | 40 | 6.23 | |
| Precuneus | 3.5% in L hIP2 | −4 | −68 | 52 | 7.08 | |||
| Right precuneus | 4 | −66 | 54 | 5.17 | ||||
| Superior parietal lobule | −26 | −74 | 46 | 6.71 | ||||
| Inferior parietal lobule (hIP1*) | 40* | −36 | −48 | 44 | 7.09 | |||
| Angular gyrus (hIP1*) | 40* | −42 | −56 | 42 | 5.72 | |||
| R | Middle occipital gyrus | 138 | 36 | −72 | 38 | 5.78 | ||
| Superior occipital gyrus | 32 | −74 | 46 | 5.42 | ||||
| Angular gyrus | 38 | −74 | 40 | 5.63 | ||||
| Superior parietal lobule | 26 | −78 | 50 | 5.18 | ||||
| R | Superior parietal lobule | 43 | 42 | −54 | 56 | 5.15 | ||
| Inferior parietal lobule | 48 | −52 | 52 | 5.37 | ||||
| R | Superior parietal lobule | 399 | 7.2% in R hIP1 | 30 | −64 | 54 | 5.95 | |
| Inferior parietal lobule (hIP1*) | 40* | 1.7% in R hIP2 | 38 | −46 | 40 | 6.14 | ||
| Angular gyrus | 42 | −66 | 46 | 5.74 | ||||
| R | Precuneus | 73 | 10 | −70 | 60 | 6.14 | ||
| L/R | SMA (6*) | 50* | 277 | 35.6% in L area 6 | −2 | 16 | 50 | 6.63 |
| SMA (6) | 10.6% in R area 6 | 8 | 18 | 46 | 6.28 | |||
| L | Superior frontal gyrus | 8 | −26 | 4 | 64 | 5.17 | ||
| L | Precentral gyrus | 133 | −36 | 2 | 58 | 5.99 | ||
| Middle frontal gyrus | −40 | 4 | 56 | 5.88 | ||||
| L | Precentral gyrus (6*) | 40* | 48 | 7.5% in L area 6 | −46 | 4 | 48 | 5.71 |
| L | Precentral gyrus (6*) | 80* | 24 | 98.8% in L area 6 | −54 | −2 | 42 | 5.46 |
| L | Middle frontal gyrus | 120 | −36 | 52 | 10 | 5.75 | ||
| L | Middle frontal gyrus | 12 | −34 | 10 | 60 | 5.58 | ||
| L | Precentral gyrus | 646 | 49.6% in L area 44 | −44 | 8 | 30 | 6.33 | |
| Middle frontal gyrus | 11.4% in L area 45 | −46 | 26 | 36 | 6.26 | |||
| Middle frontal gyrus (44*) | 40* | −46 | 10 | 36 | 5.68 | |||
| IFG pars opercularis (44*) | 40* | −54 | 10 | 12 | 5.97 | |||
| IFG pars triangularis (45*) | 60* | −52 | 26 | 28 | 5.13 | |||
| L | Insula lobe | 38 | −32 | 24 | −4 | 5.95 | ||
| L | Insula lobe | 63 | 37.5% in L area 44 | −42 | 18 | 0 | 5.20 | |
| IFG pars triangularis (44*) | 40* | 13% in L area 45 | −48 | 16 | 4 | 5.84 | ||
| R | Superior frontal gyrus | 72 | 26 | 16 | 62 | 6.10 | ||
| R | Middle frontal gyrus | 4 | 34 | 58 | 14 | 5.30 | ||
| R | IFG pars opercularis (44) | 53 | 40 | 8 | 32 | 5.81 | ||
| R | IFG pars triangularis | 18 | 44 | 34 | 26 | 5.48 | ||
| R | IFG pars orbitalis | 6 | 34 | 28 | −6 | 5.10 | ||
| L/R | Cerebellar vermis | 109 | 0 | −60 | −30 | 6.36 | ||
| L/R | Cerebellar vermis | 28 | 2 | −36 | −20 | 5.33 | ||
| L | Cerebellum (IV-V) | 8 | −22 | −32 | −34 | 5.45 | ||
| L | Cerebellum (Crus 1) | 203 | −26 | −66 | −32 | 6.34 | ||
| L | Cerebellum (Crus 1) | 100 | −14 | −76 | −30 | 6.05 | ||
| Cerebellum (Crus 2) | −8 | −82 | −26 | 5.75 | ||||
| R | Cerebellum (VI) | 889 | 30 | −64 | −32 | 7.04 | ||
| Cerebellum (Crus 1) | 40 | −62 | −34 | 6.61 | ||||
| Cerebellum (Crus 2) | 8 | −86 | −32 | 6.58 | ||||
| L | Thalamus | 41 | −12 | −26 | 12 | 5.59 | ||
| R | Thalamus | 40 | 10 | −20 | 12 | 5.89 | ||
Regions showing a significant linear increase of cerebral activation related to WM-load in post-treatment OSA patients (p < 0.05 FWE corrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann Area, ATp = most probable anatomical regions (where available) in the Anatomy Toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), hIP = human Intra-Parietal area, SMA = Supplementary Motor Area, IFG = Inferior Frontal Gyrus.
Table S4.
Parametric Effects of WM-Load: Conjunction Pre-Treatment and Post-Treatment OSA Patients
| H | Anatomical region (BA) Pre-treatment and Post-treatment OSA | ATp | K | Cluster labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| L | Superior parietal lobule | 584 | 25.1% in L hIP1 | −26 | −74 | 46 | 6.40 | |
| Inferior parietal lobule (hIP1*) | 40* | 5.5% in L hIP2 | −36 | −48 | 44 | 7.04 | ||
| Angular gyrus (hIP1*) | 40* | −44 | −56 | 44 | 5.64 | |||
| R | Superior occipital gyrus | 31 | 30 | −72 | 46 | 5.18 | ||
| Angular gyrus | 38 | −74 | 40 | 5.64 | ||||
| R | Superior parietal lobule | 12 | 42 | −54 | 56 | 5.15 | ||
| Inferior parietal lobule | 48 | −52 | 52 | 5.37 | ||||
| R | Superior parietal lobule | 202 | 7.2% in R hIP1 | 26 | −72 | 58 | 5.29 | |
| Inferior parietal lobule (hIP1*) | 40* | 1.9% in R hIP2 | 38 | −46 | 40 | 6.14 | ||
| Angular gyrus | 42 | −66 | 46 | 5.64 | ||||
| R | Inferior parietal lobule | 9 | 50 | −56 | 44 | 5.25 | ||
| L | Precuneus | 255 | −4 | −70 | 52 | 6.98 | ||
| Precuneus | −8 | −64 | 62 | 5.46 | ||||
| R | Precuneus | 14 | 10 | −70 | 60 | 5.84 | ||
| L/R | SMA (6*) | 50* | 148 | 35.5% in L area 6 | −2 | 16 | 50 | 6.63 |
| SMA | 11.1% in R area 6 | 6 | 20 | 46 | 6.25 | |||
| L | Middle frontal gyrus | 14 | −40 | 4 | 56 | 5.66 | ||
| L | Middle frontal gyrus | 327 | 51.7% in L area 44 | −46 | 26 | 36 | 6.26 | |
| Precentral gyrus | 9.2% in L area 45 | −44 | 8 | 30 | 5.90 | |||
| IFG pars opercularis (44*) | 40* | −54 | 10 | 12 | 5.97 | |||
| IFG pars triangularis (45*) | 40* | −46 | 22 | 30 | 5.62 | |||
| L | Middle frontal gyrus | 19 | −38 | 54 | 10 | 5.58 | ||
| L | Insula lobe | 26 | −32 | 24 | −4 | 5.86 | ||
| L | Insula lobe | 25 | 36.5% in L area 44 | −42 | 18 | 0 | 5.20 | |
| IFG pars triangularis (44*) | 40* | 10% in L area 45 | −48 | 16 | 4 | 5.84 | ||
| R | IFG pars opercularis (44) | 7 | 38 | 8 | 32 | 5.46 | ||
| R | IFG pars triangularis (45) | 7 | 44 | 34 | 26 | 5.32 | ||
| L/R | Cerebellar vermis | 29 | 0 | −60 | −30 | 5.87 | ||
| L | Cerebellum (Crus1) | 109 | −26 | −66 | −32 | 6.34 | ||
| L | Cerebellum (Crus1) | 51 | −12 | −78 | −28 | 5.84 | ||
| R | Cerebellum (VI) | 471 | 30 | −64 | −32 | 6.81 | ||
| Cerebellum (Crus1) | 40 | −62 | −34 | 6.57 | ||||
| Cerebellum (Crus2) | 10 | −84 | −32 | 6.24 | ||||
Regions showing a significant linear increase of cerebral activation related to WM-load in both pre-treatment and post-treatment OSA patients (p < 0.05 FWE corrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann Area, ATp = most probable anatomical regions (where available) in the Anatomy Toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), hIP = human Intra-Parietal area, SMA = Supplementary Motor Area, IFG = Inferior Frontal Gyrus.
Posttreatment Analyses: Controls vs. Posttreatment OSA Patients
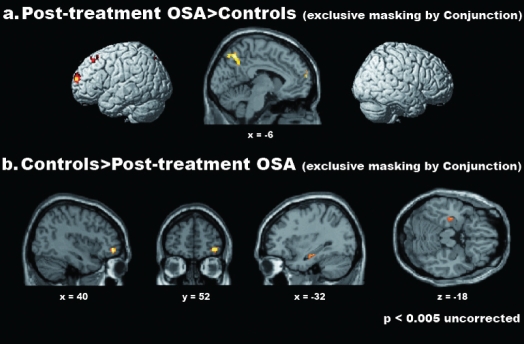
Both controls and posttreatment OSA patients showed WM-load related activity in a frontolateral-frontomedial-parietal network including the posterior parietal cortex bilaterally, the supplementary motor area, the left precentral gyrus and inferior frontal gyrus, and the anterior insula bilaterally (Figures 2 and 3). Further common activations were noticed in the cerebellum and thalamus, bilaterally (Table S5). Direct comparisons between controls and posttreatment OSA patients showed no significant difference between groups within the commonly activated regions (i.e., inclusive masking by the conjunction) (Table S6a, c). Among the regions that were not commonly activated (i.e., exclusive masking by the conjunction), stronger activations in posttreatment OSA than controls were observed in the medial precuneus and in the left frontal cortex, including foci located in the superior, middle and inferior frontal gyri (Figure 5a, Table S6d). In the opposite comparison, the right middle orbital gyrus and the left hippocampus were significantly less activated in posttreatment OSA patients than controls (Figure 5b, Table S6b). Based on the results provided by Thomas et al.,19 we applied a small-volume correction44 on the same right lateral prefrontal region that we also found to be less activated in pretreatment OSA than controls (4-mm radius sphere centered on the coordinates x = 52, y = 12, z = 32). We thereby observed that, despite a small increase with regard to pretreatment session, activity in this region was still significantly reduced in posttreatment OSA than controls (P < 0.05 FWE small-volume-corrected, see Figure 6).
Table S5.
Parametric Effects of WM-Load: Conjunction Controls and Post-Treatment OSA Patients
| H | Anatomical region (BA) Controls and Post-treatment OSA | ATp | K | Cluster labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| L | Precuneus (*SPL, 7p) | *40 | 6 | 100% in L 7p | −12 | −74 | 50 | 5.11 |
| L | Inferior parietal lobule (*hIP1) | *30 | 246 | 32.6% in L hIP1 | −36 | −52 | 46 | 6.30 |
| L | Inferior parietal lobule (*hIP2) | *40 | 27.6% in L hIP2 | −48 | −46 | 48 | 5.57 | |
| L | Inferior parietal lobule (*hIP3) | *50 | 23.4% in L hIP3 | −30 | −54 | 40 | 5.05 | |
| L | Inferior parietal lobule | 106 | 17.3% in L PGa | −30 | −72 | 44 | 5.78 | |
| L | Inferior parietal lobule (*PGa) | *30 | 9 | 50% in L hIP3 | −36 | −60 | 50 | 5.08 |
| R | Inferior parietal lobule (*hIP1) | *40 | 47 | 64.4% in R hIP1 | 38 | −46 | 40 | 5.87 |
| 18.3% in R hIP2 | ||||||||
| R | Inferior parietal lobule (*PFm) | *70 | 24 | 100% in R PFm | 48 | −50 | 50 | 5.38 |
| L | SMA (*6) | *30 | 21 | 68.8% in L area 6 | −2 | 12 | 52 | 5.36 |
| R | SMA (*6) | *40 | 22 | 48.8% in R area 6 | 4 | 20 | 50 | 5.51 |
| L | Precentral gyrus (6) | 20 | 4 | 8.3% in L area 6 | −46 | 4 | 50 | 5.16 |
| L | IFG pars triangularis (*45) | *40 | 41 | 53.7% in L area 45 | ||||
| 19.8% in L area 44 | ||||||||
| L | Insula lobe | 47 | −32 | 22 | −4 | 6.06 | ||
| R | Insula lobe | 26 | 34 | 20 | 0 | 5.76 | ||
| L | Cerebellum (Crus 1) | 350 | −40 | −72 | −32 | 6.64 | ||
| R | Cerebellum (Crus 1) | 220 | 28 | −64 | −34 | 6.85 | ||
| R | Cerebellum (Crus 2) | 46 | 6 | −86 | −32 | 5.92 | ||
| L/R | Cerebellar vermis (9) | 10 | −2 | −60 | −36 | 5.29 | ||
| L | Thalamus | 20 | −14 | −12 | 14 | 5.79 | ||
| R | Thalamus | 14 | 14 | −6 | 14 | 5.30 | ||
Regions showing a significant linear increase of cerebral activation related to WM-load in both controls and post-treatment OSA patients (p < 0.05 FWE corrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann Area, ATp = most probable anatomical regions (where available) in the Anatomy Toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), hIP = human Intra-Parietal area, SMA = Supplementary Motor Area, IFG = Inferior Frontal Gyrus.
Table S6.
Differential Parametric Effects of WM-Load in Controls and Post-Treatment OSA Patients
| H | Anatomical region (BA) | ATp | K | Cluster labeling | MNI |
Z-score | ||
|---|---|---|---|---|---|---|---|---|
| a. Controls > Post-treatment OSA, Inclusively masked by Conjunction | x | y | z | |||||
| No significant clusters | ||||||||
| b. Controls > Post-treatment OSA, Exclusively masked by Conjunction | x | y | z | |||||
| R | Middle orbital gyrus (47/46) | 156 | 40 | 48 | −6 | 4.51 | ||
| L | Hippocampus | 4 | −30 | −18 | −16 | 3.19 | ||
| c. Post-treatment OSA > Controls, Inclusively masked by Conjunction | x | y | z | |||||
| No significant clusters | ||||||||
| d. Post-treatment OSA > Controls, Exclusively masked by Conjunction | x | y | z | |||||
| L/R | Precuneus (SPL, *7p) | *40 | 84 | 37.4% in L SPL (7p) | −4 | −64 | 50 | 4.08 |
| 35.5% in L SPL (7a) | ||||||||
| L | Precuneus (7) | 15 | −12 | −58 | 34 | 3.54 | ||
| L | Superior medial gyrus (8/6) | 103 | −12 | 64 | 18 | 3.99 | ||
| Superior frontal gyrus (8/6) | −12 | 62 | 22 | 3.92 | ||||
| L | Superior frontal gyrus (8/6) | 8 | −24 | 62 | 16 | 3.51 | ||
| L | Superior frontal gyrus (8/6) | 131 | −16 | 30 | 46 | 3.89 | ||
| Middle frontal gyrus (8) | −20 | 30 | 34 | 3.64 | ||||
| L | Middle frontal gyrus (8) | 18 | −36 | 22 | 34 | 3.62 | ||
| L | IFG pars triangularis (*45) | *40 | 4 | 20.8% in L area 44 | −48 | 18 | 4 | 3.28 |
| 12.5% in L area 45 | ||||||||
From top to bottom, cerebral regions showing significantly different parametric effects of WM-load in controls vs. post-treatment OSA within, and outside, the commonly activated regions (a and b, respectively), and in post-treatment OSA vs. controls within, and outside, the commonly activated regions (c and d, respectively) (p < 0.001 uncorrected for multiple comparisons, minimum cluster size K = 4 voxels). H = Hemisphere, L = Left, R = Right, BA = estimated Brodmann Area, ATp = most probable anatomical regions (where available) in the Anatomy Toolbox [Eickhoff et al., 2005; asterisks denote assignment], K = cluster-extension in number of voxels (2 × 2 × 2 mm3), IFG = Inferior Frontal Gyrus.
Figure 5.
Differential parametric effects of WM load in healthy controls and posttreatment OSA patients. From top to bottom, the regions showing significantly stronger parametric effects of WM-load on cerebral activity (i.e., increase) in posttreatment OSA patients vs. controls (a) and the regions that were more strongly activated in controls than posttreatment OSA patients (b), outside those highlighted by their Conjunction. Only for graphical purposes, activations are shown at an uncorrected threshold of P < 0.005. Activations were superimposed onto 3D-renderings of the MNI template and representative slices from the same brain.
ROI Analysis Of Correlation Between Activation Change And Cognitive Change With Treatment
Table 7 shows the pre- and posttreatment scores on cognitive tests as well as their relative correlations with activation change in the specified regions of interest (ROIs). Regions were chosen based upon the identified changes with treatment in the group analysis (see Tables 6, 7). Change in ESS and the Rey list learning test were not correlated with change in activation for any ROI. There was a positive correlation between PASAT errors and activation in the left and right hippocampus from pre- to posttreatment. This indicates a decrease in activation in the hippocampus associated with a decrease in errors on this test. There were also significant relationships between a decrease in the time to complete the Stroop test and a reduction in activation in the areas of the left IFG and the right SMA. No other relationships were noted.
DISCUSSION
The present study supports the notion that significant differences in cerebral activity can be observed during a working-memory task in patients with OSA compared to normal controls. Furthermore, PAP treatment results in significant changes of brain activation in the patient group after 3 months of clinically effective treatment.
At baseline, there was a large overlap in the pattern of brain activation, reflecting the effects of an increasing WM load, observed in patients and normal controls. The particular pattern of brain activation is consistent with other studies based on the n-back task.25 The main difference of activation in OSA patients was an over-recruitment of brains regions suggesting that additional brain regions were needed in order to support a performance level which was comparable to normal controls. Similar patterns of over-recruitment have been observed in normal aging39 and early Alzheimer's disease,40 and have been interpreted as reflecting compensatory mechanisms supporting performance in the context of incipient brain dysfunction. This includes a number of prefrontal areas, as well as the cerebellum and hippocampal region, which can be considered to be part of an extended working memory network (see below for a discussion of the role of the hippocampus in WM). Less expected was the increased activation of the precuneus. This region is part of the so-called “default network” of brain activity, i.e., a set of functionally connected brain regions which typically shows deactivation during performance of cognitive tasks.51 A reduction in its functioning in normal aging as well as in several pathological conditions has been associated with defective integration of brain activity and to defective allocation of resources during working memory tasks.52,53 Similar evidence of overactivation in OSA has been reported by Ayalon et al., using a verbal learning task.20 Namely, an overactivation of the bilateral inferior and middle frontal gyri, cingulate gyrus, the temporoparietal junction, the thalamus, and the cerebellum was found in patients with OSA compared to controls.20
Some regions were less active in OSA patients compared to controls. Reduced activation is generally interpreted as reflecting a defective response in dysfunctional brain regions. A combined pattern of reduced and increased activation is commonly observed in functional MR studies of patient populations.47 In particular, the left middle occipital gyrus, the right middle orbital gyrus and the caudal pons were less activated in patients than in controls. The latter finding seems to be particularly remarkable, as brainstem mechanisms of respiratory control are considered to be involved in the pathogenesis of OSA.42 It is noteworthy that a recent MR T2 relaxation study showed medial pontine damage in symptomatic OSA patients.43
It is difficult to compare the present findings with the results of Thomas et al.,19 based on the same task used in the present study, because of the significantly lower accuracy and speed of the patients compared to the controls. The impaired performance was associated with a decrease in activation of the dorsolateral prefrontal and posterior parietal cortices compared to normal controls.19 Besides the difference in behavioural performance, another notable difference with our study is that Thomas et al. trained the OSA patients on the cognitive task.19 Training on working-memory tasks has been shown to increase brain activation in normal subjects.41 It may be speculated that the same mechanism may not be available to the OSA brain, explaining the discrepancy with our findings.
The reduction in brain activation seen with successful treatment of OSA supports the compensation hypothesis. The changes in functional recruitment of the left IFG, the left anterior cingulate cortex, the right SMA, as well as the hippocampus may reflect the reduced requirements for additional resources to support the preserved performance in the working memory task, with a return to an activation pattern similar to the controls. The only additional evidence available for treatment effects comes from the Thomas et al study, in which the follow-up of a subsample of 6 participants showed an activation increase in the posterior parietal cortex. This change was however not associated to significant improvements in task performance, which remained impaired.
Additional evidence for the compensatory role of cerebral over-recruitment comes from the finding that the reductions of activation between baseline and follow-up were related to significant improvements in cognitive functioning on tests of working-memory and executive functioning. Specifically, improvements in the PASAT, a working-memory test, were associated with reductions of BOLD signal in hippocampal regions. The role of the hippocampus in human working memory is a matter of debate (for a discussion, see45). On the basis of fMRI evidence, it has been suggested that the hippocampal role in long-term retrieval processes may extend to working memory.46 This is in line with the hypothesis that the hippocampal activations observed at baseline may reflect compensatory mechanisms, which regress with effective improvements. The same hypothesis may apply to the improvements in executive functioning, associated with reductions of cerebral activity in the IFG and the SMA, areas which have been implicated with high selectional and monitoring demands during cognitive tasks.48,49 It is thus surprising that we failed to find a comparable correlation between Rey list learning and hippocampal activation, given the notable improvement associated with treatment. This negative finding suggests the opportunity to include a more extensive evaluation of episodic memory performance, including tests which may be more sensitive to mild hippocampal dysfunction than list learning.50 The lack of significant correlations with subjective sleepiness is less surprising, as the effects of the improvements can be expected to be global, rather than related to a specific brain region.
There are limitations to our study. First, the normal controls were not re-evaluated at the 3-month follow-up. This restriction was due to practical consideration in terms of study cost and subject availability for repeated scanning. A second limitation could be the use of an easy working-memory task. We considered employing a more challenging 3-back task, but previous studies have indicated that this may be too difficult for clinical studies.23 In addition, the requirements of the n-back working memory task within the time-frame of a functional MR experiment may not reflect an important aspect of cognitive challenge in OSA patients, i.e., the ability to sustain effective performance over time. Finally, while our sample was more obese than the control group, it may not be obese enough to be truly representative of the general population of patients with OSA. Obesity is a common aspect of OSA and, at present, it is difficult to disentangle the effects of one versus the other on outcome measures like cognitive functioning. While the present findings may not be generalizable to an obese OSA population, we were limited by practical considerations (space limitation of the scanner).
In conclusion, the present findings indicate that an over-recruitment of brain regions can be observed in OSA patients in comparison with normal controls showing a comparable level of working memory performance in an n-back task. This finding may reflect a compensatory mechanism in participants that show a subclinical pattern of neuropsychological dysfunction. Effective PAP treatment is associated with a reduction of activation in prefrontal and hippocampal areas, which parallels an improvement of neuropsychological test performance.
DISCLOSURE STATEMENT
Financial support by Respironics Foundation, Pittsburgh, USA. Dr. Aloia is an employee of Philips Respironics and holds stock in the company. Dr. Castronovo has consulted for Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We would like to acknowledge the help of Mr Daniele Bizzozero, Mr Antonio Massimo and Miss Cristina Martinelli for their help in recording patients.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 4.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 5.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40-64, years: a population-based survey. Sleep. 1997;20:65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedner J, Bengtsson-Bostrom K, Peker Y, Grote L, Rastam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J. 2006;27:564–70. doi: 10.1183/09031936.06.00042105. [DOI] [PubMed] [Google Scholar]
- 7.Aloia MS, Arnedt JT, Smith L, Skrekas J, Stanchina M, Millman RP. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005;6:115–21. doi: 10.1016/j.sleep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 9.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 10.Aikens JE, Caruana-Montaldo B, Vanable PA, Tadimeti L, Mendelson WB. MMPI correlates of sleep and respiratory disturbance in obstructive sleep apnea. Sleep. 1999;22:362–9. doi: 10.1093/sleep/22.3.362. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C, Robinson A. Sleep-disordered breathing and hypertension: past lessons, future directions. Sleep. 1997;20:806–11. doi: 10.1093/sleep/20.9.806. [DOI] [PubMed] [Google Scholar]
- 12.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27:453–8. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 13.Brown WD. The psychosocial aspects of obstructive sleep apnea. Semin Respir Crit Care Med. 2005;26:33–43. doi: 10.1055/s-2005-864199. [DOI] [PubMed] [Google Scholar]
- 14.Lanfranchi P, Somers VK. Obstructive sleep apnea and vascular disease. Respir Res. 2001;2:315–9. doi: 10.1186/rr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med. 2006;2:461–71. [PubMed] [Google Scholar]
- 16.Ayalon L, Peterson S. Functional central nervous system imaging in the investigation of obstructive sleep apnea. Curr Opin Pulm Med. 2007;13:479–83. doi: 10.1097/MCP.0b013e3282f0e9fb. [DOI] [PubMed] [Google Scholar]
- 17.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 18.Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–4. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 19.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–34. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 20.Ayalon L, Ancoli-Israel S, Klemfuss Z, Shalauta MD, Drummond SPA. Increased brain activation during verbal learning in obstructive sleep apnea. Neuroimage. 2006;31:1817–25. doi: 10.1016/j.neuroimage.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97, 113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 22.Lezak MD, Howieson DB, Loring DW. New York: Oxford University Press; 2004. Neuropsychological assessment. [Google Scholar]
- 23.Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–6. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 24.Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11:424–46. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- 25.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baddeley A. New York: Oxford University Press; 1986. Working memory. [Google Scholar]
- 27.Cohen JD, Perlstein WM, Braver TS, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–8. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 28.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 29.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 30.Buchel C, Wise RJ, Mummery CJ, Poline JB, Friston KJ. Nonlinear regression in parametric activation studies. Neuroimage. 1996;4:60–6. doi: 10.1006/nimg.1996.0029. [DOI] [PubMed] [Google Scholar]
- 31.Zhu DF, Wang ZX, Zhang DR, et al. fMRI revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain. 2006;129:2923–30. doi: 10.1093/brain/awl215. [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;3:165–89. [Google Scholar]
- 33.Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–19. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- 34.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- 36.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox 8th International Conference on Functional Mapping of the Human Brain; 2002; Sendai, Japan. [Google Scholar]
- 37.Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain Stuttgart. Thieme. 1988 [Google Scholar]
- 38.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Grady CL, Yu H, Alain C. Age-related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb Cortex. 2008;18:189–99. doi: 10.1093/cercor/bhm045. [DOI] [PubMed] [Google Scholar]
- 40.Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer's disease. Eur Radiol. 2006;16:193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]
- 41.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–9. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 42.Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol. 2008;160:1–7. doi: 10.1016/j.resp.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cross RL, Kumar R, Macey PM, et al. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31:1103–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston K, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cereb Cortex. 2005;15:303–16. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]
- 46.Karlsgodt KH, Shirinyan D, van Erp TG, Cohen MS, Cannon TD. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25:1224–31. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 47.Petrella JR, Wang L, Krishnan S, et al. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245:224–35. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- 48.Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–24. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Rushworth MF, Kennerley SW, Walton ME. Cognitive neuroscience: resolving conflict in and over the medial frontal cortex. Curr Biol. 2005;15:R54–6. doi: 10.1016/j.cub.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 50.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–3. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 51.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambataro F, Murty VP, Callicott JH, et al. Age-related alterations in default mode network: Impact on working memory performance. Neurobiol Aging. 2008 Jul 30; doi: 10.1016/j.neurobiolaging.2008.05.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]