Abstract
Study Objectives:
To assess the association between sleep duration in children and different markers of body fat by age and weight status.
Design:
Nation-wide health survey. Measurement of BMI and body fat percentage (KFA) calculated from weight, height, skin fold thickness, age, and sex. Sleep duration and potential confounding variables were assessed in a parent questionnaire.
Setting:
N/A
Participants:
7767 German resident children from 3 to 10 years of age.
Interventions:
N/A
Measurements and Results:
Prolongation of sleep duration from the lowest to the highest percentile accounted for a similar mean decrease in BMI (−0.235, 95%-CI −0.321; −0.149) and KFA (−0.182, 95% CI −0.271; −0.092) z-scores. The given association is adjusted for confounding variables and did not show a systematic age dependency. The greatest effects of sleep duration were seen for the upper tails of the BMI and KFA distributions, which were about four as high as the lower tails.
Conclusions:
The association between sleep duration and weight status is of similar size through ages 3 to 10 years. The sleep-associated changes in BMI are likely to be a consequence of higher body fat and primarily affect children whose BMI or KFA is already elevated. These findings favor hormonal pathways nurturing adipose tissue playing a key role in the underlying physiological mechanisms.
Citation:
Bayer O; Rosario AS; Wabitsch M; von Kries R. Sleep duration and obesity in children: is the association dependent on age and choice of the outcome parameter? SLEEP 2009;32(9):1183-1189.
Keywords: Sleep, overweight, obesity, body fat, children
A GROWING BODY OF EVIDENCE THAT SLEEP DURATION AFFECTS WEIGHT STATUS IN CHILDREN HAS BEEN SUMMARIZED IN 3 RECENT REVIEWS.1–3 A meta-analysis by Chen and colleagues calculated a 9% decrease in overweight/obesity risk for a one-hour increase in sleep duration in children up to 10 years of age.
There is still considerable variability in the effects reported by different individual studies, which could be explained by different ages included. This issue has never been systematically assessed in publications the authors are aware of. The majority of studies use measures of overweight/obesity risk, while BMI is reported less frequently. The changes in BMI distributions have merely been addressed, although they might have implications for choosing the appropriate outcome measure and are interesting for physiological and epidemiological reasons. Furthermore, there is limited evidence on whether sleep duration is linked to body fat in children.4–6 A more detailed analysis of epidemiological data with respect to these issues may have implications for further research regarding underlying physiological mechanisms.
The aims of this study were
to describe the sleep duration typical for children 3 to 10 years of age in a nationally representative sample
to assess whether the effects of sleep duration on body mass are age dependent
to investigate if these effects are also seen on body fat (KFA) as outcome
to analyze how the distribution of BMI and KFA is changed in relation to sleep duration
SUBJECTS AND METHODS
Data Collection
The German Health Interview and Examination Survey for Children and Adolescents (KiGGS) was conducted from May 2003 to May 2006 by the Robert Koch Institute and surveyed 17,641 children and adolescents from 0 to 17 years of age. Data were collected with the aim of establishing a nationally representative sample.7 Different age-appropriate questionnaires were used, and the present study included children from 3 to 10 years of age, for whom information on duration of sleep was collected in a uniform manner.
Participants were invited to one of 167 local study centers, where they filled in questionnaires followed by a physical examination. The local study teams were thoroughly trained and each consisted of 5 members, led by a physician experienced in pediatrics.8 Children's height (without shoes) was measured with an accuracy of 0.1 cm, using a portable Harpenden stadiometer (Holtain Ltd., Crymych, UK). Body weight (while wearing underwear) was measured with an accuracy of 0.1 kg with a calibrated electronic scale (SECA, Birmingham, UK). For non-German families with poor command of the German language, questionnaires in the native languages were provided. Sleep duration was assessed in the self-administered parent questionnaire asking for the average hours of sleep per day (discrete value, allowing no decimal places after the comma).
Data Analysis
BMI was modelled as an age and gender-specific z-score obtained from the sample. Overweight and obesity were defined according to the International Obesity Taskforce (IOTF)9 .This definition is based on centile curves drawn to pass through the widely used cutoff points of 25 and 30 kg/m2 at age 18. The data for these curves were obtained from 6 large nationally representative cross-sectional growth studies of international origin. KFA (in %) was computed from the sum of skinfold thickness at the back and triceps region using Slaughter's formula.10 As with BMI, it was transformed to a z-score.
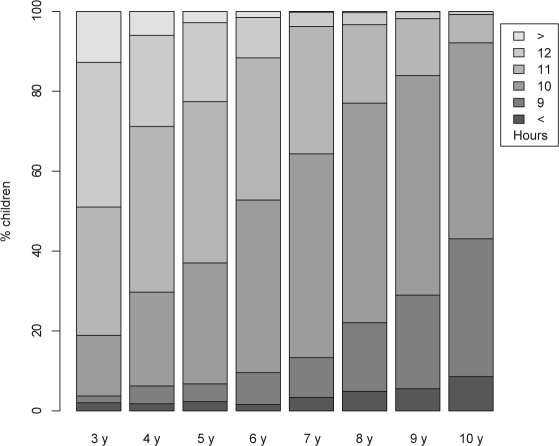
Sleep needs vary with age.11,12 As can be seen from Figure 1, it is hardly possibly to find cutpoints for sleep duration comparable through all age groups. For example, tertiles could be well applied in the 4-year-old children but do not fit in the 6-year-old children. To facilitate modelling we constructed a common measure sleep duration percentile (SDP): For each age, the cumulative frequency for the ordered discrete values of hours of sleep were computed. 11 hours of sleep in a 3-year-old child can be assigned to a cumulative frequency of 0.51, indicating, that such a child sleeps less than 1 – 0.51 = 49% and more than 18.9% (see leftmost bar in Fig. 1) of the population of his or her age. The real value for hours of sleep in a child given the discrete value of 11 h may vary between 10.5 and 11.5 h. In consequence, SDP was finally computed as the center of the adjacent cumulative frequencies, resulting in (0.189 + 0.510)/2 = 0.349 for the given example. Thus, SDP can be interpreted as a percentile, identfying the proportion of children with fewer hours of sleep. It should be re-emphasized that sleep duration was originally collected as discrete values (see end of Data collection), and the transformation does not result into a loss of precision.
Figure 1.
Sleeping hours per day for each age.
To model the association between sleep and body mass as precisely as possible, we screened a list of 24 potentially confounding variables, concerning parental BMI, social class, township size, smoking during pregnancy, breastfeeding, birth, leisure time sport activity, and electronic media consumption. Among variables of similar content (e.g., socioeconomic status (SES) of father vs. SES of mother, or exclusively breastfed until 4th vs. 6th month) the variable was selected whose inclusion in the model led to the greatest change in estimate for the regression parameter of SDP. Further selection criteria were completeness and additional variance explained. The variables finally included in our multivariable model are SES of father, classified as lower, middle (reference), or upper class according to Winkler's index13; BMI of mother (or father if BMI of mother was not available); smoking during pregnancy, categorized as “never” (reference), “yes, from time to time” or “yes, regularly”; exclusively breastfed until 4 months; birth weight in grams; and electronic media consumption classified as low (reference), intermediate, or high.
The variable electronic media consumption was used as provided by the Robert Koch Institute, which for ages 3–10 was compiled as follows: Hours watching TV and hours playing computer games were asked separately for weekdays and weekends by 4 multiple-choice questions, each allowing 1 of 5 answers, between never and > 4 h per day. The answers were assigned to values between zero and 5 hours and then added up over the 4 questions to estimate the electronic media consumption in hours per week. Finally, these were classified as low, intermediate, or high, using approximate tertiles for each age as cutoff values.
Statistical Methods
To provide adjusted effect estimates for sleep duration on z-scores for BMI and KFA, multivariable linear regression models were applied. Accordingly, adjusted effect estimates for sleep duration on overweight and obesity were calculated applying multivariable logistic regression. The correlations between potential confounders and the explanatory/outcome variables were quantified using Pearson r. While linear (ordinary least squares) regression models the conditional mean of an outcome variable Y for its whole distribution, quantile regression allows to model the conditional mean at any part of the distribution in relation to the exposure of interest. This method is warranted, if the effects of the exposure X are not homogeneous among the distribution of Y.14 This method was applied to identify potential causes for possible differences in the observed effects when using different outcome measures (metric vs. categorical outcomes).
Statistical analysis was done using R version 2.6.2 on Linux.15–18
RESULTS
The overall response in the KiGGS study was 66.6% (49:51 female-to-male)7 resulting in 17,641 children participating. Basic health-related variables were similar among responders and non-responders; in particular BMI and “smoking mother” showed no significant differences. Participation was homogeneous with regard to age (ranging from 63% to 70%) and gender (67% vs. 66% female vs. male). On average, mothers of responders completed more education than mothers of non-responders. The lower response proportion in resident aliens was compensated by oversampling.19 Of participating children, 8023 were between 3 and 10 years of age (Table 1). For 7767 of these, both SDP and BMI z-score were available, and for 7641, both SDP and KFA z-score were available, leaving between 844 and 1036 in each age. Figure 1 shows the amount of sleep decreasing with age.
Table 1.
Number of Observations, Proportion Male, Overweight, Obese, and Mean (± std) BMI and KFA for the Study Population
| age (years) | n | % male | % overweight | % obese | BMI girls boys | KFA girls boys |
|---|---|---|---|---|---|---|
| 3 | 934 | 51.2 | 8.9 | 1.6 | 15.80 (± 1.42) | 16.96 (± 3.33) |
| 15.93 (± 1.17) | 15.83 (± 3.33) | |||||
| 4 | 982 | 51.4 | 9.7 | 2.5 | 15.72 (± 1.66) | 17.10 (± 3.79) |
| 15.70 (± 1.56) | 15.39 (± 3.98) | |||||
| 5 | 953 | 51.1 | 12.7 | 2.7 | 15.62 (± 1.67) | 17.11 (± 4.05) |
| 15.66 (± 1.61) | 15.19 (± 4.46) | |||||
| 6 | 1006 | 51.4 | 13.5 | 3.5 | 15.88 (± 1.81) | 17.26 (± 4.53) |
| 16.03 (± 2.08) | 15.69 (± 5.36) | |||||
| 7 | 1026 | 51.4 | 17.1 | 5.1 | 16.24 (± 2.24) | 18.24 (± 5.56) |
| 16.57 (± 2.59) | 16.66 (± 6.28) | |||||
| 8 | 1037 | 51.2 | 20.7 | 6.3 | 16.85 (± 2.49) | 19.46 (± 6.16) |
| 17.10 (± 2.86) | 18.03 (± 7.35) | |||||
| 9 | 1067 | 51.4 | 20.1 | 5.2 | 17.64 (± 2.99) | 21.46 (± 7.41) |
| 17.45 (± 2.74) | 18.81 (± 7.85) | |||||
| 10 | 1018 | 51.4 | 22.6 | 4.9 | 18.29 (± 3.37) | 22.61 (± 7.61) |
| 18.37 (± 3.17) | 21.19 (± 9.26) |
Table 2 shows the associations of the primary explanatory variable SDP, the outcome measures, and selected potential confounders. Maternal BMI and birth weight were both inversely correlated with SDP and correlated with the outcome measures BMI and KFA z-score. In effect, if not adjusted, this can lead to an overestimation of the (inverse) association of sleep duration and body fat and mass. However, these correlations were weak. The KiGGS data confirm BMI and KFA increasing with decreasing SES. Children with a SES rated as middle showed the longest age specific sleep durations. High electronic media consumption was associated with lower sleep duration. Looking at the outcome measures, dose-response relationships to smoking in pregnancy and electronic media consumption are seen. The BMI and KFA z-scores correlated with r = 0.837 (P < 0.0001).
Table 2.
Potential Confounders of the Association Sleep Duration Percentile (SDP)–Overweight
| Confounder | SDP r, P | BMI z-score r, P | KFA z-score r, P | |||
|---|---|---|---|---|---|---|
| maternal BMI | −0.017, 0.124 | 0.253, <0.0001 | 0.227, <0.0001 | |||
| birth weight | −0.025, 0.027 | 0.175, <0.0001 | 0.094, <0.0001 | |||
| mean ± SEM | mean ± SEM | mean ± SEM | ||||
| SES | P = 0.002 | P <0.0001 | P <0.0001 | |||
| upper | 0.499 ± 0.006 | −0.185 ± 0.014 | −0.187 ± 0.015 | |||
| middle | 0.512 ± 0.005 | −0.032 ± 0.013 | −0.021 ± 0.013 | |||
| lower | 0.486 ± 0.006 | 0.149 ± 0.016 | 0.140 ± 0.016 | |||
| Smoking in pregnancy | P = 0.080 | P <0.0001 | P <0.0001 | |||
| no | 0.499 ± 0.003 | −0.049 ± 0.009 | −0.041 ± 0.009 | |||
| rarely | 0.515 ± 0.009 | 0.195 ± 0.025 | 0.160 ± 0.025 | |||
| regular | 0.481 ± 0.015 | 0.304 ± 0.048 | 0.244 ± 0.043 | |||
| Excl. breastfed | P = 0.244 | P <0.0001 | P <0.0001 | |||
| <4 m | 0.508 ± 0.005 | 0.048 ± 0.012 | 0.077 ± 0.012 | |||
| ≥ 4 m | 0.501 ± 0.004 | −0.083 ± 0.012 | −0.109 ± 0.012 | |||
| electronic media consumption | P <0.0001 | P <0.0001 | P <0.0001 | |||
| low | 0.531 ± 0.005 | −0.116 ± 0.013 | −0.135 ± 0.013 | |||
| middle | 0.513 ± 0.006 | −0.038 ± 0.014 | −0.024 ± 0.014 | |||
| high | 0.457 ± 0.006 | 0.145 ± 0.016 | 0.155 ± 0.016 |
For metric variables the correlation coefficients, for categorical variables means for SDP, body mass index (BMI), and proportion body fat (KFA) z-score by level of the confounder are given. Symbols: correlation coefficient r, its P-value P, standard error of mean SEM.
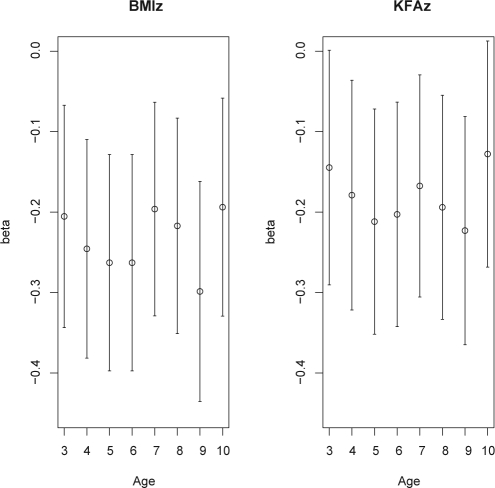
Results from the multivariable regression models for the BMI z-score as outcome are presented in Figure 2 (left part) and Table 3. Figure 2 illustrates, that the effects of SDP do not follow a systematic age dependency. Likewise, the interaction SDP*age is not significant (P = 0.873). It therefore seems justified to drop the interaction from the final model and to report a common effect estimate for all ages. The same applies to the model for the KFA z-score as outcome (see Fig. 2 [right part] and Table 4). For both outcomes, there was a significant association with SDP adjusted for confounders.
Figure 2.
Regression estimates with 95%-CI for the effect of sleep duration on BMI and KFA z-scores for each age. Covariates adjusted for are the same as listed in Table 3 adding SDP*age to allow for age dependent effects.
Table 3.
Multivariable Regression Results for the BMI z-score
| Explanatory variable | value/unit | β | standard error (β) | P-value |
|---|---|---|---|---|
| SDP | −0.235 | 0.044 | <0.0001 | |
| social class | Upper | −0.042 | 0.029 | 0.001 |
| middle (reference) | ||||
| Lower | 0.074 | 0.028 | ||
| (maternal) BMI | in kg/m2 | 0.045 | 0.003 | <0.0001 |
| smoking in pregnancy | no (reference) | <0.0001 | ||
| from time to time | 0.220 | 0.036 | ||
| Regularly | 0.259 | 0.056 | ||
| exclusively breastfed at least until 4th month | no (reference) | 0.105 | ||
| Yes | −0.039 | 0.024 | ||
| Birthweight | in 500 g | 0.130 | 0.010 | <0.0001 |
| electronic media | low (reference) | <0.0001 | ||
| intermediate | 0.072 | 0.028 | ||
| high | 0.127 | 0.030 |
For comparison, the unadjusted β for sleep duration percentile (SDP) is −0.273 [−0.356; −0.190].
Table 4.
Multivariable Regression Results for the Proportion Body Fat (KFA) z-score
| Explanatory variable | value/unit | β | standard error (β) | P-value |
|---|---|---|---|---|
| SDP | −0.182 | 0.046 | <0.0001 | |
| social class | Upper | −0.075 | 0.030 | <0.0001 |
| middle (reference) | ||||
| Lower | 0.085 | 0.029 | ||
| (maternal) BMI | in kg/m2 | 0.039 | 0.003 | <0.0001 |
| smoking in pregnancy | no (reference) | <0.0001 | ||
| from time to time | 0.125 | 0.037 | ||
| Regularly | 0.214 | 0.058 | ||
| exclusively breastfed at least until 4th month | no (reference) | <0.0001 | ||
| yes | −0.107 | 0.025 | ||
| birthweight | in 500 g | 0.062 | 0.011 | <0.0001 |
| electronic media | low (reference) | <0.0001 | ||
| intermediate | 0.104 | 0.029 | ||
| high | 0.152 | 0.031 |
For comparison, the unadjusted β for sleep duration percentile (SDP) is −0.234 [−0.317; −0.150].
Interpretation of β SDP
Since the continuous variable SDP ranges from 0 to 1, its coefficient β compares the children with the highest to those with the lowest sleep duration in an age group. To get a more intuitive measure, the following approximation is suggested: one hour of additional sleep which roughly corresponds to a upward shift of 30 points on the percentile scale is associated with a 0.30 *β = 0.30 * (−0.235) = 0.071 lower BMI z-score. For a 10-year-old girl, this would equal to a change in BMI of −0.237, given a 3.37 standard deviation for BMI in 10-year-old females.
For the sake of easier interpretation of the effect size of sleep on BMI and KFA z-score, we fit the same model using sleep duration in hours, as originally collected, instead of SDP. Since this raises the problem of confounding by age (which is circumvented by SDP) we had to add age to the model. Again, there was no systematic age dependency seen when plotting the effects of sleep duration for each age (in the same manner as in Fig. 2), and the interaction sleep duration*age was not significant and was therefore dropped from the final model. The resulting β-coefficients for sleep duration were −0.064 [95 % confidence interval −0.088; −0.039] and −0.054 [−0.080; −0.029], indicating a decrease of 0.064 in BMI z-score, and 0.054 in KFA z-score per additional hour of sleep. The unadjusted β-coefficients were −0.054 [−0.074; −0.035] and −0.050 [−0.070; −0.030], respectively.
Does Longer Sleep Duration Shift the Entire or Only Parts of the BMI/KFA Distribution?
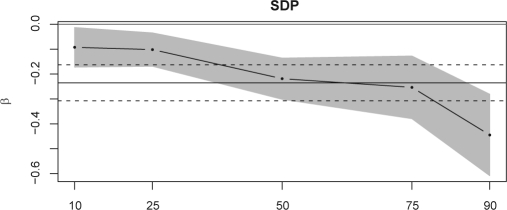
To assess whether the association of sleep duration and BMI is of the same sign and strength among the entire BMI distribution, we performed a quantile regression. Again taking the z-scores for all children from 3–10 y together, it seems that the middle and upper tail of the distribution are most affected by sleep duration (illustrated in Fig. 3, Table 5). The same is true for the distribution of KFA z-scores (Table 5). Table 5 lists quantile regression coefficients for the age-independent measure SDP as well as for sleeping hours as explanatory variables.
Figure 3.
Quantile regression results: effect of SDP (β) on the 10, 25, 50, 75, and 90% quantiles of the BMI z-score with 95%-CI (shaded area). In comparison, the horizontal lines denote the effect measure and its 95%-CI (dashed lines) obtained from ordinary regression. Covariates adjusted for are the same as in Table 3.
Table 5.
Quantile Regression Results: Effects (β) of SDP (left part) and sleeping hours (right part) on different quantiles of BMI (upper part) and KFA (lower part) z-score
| Quantile | SDP |
sleeping hours |
||||
|---|---|---|---|---|---|---|
| β | 95% CI |
β | 95% CI |
|||
| BMI z-score | ||||||
| 10% | −0.093 | −0.172 | −0.013 | −0.020 | −0.041 | 0.002 |
| 25% | −0.102 | −0.169 | −0.035 | −0.029 | −0.049 | −0.009 |
| 50% | −0.219 | −0.301 | −0.136 | −0.055 | −0.078 | −0.031 |
| 75% | −0.253 | −0.379 | −0.128 | −0.072 | −0.104 | −0.040 |
| 90% | −0.445 | −0.608 | −0.281 | −0.128 | −0.167 | −0.090 |
| KFA z-score | ||||||
| 10% | −0.064 | −0.131 | 0.003 | −0.019 | −0.036 | −0.001 |
| 25% | −0.062 | −0.133 | 0.009 | −0.011 | −0.033 | 0.010 |
| 50% | −0.128 | −0.220 | −0.036 | −0.038 | −0.065 | −0.011 |
| 75% | −0.240 | −0.373 | −0.107 | −0.060 | −0.097 | −0.022 |
| 90% | −0.222 | −0.416 | −0.027 | −0.074 | −0.122 | −0.026 |
Covariates adjusted for are the same as in Table 3. When using sleeping hours as explanatory variable, additional adjustment for age was done as explained under Interpretation of β SDP, 2nd paragraph.
Since most studies on sleep duration and weight status report risk estimates, we fitted a logistic model, using the same covariates as presented earlier to provide odds ratios for being overweight (OR = 0.56 [0.42; 0.74]) or obese (OR = 0.51 [0.31; 0.84]). Analogous to the paragraph “Interpretation of β SDP” the OR for being overweight associated with an increase in sleep duration equivalent to 0.30 on the percentile scale can be computed as 0.560.30 = 0.84 [0.76; 0.91]. If the same model is fitted using sleep duration instead of SDP, the resulting OR for being overweight per additional hour of sleep is 0.84 [0.78; 0.91], which is in excellent agreement with the “rule of thumb” for the interpretation of β SDP given above. For obesity, this OR = 0.82 [0.70; 0.95].
DISCUSSION
Our data support the hypothesis that sleep duration is inversely associated with obesity in children between 3 and 10 years of age. To assess whether this effect is age specific, we constructed a measure SDP enabling us to compare sleep durations over age groups. When taking BMI or KFA z-score as outcome, multivariable regression analysis revealed no systematic age dependency of the effect of sleep duration. Additionally, there are similar effects on BMI and KFA.
Interestingly, sleep duration is not homogeneously associated with BMI and KFA but mainly affects the upper tails of their distributions. This explains the different effect sizes obtained for sleep duration: the OR for overweight/obesity are pronounced while the changes in mean BMI are not impressive. It seems justified to publish OR as done in most publications on the subject from a biometric point of view. From the clinical perspective, the distinction of normal from not normal weight status also makes sense. However, if BMI itself is seen as a risk factor for e. g. cardiovascular diseases, it could be argued that even small shifts of the entire BMI distribution have great impact on the outcome on the population level. Therefore, modelling BMI as a metric outcome is interesting from a public health point of view. Furthermore, the fact, that sleep duration is associated with body composition primarily in children with higher BMI and also is reflected by KFA helps to generate hypotheses or to trade off already proposed hypotheses on the underlying physiological mechanisms.
The similar findings in BMI and KFA support the hypothesis that it is actually fat (and not other tissues) that is mainly influenced by sleep. Also, children with higher BMI are likely to eat more and be less physically active. Therefore they might be more sensitive to hormonal imbalances caused by short sleep, because the metabolic dysregulation multiplies the opulence of food supply.
Not surprisingly, many previous hypotheses on potential mediators focus on hormonal changes induced by sleep duration: Growth hormone (GH) is secreted during sleep and promotes body height. Sleep deprived subjects show lower GH levels.20 This could result in lower height and thus higher BMI. However, there was no positive association between SDP and height z-score (age and sex specific) in our data. Thus, the effect of sleep duration on weight status seems not to be mediated by length growth. Another effect of GH is the inhibition of adipose tissue lipoprotein lipase activity.21 It would be plausible, if subjects with higher body fat mass and thus higher amounts of this enzyme showed greater response to sleep induced GH changes. In summary, potential effects of decreased GH secretion related to sleep are more likely to be related to impact on lipoprotein lipase activity than length growth.
Ghrelin, an appetite stimulating hormone reported to be elevated by habitual short sleep duration,20 is discussed as a potential mediator of the association of sleep duration and weight status. However, with ghrelin being secreted by the stomach and being lowered in obese subjects,20 it can hardly explain the predominant effect of sleep duration on overweight subjects as found in the present study. While this does not rule out ghrelin being involved, our findings point to the relevance of other pathways regarding the association of sleep duration and weight status.
Leptin, an appetite lowering hormone, primarily secreted by adipose tissue is elevated in obesity and lowered in sleep deprivation. This mechanism is augmented by higher circulating levels of C-reactive protein (CRP), which binds leptin, found in obese and sleep-deprived subjects.22,23 Finally, this could mediate the stronger effect of sleep duration in subjects with higher body fat, as observed in our epidemiological data.
Regarding the last three pathways mentioned, a reanalysis of the data from previous laboratory studies stratified by weight status would be promising (in particular GH–lipoprotein lipase and leptin).
A strength of this study is the large sample size obtained from a survey nationally representative for Germany, a country with raising childhood obesity prevalence typical for the industrialized world.
The following limitations to our study have to be noted: sleep duration has been obtained from parental interview. The use of actigraphy or 24-h recalls are alternatives that might result in a more valid measurement but are not feasible in such a large scale study. For the same reason we had to rely on a simple measure of body fat by skin fold thickness, which despite of some imprecision on the individual level correlates well with reference methods and is established in epidemiological studies.24–26
A comprehensive discussion on the causality of sleep duration and obesity is beyond the scope of this article and can be found in a review by Marshall and colleagues.27 Since the present study is cross-sectional in design, reverse causation might be an issue. However, results of a recent longitudinal study in younger children support short sleep duration as a causal factor.5
Conclusions
Sleep needs change substantially within the first decade. The present study introduces a method to quantitatively compare the effect of sleep duration on weight status between different ages. Applied on data from a large, nationally representative sample it reveals no significant age dependency of the effect, which—in agreement with a recent review2—is seen up to an age of 9 to 10 years. Sleep duration is associated with higher body fat mass resulting in higher BMI and mainly affects heavier children, who are in the focus of clinical interest. Further research to understand the association of sleep duration and obesity should consider the alignment of physiological hypotheses with these epidemiological findings, especially the interaction with weight.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Wabitsch has participated in speaking engagements for Pfizer. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Andreas Beyerlein and Simon Röuckinger for helpful discussions. The KiGGS study was funded by the German Ministry of Health, the Ministry of Education and Research, and the Robert Koch Institute. O. Bayer is supported by LMUinnovativ research priority project MCHealth (sub-project II).
REFERENCES
- 1.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–74. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Taggart FM, Kandala N.-B, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nixon GM, Thompson JMD, Han DY, et al. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31:71–8. doi: 10.1093/sleep/31.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–11. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padez C, Mourao I, Moreira P, Rosado V. Long sleep duration and childhood overweight/obesity and body fat. Am J Hum Biol. 2009;21:371–6. doi: 10.1002/ajhb.20884. [DOI] [PubMed] [Google Scholar]
- 7.Kurth B.-M, Kamtsiuris P, Hölling H, et al. The challenge of comprehensively mapping children's health in a nation-wide health survey. Design of the German KiGGS-Study BMC Public Health. 2008;8:196. doi: 10.1186/1471-2458-8-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hölling H, Kamtsiuris P, Lange M, Thierfelder W, Thamm M, Schlack R. [The German Health Interview and Examination Survey for Children and Adolescents (KiGGS): study management and conduct of fieldwork] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50:557–66. doi: 10.1007/s00103-007-0216-8. [DOI] [PubMed] [Google Scholar]
- 9.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–23. [PubMed] [Google Scholar]
- 11.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 12.Russo PM, Bruni O, Lucidi F, Ferri R, Violani C. Sleep habits and circadian preference in Italian children and adolescents. J Sleep Res. 2007;16:163–9. doi: 10.1111/j.1365-2869.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 13.Winkler J, Stolzenberg H. [Social class index in the Federal Health Survey] Gesundheitswesen. 1999:S178–S183. [PubMed] [Google Scholar]
- 14.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62:511–7. doi: 10.1016/j.jclinepi.2008.05.015. e1. [DOI] [PubMed] [Google Scholar]
- 15.R-Development Core-Team. R: A Language and Environment for Statistical Computing. 2008. ISBN 3-900051-07-0 http://www.R-project.org. [Google Scholar]
- 16.Fox J, et al. car: Companion to Applied Regression. R package version 1.2-8. 2008. http://www.r-project.org, http://socserv.socsci.mcmaster.ca/jfox/
- 17.Harrel FE, et al. Hmisc: Harrell Miscellaneous. R package version 3.4-3. 2007. http://biostat.mc.vanderbilt.edu/s/Hmisc, http://biostat.mc.vanderbilt.edu/twiki/pub/Main/RS/sintro.pdf, http://biostat.mc.vanderbilt.edu/twiki/pub/Main/StatReport/summary.pdf, http://biostat.mc.vanderbilt.edu/trac/Hmisc.
- 18.Koenker R. quantreg: Quantile Regression. R package version 4.17. 2008. http://www.r-project.org.
- 19.Kamtsiuris P, Lange M, Rosario AS. [The German Health Interview and Examination Survey for Children and Adolescents (KiGGS): sample design, response and nonresponse analysis] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50:547–556. doi: 10.1007/s00103-007-0215-9. [DOI] [PubMed] [Google Scholar]
- 20.Wright KP., Jr Too little sleep: A risk factor for obesity? Obesity Management. 2006:140–145. [Google Scholar]
- 21.Richelsen B, Pedersen SB, Kristensen K, et al. Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism. 2000;49:906–11. doi: 10.1053/meta.2000.6738. [DOI] [PubMed] [Google Scholar]
- 22.Frey DJ, Fleshner M, Wright KP. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Knutson KL, Spiegel K, Penev P, Cauter EV. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91:612–17. doi: 10.1136/adc.2005.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goran MI. Measurement issues related to studies of childhood obesity: assessment of body composition, body fat distribution, physical activity, and food intake. Pediatrics. 1998;101:505–18. [PubMed] [Google Scholar]
- 26.Paineau D, Chiheb S, Banu I, et al. Comparison of field methods to estimate fat mass in children. Ann Hum Biol. 2008;35:185–97. doi: 10.1080/03014460801914874. [DOI] [PubMed] [Google Scholar]
- 27.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–98. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]