Abstract
Study objectives:
The ultradian NREM-REM sleep cycle and the circadian modulation of REM sleep sum to generate dreaming. Here we investigated age-related changes in dream recall, number of dreams, and emotional domain characteristics of dreaming during both NREM and REM sleep.
Design:
Analysis of dream recall and sleep EEG (NREM/REM sleep) during a 40-h multiple nap protocol (150 min of wakefulness and 75 min of sleep) under constant routine conditions.
Setting:
Centre for Chronobiology, Psychiatric Hospital of the University of Basel, Basel, Switzerland.
Participants:
Seventeen young (20-31 years) and 15 older (57-74 years) healthy volunteers
Interventions:
N/A.
Measurements and Results:
Dream recall and number of dreams varied significantly across the circadian cycle and between age groups, with older subjects exhibiting fewer dreams (P < 0.05), particularly after naps scheduled during the biological day, closely associated with the circadian rhythm of REM sleep. No significant age differences were observed for the emotional domain of dream content.
Conclusions:
Since aging was associated with attenuated amplitude in the circadian modulation of REM sleep, our data suggest that the age-related decrease in dream recall can result from an attenuated circadian modulation of REM sleep.
Citation:
Chellappa SL; Möunch M; Blatter K; Knoblauch V; Cajochen C. Does the circadian modulation of dream recall modify with age? SLEEP 2009;32(9):1201-1209.
Keywords: Dream recall, sleep mentation, NREM/REM sleep, melatonin, ageing, circadian rhythms
DREAMS HAVE AN INTERNAL STRUCTURE THAT REFLECTS ONGOING LARGE-SCALE NEURAL NETWORKS,1 WHICH MAINLY COMPRISE THE ULTRADIAN NREM-REM sleep cycle and the circadian activation of REM sleep. These combine to generate the main characteristics of dreaming.2 Accordingly, in periods of high sensory thresholds, the general cortical activation modulated by both circadian and NREM/REM sleep cycles creates an overall level of cortical activation that leads to dreaming. Therefore, circadian-driven changes in cortical activity can play a critical role in dream activation.3–5
In a study purported to control both ultradian and circadian components of dreaming, both sleep onset and offset were intentionally delayed by 3 hours.3 As a consequence, REM and NREM dreams occurred later than usual in order to coincide with the rising phase of the hypothesized circadian influence on dreaming, near the time of core body temperature nadir. A comparison of both REM and NREM dream reports from the phase-delayed condition with control reports from non-delayed sleep indicated that delayed dream reports were more visually and emotionally intense, particularly when collected later at night. Thus, the relative influence of time of day in relation to sleep stage on dream characteristics appears to play a pivotal role in dreaming. Furthermore, the emotional domain of dreaming appears to be dependent on sleep-stage specific neural activation patterns.6 It seems likely that motivation and emotionality, considered more prominent in REM dreams, may be linked to regional brain activation patterns specific to REM sleep.7 One of the theoretical underpinnings for this assumption builds on recent imaging findings confirming that general cortical activation, as measured by global cerebral blood flow, is greater in REM sleep than in NREM sleep.8 Limbic areas such as the amygdala were described as more active in REM sleep than in NREM sleep wake.1,8 While this does not necessarily imply that NREM sleep cannot be associated with dreaming, it may account for some of the differences in REM/NREM dream recall.7
Regarding age-related changes in dream recall, there is evidence for a dream recall decline with advancing age.9–12 Given that circadian rhythms have decreased amplitude and may be phase advanced in older subjects,13 it can be hypothesized that the circadian-coupled peak in dream intensification might be attenuated and occur earlier than in young subjects during the sleep episode. This may partly explain why dream recall is decreased in older individuals. Albeit the strong link between dreaming and endogenous rhythmic events that define REM sleep, there are few stringent studies that link circadian rhythms, aging and dreaming per se. Thus, in the current study, we investigated age-related changes in the circadian modulation of dream recall, number of dreams and emotional domain characteristics of dreaming, during both NREM sleep and REM sleep, under stringently controlled laboratory conditions.
METHODS
Study participants
All study participants were recruited via advertisements at different Swiss universities and in newspapers. Only candidates with a Pittsburgh sleep quality index (PSQI) score ≤ 514 and no extreme chronotype, (ratings between 14 and 21 points on the morning-evening M/E questionnaire15) were selected. All potential study participants were questioned about their sleep quality, life habits and health state. Exclusion criteria were smoking, medication or drug consumption, shift work within the last 3 months, and transmeridian flights during the month prior to the study. Volunteers underwent physical examination, interview, neuropsychological assessment (only for the older cohort to exclude motor, attention or memory impairments), and polysomnographically recorded adaptation night, in order to exclude sleep disorders. Inclusion criteria were sleep efficiency ≥ 80%, < 10 periodic leg movements per hour and an apnea-hypopnea index < 10. Only participants without any medication (with the exception of 4 young women using oral contraceptives) were included in the study. Young females started the study on day 1–5 after menses onset during the follicular phase of their menstrual cycle.
Seventeen healthy young (9 women, 8 men, age range 20–31 years, mean: 25.0 ± 3.3 SD) and 15 healthy older volunteers (7 women, 8 men, age range 57–74 years, mean: 65.1 ± 5.6 SD) were included in the study. All participants gave written informed consent. The study protocol, screening questionnaires and consent form were approved by the local ethics committee and conformed to the Declaration of Helsinki.
Study Design
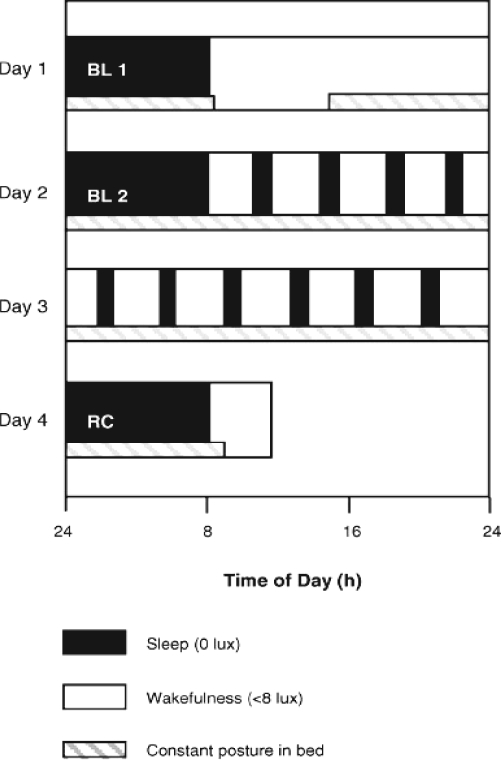
One week prior to the study (baseline week), the participants were requested to abstain from excessive caffeine and alcohol consumption (one caffeine-containing beverage per day at most and < 5 alcoholic beverages per week). They were instructed to keep a regular sleep-wake schedule during the baseline week at home (bedtimes and wake times within ± 30 minutes of a self-selected target time between 22:00 and 02:00) prior to admission to the laboratory. Compliance was checked by sleep logs and ambulatory activity measurements (wrist activity monitor, Cambridge Neurotechnology Ltd, UK). The timing of the sleep-wake schedule during the protocol was adjusted to individual habitual bedtimes. For each participant, habitual bedtime was calculated by centering the approximately 8-h sleep episodes during the baseline week at their midpoint. The inpatient part of the protocol comprised 2 baseline sleep episodes in the chronobiology laboratory, followed by a 40-h multiple nap protocol, with 10 alternating sleep-wake cycles of 75/150 minutes duration each and one recovery sleep episode (Figure 1). Baseline and recovery nights were scheduled at individual habitual bedtimes. Polysomnographic recordings and constant posture started in the afternoon after the first baseline night. Thereafter, participants remained under constant routine conditions (constant dim light levels < 8 lux during scheduled wakefulness, semi-recumbent posture in bed, food and liquid intake at regular intervals, no time cues).16–18 During scheduled sleep episodes a minor shift (45 degrees up) in the supine posture was allowed, and the lights were off (0 lux). Older participants received a daily low-dose subcutaneous heparin injection (Fragmin, 0.2 mL, 2500 IE/UI, Pfizer AG, Switzerland) to prevent potential venous thrombosis.
Figure 1.
Overview of the 4-day inpatient part of the study protocol. Black bars (0 lux) indicate the sleep episodes and white bars the wake episodes (< 8 lux). The hatched bars indicate controlled posture (semi-recumbent during wakefulness and supine during sleep). BL = baseline night, RC = recovery night (modified from Munch et al., 200518).
Polysomnographic Measures
Sleep was polysomnographically recorded with the VITAPORT ambulatory system (Vitaport-3 digital recorder, TEMEC Instruments B.V., Kerkrade, the Netherlands). Twelve EEGs channels, 2 electroculograms, a submental electromyogram, and an electrocardiogram were recorded. All signals were low-pass filtered at 30 Hz (fourth order Bessel type anti-aliasing, total 24 dB/Oct) at a time constant of 1.0 s. After online digitization by using a 12 bit AD converter (0.15V/bit) in the range of 610V and a sampling rate at 128 Hz for the EEG, the raw signals were stored on a Flash RAMCard (Viking, USA) and later downloaded to a PC hard drive. Sleep stages were visually scored per 20-s epochs (Vitaport Paperless Sleep Scoring Software) according to standard criteria.19
A nap trial that contained only REM sleep in the last 15 minutes of a scheduled 75-minute nap was defined as a REM nap and a nap trial with NREM sleep in the last 15 minutes was defined as a NREM nap. “Wakefulness naps” were defined as nap trials not containing either NREM or REM sleep stages and were excluded from further analyses. This criterion was based on a prior definition of NREM and REM naps, in which 20-min naps were employed.20 Since the current study included 75-min naps, only the last 15 min were considered for the REM sleep and NREM sleep stages, instead of 20 minutes, since the likelihood of having 20-min naps exclusively with REM sleep would be substantially reduced. Dreams from NREM and REM naps were expressed as dream recall, number of dreams, and dream characteristics on a point-scale of the Sleep Mentation Questionnaire ranging from 0 (less) to 3 (more), at each given nap (see methods section on dream recall).
Sleep stages were visually scored per 20-s epochs. NREM sleep (i.e., stages 1-4) and REM sleep were expressed as the percentage of total sleep time (i.e., stages 1-4 and REM sleep) per nap before averaging over subjects.
Subjective Sleepiness Ratings
Subjective sleepiness was assessed by the Karolinska Sleepiness Scale (KSS) on a point scale ranging from 1 (very alert) to 9 (very sleepy) every 30 min during scheduled wakefulness.21 To test possible repercussions of age-related changes in sleep inertia on dream recall, only the very first KSS rating taken (5 min after lights on) after each nap was considered for analysis.
Salivary Melatonin and Classification of Biological Day and Night
Saliva collections were scheduled during wakefulness at the same time intervals (every 30 min). A direct double-antibody radioimmunoassay was utilized for the melatonin assay (by gas chromatography–mass spectroscopy with an analytical least detectable dose of 0.65 pm/mL; Böuhlmann Laboratory, Schöonenbuch, Switzerland). 22 For mean melatonin levels, values of all samples between the upward- and downward-mean crossing points were averaged per subject and age group. The mean melatonin concentration was calculated for each subject. A nap was classified as a night nap (biological night) if the melatonin concentration of the last saliva sample prior to the nap was above the individual mean; otherwise, it was classified as a day nap (biological day).18,23
Dream Recall
Dream recall was assessed immediately at the end of each nap trial (10 naps in total) with the Sleep Mentation Questionnaire, which addresses main characteristics of dream recall, such as number of dreams, emotionality, vividness, pleasantness, hostility, and colourfulness. Questions were not asked about detailed dream content, as this could have influenced dream reports at successive nap trials. The dream recall questionnaire comprised the following questions:
-
Q1.
“How much did you dream?” (1: greatly, 2: fairly, 3: relatively little, 4: not at all)
-
Q2.
“How many different dreams can you remember having?” (none [0], 1, 2, 3, 4, 5, 6, more than 6). When the reply to Q1 and Q2 was 4 and 0, respectively, Q3-7 were not asked. Otherwise, Q2 was followed by Q3-7. Hence, participants were considered to have had dream recall if their response to Q1 and Q2 was not, respectively, 4 and 0.
-
Q3.
“How emotional was your dream?” (1: greatly, 2: moderately, 3: little, 4: not at all)
-
Q4.
How vivid was the dream?” (1: very vivid, 2: moderately, 3: little, 4: not vivid)
-
Q5.
“How pleasant was the dream?” (1: very pleasant, 2: moderately pleasant, 3: moderately unpleasant, 4: very unpleasant)
-
Q6.
“How much hostility was in your dream?” (1: greatly, 2: fairly, 3: relatively little, 4: not at all)
-
Q7.
“Did you dream in colour?” (1: greatly, 2: fairly, 3: relatively little, 4: not at all)
The participants' responses to Q1 and Q2 were averaged separately for REM naps and NREM naps. Likewise, participants' mean scores for Q3-Q7 after REM and NREM naps were calculated.
For analysis of the dream variables, the point scale from 1 (more) to 4 (less) of this dream questionnaire was inverted from 0 (less) to 3 (more) for statistical comparisons. Afterwards, for the analysis of dream characteristics, an emotional composite score was built with the following 5 items: emotionality, vividness, pleasantness, hostility, and colourfulness. Since dream characteristics rely on number of dreams, the emotional composite score was adjusted to the individual mean values of number of dreams.
Statistical Analysis
For all analysis, the statistical packages SAS (SAS Institute Inc., Cary, NC, USA; Version 6.12) and Statistica (Stat-Soft Inc., 2000–2004, STATISTICA for Windows, Tulsa, OK, USA) were utilized. Visually scored sleep stages per nap sequence and the dream characteristics after naps and baseline sleep episode were tested with a Mann–Whitney U test for the age group comparisons, since the data did not reach the criterion for a parametric distribution. For the comparison of recall, number, and the emotional composite score of dreams across naps with baseline values in the young cohort and in the older cohort, the Wilcoxon matched pairs test was utilized. For group differences of dream variables both in relation to baseline and within naps, the mixed-model analyses of variance for repeated measures, r-ANOVA (PROC Mixed), with factors “age” (young and older) and “time” (10 naps) was performed. For group differences of dream variables during the biological day and biological night, PROC Mixed was utilized considering factors “age” (young and older) and “condition” (subjective day/night). REM and NREM sleep duration were included as covariates in this model, in which the same factors were utilized. For group differences of the KSS in relation to the naps, PROC Mixed was performed considering factors “age” (young and older) and “time” (10 naps). For dream recall after NREM and REM naps averaged across the 10 naps, PROC Mixed was performed considering factors “age” (young and older) and “type” (NREM and REM naps). Contrasts were assessed with the LSMEANS statement and all P-values for the r-ANOVA were based on the Kenward-Roger corrected degrees of freedom. For the adjustment for post-hoc multiple comparisons, the Tukey-Kramer test for unbalanced data was utilized in the PROC Mixed.
RESULTS
Age-Related Changes in Dream Recall After Baseline Sleep and Nap Episodes
Baseline Sleep Episode
Baseline dream values, conducted immediately after baseline night, encompassed dream recall, number of dreams, and dream emotionality on the sleep mentation questionnaire. No significant age-related differences were observed with respect to dream recall (older: 1.1 ± 0.9, young: 1.7 ± 0.9, Mann-Whitney U test, P > 0.1) and number of dreams (older: 0.9 ± 0.9, young: 1.3 ± 1.1, Mann-Whitney U test, P > 0.1). However, older individuals exhibited a comparatively lower emotional composite score (older: 0.78 ± 0.6, young: 1.0 ± 0.7, Mann-Whitney U test; P < 0.1).
Dream recall, number of dreams and the emotional composite score expressed as percentage of dream recall averaged across the 10 naps in relation to baseline recall (relative values) are shown in Table 1. Baseline values were deemed as 100% for both age groups. Comparison between baseline and nap values for the young group yielded a significant decrease for dream recall and an increase for number of dreams (Wilcoxon paired test, P < 0.05), with no differences for the emotional composite score. For the older cohort, a trend for lesser dream recall was elicited for the naps in comparison to baseline (Wilcoxon paired test P < 0.1), with no further differences for the other dream variables (Table 1). Comparison between age groups yielded no significant differences for any of the aforementioned dream variables (Mann-Whitney U test, P > 0.1). Therefore, when adjusted for baseline levels, both age groups recalled dreams similarly, and the emotional composite score of their dreams did not significantly differ. No gender differences were seen in baseline values or in the remainder of this data set.
Table 1.
Dream Recall by Age Group Averaged Across Naps as Percentage of Baseline Values for Dream Recall
| Young | Older | Young vs. Older** | Difference from baseline: Young*** | Difference from baseline: Older**** | |
|---|---|---|---|---|---|
| Baseline | 100 | 100 | |||
| Dream recall (Q1) | 82 ± 7.2* | 80 ± 7.3 | n.s | <0.05 | 0.07 |
| Number of dreams (Q2) | 138 ± 9.6 | 115 ± 6 | n.s | <0.05 | n.s |
| Emotional composite (EC) | 111 ± 8.9 | 103 ± 8.2 | n.s | n.s | n.s |
Mean ± Standard error of mean;
P-values for comparison of Q1, Q2, and EC between the young and older cohort (Mann-Whitney U test; n.s = P > 0.1);
P-values for comparison of Q1, Q2, and EC across naps with baseline values in the young cohort (Wilcoxon matched pairs test);
P-values for comparison of Q1, Q2, and EC across naps with baseline values in the older cohort (Wilcoxon matched pairs test)
Naps
In a second step, age-group specific differences were compared with r-ANOVA with factors “age” and “time (naps).” For dream recall, significant differences were elicited for main effects “age” (r-ANOVA, F1,60 = 17.3, P < 0.001) and “time” (r-ANOVA, F9,250 = 5.1, P < 0.001). Likewise, number of dreams yielded significant differences for main effects “age” (r-ANOVA, F1,80 = 12.8, P < 0.001) and “time” (r-ANOVA, F9,246 = 3.2, P < 0.001). For the emotional composite only the main factor “time” yielded significance (r-ANOVA, F9,205 = 4.1, P < 0.001). The interaction “age” × “time” yielded no significant differences for any of the dream variables.
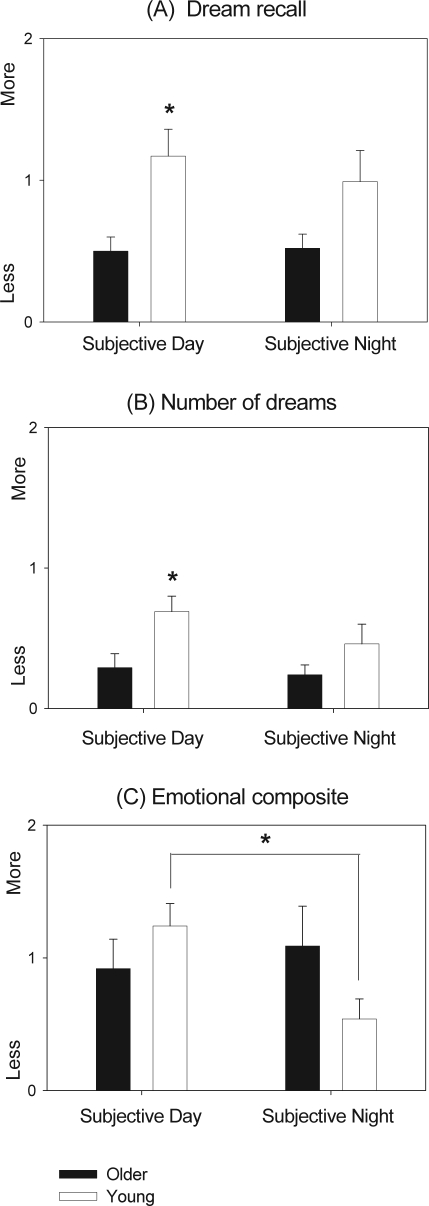
In this step, dream recall was deemed as the amount of recall at each of the given 10 naps on a same point-scale questionnaire value (0-3) for each dream variable in both age groups, with the emotional composite score adjusted to the individual mean number of dreams. Averaging these data for young and older subjects and for subjective day and night, respectively when endogenous melatonin levels are lowest and highest (Figure 2), indicated that dream recall (r-ANOVA: main effect “age”; F1,60 = 11.51, P < 0.05) and number of dreams (r-ANOVA: main effect “age”; F1,60 = 8.21, P < 0.05) were significantly higher in young individuals during subjective day in detriment to older subjects. However, no significant interactions were elicited for either of these variables. Regarding the emotional composite score, there was a significant interaction (age × subjective day/night) between young and older individuals (r-ANOVA: interaction “age”; × “condition” F1,56 = 4.32, P < 0.05), with younger subjects exhibiting a higher emotional composite score during the subjective day than the subjective night. Figure 3 illustrates the time course of dream recall, number of dreams, and emotional composite score for both age groups.
Figure 2.
Dream recall, number of dreams, and emotional composite score averaged for young (white bars, n= 17) and older subjects (black bars, n= 15) for subjective day and subjective night. Scores are presented as mean values ± SEM. *P < 0.05; °P < 0.1.
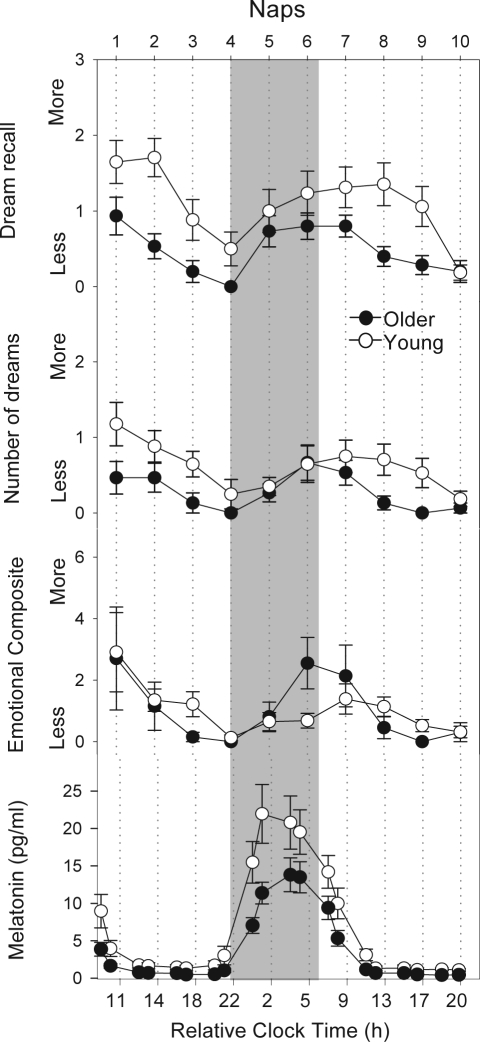
Figure 3.
Time course of dream recall, number of dreams, emotional composite score and mean ± SEM. values of melatonin secretion in both age groups during the 40-h nap protocol between young (white circles, n = 17) and older volunteers (black circles, n = 15). The grey bar illustrates biological night. Scores are presented as mean values ± SEM.
Time Course of Salivary Melatonin
The time course of melatonin concentrations throughout the naps is illustrated in Figure 3 (last panel). Older participants had significantly lower nighttime salivary melatonin levels in relation to the young cohort (t-test 2-tailed for independent samples; 11.4 ± 6.1 older vs.18.9 ± 12.6 pg/mL young group; mean ± SEM; P < 0.05). When considering dream recall, the significant age-related differences were mostly observed in naps 1, 2, 3, and 9 for dream recall and dream characteristics. Interestingly, these significant differences occurred exclusively during the biological day, when saliva melatonin was at the lowest levels.
Time Course of TST, NREM Sleep, and REM Sleep Within Naps
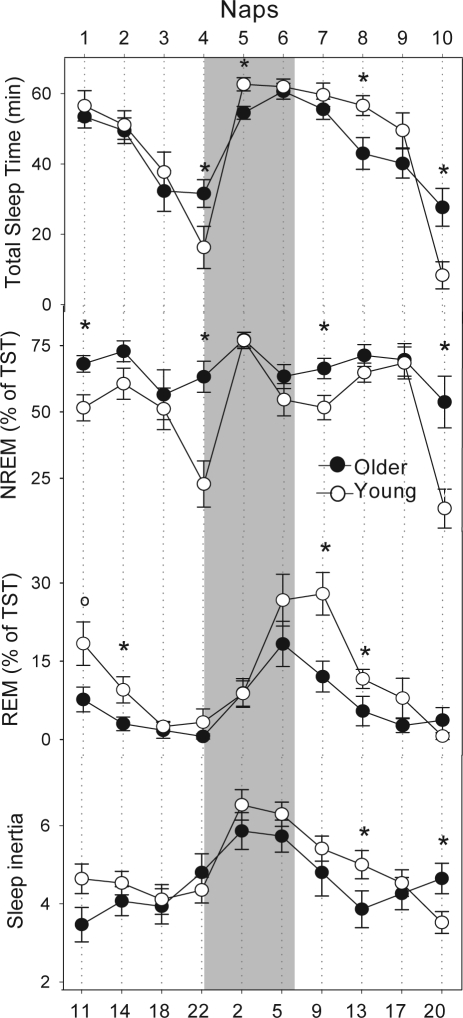
The analysis of total sleep time (TST) revealed that the young cohort slept significantly less during naps 4 and 10 (wake maintenance zone) and more during naps 5 and 8 (Mann-Whitney U test; P < 0.05; Figure 4, first panel). Older subjects exhibited comparatively more NREM sleep during naps 1, 4, 7 and 10 than the young cohort (P < 0.05; Figure 4, second panel). On the other hand, older volunteers had significantly less REM sleep than young volunteers during naps 2, 7, and 8 (P < 0.05; Figure 4, third panel) and a tendency during the first nap (P < 0.1). Nonetheless, both age groups exhibited an apparent circadian modulation of REM sleep. For more detailed information on age-related changes on sleep structure and sleep EEG characteristics, please see Möunch et al. 2005.18
Figure 4.
The first panel shows total sleep time (TST), the second depicts NREM sleep, the third shows the REM sleep and the last panel represents the Karolinska Sleepiness Scale (KSS) ratings across the 40-h nap protocol between young (white circles, n = 17) and older volunteers (black circles, n = 15). The grey bar illustrates the biological night. Mean ± SEM. *P < 0.05.
In order to discriminate the contributions of REM and NREM sleep duration during the last 15 minutes of a given nap on dream recall, these sleep parameters were included as covariates for both age groups and averaged across the naps. The analysis of covariance of REM and NREM sleep duration revealed that NREM sleep significantly modulated dream recall (F1,250 = 11.8, P < 0.001), albeit not number of dreams (F1,286 = 0.14, P > 0.1) or the emotional composite score (F1,244 = 1.6, P > 0.1). REM sleep significantly modulated dream recall (F1,258 = 21.4, P < 0.001), number of dreams (F1,291 = 58.7, P < 0.001) and the emotional composite score (F1,244 = 7.3, P < 0.05). In addition, all these dream variables varied significantly across the circadian cycle (r-ANOVA, main factor: “time” (naps): P < 0.05), with a circadian modulation closely associated with the time course of REM sleep throughout the naps.
Time Course of Sleep Inertia
The very first sleepiness rating (KSS) after each nap, as an index for sleep inertia, of both age groups exhibited a clear circadian modulation, with highest sleepiness levels occurring at around naps 5 to 7 (time of day: day 1: 22:00 to day 2: 05:00) (Figure 4, fourth panel). Although older subjects appeared to be comparatively less sleepy, no significant age-related effects were observed (2-way r-ANOVA, factor “age,” F1,59.5 = 1.57; P = 0.21). The time course of KSS ratings yielded significance for naps (2-way r-ANOVA, factor “time” [naps], F9,259 = 7.16; P < 0.001) as well as for the interaction “age” × “time” (2-way r-ANOVA; F9,259 = 2.06, P = 0.03). Sleep inertia after nap 8 was significantly higher in younger subjects, whereas after nap 10 (wake maintenance zone), older subjects experienced more sleep inertia than young individuals.
Dream Recall from NREM and REM Naps
Taken together, the young (n = 17) and older (n = 15) cohorts had a total of 170 and 150 scheduled naps, respectively. According to our criteria, naps among the young cohort included 48.3% NREM naps, 27.9% REM naps, and 23.8% wakefulness naps. The older cohort had 61.2% NREM naps, 14.3% REM naps, and 24.5% wakefulness naps. When comparing the types of naps between age groups, older subjects had significantly fewer REM naps than young subjects (Mann-Whitney U test; P < 0.05), while having more NREM naps instead (Mann-Whitney U test; P < 0.1). There were no significant age-related differences for wakefulness naps. Dream recall analysis from REM and NREM naps revealed an overall recall rate of 84% and 57% for REM and NREM naps, respectively.
Age-group specific differences were compared by r-ANOVA with factors “age” and “type” (NREM sleep/REM sleep). For dream recall, the main effects “age” (r-ANOVA, F1,79 = 7.9, P < 0.05) and “type” (r-ANOVA, F2,79 = 19, P < 0.001) yielded significant differences. Similarly, for number of dreams, significant differences were elicited for main effects “age” (r-ANOVA, F1,79 = 5.6, P < 0.05) and “type” (r-ANOVA, F2,79 = 9.8, P < 0.001). The interaction “age” × “type” yielded significant differences only for dream recall (r-ANOVA, F2,79 = 3.1, P < 0.05). With respect to the emotional composite score, factor “type” was significant (r-ANOVA, F2,75 = 5.1, P < 0.05).
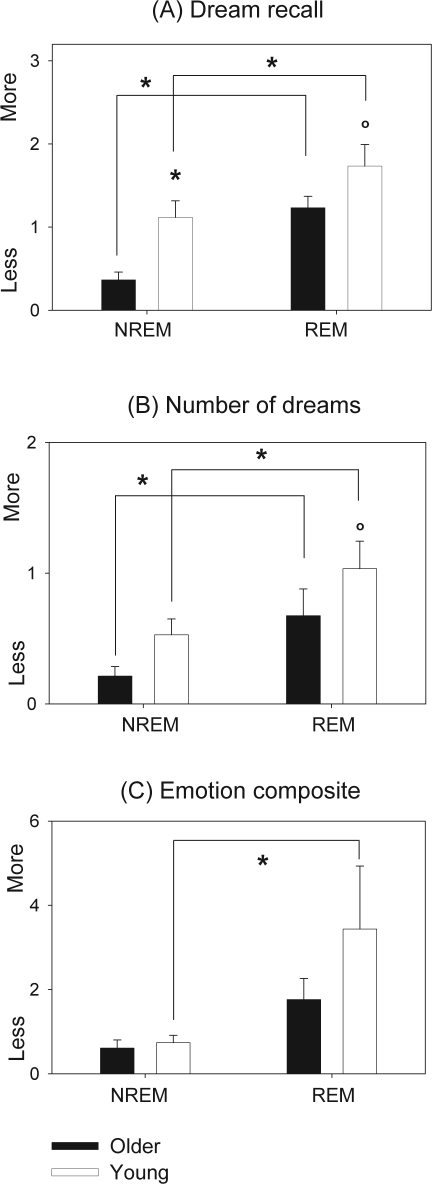
Older individuals had significantly less dream recall after NREM naps (older: 0.36 ± 0.09, young: 1.11 ± 0.2, r-ANOVA main factor “age,” P < 0.05) and a tendency for less dream recall after REM naps (older: 1.23 ± 0.13, young: 1.73 ± 0.25, r-ANOVA main factor “age,” P < 0.1) (Figure 5A). Additionally, both older (r-ANOVA interaction “age” × “time” [naps], P < 0.001), and young individuals (r-ANOVA interaction “age” × “time” [naps], P < 0.05) had significantly less dream recall after NREM naps than after REM naps (Figure 5A). A trend for fewer dreams was yielded for older individuals after REM naps (older: 0.67 ± 0.2, young: 1.03 ± 0.21, r-ANOVA main factor “age,” P < 0.1) (Figure 5B). Furthermore, both older and young individuals had significantly fewer dreams after NREM naps than REM naps (P < 0.05) (Figure 5B). No age-related differences were observed for the emotional composite score after NREM and REM naps (Figure 5C). However, young individuals exhibited a significantly higher emotional composite score after REM naps than NREM naps (P < 0.001) (Figure 5C).
Figure 5.
Dream recall, number of dreams and the emotional composite score for NREM and REM naps in young (white bars) and older volunteers (black bars). Scores are presented as mean values ± SEM. *P < 0.05.
DISCUSSION
The time course of dream recall across a 40-h nap protocol followed the clear circadian profile of sleep propensity. This circadian modulation of dream recall showed an age-dependent attenuation, as indexed by less dream recall in older subjects, particularly during naps scheduled during the biological day when endogenous melatonin levels were low. The emotional composite score, which encompasses emotionality, vividness, pleasantness, hostility and colourfulness of dream content, yielded no age-related differences. In other words, older subjects did not exhibit a decrease in the emotional domain of dreaming as compared to the young cohort, when adjusted for number of dreams. Our data indicate that the observed age-related decline in dream recall and number of dreams are related to the concomitant age-related decrease in circadian REM sleep propensity.
Is Aging Per Se Accountable for Lower Dream Recall?
Dream recall and number of dreams decreased with age, which corroborates with previous findings that support fewer dreams with increasing age.10,11 It is assumed that, while sensory input is attenuated in sleep, dreaming is generated by cortical activation driven by ultradian and circadian activation cycles.2 This is strongly supported by the fact that dreaming can fluctuate in concert with the circadian activation, irrespective of REM sleep-specific regional activation pattern.24 However, this assumption is controversial, since one could argue that dreaming processes are likely to be dependent on regional subcortical brain activation specific to REM sleep.7 It might be that the age-related changes in both sleep structure and consolidation, caused by reduction in the circadian force that opposes homeostatic pressure,18,25 may account for age-related effects in dream recall. Since dreaming depends on circadian dependent brain activation, this assumption can be further supported by the fact that, throughout the 40-h multiple-nap protocol, the older cohort showed a diminished circadian rhythm of REM sleep. As dream recall is robustly connected with REM sleep, it can be hypothesized that an attenuated circadian modulation of REM sleep in older individuals can have repercussions on dreaming process.
Is Dream Recall Modulated in a Circadian Manner?
In this study, dream recall and number of dreams varied significantly across the naps, with an age-related difference, particularly in naps 1, 2, 8, and 9, during the biological day—the time of lowest levels of saliva melatonin (respectively, time of day: day 1, 11:00, 14:00, and day 2, 13:00 and 17:00). As illustrated in the results, the age-related changes in dream recall were more apparent when a similar age difference was observed for the REM sleep, which occurred during the biological day. A possible hypothesis is that sleeping outside the time window of endogenous melatonin secretion leads to fewer sleep spindles,23 which in turn may reflect higher brain activation and, thus, an increased likelihood for dream mentation. Nevertheless, the reason for higher dream recall when melatonin levels are lowest remains to be clarified.
Older participants appeared to exhibit less sleep inertia than young individuals, mostly in nap 8 (time of day: day 2, 13:00). Evidence suggests that cognitive performance during or shortly after awakening can play a key role for dream recall, since, within this state, cognitive functioning can be impaired by the effects of sleep inertia.26 Another point to be addressed is that older subjects are more likely to experience sleepiness and increased total sleep time during the wake maintenance zone,18 since the circadian arousal signal in the evening can fail to adequately oppose the increasing homeostatic sleep pressure in older individuals.25 However, when considering only the very first KSS rating after the naps, older subjects did not appear to be sleepier than young individuals. Taken together, our results indicate that, although older subjects were less sleepy, they still had fewer dreams, which argues against an age-related decrease in dream recall caused by sleep inertia.
One aspect to be considered is that in older subjects circadian rhythms may have a decrease in amplitude and/or be phase advanced.27,28,13 Evidence that the circadian pacemaker plays a pivotal role in sleep regulation has led to the hypothesis that age differences in sleep can be mediated by changes in the circadian timing system.27,28 Accordingly, an age-related reduction in the amplitude of the circadian signal can imply an attenuation of the circadian drive for sleep in the morning hours. This can lead to an internal circadian advance, relative to the core body temperature and melatonin rhythm, of the propensity to awaken in older people.28 This may partly explain the overall decrease in retrospectively estimated dream recall with advanced age. For instance, if dream intensification is phase advanced, spontaneous morning dream recall should be lower.29 In our study, older individuals appeared to exhibit a higher emotional composite score earlier (around 05:00) than young subjects (after 05:00). Taken together, it can be speculated that older individuals have a phase advance and a decrease in amplitude in their dream recall.
What Do Age Effects in Dream Recall of NREM and REM Sleep Mirror?
Older individuals recalled fewer dreams after both NREM and REM naps than young individuals. The emotional composite score did not yield age-related differences with respect to NREM and REM naps, although the young cohort exhibited significantly more emotion scores after REM naps. Furthermore, both age groups recalled more dreams after REM naps, with a comparative decrease in the older cohort. Taken together, it can be inferred that dream recall was higher after REM naps, although it also occurred after NREM sleep.
The initial association between REM sleep and dreams30 stimulated studies designed to clarify the relationship between sleep physiology and dreams. A theoretical assumption emerged, according to which dreaming was viewed as an exclusive REM sleep domain.31 Nonetheless, various studies have challenged the REM sleep-dreaming perspective, by demonstrating dream recall from NREM sleep stages.32,33,20 As a result, this has raised the question of to what extent REM sleep-specific physiology constitutes a satisfactory explanation of dreaming. Hence, one of the current debates has shifted to how characteristics of NREM and REM dreams differ and to what might be the neurobiological basis of these differences.34
It is well-established that the regional activation pattern of REM sleep can modulate dream recall.35,8 As hypothesized, dreaming is selectively enhanced during REM sleep, probably due to the heightened limbic activity characteristic of REM sleep.35,8,1 This adds to the activation of the dorsolateral prefrontal cortex, associated with higher cognitive functions in REM sleep. Thus, the cortical activation appears to be biased toward dreaming processes in this state.36 This can explain the higher propensity for dreaming in REM sleep, which in our study was demonstrated by the fact that REM sleep significantly modulated dream recall, number of dreams and the emotional composite score. In the realm of age effects, our data indicated no age-related changes in the emotional domain of dreaming. In other words, older subjects do not necessarily have less emotional dreams than young subjects. Thus, it can be argued that the apparent decrease of these dream characteristics, as described previously,9–11 is likely to happen since older individuals report less dream recall and number of dreams.
As indicated in the covariate analysis, NREM sleep strongly modulated dream recall, albeit not with respect to number of dreams and the emotional composite score. Within a theoretical framework, it is possible that the intrinsic thalamocortical rhythms associated with NREM sleep, such as higher levels of sleep spindles and delta waves, may interfere with ongoing cognitive activity during NREM sleep, leading to fewer dreams in this state.24 Likewise, one could argue that dream recall after NREM sleep can be attributed to prior REM sleep.2 In our study, only the last 15 minutes of each nap were utilized to determine if a given nap was NREM or REM, thus not excluding the possibility of having REM sleep before a NREM nap. Does this imply that NREM sleep is not associated with dreaming? Perhaps not. For instance, dreaming scores elicited for NREM dream recall can be distributed sinusoidally across the 24-h day, with an acrophase at 08:00h20. REM dream scores were high for the entire diurnal period and then dropped markedly. Since NREM dreaming curve paralleled the REM sleep curve, as indexed by time-in-stage prior to awakening, it is likely that the dreaming output from REM and NREM sleep are influenced by the same underlying circadian oscillator.37 Thus, it might be that these cycles sum in such a fashion that NREM sleep can support dream recall when there is circadian activation for dreaming. Taken together, NREM dream recall contradicts REM sleep exclusive dream theories, which assert that dreams are generated solely in REM sleep.24
In the context of a multilevel sleep-dependent memory reprocessing, dreams represent the conscious awareness of complex brain systems involved in the reprocessing of emotions and memories during sleep,36 which can provide a functional role for REM dreaming (particularly for REM sleep-facilitated retention of emotional memories). Thus, it is tempting to speculate that the age-related dampening of the circadian rhythm of REM sleep may lead to decrements in REM sleep replay of emotional memory.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Claudia Renz, Marie-France Dattler, and Giovanni Balestrieri for their help in data acquisition; and the volunteers for participating. We also thank Carmen Schroeder and Corina Schnitzler for the medical screenings, and Silvia Frey for statistical advice. This research was supported by Swiss National Science Foundation Grants START 3100-055385.98 and 3130-054991.98 to CC, the Velux Foundation (Switzerland) and Böuhlmann Laboratories, Allschwil (Switzerland).
Footnotes
Financial support: This research was supported by Swiss National Science Foundation Grants START 3100-055385.98 and 3130-054991.98 to CC, the Velux Foundation (Switzerland) and Böuhlmann Laboratories, Allschwil (Switzerland).
REFERENCES
- 1.Schwartz S, Maquet P. Sleep imaging and the neuropsychological assessment of dreams. Trends Cogn Sci. 2003;6:23–30. doi: 10.1016/s1364-6613(00)01818-0. [DOI] [PubMed] [Google Scholar]
- 2.Wamsley EJ, Hirota Y, Tucker MA, Smith MR, Antrobus JS. Circadian and ultradian influences on dreaming: A dual rhythm model. Brain Res Bull. 2007;71:347–54. doi: 10.1016/j.brainresbull.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Antrobus J, Kondo T, Reinsel R. Dreaming in the late morning: summation of REM and diurnal cortical activation. Conscious Cogn. 1995;4:275–99. doi: 10.1006/ccog.1995.1039. [DOI] [PubMed] [Google Scholar]
- 4.Casagrande M, Violani C, Lucidi F, Buttinelli E, Bertini M. Variations in sleep mentation as a function of time of night. Int J Neurosci. 1996;85:19–30. doi: 10.3109/00207459608986348. [DOI] [PubMed] [Google Scholar]
- 5.Stickgold R, Malia A, Fosse R, Propper R, Hobson J. Brain–mind states. I. Longitudinal field study of sleep/wake factors influencing mentation report length. Sleep. 2001;24:171–9. doi: 10.1093/sleep/24.2.171. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen TA. Chronobiological features of dream production. Sleep Med Rev. 2004;8:403–24. doi: 10.1016/j.smrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Smith M, Antrobus J, Gordon E, Tucker M, Hirota Y, Wamsley EJ. Motivation and affect in REM sleep and the mentation reporting process. Conscious Cogn. 2004;13:501–11. doi: 10.1016/j.concog.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res. 2000;9:207–31. doi: 10.1046/j.1365-2869.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 9.Waterman D. Aging and memory for dreams. Percept Mot Skills. 1999;73:355–65. doi: 10.2466/pms.1991.73.2.355. [DOI] [PubMed] [Google Scholar]
- 10.Giambra LM, Jung RE, Grodsky A. Age changes in dream recall in adulthood. Dreaming. 1996;6:17–31. [Google Scholar]
- 11.Funkhouser AT, Hirsbrunner HP, Cornu C, Bahro M. Dreams and dreaming among the elderly: an overview. Aging Mental Health. 1999;3:10–20. [Google Scholar]
- 12.Zanasi M, De Persis S, Caporali M, Siracusano A. Dreams and age. Percept Mot Skills. 2005;100:925–38. doi: 10.2466/pms.100.3c.925-938. [DOI] [PubMed] [Google Scholar]
- 13.Yoon IY, Kripke DF, Elliott JA, et al. Age-related changes of circadian rhythms and sleep–wake cycles. J Am Geriatr Soc. 2003;51:1085–91. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 14.Buysse D, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Torsvall L, Akerstedt T. A diurnal type scale. Construction, consistency and validation in shift work. Scand J Work Environ Health. 1980;6:283–90. doi: 10.5271/sjweh.2608. [DOI] [PubMed] [Google Scholar]
- 16.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol Regul Integr Comp Physiol. 1999;277:640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 17.Cajochen C, Knoblauch V, Kröauchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport. 2001;12:2277–81. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- 18.Möunch M, Knoblauch V, Blatter K, et al. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol Aging. 2005;26:1307–19. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, MD: US Department of Health, Education and Welfare, Public Health Service; 1968. [Google Scholar]
- 20.Suzuki H, Uchiyama M, Tagaya H, Ozaki A, Kuriyama K, Aritake S. Dreaming during non-rapid eye movement sleep in the absence of prior rapid eye movement sleep. Sleep. 2004;27:1486–90. doi: 10.1093/sleep/27.8.1486. [DOI] [PubMed] [Google Scholar]
- 21.Gillberg M, Kecklund G, Akerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 22.Weber JM, Schwander JC, Unger I, Meier D. A direct ultrasensitive RIA for the melatonin in human saliva: comparison with serum levels. J Sleep Res. 1997;26:75. [Google Scholar]
- 23.Knoblauch V, Möunch M, Blatter K, et al. Age-related changes in the circadian modulation of sleep-spindle frequency during nap sleep. Sleep. 2005;28:1093–101. doi: 10.1093/sleep/28.9.1093. [DOI] [PubMed] [Google Scholar]
- 24.Hobson J, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23:793–821. doi: 10.1017/s0140525x00003976. [DOI] [PubMed] [Google Scholar]
- 25.Cajochen C, Möunch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23:461–74. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- 26.Schredl M, Lutzwittmann L, Ciric P, Göotz S. Factors of home dream recall: a structural equation model. J Sleep Res. 2003;12:133–41. doi: 10.1046/j.1365-2869.2003.00344.x. [DOI] [PubMed] [Google Scholar]
- 27.Duffy JF, Dijk DJ, Klerman EB. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:1478–87. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 28.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–27. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wing YK, Chiu H, Leung T. Dreaming in the elderly. J Sleep Res. 1999;8:151–5. doi: 10.1046/j.1365-2869.1999.00143.x. [DOI] [PubMed] [Google Scholar]
- 30.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena during sleep. Science. 1953;118:273–4. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 31.Foulkes D. Dreaming and REM sleep. J Sleep Res. 1993;2:199–202. doi: 10.1111/j.1365-2869.1993.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 32.Cavallero C, Cicogna P, Natale V, Occhionero M, Zito A. Slow wave sleep dreaming. Sleep. 1992;15:562–6. doi: 10.1093/sleep/15.6.562. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd SR, Cartwright RD. The collection of home and laboratory dreams by means of an instrumental response technique. Dreaming. 1995;5:63–73. [Google Scholar]
- 34.Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci. 2002;23:1083–121. doi: 10.1017/s0140525x00003988. [DOI] [PubMed] [Google Scholar]
- 35.Braun AR, Balkin T, Wesensten N, et al. Regional cerebral blood flow throughout the sleep–wake cycle: an H2 15O PET study. Brain. 1997;120:1173–97. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 36.Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–7. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen TA. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23:851–66. doi: 10.1017/s0140525x0000399x. [DOI] [PubMed] [Google Scholar]