Abstract
Background:
The classical narcolepsy patient reports intense feelings of sleepiness (with/out cataplexy), normal or disrupted nighttime sleep, and takes short and restorative naps. However, with long-term monitoring, we identified some narcoleptics resembling patients with idiopathic hypersomnia.
Objective:
To isolate and describe a new subtype of narcolepsy with long sleep time).
Setting:
University Hospital
Design:
Controlled, prospective cohort
Participants:
Out of 160 narcoleptics newly diagnosed within the past 3 years, 29 (18%) had a long sleep time (more than 11 h/24 h). We compared narcoleptics with (n = 23) and without (n = 29) long sleep time to 25 hypersomniacs with long sleep time and 20 healthy subjects.
Intervention:
Patients and controls underwent face-to face interviews, questionnaires, human leukocyte antigen (HLA) genotype, an overnight polysomnography, multiple sleep latency tests, and 24-h ad libitum sleep monitoring.
Results:
Narcoleptics with long sleep time had a similar disease course and similar frequencies of cataplexy, sleep paralysis, hallucinations, multiple sleep onset in REM periods, short mean sleep latencies, and HLA DQB1*0602 positivity as narcoleptics with normal sleep time did. However, they had longer sleep time during 24 h, and higher sleep efficiency, lower Epworth Sleepiness Scale scores, and reported their naps were more often unrefreshing. Only 3/23 had core narcolepsy (HLA and cataplexy positive).
Conclusions:
The subgroup of narcoleptics with a long sleep time comprises 18% of narcoleptics. Their symptoms combine the disabilities of both narcolepsy (severe sleepiness) and idiopathic hypersomnia (long sleep time and unrefreshing naps). Thus, they may constitute a group with multiple arousal system dysfunctions.
Citation:
Vernet C; Arnulf I. Narcolepsy with long sleep time: a specific entity? SLEEP 2009;32(9):1229-1235.
Keywords: Narcolepsy, hypersomnia, sleep drunkenness, long sleep time, cataplexy
THE CLINICAL SYMPTOMS OF NARCOLEPSY INCLUDE CHRONIC, SEVERE, OBJECTIVE DAYTIME SLEEPINESS WITH MULTIPLE DAYTIME SLEEP ONSET REM PERIODS. These markers are associated, to various extents, with REM sleep-associated phenomena (i.e., cataplexy, hypnagogic hallucinations, sleep paralysis, REM sleep behavior disorders), disturbed nocturnal sleep, periodic leg movements, depressive mood, and increased weight.1 During the night, the most specific feature of nocturnal sleep is a REM sleep period at sleep onset, which is observed in only 25% of patients during the first monitored night. In addition, sleep efficiency in narcoleptics tends be lower than in controls.2 Up to 71% narcoleptics are unable to sleep without awakening, and 83% complain of early awakenings.3 Recently, much emphasis has been placed on the frequent nighttime awakenings, which may follow an ultradian rhythm in narcoleptics. The existence of narcoleptic dyssomnia has, in part, led to the recent treatment of sodium oxybate given before and during sleep in narcoleptics, as a means to obtaining more continuous and deep sleep. It also helps alleviate cataplexy and daytime sleepiness.
Disrupted nighttime sleep in human narcolepsy parallels the fragmented NREM sleep observed in Doberman Pinschers with genetically abnormal hypocretin-2 receptors, though the total duration of sleep is not different from control dogs.4 In murine narcolepsy, sleep episodes are similarly fragmented, though the total time asleep in these transgenic animals (823 min) is not significantly different from the 783 min observed in wild animals.5 In human narcolepsy, total sleep time was initially measured during a 24-h period, before the development of the multiple sleep latency test.6 Sleep duration was not found to be different between narcoleptic patients and controls, because daytime naps were balanced by ultradian intra-sleep awakenings in narcoleptic patients.7 Combined, these results led to the primary theory of sleep-state instability in narcolepsy, which refers to a difficulty in consolidating either wakefulness (that is, an inability to maintain wakefulness over long periods of the daytime) or sleep (referring to an inability to maintain continuous sleep, with frequent awakening). As narcolepsy is primarily caused by hypocretin deficiency, it has been hypothesized that hypocretin could stabilize the wake-sleep switches.8
Although most narcoleptics fit this framework (that is, they generally show disrupted sleep and short, restorative naps), we observed in our clinical practice a subgroup of patients with narcolepsy who reported abnormally long nighttime sleep, with feelings of sleep drunkenness and unrefreshing long naps. These clinical features were similar to those observed in idiopathic hypersomnia with long sleep time. Idiopathic hypersomnia is now further divided into hypersomnia with and without long sleep time.9 Hypersomnia with long sleep time is characterized by a prolonged (> 10 h) nighttime sleep, with frequent sleep drunkenness and long, unrefreshing naps, usually sleeping more than 11 to 12 h per day. Recently, we found that hypersomniacs (and no healthy controls) slept longer than 11 h when monitored continuously during night and day.10 Hypersomnia without long sleep time is characterized by normal (< 10 h) sleep time, short (< 8 min) mean daytime latency during multiple sleep latency tests (MSLTs) and fewer than 2 sleep onset in REM periods (SOREMPs). This recent category, resembling narcolepsy without REM sleep-associated abnormalities, has been isolated after studying a group of patients with idiopathic hypersomnia.11 Here, we systematically studied the narcoleptics with long sleep time (> 11 h /24 h) and contrasted them with patients suffering a classical narcolepsy and patients suffering from idiopathic hypersomnia with long sleep time.
METHODS
Subjects
Between 2005 and 2008, all (n = 900) patients referred for excessive daytime sleepiness without sleep disordered breathing (determined by respiratory polygraphy) underwent the same, routine 48-h protocol (see below) in the sleep disorders unit, a tertiary care university hospital with a biased over-recruitment of neurological cases. In this series, we identified patients with hypersomnia of central origin who met the following inclusion criteria: (1) complaints of excessive daytime sleepiness occurring daily for ≥ 3 months; and (2) no improvement with an increase of the nighttime length for 15 days; and (3) a mean sleep latency during MSLT < 8 min; or (4) a total sleep time > 10 h (600 min) during nighttime sleep monitoring. We excluded the patients with: (1) sleep disordered breathing, defined by a respiratory disturbance index > 10/h (this index included apnea, hypopnea, and respiratory effort related arousal events; flow limitation was measured on the nasal cannulae, and threshold was set at a minimum of 5 apneas/hour); and (2) narcolepsy due to a medical condition (e.g., Parkinson disease, lupus, genetic disease, depression); and (3) hypersomnia due to drug or substance use. No patient received any psychotropic drug during the period of sleep monitoring. In previously treated patients, special care was paid to withdraw any psychotropic drugs for at least 15 half-lives.
In this group of 312 consecutive patients with hypersomnia of central origin, we identified 160 patients with narcolepsy, based on classical criteria (that is, with a mean sleep latency during MSLT < 8 min and more than one sleep-onset REM period or clear-cut cataplexy).9 Among these 160 narcoleptics, we isolated 29 (18.1%) patients with a diagnosis of narcolepsy and long sleep time (that is, with a total sleep time > 11 h during 24-h, long-term sleep monitoring). Among these 29 patients, only 23 fully completed the various clinical measures. We contrasted their clinical, demographic, and polygraphic characteristics with a random sample of 29 patients demonstrating a classical narcolepsy (short MSLT, multiple SOREMPs, or cataplexy), and 25 randomly selected patients with idiopathic hypersomnia with long sleep time (defined as ≤ 1 SOREMP during MSLT and a total sleep time > 11 h on 24-h sleep monitoring). Using a threshold of 11 h to define long sleep time was previously piloted while monitoring 35 healthy volunteers during the 48-h procedure.11 In the International Classification of Sleep Disorders, second edition, a cut-off of 10 h nighttime sleep and daytime sleepiness is used for defining hypersomnia with long sleep time, while more than 11–12 h of total sleep per 24 h is indicated as a typical finding when long-term sleep monitoring is performed in patients with hypersomnia with long sleep time.
Twenty healthy subjects volunteered to take part as controls after recruitment by advertisement. They were matched by age and sex with the group of narcoleptics with long sleep time. The subjects were selected after a medical interview for having no complaints regarding their sleep, no excessive daytime sleepiness (defined as the absence of spontaneous or elicited complaint and an Epworth Sleepiness Scale score < 11), no chronic sleep deprivation (determined using a questionnaire on sleep habits), no shift or night work, no severe medical illness, and no use of medications known to modify sleep and wakefulness. The control group took part in the 48-h sleep protocol. Control subjects complied adequately with study requirements, signed informed consents, and were paid. The study was approved by the local ethics committee.
Investigations
Participants were instructed to follow a regular sleep-wake rhythm, with ≥ 8 h in bed during the week preceding the 48-h investigations in the sleep disorders unit. They underwent a face to face interview about sleep symptoms (cataplexy, sleep drunkenness, sleep paralysis, restorative naps) and completed a standardized comprehensive sleep questionnaire including the Epworth Sleepiness Scale,12 the Horne-Ostberg eveningness-morningness scale,13 the Pichot fatigue scale,14 the Fatigue Severity Score,15 and the hospital depression and anxiety (HAD) rating scale.16 The class-II human leukocyte antigen (HLA) genotype was determined for all patients and controls. The sleep and wake monitoring procedure included (i) a habituation night with sleep and respiratory monitoring from 23:00 to 06:30, followed the next day by (ii) an MSLT with naps at 08:00, 10:00, noon, 14:00, and 16:00, that were terminated after 20 minutes if no sleep occurred, and after 15 min asleep if sleep occurred17; (iii) followed the next evening by a long term (24-h) sleep monitoring. The aim of long-term sleep monitoring was to elicit the maximum spontaneous amount of sleep in relaxed and quiet, but not totally abnormal conditions; no sleep episode, whether at night or during daytime, was interrupted by the technicians. Controls and patients were in the sleep unit for 24 h. They received dinner at 19:00. TV, computer, and visits from friends were forbidden; but books, newspapers, watches, and daylight were allowed. After 21:00, the participants were free to determine when they wanted switch lights off for the evening, and were allowed to sleep until they spontaneously awakened (and turned lights on) the next day. In addition, all subjects were asked to lay down in the dark for 2 naps during the morning and the afternoon. The nap attempts were discontinued by the subjects after 30 min if they could not sleep, and were continued ad libitum when they fell asleep. Tests were stopped at 17:00 the last day. In total, this procedure provided a 20-h opportunity to sleep for all subjects. Subjects received a breakfast after waking up and lunch if they were awake. This procedure is highly recommended in order to diagnose idiopathic hypersomnia9,18 but does not yield standardized values for healthy subjects. As part of our current study, we present normative values.
Polysomnographic recordings included electroencephalography (Fp1-A2, C3-A2, O1-A2), left and right electro-oculograms; levator menti, and bilateral tibialis anterior surface electromyography; nasal pressure trough cannulae; respiratory efforts using thoracic and abdominal belts; position; tracheal sounds; pulse rate; and transcutaneous oximetry (Medatec Ltd, France) during the first night. The respiratory sensors were removed during the MSLT and the 24-h sleep monitoring. Sleep stages, arousals, periodic leg movements, and respiratory events were scored visually according to standard criteria.19–22 The total sleep time, total sleep period, sleep and REM sleep latencies, the durations and percentages of NREM sleep stages 1, 2, 3-4, and REM sleep were determined during Night 1 and Night 2 and during the 24-h monitoring procedure. The indexes of sleep fragmentation (that is, the arousal index, periodic leg movements, periodic leg movement-associated arousal index, and apnea-hypopnea index), and minimal oxygen saturation during sleep were measured during Night 1. In order to determine whether it was correlated with sleep drunkenness, we noted the time of offset of the last slow wave sleep (SWS) episode that lasted longer than 5 minutes during the second night sleep.
Statistical Analysis
The patients with narcolepsy with long sleep time were compared as a group to each of the other studied groups, to determine if they were more similar to those with classical narcolepsy or those with idiopathic hypersomnia with long sleep time. We analyzed our between-group dichotomous variables using χ2 tests, and analyzed our continuous variables using analysis of variance (Statistica 7.1, Stat Soft Inc, Tulsa, OK). A P value less than 0.05 was considered to be significant (with corrections for repeated measures). Values are presented as mean ± SD (unless otherwise specified).
RESULTS
Clinical Differences Between Narcoleptics with Long Sleep Time and Other Groups
Among 160 narcoleptics, we isolated 29 (18.1%) patients with a diagnosis of narcolepsy with long sleep time (that is, with a total sleep time > 11 h on 24-h, long-term sleep monitoring). None of these participants had a personal or family history of long sleeper prior to the sleepiness onset. Their demographic and clinical characteristics compared to other participant groups are summarized in Table 1. The age, body mass index, and sex ratio of narcoleptics with long sleep time did not differ from controls (as expected by the matching procedure), nor did they differ from classical narcoleptics. The age at symptom onset was similar in narcoleptics with (18.1 ± 5.3 y) and without (19.9 ± 6.2 y, P = 0.46) long sleep time. At the time of the polysomnography, the disease course was similar in narcoleptics with (9 ± 8 y, range: 1±21 y) and without (8 ± 8 y, range: 1–24 y, P = 0.77) long sleep time.
Table 1.
Clinical and Biological Characteristics of Narcoleptics with Long Sleep Time (Total Sleep Time During 24-h Monitoring > 11 h) Compared with Patients with Narcolepsy without Long Sleep Time, Idiopathic Hypersomnia with Long Sleep Time, and Controls
| Patients | Narcolepsy with long sleep time | Narcolepsy without long sleep time | Idiopathic hypersomnia with long sleep time | Controls |
|---|---|---|---|---|
| Number | 23 | 29 | 25 | 20 |
| Age, y | 25.7 ± 10.6 | 30.8 ± 10.1 | 29.6 ± 12.5 | 29.4 ± 8.7 |
| Body mass index, kg/m2 | 25.3 ± 6.0 | 24.5 ± 6.2 | 23.2 ± 3.6 | 23.9 ± 4.8 |
| Women, % | 61 | 56 | 72 | 65 |
| Hypnagogic hallucinations, % | 44 | 68 | 32 | 10* |
| Sleep paralysis, % | 37 | 47 | 37 | 0* |
| Non refreshing naps, % | 80 | 33* | 59 | 0* |
| Sleep drunkenness, % | 54 | 42 | 72 | 0* |
| Clear-cut cataplexy, % | 29 | 44 | 0* | 0* |
| HLA DQB1*0602 positive, % | 53 | 54 | 30 | 10* |
| Core narcolepsy, % † | 13 | 24 | NA | NA |
| Epworth Sleepiness score (0-24) | 14.8 ± 3.4 | 17.1 ± 3.4* | 15.1 ± 4.5 | 5.8 ± 2.0* |
| Fatigue Severity Score (0-70) | 50.3 ± 10.6 | 55.1 ± 6.3 | 51.1 ± 10.7 | 31.6 ± 9.2* |
| Pichot Fatigue Score (0-32) | 27.9 ± 7.8 | 27.2 ± 7.2 | 24.9 ± 9.0 | 11.0 ± 3.5* |
| HAD anxiety (0-21) | 10.5 ± 5.7 | 11.9 ± 4.0 | 9.5 ± 4.5 | 5.6 ± 3.3 |
| HAD depression (0-21) | 6.5 ± 3.1 | 5.3 ± 2.8 | 6.4 ± 5.0 | 3.9 ± 2.8* |
| Horne-Ostberg score | 50.6 ± 10.1 | 46.1 ± 14.7 | 39.3 ± 13.4* | 54.8 ± 7.6 |
Core narcolepsy: simultaneous presence of clear-cut cataplexy and HLA DQB1*0602 positivity, which yields 88% to 93% sensitivity for hypocretin deficiency.
P < 0.05 for a difference between narcolepsy with long sleep time, HLA: human leukocyte antigen; HAD: score at the hospital anxiety and depression scale
Hypnagogic hallucinations, cataplexy, sleep paralysis, as well as sleep drunkenness and HLA DQB1*0602 positivity were as frequent in narcoleptics with and without long sleep time, but the naps were more frequently unrefreshing in the narcoleptics with long sleep time, and the Epworth score was lower. All these symptoms (except cataplexy) were observed in patients with idiopathic hypersomnia with long sleep time, but not in controls. As for what can be defined as core narcolepsy (i.e., the association in the same patient of clear cut cataplexy, short MSL, multiple SOREMPs, and HLA positivity), there were 3/23 (13%) patients with core narcolepsy in the group of narcoleptics with long sleep time, and 7/29 (24%) in the group of narcolepsy without long sleep time. These percentages were not different. The demographic, clinical, and sleep characteristics of this subgroup is displayed in the Supplementary Table A. Sleepiness and fatigue scores were similar for the 3 patient groups, and all 3 patient groups scored higher than those in the control group did. The same pattern was observed for anxiety and depression scores, which were slightly higher in patients than in controls. As evidenced by their Horne-Ostberg scores, narcoleptics with long sleep time had a more advanced sleep phase than patients with idiopathic hypersomnia but were not different from controls and from other narcoleptics.
Supplementary Table A.
Clinical, Biological Characteristics and Sleep Measures During Night-Time, MSLT and 24-Hour Long Monitoring in Core Narcoleptics† with and without Long Sleep Time (Total Sleep Time During the 24-Hours Monitoring > 11 Hours)
| Patients | Core narcolepsy† with long sleep time | Core narcolepsy† without long sleep time |
|---|---|---|
| Number | 3 | 7 |
| Age, y | 27.3 ± 7.1 | 26.7 ± 7.0 |
| Body mass index, kg/m2 | 20.8 ± 2.8 | 25.9 ± 8.0 |
| Clear-cut cataplexy, % | 100 | 100 |
| HLA DQB1*0602 positive, % | 100 | 100 |
| Total night-time sleep time, min | 605 ± 63 | 474 ± 72 * |
| Sleep efficiency, % | 88.4 ± 4.0 | 84.4 ± 13.7 |
| Latency to, min | ||
| Sleep onset | 6 ± 3 | 15 ± 10 |
| REM sleep | 27 ± 40 | 48 ± 31 |
| Arousals, n/h | 12.2 ± 5.8 | 10.7 ± 5.0 |
| Periodic legs movements, n/h | 7.9 ± 7.8 | 4.5 ± 5.0 |
| Daytime sleep time, min | 117 ± 34 | 85 ± 65 |
| Total sleep time during 24 hour monitoring, min | 722 ± 57 | 559 ± 36 * |
| Multiple sleep latency test | ||
| Mean sleep latency ± SE, min | 3.7 ± 0.4 | 8.5 ± 2.0 * |
| Number of SOREMPs | 5.0 ± 0.0 | 2.1 ± 1.9 * |
Core narcolepsy: simultaneous presence of clear-cut cataplexy and HLA DQB1*0602 positivity, which yields a 88–93% sensitivity for hypocretin deficiency.
<0.05 for a difference between narcolepsy with long sleep time; SWS: slow wave sleep (non REM sleep stages 3–4); SOREMP: sleep onset in rapid eye movements sleep period.
Polysomnographic Differences Between Narcoleptics with Long Sleep Time and Other Groups
The polysomnographic measures for all groups are displayed in Table 2. As expected, nighttime sleep time was 2 h longer in narcoleptics with long sleep time than those without; it was 1 h 30 min longer than for control subjects but 50 min shorter than for patients with idiopathic hypersomnia. Accordingly, this difference corresponded to one additional sleep cycle in narcoleptics with long sleep time and hypersomniacs, as compared to the other groups, while sleep cycle durations were similar across all groups. Sleep efficiency was higher in narcoleptics with (92.4%) than without long sleep time (87.1%, P = 0.04). In all groups, sleep onset latency was similar, though REM sleep latency was shorter in narcoleptics than in controls. As concerns sleep architecture, there were no differences between narcoleptics with long sleep time and any other group for percentage of sleep stages 1, 2, 3-4, and REM sleep. All groups contained similar numbers of subjects with SWS after 06:00, though the last SWS episode for the narcolepsy with long sleep time group occurred later than in controls and earlier than in patients with idiopathic hypersomnia. As concerns sleep fragmentation and movements, narcoleptics with long sleep time did not differ from other patient groups, but had fewer arousals and more frequent periodic leg movements than did controls. The respiratory disturbances indexes were generally low and were not different between groups.
Table 2.
Sleep Measures During Night-Time, MSLT, and 24-h Monitoring in Narcoleptics with Long Sleep Time (Total Sleep Time During 24-h Monitoring > 11 h) Compared with Patients with Narcolepsy without Long Sleep Time, Idiopathic Hypersomnia with Long Sleep Time, and Controls
| Patients | Narcolepsy with long sleep time | Narcolepsy without long sleep time | Idiopathic hypersomnia with long sleep time | Controls |
|---|---|---|---|---|
| Number | 23 | 29 | 25 | 20 |
| Nighttime sleep | ||||
| Total sleep time, min | 607 ± 61 | 477 ± 61* | 655 ± 57* | 517 ± 60* |
| Sleep efficiency, % | 92.4 ± 6.7 | 87.1 ± 11.0* | 93.9 ± 4.5 | 91.1 ± 4.6 |
| Latency to, min | ||||
| Sleep onset | 25 ± 25 | 21 ± 30 | 28 ± 37 | 34 ± 21 |
| REM sleep | 53 ± 34 | 55 ± 35 | 70 ± 30* | 78 ± 32* |
| Sleep stages, % total | ||||
| Stages 1–2 | 53.3 ± 9.7 | 54.9 ± 8.1 | 55.1 ± 7.1 | 52.0 ± 7.4 |
| Stages 3–4 | 22.1 ± 11.1 | 22.5 ± 5.3 | 20.7 ± 8.7 | 24.9 ± 6.1 |
| REM sleep | 24.6 ± 6.4 | 22.2 ± 5.2 | 24.1 ± 6.9 | 22.8 ± 4.2 |
| Sleep cycle numbers | 6.5 ± 1.1 | 5.2 ± 0.9* | 6.5 ± 0.7 | 5.5 ± 0.8* |
| Sleep cycle duration, min | 95 ± 15 | 97 ± 13 | 103 ± 11 | 94 ± 11 |
| End of the night | ||||
| SWS after 06:00 % patients | 60.9 | 48.2 | 80.9 | 45.0 |
| Time of last SWS episode, am | 8:03 ± 1.03 | 8:04 ± 1:08 | 9:47 ± 2:01* | 6:21 ± 1:37* |
| Sleep fragmentation | ||||
| Arousals, n/h | 11.6 ± 5.0 | 14.0 ± 6.8 | 10.5 ± 6.0 | 21.8 ± 10.6* |
| Periodic legs movements, n/h | 6.8 ± 7.2 | 8.2 ± 14.4 | 9.4 ± 17.0 | 1.3 ± 1.5* |
| Periodic legs movement arousals, n/h | 1.3 ± 1.1 | 1.9 ± 3.4 | 1.2 ± 2.2 | 0.5 ± 0.7* |
| Apnea/hypopnea, n/h | 1.8 ± 2.9 | 1.9 ± 2.7 | 1.6 ± 3.6 | 2.3 ± 2.9 |
| Daytime sleep time, min | 131 ± 52* | 86 ± 57* | 90 ± 62* | 47 ± 33* |
| Sleep during 24-h monitoring | ||||
| Total sleep time, min | 738 ± 58 | 562 ± 66* | 745 ± 72 | 564 ± 61* |
| Sleep stages, % total | ||||
| Stages 1–2 | 54.1 ± 9.5 | 55.3 ± 7.6 | 57.6 ± 7.8 | 54.7 ± 7.1 |
| Stages 3–4 | 21.9 ± 11.0 | 22.2 ± 5.6 | 20.0 ± 8.9 | 23.9 ± 5.6 |
| REM sleep | 24.0 ± 6.1 | 22.0 ± 4.8 | 22.3 ± 6.3 | 21.3 ± 4.1 |
| Multiple sleep latency test | ||||
| Mean sleep latency ± SE, min | 5.1 ± 0.5 | 6.5 ± 0.6 | 8.7 ± 0.9* | 15.3 ± 0.8* |
| Number of SOREMPs | 3.3 ± 1.4 | 2.7 ± 1.5 | 0.2 ± 0.5* | 0.2 ± 0.6* |
P < 0.05 for a difference between narcolepsy with long sleep time; SWS: slow wave sleep (NREM sleep stages 3-4); SOREMP: sleep onset in rapid eye movements sleep period.
During the MSLTs, mean sleep latency was shorter in narcoleptics (with or without long sleep time) than in hypersomniacs or control subjects. There was no difference in SOREMPS between narcoleptics with and without long sleep time.
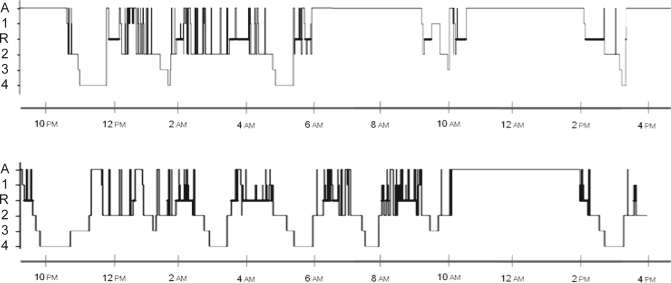
During the 24-h sleep monitoring, daytime sleep time was longer in narcoleptics with long sleep time than in all other groups. On a 24-h basis, narcoleptics with long sleep time (as expected) produced longer sleep amounts than other narcoleptics and controls, with a mean sleep amount of 738 ± 58 min (12.3 h, range:11–14.5 h), similar to the total sleep of patients with idiopathic hypersomnia (with no change in the breakdown of sleep stages). That is, narcoleptics with long sleep time slept 3 h more per 24 h than the other narcoleptics. An example of 24-h sleep hypnograms in patients with narcolepsy/cataplexy with and without long sleep time is displayed in Figure 1.
Figure 1.
Hypnogram during night and day of two patients with narcolepsy/cataplexy without (top, 583 min) and with (bottom, 783 min) long sleep time. A, R, 1, 2, 3 and 4 are respectively: Awake, REM sleep, non REM sleep stages 1, 2, 3 and 4.
If one isolates the narcoleptics sleeping > 10 h per night, they represent only 12/23 patients. Although they were younger (23.8 ± 6.7 y) than the other narcoleptics (31.2 ± 10.1 y, P = 0.03, a difference not seen when the threshold for long sleep time is 11h/24), differences among the 3 other groups were similar to what we found before (supplementary Tables B and C). Compared to other narcoleptics, they had longer sleep at night and during 24-h monitoring, with higher sleep efficiency. Compared to controls, they had more frequent HLA positivity and hypnagogic hallucinations, and higher subjective scores of sleepiness, fatigue, anxiety, and depression. Compared to patients with idiopathic hypersomnia, they had higher Horne-Ostberg scores.
Supplementary Table B.
Clinical and Biological Characteristics of Narcoleptics with Long Night Time Sleep (Total Night Time Sleep During the Second Night > 10 Hours) Compared with Patients with Narcolepsy without Long Night Time Sleep, Idiopathic Hypersomnia with Long Night Time Sleep and Controls
| Patients | Narcolepsy with long night time sleep | Narcolepsy without long night time sleep | Idiopathic hypersomnia with long night time sleep | Controls |
|---|---|---|---|---|
| Number | 12 | 28 | 25 | 20 |
| Age, y | 23.8 ± 6.7 | 31.2 ± 10.1 * | 29.6 ± 12.5 | 29.4 ± 8.7 * |
| Body mass index, kg/m2 | 24.1 ± 5.0 | 24.8 ± 6.1 | 23.2 ± 3.6 | 23.9 ± 4.8 |
| Women, % | 67 | 50 | 72 | 65 |
| Hypnagogic hallucinations, % | 64 | 68 | 32 | 10 * |
| Sleep paralysis, % | 36 | 47 | 37 | 0 * |
| Non refreshing naps, % | 50 | 60 | 59 | 0 * |
| Sleep drunkenness, % | 60 | 42 | 72 | 0 * |
| Clear-cut cataplexy, % | 18 | 46 | 0 * | 0 * |
| HLA DQB1*0602 positive, % | 50 | 52 | 30 | 10 * |
| Core narcolepsy, %† | 8 | 29 | NA | NA |
| Epworth Sleepiness score (0–24) | 15.1 ± 2.1 | 17.1 ± 3.4 | 15.1 ± 4.5 | 5.8 ± 2.0 * |
| Fatigue Severity Score (0–70) | 48.9 ± 8.3 | 55.1 ± 6.3 | 51.1 ± 10.7 | 31.6 ± 9.2 * |
| Pichot Fatigue Score (0–32) | 27.4 ± 7.1 | 27.2 ± 7.2 | 24.9 ± 9.0 | 11.0 ± 3.5 * |
| HAD anxiety (0–21) | 9.1 ± 6.1 | 10.7 ± 4.0 | 9.5 ± 4.5 | 5.6 ± 3.3 |
| HAD depression (0–21) | 6.9 ± 2.7 | 5.2 ± 2.8 | 6.4 ± 5.0 | 3.9 ± 2.8 * |
| Horne-Ostberg score | 48.2 ± 7.6 | 46.1 ± 14.7 | 39.3 ± 13.4 * | 54.8 ± 7.6 |
Core narcolepsy: simultaneous presence of clear-cut cataplexy and HLA DQB1*0602 positivity, which yields a 88-93% sensitivity for hypocretin deficiency.
P<0.05 for a difference between narcolepsy with long sleep time, HLA: human leukocyte antigen; HAD: score at the hospital anxiety and depression scale
Supplementary Table C.
Sleep Measures During Night-Time, MSLT and 24-Hour Long Monitoring in Narcoleptics with Long Night Time Sleep (Total Night Time Sleep During the Second Night > 10 Hours) Compared with Patients with Narcolepsy without Long Night Time Sleep, Idiopathic Hypersomnia with Long Night Time Sleep and Controls
| Patients | Narcolepsy with long night time sleep | Narcolepsy without long night time sleep | Idiopathic hypersomnia with long night time sleep | Controls | |
|---|---|---|---|---|---|
| Number | 12 | 28 | 25 | 20 | |
| Total night-time sleep time, min | 654 ± 46 | 475 ± 61 * | 655 ± 57 | 517 ± 60 * | |
| Sleep efficiency, % | 94.8 ± 2.3 | 86.8 ± 11.0 * | 93.9 ± 4.5 | 91.1 ± 4.6 * | |
| Latency to, min | |||||
| Sleep onset | 24 ± 28 | 21 ± 30 | 28 ± 37 | 34 ± 21 | |
| REM sleep | 53 ± 34 | 55 ± 36 | 70 ± 30 | 78 ± 32 * | |
| Arousals, n/h | 10.8 ± 4.9 | 14.2 ± 6.9 | 10.5 ± 6.0 | 21.8 ± 10.6 * | |
| Periodic legs movements, n/h | 6.1 ± 6.1 | 8.6 ± 14.6 | 9.4 ± 17.0 | 1.3 ± 1.5 * | |
| Periodic legs movement arousals, n/h | 0.9 ± 0.8 | 1.9 ± 3.5 | 1.2 ± 2.2 | 0.5 ± 0.7 | |
| Daytime sleep time, min | 99 ± 30 | 87 ± 58 | 90 ± 62 | 47 ± 33 * | |
| Total sleep time during 24 hour monitoring, min | 753 ± 61 | 562 ± 66 * | 745 ± 72 | 564 ± 61 * | |
| Multiple sleep latency test | |||||
| Mean sleep latency ± SE, min | 5.8 ± 0.7 | 6.6 ± 0.7 | 8.7 ± 0.9 * | 15.3 ± 0.8 * | |
| Number of SOREMPs | 2.9 ± 1.2 | 2.7 ± 1.5 | 0.2 ± 0.5 * | 0.2 ± 0.6 * |
P<0.05 for a difference between narcolepsy with long sleep time; SWS: slow wave sleep (non REM sleep stages 3-4); SOREMP: sleep onset in rapid eye movements sleep period.
As a spinal tap is not considered a routine procedure for the diagnosis of narcolepsy when it is possible by other means, we could gather only a single measure of cerebrospinal hypocretin-1. This procedure was performed in Stanford University in one of our narcoleptic with long sleep time and without typical cataplexy. The hypocretin-1 level was undetectable (that is, it was < 40 pg/mL).
DISCUSSION
The subgroup of narcoleptics with long sleep time represents 18% of a consecutive series of narcoleptics in a tertiary sleep disorders unit. This subgroup shares many similarities with classical narcolepsy (namely, similar rates of cataplexy, sleep paralysis, hypnagogic hallucinations, short daytime mean sleep latency with multiple sleep onset in REM periods, 50% rate of HLA positivity). This group, however, has per definition, longer sleep time during the night and day, with higher sleep efficiencies. As for the increased sleep amounts, this group most resembles the patients with idiopathic hypersomnia and long sleep time, with both groups sleeping a mean 12 h per day. The three patient groups have similar scores of sleepiness and fatigue, and mild scores of anxiety and depression, which clearly differentiate them from healthy controls.
One may object that narcoleptics with long sleep time could be some former long sleepers that later developed narcolepsy, and hence present with a mixture of a physiological trait (long sleeper) and disease (narcolepsy). This is not the case here, as our patients had no previous personal or family history of long sleep time. Acute recent onset narcolepsy has been occasionally associated with increased sleep amount, especially in children.23 However, in our series, there were no children or patients with recent onset (< 1 year) narcolepsy, and no different disease course between narcoleptics with and without long sleep time was observed. This suggests that the long sleep time phenotype may last beyond the first years of disease.
In the past, several groups concluded that narcoleptics fail to demonstrate an excess need for sleep when given a chance to sleep uninterrupted for 24 h.7,24 In contrast, one patient with narcolepsy slept 17 hours during 24-h sleep monitoring, while 3 other narcoleptics slept more than 11 h in a series of 8 narcoleptics monitored during bed rest.25 In a large study of 157 Italian patients with narcolepsy, narcoleptics slept an hour more (per 24 h) than controls, with standard deviations suggesting that some of them would sleep more than 11 h.26 The occasional presence of narcoleptics with sleep drunkenness and unrefreshing naps has not been totally overlooked in the history of narcolepsy. Daniels early stated that “Although patients with narcolepsy are usually fresher early in the morning than during the remainder of the day, some become very drowsy shortly after arising from a good night's rest; an unusually long night's sleep may even increase the diurnal drowsiness.”27 In a group of 41 patients with narcolepsy/cataplexy, 59% did not feel refreshed in the morning, 39% had an incomplete awakening suggestive of sleep drunkenness, while 5% had non-refreshing naps.28
The possibility of isolating a group of narcoleptics with long sleep time is of both clinical and pathophysiological interest. Narcoleptics with long sleep time may have more daytime problems than patients with classical narcolepsy do, because they share the main features of narcolepsy, but have additional difficulties waking up refreshed, being on time at work or at school in the morning, and do not benefit from short, refreshing naps. In our experience, they are the most difficult to treat, although this aspect was not formally addressed in this paper.
The main limitation of this series is the absence of measure of the CSF hypocretin-1 levels, except in one narcoleptic. Though this narcoleptic was hypocretin-1 deficient and had a long sleep time, we cannot yet determine how many narcoleptics with long sleep time are also hypocretin-1 deficient; further research is required. As hypocretin-1 is deficient in 88% to 93% of narcoleptics with clear-cut cataplexy, HLA positivity, and no family history of narcolepsy,29,30 it has been suggested that these clinical and biological characteristics define a homogenous subgroup of core narcolepsy. This core narcolepsy is, however, also found in 13% of patients with long sleep time. The pathophysiology of narcolepsy with long sleep time phenotype is unknown, as is the cause of idiopathic hypersomnia, which challenges our concept of hypocretin as consolidating the sleep-wake transitions. Hypocretin-deficient animals do not, however, display increased amounts of sleep, suggesting that additional deficits in arousal systems must be necessary for developing a true hypersomnia. Animals with double invalidation of genes coding for neurotransmitters involved in regulating arousal (e.g., hypocretin and histamine) could be useful for investigating these hypotheses. Our patients may have a double dysfunction in their arousal systems. The observation of long sleep time during acute onset narcolepsy, disappearing years later, suggests that some (but not all) patients compensate for this deficit, with differences being potentially dependent on their genetics. Furthermore, recent measures of CSF histamine levels in narcoleptics, controls, and patients with idiopathic hypersomnia suggest that the histamine arousal systems can also be deficient in some of these subjects.30,31 Demographic and clinical data on sleep excess, as well as animal models with excess sleep amounts, are lacking.32
After the recent sleep disorders reclassification, the narcolepsy/hypersomnia landscape is becoming richer with the addition of syndromes previously considered atypical, including narcolepsy without cataplexy and hypersomnia without long sleep time (which resembles narcolepsy but does not involve REM sleep abnormalities). Mirroring this subgroup, narcolepsy with long sleep time resembles idiopathic hypersomnia with long sleep time but contains numerous REM sleep abnormalities. To illustrate this concept, we suggest a table classifying the 4 groups, depending on sleep time, MSLT results, and SOREMPs (Table 3). Patients suffering this form of narcolepsy are more disabled than others, with shorter mean sleep latencies and a trend for more frequent sleep drunkenness and unrefreshing naps; hence, they may comprise a clinical spectrum that deserves specific medical attention.
Table 3.
Suggested Classification of Four Groups of Patients without Cataplexy, Depending on Sleep Time, MSLT Results and SOREMPs
| Total sleep time < 10 h and MSL < 8 min | Total sleep time > 10 h | |
|---|---|---|
| 0 or 1 SOREMP | Idiopathic hypersomnia without long sleep time | Idiopathic hypersomnia with long sleep time |
| 2 or more SOREMPs | Narcolepsy | Narcolepsy with long sleep time |
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Arnulf has received research support from Bioprojet and has participated in speaking engagements for Boehringer Ingelheim, Actelion, and UCB. Dr. Vernet has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Cyrille Vernet received three unrestricted grants from ANTADIR AO2006, UCB-Pharma Ltd AO2007 and CARDIF AO2007. Part of this work is financed by the grant of PHRC 2007-P070138.
REFERENCES
- 1.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 2.Nykamp K, Rosenthal L, Helmus T, et al. Repeated nocturnal sleep latencies in narcoleptic, sleepy and alert subjects. Clin Neurophysiol. 1999;110:1531–4. doi: 10.1016/s1388-2457(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 3.Plazzi G, Serra L, Ferri R. Nocturnal aspects of narcolepsy with cataplexy. Sleep Med Rev. 2008;12:109–28. doi: 10.1016/j.smrv.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Mitler MM, Dement WC. Sleep studies on canine narcolepsy: pattern and cycle comparisons between affected and normal dogs. Electroencephalogr Clin Neurophysiol. 1977;43:691–9. doi: 10.1016/0013-4694(77)90084-0. [DOI] [PubMed] [Google Scholar]
- 5.Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 6.Guilleminault C. [Narcolepsy] Rev Neuropsychiatr Infant. 1972;20:857–61. [PubMed] [Google Scholar]
- 7.Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol. 1988;70:473–81. doi: 10.1016/0013-4694(88)90145-9. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Sleep Medicine. Diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd ed. [Google Scholar]
- 10.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32:753–9. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassetti C, Aldrich M. Idiopathic hypersomnia. A series of 42 patients. Brain. 1997;120:1423–35. doi: 10.1093/brain/120.8.1423. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 13.Horne J, Ostberg O. A self-assesment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 14.Pichot P, Brun JP. [Brief self-evaluation questionnaire for depressive, asthenic and anxious dimensions] Ann Med Psychol (Paris) 1984;142:862–5. [PubMed] [Google Scholar]
- 15.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Carskadon MA, Mitler MM, Roth T. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 18.Billiard M, Dauvilliers Y. Idiopathic Hypersomnia. Sleep Med Rev. 2001;5:349–58. doi: 10.1053/smrv.2001.0168. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 20.American Sleep Disorders Association. EEG arousals: Scoring rules and examples. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 21.American Sleep Disorders Association. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances. Sleep. 1995;18:511–3. doi: 10.1093/sleep/18.6.511. [DOI] [PubMed] [Google Scholar]
- 22.American Sleep Disorders Association Atlas Task Force. Recording and scoring leg movements. Sleep. 1993;16:749–59. [PubMed] [Google Scholar]
- 23.Kotagal S, Hartse KM, Walsh JK. Characteristics of narcolepsy in preteenaged children. Pediatrics. 1990;85:205–9. [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Wolpert EA, Dement WC, Mitchell SA, Fisher C. Nocturnal sleep of narcoleptics. Electroencephalogr Clin Neurophysiol. 1963;15:599–609. doi: 10.1016/0013-4694(63)90032-4. [DOI] [PubMed] [Google Scholar]
- 25.Passouant P, Popoviciu L, Velok G, Baldy-Moulinier M. [Polygraphic study of narcolepsy during the nycthemeral period] Rev Neurol (Paris) 1968;118:431–41. [PubMed] [Google Scholar]
- 26.Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. How age influences the expression of narcolepsy. J Psychosom Res. 2005;59:399–405. doi: 10.1016/j.jpsychores.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 27.Daniels L. Narcolepsy. Medicine. 1934;13:1–122. [Google Scholar]
- 28.Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 29.Bourgin P, Zeitzer JM, Mignot E. CSF hypocretin-1 assessment in sleep and neurological disorders. Lancet Neurol. 2008;7:649–62. doi: 10.1016/S1474-4422(08)70140-6. [DOI] [PubMed] [Google Scholar]
- 30.Kanbayashi T, Kodama T, Kondo H, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep. 2009;1(32):181–7. doi: 10.1093/sleep/32.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino S, Sakurai E, Nevsimalova S, et al. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep. 2009;1(32):175–80. doi: 10.1093/sleep/32.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mignot E. Excessive daytime sleepiness: Population and etiology versus nosology. Sleep Med Rev. 2008;12:87–94. doi: 10.1016/j.smrv.2007.12.006. [DOI] [PubMed] [Google Scholar]