Abstract
Vaccinating school-aged children against influenza can reduce age-specific and population-level illness attack rates. Using a stochastic simulation model of influenza transmission, the authors assessed strategies for vaccinating children in the United States, varying the vaccine type, coverage level, and reproductive number R (average number of secondary cases produced by a typical primary case). Results indicated that vaccinating children can substantially reduce population-level illness attack rates over a wide range of scenarios. The greatest absolute reduction in influenza illness cases per season occurred at R values ranging from 1.2 to 1.6 for a given vaccine coverage level. The indirect, total, and overall effects of vaccinating children were strong when transmission intensity was low to intermediate. The indirect effects declined rapidly as transmission intensity increased. In a mild influenza season (R = 1.1), approximately 19 million influenza cases could be prevented by vaccinating 70% of children. At most, nearly 100 million cases of influenza illness could be prevented, depending on the proportion of children vaccinated and the transmission intensity. Given the current worldwide threat of novel influenza A (H1N1), with an estimated R of 1.4–1.6, health officials should consider strategies for vaccinating children against novel influenza A (H1N1) as well as seasonal influenza.
Keywords: communicable disease control; influenza, human; influenza vaccines; mass immunization
School-aged children have high influenza illness attack rates and play a key role in influenza transmission. In addition, vaccinating children in this age group has been shown to reduce population-level influenza illness attack rates (1–5). As a result, targeted vaccination of school-aged children has the potential to substantially reduce the overall morbidity and mortality associated with influenza illness and should be evaluated further.
In 2008, the US Advisory Committee on Immunization Practices recommended yearly seasonal influenza vaccination for all children aged 6 months to 18 years (6, 7). Currently, 2 types of influenza vaccine are licensed in the United States. Trivalent inactivated influenza vaccine (TIV), administered intramuscularly, is recommended for use in all children 6 months of age or older. Trivalent live, attenuated influenza vaccine (LAIV), administered by the intranasal route, is recommended for use in healthy children over 2 years of age (6, 7).
The ability of a vaccination strategy to prevent illness in a population is determined by a number of factors. The efficacy of the vaccine in directly protecting against infection and illness and in reducing infectiousness is key. The baseline intensity of transmission, described by the reproductive number R, and the level of vaccine coverage are also important.
A recent analysis based on challenge studies, community-based trials, and field studies estimated the efficacy of LAIV and TIV (8). Overall, on the basis of the analysis of challenge studies, both vaccines demonstrated a comparable, moderate level of efficacy against infection. Although the differences were not statistically significant, there was some evidence that, compared with inactivated vaccine, LAIV had higher efficacy against infection and illness, illness given infection, and infectiousness.
In the present study, we determined the effect of vaccinating school-aged children in the United States against influenza illness by modeling several different vaccination strategies. In this paper, we compare the reduction in overall and age-specific influenza illness attack rates and the number of influenza cases that could be prevented by implementing these strategies. In addition, the indirect effects of vaccination are quantified under these scenarios.
The recent outbreak of novel influenza A (H1N1) began in Mexico and had spread to over 70 countries, resulting in more than 27,700 cases and 140 deaths, by early June 2009 (9). Plans to produce a vaccine are under way, which highlights the urgent need for a vaccination strategy that produces the greatest reduction in influenza illness attack rates. In this context, and with the ever-present need to reduce the morbidity and mortality caused by seasonal influenza, we consider strategies for vaccinating children to reduce overall population-level influenza attack rates.
MATERIALS AND METHODS
Stochastic simulation model
We use a stochastic simulation model of influenza transmission based on a model described previously (10, 11). Briefly, we modeled the spread of influenza in the population of Los Angeles County, California, a metropolitan area of approximately 11 million residents, using employment rates and commuting data for Los Angeles County from the 2000 US Census (12, 13) and population estimates from Walter R. McDonald & Associates, Inc. (14), to which we added an estimated 776,000 undocumented people in Los Angeles County (15). Using this population, we calculated the illness attack rates in Los Angeles County and extrapolated the results to the 2009 US population, an estimated 305.5 million people (16).
Each individual in the simulation is assigned an age and a set of social contact groups including family, household cluster, neighborhood, community, workplace, neighborhood playgroup, and school, as appropriate. Influenza is transmitted from infected individuals to susceptible ones based on the probabilities of contact between the 2 individuals, determined by their membership in common social groups, and on the state of the 2 individuals, affected by duration of infection and their vaccination status. Individuals were randomly selected from the population and infected to simulate a continuous random seeding process.
The dynamics of influenza infection, illness, and infectiousness reflect our current understanding of the natural history of influenza (17). Once infected, an individual becomes infectious according to a probability distribution based on viral shedding. Thirty-three percent of those infected do not become symptomatic, while the other 67% become symptomatic after a 1–3-day incubation period (18, 19). Symptomatic individuals are twice as infectious as asymptomatic individuals. After 6 days, infected individuals are no longer infectious or susceptible.
The model was run 5 times for each scenario, and the average illness attack rates and numbers of cases are reported here. There was little stochastic variability across the runs because of the large population size, indicating that 5 runs are adequate.
Vaccine efficacy model parameters
In general, vaccine efficacy is defined as vaccine efficacy = 1 − relative risk, where the relative risk compares an outcome in the vaccinated group with the same outcome among the controls. Depending upon the specific outcome of interest, a measure of vaccine efficacy can quantify protection against infection, illness, illness given infection, or reduction in infectiousness among infected individuals (20). Specifically, vaccine efficacy for susceptibility (VES) estimates the ability of the vaccine to prevent infection. Vaccine efficacy for infection-confirmed symptomatic illness (VESP) quantifies the ability of the vaccine to prevent infection-confirmed symptomatic illness. Vaccine efficacy for symptomatic illness given infection (VEP) estimates the degree to which the vaccine prevents an infected individual from developing symptoms or reduces pathogenicity. Vaccine efficacy for infectiousness (VEI) estimates the reduction in the probability that an infected, vaccinated person compared with an infected, unvaccinated person will transmit the infection to another individual. Both VEP and VEI condition on being infected; VES and VESP do not condition on infection. The combined vaccine efficacy VEC is a function of these components of vaccine efficacy and quantifies the reduction in transmission in the entire population due to vaccination (8).
The values for VES, VESP, VEP, and VEI for LAIV and TIV used in the model are shown in Table 1 and were drawn from our best estimates based on previous work (8). Given these efficacy values, LAIV and TIV would provide the same moderate protection against infection for both homologous (VES = 40%) and heterologous (VES = 30%) seasons. Vaccine efficacy for symptomatic illness given infection (VEP), for infection-confirmed symptomatic illness (VESP), and for infectiousness (VEI), based on these parameters, would be somewhat higher for LAIV compared with TIV, although not significantly so, when the vaccines are both well matched and poorly matched.
Table 1.
Expected Vaccine Efficacies (%) for LAIV and TIV When the Vaccines Are Homologous and Heterologous Based on Challenge Study, Community-based Trial, and Field Study Data, as Reported by Basta et al. (8)
| LAIV |
TIV |
|||
| Homologous | Heterologous | Homologous | Heterologous | |
| VES | 40 | 30 | 40 | 30 |
| VEP | 83 | 57 | 67 | 14 |
| VESP | 90 | 70 | 80 | 40 |
| VEI | 50 | 30 | 40 | 20 |
| VEC | 83 | 68 | 78 | 56 |
Abbreviations: LAIV, trivalent live, attenuated influenza vaccine; TIV, trivalent inactivated influenza vaccine; VEC, combined vaccine efficacy; VEI, vaccine efficacy for infectiousness; VEP, vaccine efficacy for symptomatic illness given infection; VES, vaccine efficacy for susceptibility; VESP, vaccine efficacy for infection-confirmed symptomatic illness.
Indirect, total, and overall vaccine effects
In this study, our aim was to determine how an intervention strategy of vaccinating children would alter population-level influenza illness rates. In this context, the intervention is to vaccinate children. To determine the indirect, total, and overall vaccine effects, we compare the attack rates in the population that received the intervention with those in the nonintervention population, which is the baseline population. Individuals in the baseline population have some level of preexisting immunity because of previous vaccination or natural infection. We compare outcomes in these 2 populations for different values of the reproductive number R. We define R as the average number of secondary cases produced by a typical primary case in a population with a certain level of preexisting partial immunity. The basic reproductive number, R0, is the reproductive number in a population with no preexisting immunity. Because our intervention of vaccinating children is in addition to any preexisting immunity in the population, conceptually we have R = α R0, where 1 − α is the proportional reduction in susceptibility of exposed people, that is, the leaky model of immunity (20), that accounts for the degree of preexisting immunity.
Comparing illness attack rates in the intervention population with those in a comparable population in which no intervention has taken place provides further insight into the ability of the vaccination strategy to reduce influenza illness. Again, vaccine efficacy takes the form 1 − relative risk, but here we are interested in quantifying the population-level effects of vaccination. The indirect effects of vaccination, VEIndirect, compare the attack rates in those who did not receive the intervention in the population in which children were vaccinated with those in the nonintervention population. The total effectiveness of vaccination and the vaccination program, VETotal, compares the attack rates in the vaccinated children in the intervention population with those in the nonintervention population. Finally, the overall effectiveness of the vaccination program, VEOverall, compares the average attack rate in the intervention community with the overall attack rate in the nonintervention population (21).
Vaccination strategies and outcomes
We evaluated several strategies for vaccinating children against influenza by varying the type of vaccine used (LAIV or TIV), the level of vaccination coverage (30%, 50%, or 70% of all children), R (the average number of secondary cases produced by a typical primary case, ranging from 1.1 to 2.4), and whether the vaccine strain was well matched (homologous) or poorly matched (heterologous) to the circulating influenza strain. We assessed how combinations of these factors altered overall population-level attack rates, the total number of influenza cases expected in the entire US population in a single season, and the age-specific attack rates compared with a baseline scenario with no additional intervention. The absolute measures of the effect of vaccinating children, given by the difference in the number of cases, provide important information useful for assessing the public health impact of the intervention. In all of the scenarios, the intervention consists of vaccinating children, although the proportion vaccinated varies. Approximately 20% of children aged 6 months to 18 years are expected to be ineligible to receive LAIV because of contraindications such as a history of asthma or age less than 2 years (22). Therefore, 20% of vaccinated children in the LAIV scenarios were randomly selected to receive inactivated vaccine instead of the live, attenuated formulation. We calculated the indirect, total, and overall effects of vaccinating children at the population level, as described by Halloran et al. (20), stratifying by age (children aged ≤18 years vs. adults aged >18 years) when appropriate.
RESULTS
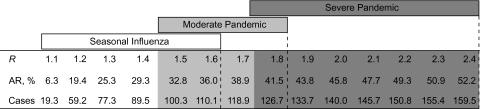
Figure 1 presents the overall illness attack rates and numbers of cases of influenza illness expected during a single influenza season in the US population under the baseline scenario for each value of R in 0.1 increments from 1.1 to 2.4. On the basis of estimates derived from past influenza seasons, a seasonal influenza outbreak with R ranging from 1.1 to 1.6 would correspond to a mild, moderate, or severe seasonal outbreak. A pandemic influenza outbreak with R ranging from 1.5 to 1.8 would likely be considered a moderate pandemic, and a pandemic influenza outbreak with an R of 1.8–2.4 would be severe (23–27). Our model indicates that approximately 19.3–59.2 million cases of influenza (a 6.3%–19.4% illness attack rate) would occur among the 305.5 million people in the United States during a mild seasonal influenza outbreak (R = 1.1–1.2) at baseline. These numbers are consistent with previous estimates. For example, Molinari et al. (28) estimated that 24.7 million cases occur annually in a typical influenza season (an 8.5% illness attack rate based on the 2003 US population). Under a severe pandemic scenario, if R = 2.4, nearly 160 million cases would be expected in the United States (a 52.2% illness attack rate) based on our model.
Figure 1.
Baseline influenza illness attack rates (AR, %) and number of cases (millions) based on the model and a US population of 305.5 million people for reproductive number (R) values ranging from 1.1 to 2.4.
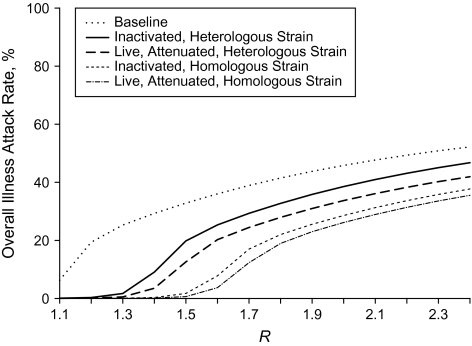
The higher the vaccine coverage in children, the greater the reduction in the overall attack rate, the age-specific attack rates, and the number of cases for a given value of R, regardless of whether the vaccine is well matched or poorly matched to the circulating strain (Tables 2 and 3, Figures 2 and 3). The relation between increasing values of R and the absolute difference between illness attack rates for a given level of vaccine coverage compared with baseline is nonmonotonic. The greatest absolute difference in overall attack rates due to vaccination compared with baseline occurs at low to moderate values of R (R = 1.2–1.5). Within this range, as vaccination coverage increases, the greatest absolute benefit of vaccination occurs at slightly higher values of R. If the vaccines are well matched to the circulating influenza strain, vaccinating 70% of children could prevent as many as 98.7 million cases during a severe seasonal influenza outbreak or a moderate pandemic, depending on the transmission intensity (Table 2; 70% vaccination coverage, LAIV, R = 1.6). If the vaccines are poorly matched, vaccinating 70% of children could prevent as many as 78.7 million cases during a severe seasonal influenza outbreak, depending on the transmission intensity (Table 3; 70% vaccination coverage, LAIV, R = 1.4).
Table 2.
Homologous Vaccine: Comparison of Numbers of Influenza Cases (Millions)a Under 6 Different Vaccination Strategies as the Reproductive Number (R) Ranges From 1.1 to 2.4
|
R |
||||||||||||||
| 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 | |
| Baseline | 19.3 | 59.2 | 77.3 | 89.5 | 100.3 | 110.1 | 118.9 | 126.7 | 133.7 | 140.0 | 145.7 | 150.8 | 155.4 | 159.5 |
| 30% vaccine coverage | ||||||||||||||
| TIV | 1.0 | 5.4 | 30.4 | 59.6 | 74.1 | 85.3 | 95.4 | 104.4 | 112.4 | 119.6 | 126.1 | 131.9 | 137.1 | 141.8 |
| LAIV | 0.8 | 3.9 | 23.6 | 54.4 | 70.8 | 82.2 | 92.2 | 101.3 | 109.5 | 116.7 | 123.3 | 129.1 | 134.4 | 139.1 |
| Additional cases prevented by LAIV | 0.2 | 1.5 | 6.8 | 5.1 | 3.3 | 3.1 | 3.2 | 3.0 | 2.9 | 2.9 | 2.8 | 2.9 | 2.7 | 2.7 |
| 50% vaccine coverage | ||||||||||||||
| TIV | 0.2 | 0.6 | 2.8 | 17.1 | 46.8 | 65.4 | 77.2 | 87.3 | 96.4 | 104.4 | 111.7 | 118.1 | 123.9 | 129.1 |
| LAIV | 0.2 | 0.4 | 1.4 | 9.3 | 35.4 | 58.5 | 71.7 | 82.0 | 91.2 | 99.4 | 106.7 | 113.3 | 119.2 | 124.4 |
| Additional cases prevented by LAIV | 0.0 | 0.2 | 1.3 | 7.7 | 11.3 | 6.9 | 5.5 | 5.3 | 5.2 | 5.0 | 5.0 | 4.8 | 4.7 | 4.7 |
| 70% vaccine coverage | ||||||||||||||
| TIV | 0.1 | 0.2 | 0.3 | 1.0 | 5.3 | 23.8 | 52.0 | 67.2 | 78.1 | 87.3 | 95.6 | 102.9 | 109.5 | 115.2 |
| LAIV | 0.1 | 0.1 | 0.2 | 0.5 | 1.9 | 11.4 | 37.7 | 58.0 | 70.3 | 80.0 | 88.4 | 95.9 | 102.6 | 108.6 |
| Additional cases prevented by LAIV | 0.0 | 0.0 | 0.1 | 0.5 | 3.4 | 12.4 | 14.3 | 9.3 | 7.8 | 7.4 | 7.2 | 7.0 | 6.8 | 6.7 |
Abbreviations: LAIV, trivalent live, attenuated influenza vaccine; TIV, trivalent inactivated influenza vaccine.
The expected number of cases is based on a US population of 305.5 million.
Table 3.
Heterologous Vaccine: Comparison of Numbers of Influenza Cases (Millions)a Under 6 Different Vaccination Strategies as the Reproductive Number (R) Ranges From 1.1 to 2.4
|
R |
||||||||||||||
| 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 | |
| Baseline | 19.3 | 59.2 | 77.3 | 89.5 | 100.3 | 110.1 | 118.9 | 126.7 | 133.7 | 140.0 | 145.7 | 150.8 | 155.4 | 159.5 |
| 30% vaccine coverage | ||||||||||||||
| TIV | 2.6 | 18.8 | 54.9 | 74.7 | 87.1 | 98.1 | 107.9 | 116.6 | 124.5 | 131.5 | 137.8 | 143.4 | 148.5 | 152.9 |
| LAIV | 2.0 | 12.4 | 45.9 | 68.6 | 81.1 | 92.0 | 101.7 | 110.5 | 118.3 | 125.3 | 131.5 | 137.2 | 142.1 | 146.7 |
| Additional cases prevented by LAIV | 0.6 | 6.4 | 9.1 | 6.1 | 6.0 | 6.1 | 6.2 | 6.1 | 6.2 | 6.2 | 6.2 | 6.2 | 6.4 | 6.2 |
| 50% vaccine coverage | ||||||||||||||
| TIV | 0.8 | 4.1 | 24.0 | 59.7 | 76.6 | 88.7 | 99.4 | 108.9 | 117.4 | 125.0 | 131.8 | 137.8 | 143.3 | 148.2 |
| LAIV | 0.5 | 2.1 | 12.3 | 44.9 | 66.0 | 78.4 | 89.1 | 98.5 | 107.0 | 114.6 | 121.3 | 127.4 | 132.8 | 137.7 |
| Additional cases prevented by LAIV | 0.3 | 2.0 | 11.7 | 14.8 | 10.6 | 10.3 | 10.4 | 10.4 | 10.4 | 10.4 | 10.4 | 10.4 | 10.5 | 10.5 |
| 70% vaccine coverage | ||||||||||||||
| TIV | 0.3 | 1.0 | 5.2 | 27.8 | 60.6 | 77.4 | 89.6 | 100.1 | 109.5 | 117.8 | 125.1 | 131.7 | 137.6 | 142.9 |
| LAIV | 0.2 | 0.4 | 1.6 | 10.8 | 38.5 | 61.9 | 74.7 | 85.3 | 94.7 | 103.0 | 110.3 | 117.0 | 122.9 | 128.1 |
| Additional cases prevented by LAIV | 0.1 | 0.5 | 3.6 | 17.0 | 22.1 | 15.5 | 14.9 | 14.7 | 14.8 | 14.7 | 14.8 | 14.8 | 14.7 | 14.8 |
Abbreviations: LAIV, trivalent live, attenuated influenza vaccine; TIV, trivalent inactivated influenza vaccine.
The expected number of cases is based on a US population of 305.5 million.
Figure 2.
Influenza illness attack rates, at baseline and after 70% of children are vaccinated, for a range of values of the reproductive number (R) for both homologous and heterologous vaccine.
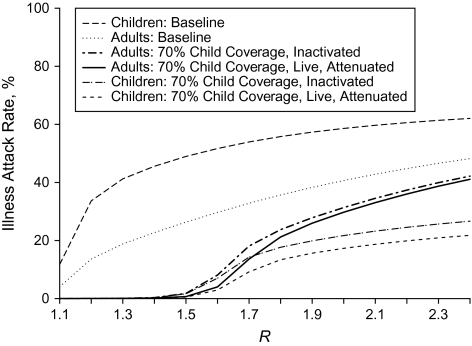
Figure 3.
Age-specific influenza illness attack rates for adults and children, at baseline and after 70% of children are vaccinated, for a range of values of the reproductive number (R) when the vaccine is homologous.
There is also a nonmonotonic relation between the value of R and the absolute difference in the reduction in the number of cases when comparing vaccinating children with LAIV and with TIV. Again, the greatest difference in the number of cases prevented when comparing the 2 vaccines was observed at intermediate values of R for all scenarios. For example, if the vaccines are poorly matched to the circulating influenza strain and vaccine coverage is high (70%), LAIV could prevent an additional 22.1 million influenza cases in the population beyond the number of cases prevented by TIV alone (Table 3; R = 1.5), given the efficacies modeled. In our model, LAIV, compared with TIV, was assigned higher VESP, VEP, and VEI values based on our best guesses for these efficacies from previous work (8). The higher the vaccine efficacies, the greater the numbers of influenza cases that can be prevented.
Regardless of transmission intensity, children have higher attack rates than adults at baseline (Figure 3). Vaccinating 70% of children with a well-matched influenza vaccine substantially reduces the attack rates for both children and adults at low to moderate levels of transmission intensity (R = 1.1−1.5). The difference between the baseline attack rates and the age-specific attack rates at higher levels of transmission intensity is greater for children than for adults. Children experience a greater reduction in age-specific attack rates with the intervention modeled because they benefit from the direct protection of vaccination and the indirect effects, whereas the reduction in the attack rates for adults results from only the indirect effects of vaccinating children. The age-specific illness attack rates at lower vaccination coverage levels are qualitatively similar to those at higher coverage levels, but the rise in the attack rates occurs at lower values of R for lower vaccination coverage levels and increases more rapidly (results not shown).
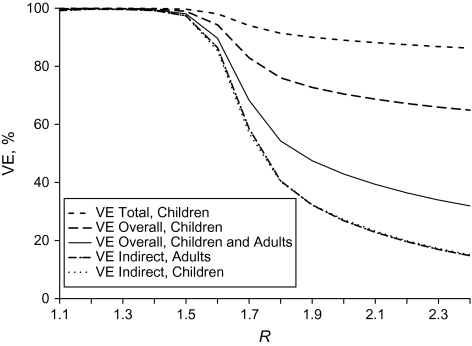
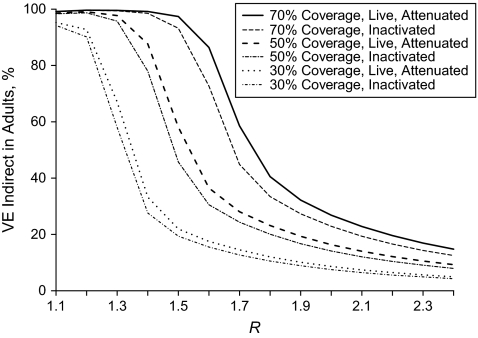
Finally, we observed strong indirect, total, and overall effects of influenza vaccination at lower values of R with high vaccination coverage (Figure 4). The indirect effects in both adults and children were similar regardless of transmission intensity. The indirect vaccine effects in both groups declined rapidly after R = 1.2−1.6, depending on the coverage, indicating that as the level of transmission increased, the unvaccinated were not as well protected indirectly. At values of R ≥ 2.0, the intensity of transmission effectively overwhelms the ability of the vaccine to protect indirectly, given the greater level of exposure to infection. The value of R at which the indirect effects began to decline depends on the vaccination coverage. With lower vaccination coverage in children, the decline in indirect vaccine effects occurs at lower values of R. This shift in the decline of the indirect effects in adults is illustrated for all 3 levels of vaccine coverage when both LAIV and TIV are well matched to the circulating influenza strain (Figure 5). The indirect effects of TIV were slightly lower than the VEIndirect observed for LAIV regardless of transmission intensity, but the shape of the trends was qualitatively similar.
Figure 4.
Indirect, overall, and total vaccine effects (VE) for adults and children when 70% of children are vaccinated with live, attenuated vaccine for a range of values of the reproductive number (R) when the vaccine is homologous.
Figure 5.
Indirect vaccine effects (VE) for adults after vaccinating children with influenza vaccine for a range of values of the reproductive number (R) when the vaccine is homologous.
The total effect of vaccination in children remained relatively high regardless of the severity of the outbreak or the coverage level when the vaccines were well matched to the influenza strain. Both the VEOverall in children and the VEOverall in the entire population decreased as R increased, with the initial decline occurring at lower levels of transmission intensity for lower levels of vaccination coverage. The overall and total effects were higher than the indirect effects because they account for the direct effect of the vaccine in the vaccinated children in the population as well as the indirect effects of the vaccination intervention.
When the vaccines were poorly matched to the circulating influenza strains, the same qualitative trends were observed. However, the decline in indirect, total, and overall vaccine effects occurred at lower levels of transmission intensity and decreased more rapidly compared with the well-matched scenario (results not shown).
DISCUSSION
The results of our stochastic simulation model demonstrate that vaccinating children against influenza can substantially reduce influenza illness in the overall population given a wide range of scenarios. In a mild influenza season (R = 1.1), approximately 19 million cases of influenza in the United States could be prevented if 70% of children were vaccinated. The greatest reduction in the number of cases compared with baseline was achieved with 70% vaccination coverage in children when the vaccine is homologous and transmission intensity is intermediate (R = 1.6). In this instance, approximately 98.7 million cases in the United States could be prevented. Even at low levels of vaccination, our model predicts that millions of cases of influenza can be prevented by targeted vaccination of children.
Vaccinating children against influenza produces both direct benefits in the children vaccinated and indirect benefits in the rest of the population. The indirect effects for both children and adults were high at low to intermediate values of R, indicating that influenza vaccination can provide substantial herd immunity when transmission intensity is low to intermediate. As transmission intensity increases, the indirect effects of vaccinating children decline rapidly, even at high vaccination coverage levels.
The benefit of vaccination depends upon the components of vaccine efficacy for a given vaccine. In our model, LAIV and TIV were assigned the same VES and relatively similar VESP and VEI. However, for LAIV, the VEP assigned in both homologous and heterologous seasons was higher. The higher VEP reduces influenza illnesses directly, by preventing illness, and indirectly, because asymptomatic individuals are less infectious. The vaccine efficacies used in the model were assigned based on the best available data. However, there is a need for well-designed epidemiologic field studies in which the components of vaccine efficacy can be estimated for both vaccines directly. Belshe et al. (29) reported that LAIV was 50% more efficacious than TIV against clinical infection in young children. There is some evidence that, compared with the inactivated vaccine, LAIV is easier to administer, is more acceptable to children, and induces broader cross-protective immunity (30–33).
Children experience high seasonal influenza attack rates and bear a large burden of disease (1, 2, 28). Evidence indicates that targeted vaccination of children has the benefit of reducing the burden of disease in this age group and the added public health advantage of reducing morbidity and mortality in the entire population (4, 22, 34). Strategies for vaccinating populations and allocating vaccines to reduce influenza morbidity and mortality have been modeled previously (35–40). Despite various modeling approaches and assumptions, several studies have found that vaccinating schoolchildren could reduce the overall incidence of influenza illness in the population and the overall number of deaths expected (35, 37, 38, 40). The economic benefits of vaccination strategies that target children for influenza vaccination have also been demonstrated (34, 41–43). Prospectively designed field studies that evaluate strategies for vaccinating children are needed to test these modeling results in the community, as has been noted (44).
In response to the outbreak of novel influenza A (H1N1) in the spring of 2009, health officials have called for the production of a new influenza vaccine to mitigate spread of this strain. Yet, plans for the use of such a vaccine have not been determined. Early reports indicate that the attack rate pattern for the novel influenza A (H1N1) strain is similar to spread of the Asian pandemic influenza A (H2N2) in 1957–1958, with a range in R of between 1.4 and 1.6, and with high spread among children (45). Therefore, the results of our modeling study can be used to address this situation. Indeed, our results indicate that the greatest benefit of vaccinating children is achieved at intermediate values of R, and that the benefit is maximized when vaccination coverage is high and the vaccine is well matched. With a high vaccination rate (70%) and a well-matched vaccine, as many as 95–98.7 million additional cases could be prevented by vaccinating children. Vaccinating just 30% of children with an influenza vaccine, even if poorly matched, could prevent an additional 12–20.9 million cases in the United States if R ranged from 1.4 to 1.6. The typical influenza season in the Northern Hemisphere is just months away, and health officials will soon need to decide how to best allocate a newly developed vaccine to mitigate the impending threat of novel influenza A (H1N1). Our results build upon an increasing body of research and provide strong evidence that vaccinating children against influenza can substantially reduce population-level attack rates given a wide range of scenarios.
Acknowledgments
Author affiliations: Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Nicole E. Basta); Program in Biostatistics and Biomathematics, Vaccine and Infectious Disease Institute, Fred Hutchinson Cancer Research Center, Seattle, Washington (Nicole E. Basta, Dennis L. Chao, M. Elizabeth Halloran, Laura Matrajt, Ira M. Longini, Jr.); Department of Biostatistics, School of Public Health and Community Medicine, University of Washington, Seattle, Washington (M. Elizabeth Halloran, Ira M. Longini, Jr.); and Department of Applied Mathematics, University of Washington, Seattle, Washington (Laura Matrajt).
This work was partially supported by the National Institute of General Medical Sciences MIDAS grant U01-GM070749; National Institute of Allergy and Infectious Diseases grant R01-AI32042; a contract from MedImmune (Gaithersburg, Maryland), which makes a live, attenuated influenza vaccine; and a fellowship from the Achievement Rewards for College Scientists (ARCS) Foundation Inc. (to N. E. B.).
Conflict of interest: none declared.
Glossary
Abbreviations
- LAIV
trivalent live, attenuated influenza vaccine
- R
average number of secondary cases produced by a typical primary case in a population with a certain level of preexisting partial immunity
- TIV
trivalent inactivated influenza vaccine
- VEI
vaccine efficacy for infectiousness
- VEP
vaccine efficacy for illness given infection
- VES
vaccine efficacy for susceptibility
- VESP
vaccine efficacy for infection-confirmed symptomatic illness
References
- 1.Monto AS, Kioumehr F The Tecumseh Study of Respiratory Illness. IX. Occurrence of influenza in the community, 1966–1971. Am J Epidemiol. 1975;102(6):553–563. doi: 10.1093/oxfordjournals.aje.a112193. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med. 1978;298(11):587–592. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 3.Reichert TA, Sugaya N, Fedson DS, et al. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344(12):889–896. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 4.Monto AS, Davenport FM, Napier JA, et al. Effect of vaccination of a school-age population upon the course of an A2-Hong Kong influenza epidemic. Bull World Health Organ. 1969;41(3):537–542. [PMC free article] [PubMed] [Google Scholar]
- 5.Longini IM, Jr, Halloran ME. Strategy for distribution of influenza vaccine to high-risk groups and children. Am J Epidemiol. 2005;161(4):303–306. doi: 10.1093/aje/kwi053. [DOI] [PubMed] [Google Scholar]
- 6.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Committee on Infectious Diseases. Prevention of influenza: recommendations for influenza immunization of children, 2008–2009. Pediatrics. 2008;122(5):1135–1141. doi: 10.1542/peds.2008-2449. [DOI] [PubMed] [Google Scholar]
- 8.Basta NE, Halloran ME, Matrajt L, et al. Estimating influenza vaccine efficacy from challenge and community-based study data. Am J Epidemiol. 2008;168(12):1343–1352. doi: 10.1093/aje/kwn259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Influenza A (H1N1)—Update 46. June 10, 2009. ( www.who.int/csr/don/2009_06_10a/en/index.html). (Accessed June 11, 2009) [Google Scholar]
- 10.Germann TC, Kadau K, Longini IM, Jr, et al. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103(15):5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longini IM, Jr, Halloran ME, Nizam A, et al. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–633. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Transportation. Census Transportation Planning Package 2000: Tract-Tract Worker Flow Data. Part 3 Journey-to-Work Flow Tables. ( www.fhwa.dot.gov/ctpp/tract.htm). (Accessed January 15, 2009) [Google Scholar]
- 13.US Census Bureau. Census 2000. Summary File 3. ( www.census.gov/Press-Release/www/2002/sumfile3.html). (Accessed January 15, 2009) [Google Scholar]
- 14.Walter R McDonald & Associates, Inc. (WRMA) July 1, 2007 Population Estimates. Report prepared for Urban Research, Los Angeles County CAO; 2007. [Google Scholar]
- 15.Flaming D, Haydamack B, Joassart P. Hopeful Workers, Marginal Jobs: LA's Off-the-Books Labor Force. Los Angeles, CA: Economic Roundtable; 2005. [Google Scholar]
- 16.US Census Bureau. Press Release CB08-191. 2009. ( www.census.gov/Press-Release/www/releases/archives/population/013127.html). (Accessed January 15, 2009) [Google Scholar]
- 17.Longini IM, Jr, Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 18.Halloran ME, Hayden FG, Yang Y, et al. Antiviral effects on influenza viral transmission and pathogenicity: observations from household-based trials. Am J Epidemiol. 2007;165(2):212–221. doi: 10.1093/aje/kwj362. [DOI] [PubMed] [Google Scholar]
- 19.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 20.Halloran ME, Longini IM, Jr, Struchiner CJ. Design and interpretation of vaccine field studies. Epidemiol Rev. 1999;21(1):73–88. doi: 10.1093/oxfordjournals.epirev.a017990. [DOI] [PubMed] [Google Scholar]
- 21.Halloran ME, Struchiner CJ. Study designs for dependent happenings. Epidemiology. 1991;2(5):331–338. doi: 10.1097/00001648-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Piedra PA, Gaglani MJ, Kozinetz CA, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine. 2005;23(13):1540–1548. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Chowell G, Ammon CE, Hengartner NW, et al. Estimating the reproduction number from the initial phase of the Spanish flu pandemic waves in Geneva, Switzerland. Math Biosci Eng. 2007;4(3):457–470. doi: 10.3934/mbe.2007.4.457. [DOI] [PubMed] [Google Scholar]
- 24.Chowell G, Miller MA, Viboud C. Seasonal influenza in the United States, France, and Australia: transmission and prospects for control. Epidemiol Infect. 2008;136(6):852–864. doi: 10.1017/S0950268807009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rvachev LA, Longini IM., Jr A mathematical model for the global spread of influenza. Math Biosci. 1985;75(1):3–22. [Google Scholar]
- 26.Mills CE, Robins JM, Lipsitch M. Transmissibility of 1918 pandemic influenza. Nature. 2004;432(7019):904–906. doi: 10.1038/nature03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longini IM., Jr The generalized discrete-time epidemic model with immunity: a synthesis. Math Biosci. 1986;82(1):19–41. [Google Scholar]
- 28.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 29.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 30.Rhorer J, Ambrose CS, Dickinson S, et al. Efficacy of live attenuated influenza vaccine in children: a meta-analysis of nine randomized clinical trials. Vaccine. 2009;27(7):1101–1110. doi: 10.1016/j.vaccine.2008.11.093. [DOI] [PubMed] [Google Scholar]
- 31.Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2–7 years of age. Vaccine. 2008;26(suppl 4):D10–D16. doi: 10.1016/j.vaccine.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 32.Vesikari T. Emerging data on the safety and efficacy of influenza vaccines in children. Pediatr Infect Dis J. 2008;27(11 suppl):S159–S161. doi: 10.1097/INF.0b013e31818a545d. [DOI] [PubMed] [Google Scholar]
- 33.Glezen WP. Universal influenza vaccination and live attenuated influenza vaccination of children. Pediatr Infect Dis J. 2008;27(10 suppl):S104–S109. doi: 10.1097/INF.0b013e318168b729. [DOI] [PubMed] [Google Scholar]
- 34.Weycker D, Edelsberg J, Halloran ME, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23(10):1284–1293. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 35.Vynnycky E, Pitman R, Siddiqui R, et al. Estimating the impact of childhood influenza vaccination programmes in England and Wales. Vaccine. 2008;26(41):5321–5330. doi: 10.1016/j.vaccine.2008.06.101. [DOI] [PubMed] [Google Scholar]
- 36.Riley S, Wu JT, Leung GM. Optimizing the dose of pre-pandemic influenza vaccines to reduce the infection attack rate [electronic article] PLoS Med. 2007;4(6) doi: 10.1371/journal.pmed.0040218. e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel R, Longini IM, Jr, Halloran ME. Finding optimal vaccination strategies for pandemic influenza using genetic algorithms. J Theor Biol. 2005;234(2):201–212. doi: 10.1016/j.jtbi.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 38.Mylius SD, Hagenaars TJ, Lugnér AK, et al. Optimal allocation of pandemic influenza vaccine depends on age, risk and timing. Vaccine. 2008;26(29–30):3742–3749. doi: 10.1016/j.vaccine.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 39.Dushoff J, Plotkin JB, Viboud C, et al. Vaccinating to protect a vulnerable subpopulation [electronic article] PLoS Med. 2007;4(5) doi: 10.1371/journal.pmed.0040174. e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bansal S, Pourbohloul B, Meyers LA. A comparative analysis of influenza vaccination programs [electronic article] PLoS Med. 2006;3(10) doi: 10.1371/journal.pmed.0030387. e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White T, Lavoie S, Nettleman MD. Potential cost savings attributable to influenza vaccination of school-aged children [electronic article] Pediatrics. 1999;103(6) doi: 10.1542/peds.103.6.e73. e73. [DOI] [PubMed] [Google Scholar]
- 42.Cohen GM, Nettleman MD. Economic impact of influenza vaccination in preschool children. Pediatrics. 2000;106(5):973–976. doi: 10.1542/peds.106.5.973. [DOI] [PubMed] [Google Scholar]
- 43.Luce BR, Zangwill KM, Palmer CS, et al. Cost-effectiveness analysis of an intranasal influenza vaccine for the prevention of influenza in healthy children [electronic article] Pediatrics. 2001;108(2):E24. doi: 10.1542/peds.108.2.e24. [DOI] [PubMed] [Google Scholar]
- 44.Halloran ME, Longini IM., Jr Community studies for vaccinating schoolchildren against influenza. Science. 2006;311(5761):615–616. doi: 10.1126/science.1122143. [DOI] [PubMed] [Google Scholar]
- 45.Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324(5934):1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]