Abstract
Background
Frailty in older adults, defined as a constellation of signs and symptoms, is associated with abnormal levels in individual physiological systems. We tested the hypothesis that it is the critical mass of physiological systems abnormal that is associated with frailty, over and above the status of each individual system, and that the relationship is nonlinear.
Methods
Using data on women aged 70–79 years from the Women’s Health and Aging Studies I and II, multiple analytic approaches assessed the cross-sectional association of frailty with eight physiological measures.
Results
Abnormality in each system (anemia, inflammation, insulin-like growth factor-1, dehydroepiandrosterone-sulfate, hemoglobin A1c, micronutrients, adiposity, and fine motor speed) was significantly associated with frailty status. However, adjusting for the level of each system measure, the mean number of systems impaired significantly and nonlinearly predicted frailty. Those with three or more systems impaired were most likely to be frail, with odds of frailty increasing with number of systems at abnormal level, from odds ratios (ORs) of 4.8 to 11 to 26 for those with one to two, three to four, and five or more systems abnormal (p < .05 for all). Finally, two subgroups were identified, one with isolated or no systems abnormal and a second (in 30%) with multiple systems abnormal. The latter group was independently associated with being frail (OR = 2.6, p < .05), adjusting for confounders and chronic diseases and then controlling for individual systems.
Conclusions
Overall, these findings indicate that the likelihood of frailty increases nonlinearly in relationship to the number of physiological systems abnormal, and the number of abnormal systems is more predictive than the individual abnormal system. These findings support theories that aggregate loss of complexity, with aging, in physiological systems is an important cause of frailty. Implications are that a threshold loss of complexity, as indicated by number of systems abnormal, may undermine homeostatic adaptive capacity, leading to the development of frailty and its associated risk for subsequent adverse outcomes. It further suggests that replacement of any one deficient system may not be sufficient to prevent or ameliorate frailty.
Keywords: Frailty etiology, Aging
FRAIL older adults are a group at increased risk of serious adverse clinical outcomes, including mortality and loss of independence (1–5). One conceptualization of frailty is that it is a distinct medical syndrome, which is clinically recognizable when a critical mass of symptoms and signs emerge (1,3). This has been operationalized as the concurrent presence of three or more of the following: low strength, low energy, slowed motor performance, low physical activity, or unintentional weight loss (1,2), and validated as a syndrome (3). It is hypothesized that this clinical presentation is a result of dysregulated energetics linked to abnormal levels of individual physiological systems such as a mild proinflammatory state, anemia, abnormal hormonal levels, micronutrient deficiencies, sarcopenia, and, possibly, decrements in neuromuscular control (1,6–13). Additionally, abnormal physiological systems can interact with synergistically increased risk of frailty (ie, insulin-like growth factor-1 [IGF-1] and IL-6; 10,14,15). These findings raise the question of whether the risk of frailty rises with the number of systems abnormal, over and above any specific system functioning abnormally. If so, this would provide insight into the etiology of frailty, and into treatment and prevention.
Aging itself is thought to be associated with progressive homeostatic dysregulation of our complex system that makes a resilient organism. This is theorized to result both from decreased function in multiple physiological systems and from loss of layers of feedforward and feedback mechanisms among interacting systems (1,16–22). This could result in compromise of the homeostatic physiological safety net essential to reserves and resilience. We hypothesized that frailty results when this safety net of interconnected physiological systems crosses a threshold of aggregate diminution in functioning; this could result from aging-related physiological changes, potentially exacerbated by disease. We have previously demonstrated that frailty is associated with vulnerability to adverse outcomes including mortality. We hypothesized that this could result from aggregate physiological dysfunction and resulting compromise of the stress response mechanisms that maintain homeostasis in a complex resilient organism (1,18), which simultaneously could underly the frailty phenotype itself. As a next step in assessing this theory, we evaluated whether frailty is associated, in older women, with underlying abnormalities in multiple physiological systems simultaneously, whether the aggregate dysfunction is predictive of frailty over and above the effects of each individual system, and whether the association is nonlinear—as would be anticipated in a complex system.
METHODS
The study sample consisted of participants in the Women’s Health and Aging Studies (WHAS) I and II, two complementary population-based observational studies, which together comprise the full spectrum of health and physical functioning in community-dwelling older women. WHAS I recruited from an age-stratified random sample of the Medicare eligibility lists of community-dwelling women aged 65 years and older in Baltimore, Maryland, a cohort of the one third of the most disabled women. Eligibility involved self-reported difficulty in tasks in two or more of four domains of functioning: mobility, upper extremity, household management tasks, and self-care tasks, and a Mini-Mental State Examination (MMSE) score of greater than 18 (23,24). Of the 1,409 women who met study eligibility, 1,002 agreed to participate. After written informed consent, participants were evaluated in their homes.
WHAS II studied the two third’s least disabled older women in the community. It recruited, from the same sampling frame as WHAS I, women aged 70–79 years who had either no difficulty or difficulty in tasks in only one functional domain and who had MMSE scores of 24 or greater (25). Among the screened and eligible women, 436 (49.5%) agreed to participate; these had more education and more diseases than those who refused but did not differ significantly in disability characteristics. All evaluations were performed in the Johns Hopkins Outpatient General Clinical Research Center by trained technicians. WHAS II data collection was standardized to WHAS I. Both studies conducted an extensive standardized interview, which ascertained demographic, health, frailty, and disability information and involved a standardized examination. Self-report of physician diagnosis of any of 14 diseases queried was validated by standardized adjudication of exam and medical records data plus physician questionnaire information (23).
Nonfasting blood samples were collected at 09:00 or 14:00 hours by trained phlebotomists either as an ancillary study to WHAS I or as part of the WHAS II baseline exam. Blood was initially processed at the Core Genetics Laboratory of the Johns Hopkins University School of Medicine, following standardized protocols, and then shipped to Quest Diagnostics for processing the same day. For WHAS I, we analyzed only blood samples from the first draw collected within 90 days of one of the first three 6 monthly examinations within the first year in the study, treating them as baseline measures.
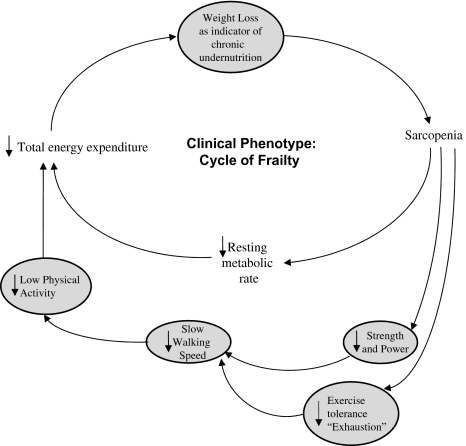
Frailty phenotype: Frailty status was assessed using a validated measure (2,3) consisting of five binary criteria: weakness, slowness, low physical activity, weight loss, and exhaustion. Frailty was defined as the presence of three or more criteria (Figure 1); those with one to two were defined as “prefrail” and those meeting none as nonfrail (2).
Figure 1.
Theorized and validated components of phenotype of frailty with aging, related in an adverse feedforward cycle. Note: Frail, three or more of five possible criteria present (2,3).
Measures of physiological dysregulation: We evaluated 12 measures from six different physiological systems (Table 1 and Appendix 1; 6–14). These were as follows—hematological: hemoglobin less then 12 g/dL; inflammatory: IL-6, top tertile, more than 4.6 pg/mL; hormonal: IGF-1 less than 74.3 μg/L, dehydroepiandrosterone-sulfate (DHEA-S) less than 0.215 mcg/mL, and hemoglobin A1c (HbgA1c) greater than 6.5%; adiposity: triceps muscle skinfold thickness less than 17 mm (lowest quartile); neuromuscular: slow fine motor speed (by Purdue Pegboard) greater than 31.9 seconds (highest quartile); and micronutrients: two or more measures abnormal or deficient, based on prior evidence that multiple micronutrient deficiencies predict frailty better than any one (26), among 25-hydroxyvitamin vitamin D (<30 mmol/L), folate (<5 ng/mL), vitamin B12 (<300 pg/mL), alpha-tocopherol (vitamin E <11.6 μmol/L), and total carotenoids (<0.45 μmol/L). Cutoffs used to define abnormality were either widely accepted criteria (eg, hemoglobin <12 g/dL for anemia by World Health Organization; 8,27) or where there are no commonly used age-specific normal ranges; we used the top (for IL-6 and Purdue Pegboard time) or the bottom quartile (of skinfold thickness) to define abnormal.
Table 1.
Associations of Abnormal Levels of Physiological Measures With Frailty and Prefrailty: Women Aged 70–79 Years in the Women’s Health and Aging Studies. (A) Means and Frequencies of System Deficits and (B) Multivariate Analysis: Associations of Individual Systems at Abnormal Levels With the Risk of Being Frail (vs Nonfrail; N = 704)*
| A |
B |
||||||
| % Abnormal |
Frail vs Nonfrail |
||||||
| System | Measure‡ | Overall (N = 704) | Nonfrail (N = 284) | Prefrail (N = 330) | Frail (N = 90) | p Value† | Odds Ratio (95% CI) |
| Anemia | Hemoglobin in g/dL, M (SD) | 13.1 (1.1) | 13.2 (1.1) | 13.2 (1.1) | 12.6 (1.1) | <.01 | 1.5 (0.7–3.4) |
| Hemoglobin <12 g/dL (%) | 13.3 | 10.1 | 13.3 | 27.0 | <.01 | ||
| Inflammation | IL-6 in pg/mL, M (SD) | 3.4 (2.0) | 3.0 (1.69) | 3.7 (2.09) | 4.7 (2.20) | <.01 | 1.2 (0.6–2.2) |
| IL-6 >4.6 pg/mL (%) | 25.3 | 19.3 | 28.3 | 38.6 | <.01 | ||
| Endocrine | IGF-1 in ng/mL, M (SD) | 111.8 (1.6) | 114.0 (1.5) | 113.2 (1.6) | 98.3 (1.6) | .04 | 1.7 (0.8–3.4) |
| IGF-1 <74.3 ng/mL (%) | 17.4 | 13.8 | 18.6 | 27.0 | .03 | ||
| DHEA-S in mcg/mL, M (SD) | 0.38 (2.18) | 0.42 (2.14) | 0.36 (2.17) | 0.31 (2.24) | <.01 | 1.4 (0.7–2.8) | |
| DHEA-S <0.215 mcg/mL (%) | 24.3 | 21.3 | 25.4 | 33.7 | .10 | ||
| Hemoglobin A1c, M (SD) | 6.1 (1.2) | 5.9 (1.1) | 6.2 (1.2) | 6.1 (1.2) | <.01 | 1.2 (0.5–2.4) | |
| Hemoglobin A1c ≥6.5 (%) | 23.5 | 16.3 | 29.3 | 29.9 | <.01 | ||
| Micronutrients | 25-Hydroxyvitamin vitamin D in nmol/L, M (SD) | 20.1 (1.6) | 20.8 (1.6) | 20.0 (1.6) | 17.6 (1.8) | .08 | 2.6 (1.3–5.0)‖ |
| Folate in ng/mL, M (SD) | 9.7 (1.8) | 10.0 (1.8) | 9.5 (1.8) | 9.6 (1.9) | .64 | ||
| Vitamin B12 in pg/mL, M (SD) | 439.9 (1.7) | 444.2 (1.6) | 440.1 (1.7) | 420.0 (1.7) | .68 | ||
| Alpha-tocopherol in μmol/L, M (SD) | 21.8 (1.5) | 22.6 (1.5) | 21.6 (1.5) | 19.7 (1.4) | .01 | ||
| Total carotenoids in μmol/L, M (SD) | 1.7 (1.6) | 1.9 (1.6) | 1.6 (1.7) | 1.4 (1.6) | <.01 | ||
| ≥2 nutritional deficits§ (%) | 22.6 | 17.9 | 24.1 | 35.4 | <.01 | ||
| Adiposity | Skinfold thickness in mm, M (SD) | 23.0 (8.3) | 23.1 (7.3) | 23.5 (8.7) | 20.4 (9.7) | .03 | 2.6 (1.3–5.2)‖ |
| Skinfold thickness <17 mm (%) | 24.8 | 19.8 | 25.9 | 40.1 | <.01 | ||
| Fine motor speed | Purdue Pegboard in s, M (SD) | 28.6 (1.2) | 27.3 (1.2) | 29.0 (1.2) | 33.5 (1.3) | <.01 | 3.4 (1.8–6.5)‖ |
| Purdue Pegboard >31.9 s (%) | 24.0 | 14.5 | 26.5 | 51.5 | <.01 | ||
Notes: CI = confidence interval. Nonfrail, zero criterion; prefrail, one to two criteria; and frail, three or more criteria present.
Multivariable adjusted polytomous regression analysis, including all systems indicated and adjusted for age, race, education, and number of chronic diseases. Analyses were restricted to the subset of women with complete data on all eight systems for valid comparison between univariate and multivariate associations. In the multivariate analysis, all the systems were entered into a model simultaneously to assess the independent association of each system with frailty and prefrailty after adjusting for the other systems.
Based on analysis of variance test for continuous variables and chi-square test for categorical variables.
Geometric mean and geometric standard deviation for all continuous measures except for skinfold thickness, where arithmetic mean and standard deviation are used instead.
Cutoffs for defining deficits: 25-hydroxyvitamin vitamin D <30 nmol/L, folate < 5 ng/mL, vitamin B12 <300 pg/mL, alpha-tocopherol <11.6 μmol/L, and total carotenoids <0.45 μmol/L.
p value <.05.
Data Analysis
Of the combined WHAS I and II sample of 829 women aged 70–79 years, 709 provided blood samples within the 90-day constraint. Five of the 709 women had frailty status missing at baseline, leaving a final sample size for this study of 704. The sample size for each physiological measure ranged from 632 (for HgbA1c) to 699 (for Purdue Pegboard); some measures were missing due to laboratory error, insufficient blood, change of assay, and so forth, unrelated to participant characteristics. All analyses incorporated sampling weights that reference study-specific analyses to population representation and obtain valid standard error estimates (23,25).
In cross-sectional analysis, means and frequencies of abnormal levels of the physiological measures were compared by frailty status at baseline using the chi-square test for proportions and analysis of variance for continuous measures. Geometric means and standard deviations were calculated for all continuous measures due to the measures’ skewed distributions. As one exception, arithmetic statistics were calculated for skinfold thickness, whose distribution appeared symmetric.
We assessed the associations of individual systems with frailty and prefrailty using multinomial logistic regression (MLR; nonfrail group as reference). MLR estimates odds ratios (ORs) of being frail or prefrail versus nonfrail, comparing those with abnormal versus normal levels of the measure of interest. We first analyzed the relationship of frailty to each of the eight measures, adjusting for age, education, race, and number of adjudicated chronic diseases. Then, in multivariable analysis, eight measures from six physiological systems were entered into a model simultaneously to assess the independent association of each system with frailty, adjusting for the other systems and confounders.
We next evaluated whether frailty was associated with an increased number of systems impaired, regardless of individually significant associations, using four complementary approaches. First, frequency distributions of number of systems at abnormal levels were compared by frailty status. Second, we plotted frailty prevalences by the number of abnormal systems. To test for potential nonlinearity of this relationship, we fit a binomial regression model with identity link and included both linear and quadratic effects of the deficit count in the model as covariates.
Third, we analyzed the association of the number of systems abnormal with frailty and with prefrailty (compared with nonfrail) using MLR. We compared the ORs of being frail versus nonfrail (or prefrail vs nonfrail) between those with one to two, three to four, or more than five systems at abnormal levels and those with no systems abnormal. To assess the independent contribution of specific system abnormalities to this frailty phenotype, above and beyond the number of systems abnormal, we included both the number of systems at abnormal levels and each individual system deficit (in separate models) in the model as predictors.
Fourth, we used latent class analysis (LCA) to identify population subsets with similar profiles of multisystem deficits and assess two competing hypotheses: whether abnormalities aggregate by distinct subgroups of systems or if counting system deficits provides reasonable identification of subgroups. Patterns of deficit co-occurrence that would support the count measure are (a) manifestation in a critical mass, without evidence of co-occurring in etiologically distinct subgroups, and (b) aggregation in a specific order; the latter would suggest a hierarchy of dysregulation via profiles containing nested subsets of abnormal systems. The number of subgroups (classes) was determined by goodness-of-fit evaluation using Pearson’s chi-square test (28), the Akaike information criterion (29), and the Bayesian information criterion (30). To ensure a stable model solution, we fit our model 100 times with randomly generated starting values for model parameters; model estimates are trustworthy if a large majority of runs results in the same solution. In this case, all runs gave identical estimates.
We then assessed the a priori hypothesis that the association between the aggregate number of abnormal physiological systems and the clinical phenotype of frailty is independent of specific system abnormalities. After LCA fitting, we randomly generated latent class memberships from their fitted “posterior” distributions given the observed abnormalities (31). The group of abnormal systems identified was analyzed for association with the clinical phenotype of frailty using MLR, adjusting for number of comorbid diseases, age, race, and education. We then augmented the MLR models to also include system-specific abnormalities, one at a time. To account for the uncertainty in the generation of latent class memberships, we repeated the process of physiotype generation and MLR fitting 100 times, and parameter estimates were combined using multiple imputation (32). Analyses were performed using STATA version 9.1 (StataCorp, College Station, TX) and MPLUS version 4.1 (Muthen & Muthen, Los Angeles, CA).
RESULTS
Study participants were women aged 70–79 years; 21% were African American, the rest Caucasian. Numbers of chronic diseases present (of 11) were as follows: one among 37%, two in 22%, and more than three in 14%. Ten percent were frail and 45% prefrail. Comparatively, 39% of those who were frail had more than three diseases, versus 20% of those prefrail and 3% of the nonfrail (p = .01), whereas 9% of those who were frail and 38% of the nonfrail reported none of these diseases (see Table 2, panel B, footnote for the 11 diseases).
Table 2.
Aggregation of System Abnormalities and Joint Association With Frailty. (A) Multisystem Deficit Profile: Conditional Probabilities of Meeting Deficit Criteria Within Latent Classes (N = 704) and (B) Independent Association of Class 2 Cluster of 2.9 System Abnormalities, on Average, With Frailty (compared with class 1 cluster with 1.3 system abnormalities)*
| A | ||||
| 1-Class Model |
2-Class Model |
|||
| System | Measure | Class 1 | Class 1 | Class 2 |
| Prevalences of abnormal level of each measure | ||||
| Anemia | Hemoglobin <12 g/dL | 0.131 | 0.071 | 0.267 |
| Inflammation | IL-6 >4.6 pg/mL | 0.253 | 0.153 | 0.485 |
| Endocrine | IGF-1 <74.3 ng/mL | 0.174 | 0.137 | 0.257 |
| DHEA-S <0.215 mcg/mL | 0.244 | 0.217 | 0.307 | |
| Glucose tolerance | Hemoglobin A1c ≥6.5% | 0.234 | 0.192 | 0.329 |
| Micronutrient | ≥2 micronutrient deficits | 0.225 | 0.188 | 0.311 |
| Body composition | Skinfold thickness <17 mm | 0.247 | 0.211 | 0.328 |
| Fine motor speed | Purdue Pegboard >31.9 s | 0.237 | 0.089 | 0.577 |
| Class prevalence (%) | 100 | 69.6 | 30.4 | |
| Mean number of systems abnormal | 1.7 | 1.3 | 2.9 | |
| Model fit statistics | LR chi square | 293.5 (p < .01) | 244.1 (p = .19) | |
| AIC | 5,573.9 | 5,534.1 | ||
| BIC | 5,685.0 | 5,557.6 | ||
| B | ||||
| Frail vs Nonfrail OR (95% CI) | ||||
| Age | 1.15 (1.04–1.28)† | |||
| Black race | 1.75 (0.92–3.31) | |||
| Education (y) | 0.81 (0.74–0.89)† | |||
| Number of diseases‡ | 2.42 (1.89–3.10)† | |||
| Multiple (ie, class 2) vs isolated abnormalities (ie, class 1) | 2.59 (1.22–5.52)§ | |||
Notes: AIC = Akaike information criterion; BIC = Bayesian information criterion; CI = confidence interval; LR= Likelihood Ratio; OR = odds ratio.
Results of polytomous multinomial logistic regression based on repeated generation of “pseudo” classes of multisystem impairments using posterior latent class probabilities.
p value <.01.
Number of adjudicated “definite” chronic conditions of 11: coronary artery disease (angina pectoris and/or myocardial infarction), congestive heart failure; peripheral arterial disease; stroke; pulmonary disease; hip fracture; diabetes mellitus; osteoarthritis of the knee, hip, and hand; rheumatoid arthritis; Parkinson’s disease; and cancer.
p value<.05.
Table 1, panel A, displays mean levels for 12 measures within six physiological systems, along with the proportion meeting criteria for abnormal levels, by frailty status. These data indicate significant dose–response or threshold associations of each measure with frailty status, with 1.8- to 3.6-fold increases in the proportion with abnormal levels in frail compared with nonfrail. Folate and vitamin B12 were the exceptions; note that this study was performed before folate fortification of flour and cereal grain products became mandated in the United States.
Combining the five micronutrients into a summary measure of number at deficient levels, we then evaluated the independent associations of abnormal levels of eight different physiological systems with frailty, adjusting for confounders. Table 1, panel B, shows that, when adjusting for each of the seven other independent variables, two or more micronutrients at deficient levels, adiposity, and slowed fine motor speed were each significantly associated with 2.6- to 3.4-fold increases in the likelihood of being frail (p < .01). The direction of association was positive for each of the other three independent variables but not significant after adjustment for the seven others. Previously, univariable analyses (adjusted only for age, education, race, and number of chronic diseases) showed similar but stronger associations of each of these measures, separately, with frailty; among these, low IGF-1 was also significantly associated with frailty (OR 2.1, 95% confidence interval [CI] 1.1–4.2; data not shown).
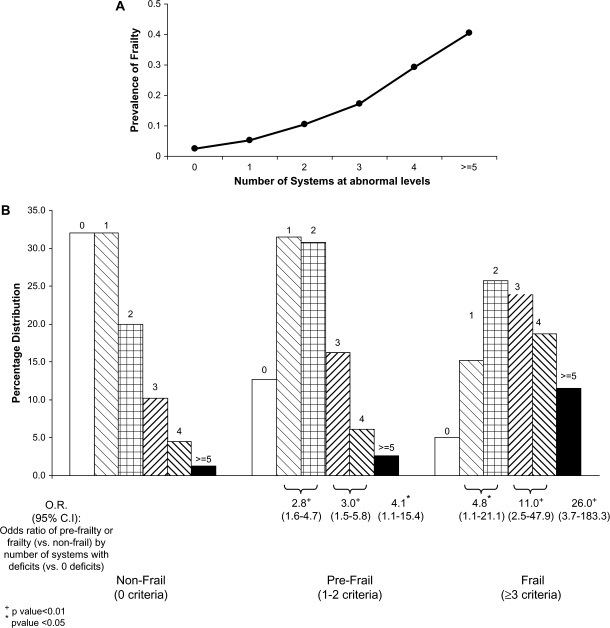
To assess whether frailty was associated with an increased number of systems impaired, regardless of individually significant associations, we took four complementary approaches. First, the mean numbers of individual systems at abnormal levels (and 95% CIs) were as follows: nonfrail, 1.3 (1.1–1.4); prefrail, 1.8 (1.7–1.9); and frail, 2.7 (2.4–3.0). Second, Figure 2A displays a nonlinear increase in frailty prevalence with increasing number of abnormal physiological systems (p < .01 for quadratic trend).
Figure 2.
Association of number of physiological systems at abnormal levels with being frail, women aged 70–79 years (p < .01 for qualitative trend). (A) Prevalence of being frail by number of dysregulated systems at baseline. (B) Number of system deficits (of eight possible) by frailty categories.
Third, we assessed the risk of frailty or prefrailty by number of systems at abnormal levels. Figure 2B indicates that those with three, four, or five or more systems at abnormal levels were most likely to be frail. Half of those frail had three or more systems at abnormal levels, compared with 25% of the prefrail and 16% of the nonfrail (p < .01, unadjusted); less than 21% of the frail had zero or one system abnormal (of eight). There was a dose–response relationship between the number of systems at abnormal levels and risk of frailty, increasing from ORs of 4.8 for those with one to two systems abnormal (compared with zero) to 11- and 26-fold increased risk for those with three to four and five or more systems at abnormal levels, respectively (95% CIs did not include 1). There was also an intermediate level of risk for each number of systems abnormal in association with prefrail status (Figure 2B).
We also evaluated whether each individual system was independently associated with frailty when the number of systems was also in the model. The number of systems abnormal was strongly predictive of the likelihood of frailty, whereas the individual systems were not, with the exception of fine motor speed (data not shown). These findings consistently indicate that the frailty phenotype is associated with multiple physiological abnormalities.
Fourth, we evaluated whether any subgroups of this population had distinct profiles of multisystem deficits, to understand whether specific clusters of deficits mattered, or the issue was aggregate burden. LCA identified a two-class model as providing an adequate fit to the observed data, based on goodness-of-fit statistics (Table 2, panel A). Estimated prevalences for these two mutually exclusive “classes,” classes 1 and 2, were 69.6% and 30.4%, respectively (Table 2, panel A). Class 1 appears to represent a subset of the population with one or no systems (ie, 1.3 systems on average) at abnormal levels, whereas the 30% in class 2 are a subset with multiple systems abnormal, that is, 2.9 on average. LCA also provides the probabilities that someone in a class will have a given level (normal or abnormal) on each of the physiological measures assessed. For example, in Table 2, panel A, considering IL-6, the conditional probability .153 means that, on average, 15.3% of the women who were in class 1 had IL-6 levels greater than 4.6 pg/mL, in contrast to 48.5% of those in class 2. The prevalence of abnormal levels of each measure increased progressively from classes 1 to 2, with the most dramatic increases in prevalence being associated with deficient fine motor speed (an increase of 6.5-fold, from .089 to .577), anemia (3.8-fold), and inflammation (3.2-fold; Table 2, panel A). Further analysis of the 256 (28) possible patterns indicated little evidence as to dominating patterns of change between classes 1 and 2 (data not shown), consistent with no subgroups of systems involved.
We then evaluated whether the two classes in the population, as aforementioned, were associated with frailty status. Table 2, panel B, shows that class 2, with multisystem decrements (vs class 1 isolated decrements, as in Table 2, panel A), was independently associated with being frail (compared with not frail), with OR of 2.6 (95% CI 1.2–5.5), adjusting for number of comorbid diseases, age, race, and education. Furthermore, the number of comorbid diseases was associated with frailty independent of multisystem abnormalities (per class 2), and the strength of association approximated those of the class 2 associations. In separate analyses, the association of class 2 with being frail remained significant after individually controlling for each of the eight system measures; the latter were not themselves significant in these models with the exception of fine motor speed (data not shown).
DISCUSSION
These analyses provide the first evidence to support the a priori hypothesis that a clinical phenotype of frailty is related, nonlinearly, to the number of abnormal physiological systems, independent of specific system abnormalities, number of chronic diseases, and chronological age. Evaluations with four different methodological perspectives yielded concordant findings: three or more systems at abnormal levels were significant predictors of being frail, and the dominating predictor was the number of systems abnormal, not any particular system. These findings, although cross-sectional and not able to prove causality, strongly suggest that when physiological dysregulations reach an aggregate critical mass, frailty becomes clinically evident.
The mechanism by which the aggregate effect of many systems abnormal seems to independently drive frailty remains to be determined. Interestingly, the physiological systems studied here are known to each affect two or more of five components of this frailty phenotype (Figure 1). With multiple systems dysregulated, there is the potential for multiple adverse influences on any single phenotypic component (eg, abnormal IGF-1, DHEA-S, IL-6, glucose intolerance, and vitamin D each independently adversely affect strength via muscle or fat mass) or simultaneously adversely affecting multiple phenotypic components (ie, the previously mentioned effects on strength and exercise tolerance via muscle are potentially compounded by the impact of anemia and DHEA-S on exercise tolerance and energy, or “exhaustion”; furthermore, fine motor speed and walking speed are weakly to moderately correlated). Thus, there is plausible biologic specificity to the relationship of these specific physiological systems to this frailty phenotype, as well as aggregate effects of increasing risk of frailty when many systems are affected.
Given that these physiological predictors themselves mutually affect each other, they could be both initiating and intermediary mechanisms in frailty. For example, sarcopenia and adiposity are themselves affected by chronic inflammation and altered hormonal inputs. Furthermore, micronutrient levels result from nutrient intake, metabolism, and intrinsic consumption (eg, oxidative stress) and themselves affect diverse cellular pathways. Thus, the systems studied here have numerous physiological interconnections with each other (17,18). Interconnected systems are essential elements of a nonlinear complex system (16–22,33,40). It has been proposed that, although senescent processes (such as those in the systems studied here) display quasilinear properties of decline over the adult life course, their aggregate effect in contributing to a complex system is nonlinear (22). The work presented here is, to our knowledge, the first to offer evidence supportive of this theory, linking aggregate decline in multiple systems with the clinical presentation of “being frail.” Separately, being frail predicts mortality, disability, and other adverse outcomes in human beings (2,3,34–37). In combination, these findings suggest that the loss of function in a complex system may affect, past a threshold, homeostatic adaptive capacity, and the latter may thus underly both clinical frailty and its adverse outcomes. Overall, these data offer insight into the effects of alteration of complex systems with aging and their culmination in a clinically recognizable state of vulnerability, frailty. Findings here may be broadly applicable, beyond frailty, to insights into the systems biology of the intact resilient human organism (16,17,22,33,38–40).
This population of older women revealed two mutually exclusive subclasses characterized by low versus high frequencies of multisystem abnormalities. That there was no evidence that certain system deficits co-occurred preferentially in specific subgroups argues against etiologically distinct subgroups or a sentinel system spurring a cascade of alterations across other systems. This observation that there was little evidence of any dominant subgroups of dysregulated systems is consistent with the expectations of a nonlinear complex system (18,19,20,22) and the systemic nature of aging (22).
Given that many of the physiological systems evaluated affect or regulate each other, alteration of one may not be independent of another. Notably, the nonlinear relationship of accelerating likelihood of frailty as the number of abnormal systems escalates (Figure 2A) suggests that there could be a threshold beyond which there is an adverse downward spiraling nature to frailty etiology and progression. This would be consistent with the concept of “majority rules” in systems biology (22,40), that past a critical level of dysregulation of physiological systems, the aggregate systems impaired may adversely affect other systems functioning at a normal level, thus bringing the whole system to a more dysregulated state. These observations of nonlinearity are further supportive of considering frailty as an outcome of depletion or dysregulation of a complex system. The clinical implications of these findings are, practically, that simple therapeutic replacement of just one system at deficient levels (eg, testosterone, estrogen, or growth hormone) is unlikely to resolve frailty or even prevent it. The exception could be to seek a single etiologic factor that is critical to creating a hierarchical cascade of dysregulation of multiple other systems or that concurrently affects multiple systems. To evaluate for such is beyond the scope of the current study.
The number of chronic diseases was also a predictor of frailty, independent of the number of physiological systems at abnormal levels. This supports frailty being a final common pathway of multiple etiologies and that the burden of disease is a factor as well as an aging-related physiological dysregulation. This finding suggests intriguing parallels between these findings and other work suggesting that frailty can be conceived as the deficits in multiple unrelated systems, which predict more distal adverse outcomes such as mortality (36,37,41). In each of these cases, there is an association of number of deficits with an adverse outcome, suggesting that critical mass of deficits matters across a range of issues; these outcomes may, themselves, turn out to be related or to point to an as-yet unidentified biologic etiology. Of course, although the definition of frailty used in this study has previously been shown to predict mortality and disability and has been validated to function as a medical syndrome, the present study is solely addressing the prediction of this syndrome of frailty—not mortality. Potentially, the vulnerability conferred by loss of reserves due to multiple illnesses may contribute to both frailty and its sequellae.
There are several limitations of this work. Cross-sectional data permit characterizing physiological status in multiple systems concurrent with a clinical presentation but do not confirm causality. Thus, our findings should be complemented by longitudinal and experimental evidence. Additionally, we analyzed biomarkers of a number of physiological systems in this population-based study. However, there are several additional systems central to maintenance of effective adaptation and resilience that we could not assess in these data. These include, particularly, glucocorticoids and the autonomic nervous system.
Overall, the findings reported here indicate an escalating nonlinear association such that the greater the number of physiological systems at abnormal levels, the stronger the likelihood of frailty. This multisystem dysregulation appears independent of the effects on frailty of chronic diseases or specific systems abnormal. These findings support the hypothesis that an aggregate loss of function in multiple interconnected and mutually adaptive physiological systems may underly the clinical presentation of frailty and may confer the vulnerability to stressors—and resulting adverse outcomes—associated with this phenotype while initiating the phenotype itself.
FUNDING
This research was supported by the National Institute on Aging (NIA), Older Americans Independence Center, grant P30 AG021334 and National Institutes of Health/NIA grant R37 AG019905.
Appendix 1
Triceps skinfold thickness was standardly measured at the midpoint of the upper right arm with Holtain skinfold calipers (Seritex, Carlstadt, NJ) to the nearest 0.2 mm (31). The Purdue Pegboard tests manual dexterity involving gross movements of arms, hands, and fingers and fine motor dexterity. The test consists of picking up small steel pegs from a well in the pegboard and placing them sequentially in 10 holes as quickly as possible. The time to complete the task was recorded in seconds (27,28). IGF-1 was measured by radioimmunoassay (RIA; Nichols Institute Diagnostics, San Juan Capistrano, CA) with ethanol extraction at the time of blood collection. The overall coefficient of variation is less than 15% and the assay sensitivity was 0.1 μg/L. DHEA-S was measured in duplicate by enzyme-linked immunosorbent assay (ELISA; American Laboratory Products Company, Windham, NH) from frozen specimens. The interassay coefficient of variation was 3.3%; assay sensitivity was 2 μg/dL (0.05 μmol/L). IL-6 was measured in duplicate by ELISA (High Sensitivity kit; R&D Systems Inc, Minneapolis, MN) from frozen specimens. The lower detection limit was 0.1 pg/mL, and the interassay coefficient of variation was 7%. Hemoglobin was measured using spectrophotometry using the cyanmethemoglobin method (Quest Diagnostics, Teterboro, NJ). Serum carotenoids and alpha-tocopherol were determined by high-performance liquid chromatography. Total carotenoids were calculated as the sum of alpha-carotene, beta-carotene, beta-cryptoxanthin, lutein/zeaxanthin, and lycopene in μmol/L. Serum concentration of 25-hydroxyvitamin D was measured using a radioreceptor assay. Serum vitamin B12 and folate were measured by RIA. Intra- and interassay coefficients of variation, respectively, were 10.0% and 23.9% for alpha-carotene, 7.0% and 19.1% for beta-carotene, 4.7% and 8.5% for beta-cryptoxanthin, 4.1% and 4.6% for lutein/zeaxanthin, 10.0% and 14.0% for lycopene, 4.1% and 9.7% for alpha-tocopherol, and 7.5% and 9.6% for 25-hydroxyvitamin D. Hemoglobin A1c was measured using a hemoglobin A1c analyzer (Cholestech GDX System, Hayward, CA), with an intra-assay coefficient of variance of 1.43% and an interassay coefficient of variance of 2.34% (General Clinical Research Center Core Laboratory, Johns Hopkins Bayview Medical Center, Baltimore, MD). The GDX system met all National Glycohemoglobin Standardization Program requirements for accuracy and precision and is Clinical Laboratory Improvement Amendments waived.
References
- 1.Fried LP, Walston J. Hazzard WR, Blass JP. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003. Frailty and failure to thrive; pp. 1487–1502. [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 5.Studenski S, Hayes RP, Leibowitz RQ, et al. Clinical global impression of change in physical frailty: development of a measure based on clinical judgement. J Am Geriatr Soc. 2004;52:1560–1566. doi: 10.1111/j.1532-5415.2004.52423.x. [DOI] [PubMed] [Google Scholar]
- 6.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167(7):635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 7.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 8.Chaves PHM, Ashar B, Fried LP. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women: should the criteria currently used to define anemia in older women in the elderly be evaluated? J Am Geriatr Soc. 2002;50(7):1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- 9.Cappola AR, Bandeen-Roche K, Wand GS, Volpato S, Fried LP. Association of IGF-1 levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86:4139–4146. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]
- 10.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 11.Michelon E, Blaum CS, Semba RD, Xue QL, Ricks MO, Fried LP. Vitamin and carotenoid status in older women: associations with the frailty syndrome. J Gerontol A Biol Sci Med Sci. 2006;61(6):600–607. doi: 10.1093/gerona/61.6.600. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 13.Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63(2):190–195. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- 14.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 15.Puts MT, Visser M, Twisk JWR, Deeg DJH, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63(4):403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 16.Csete ME, Doyle JC. Reverse engineering of biological complexity. Science. 2002;295(5560):1664–1669. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52(10 suppl 2):10–16. doi: 10.1016/s0026-0495(03)00295-6. Review. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005(31):pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 19.Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci Aging Knowledge Environ. 2004;2004(16):pe16. doi: 10.1126/sageke.2004.16.pe16. Review. [DOI] [PubMed] [Google Scholar]
- 20.Lipsitz LA, Goldberg AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267(13):1806–1809. [PubMed] [Google Scholar]
- 21.Bortz WM., 2nd A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57(5):M283–M288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 22.Yates FE. Complexity of a human being: changes with age. Neurobiol Aging. 2002;23(1):17–19. doi: 10.1016/s0197-4580(01)00261-5. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women With Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Pub. No. 95-4009. [Google Scholar]
- 24.Simonsick E, Maffeo C, Rogers S, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M264–M274. doi: 10.1093/gerona/52a.5.m264. [DOI] [PubMed] [Google Scholar]
- 25.Fried LP, Young Y, Rubin G, Bandeen-Roche K. Self-reported preclinical disability identifies older women with early declines in performance and early disease. J Clin Epidemiol. 2001;54:889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- 26.Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci. 2006;61(6):594–599. doi: 10.1093/gerona/61.6.594. [DOI] [PubMed] [Google Scholar]
- 27.Lohman T, Roche A, Mastasell R. Anthropometric Standardization Reference Manual. Champagne, IL: Human Kinetics Books; 1988. [Google Scholar]
- 28.Chernoff H, Lehmann EL. The use of maximum likelihood estimates in x2 tests for goodness of fit. Ann Math Statist. 1954;25:579–586. [Google Scholar]
- 29.Akaike H. A new look at statistical model identification. IEEE Trans Autom Control. 1974;19:716–723. [Google Scholar]
- 30.Schwarz G. Estimating the dimensions of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 31.Bandeen-Roche K, Miglioretti D, Zeger SL, Rathouz P. Latent variable regression for multiple discrete outcomes. J Am Stat Assoc. 1997;92(440):1375–1386. [Google Scholar]
- 32.Rubin DB. Multiple Imputation for Nonresponse in Survey Research. New York, NY: Wiley; 1987. [Google Scholar]
- 33.Kitano H. Systems biology: a brief overview. Science. 2002;295(5560):1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 34.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 35.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 36.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59(6):627–632. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 37.Kulminsky AM, Ukraintseva SV, Culminskaya IV, et al. Cumulative deficits and physiological indices as predictors of mortality and long life. J Gerontol A Biol Sci Med Sci. 2008;63(10):1053–1059. doi: 10.1093/gerona/63.10.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gavrilov LA, Gavrilova NS. The reliability-engineering approach to the problem of biological aging. Ann N Y Acad Sci. 2004;1019:509–512. doi: 10.1196/annals.1297.094. Review. [DOI] [PubMed] [Google Scholar]
- 39.Seely AJ, Christou NV. Multiple organ dysfunction syndrome: exploring the paradigm of complex nonlinear systems. Crit Care Med. 2000;28(7):2193–2200. doi: 10.1097/00003246-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Amaral LA, diaz-Guilera A, Moreia AA, Goldberger AL, Lipsitz LA. Emergence of complex dynamics in a simple model of signaling networks. Proc Natl Acad Sci U S A. 2004;101(44):15551–15555. doi: 10.1073/pnas.0404843101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fried LP, Kronmal RA, Bild D, et al. Risk factors for five-year mortality in older adults: The Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]