Abstract
Background
Our objective was to perform a systematic review and meta-analysis of the research literature that compared muscle strength in postmenopausal women who were and were not on estrogen-based hormone therapy (HT).
Methods
Twenty-three relevant studies were found. Effect sizes (ESs) were calculated as the standardized mean difference, and meta-analyses were completed using a random effects model.
Results
HT was found to result in a small beneficial effect on muscle strength in postmenopausal women (overall ES = 0.23; p = .003) that equated to an ∼5% greater strength for women on HT. Among the 23 studies, various muscle groups were assessed for strength, and those that benefitted the most were the thumb adductors (ES = 1.14; p < .001). Ten studies that compared muscle strength in rodents that were and were not estradiol deficient were also analyzed. The ES for absolute strength was moderate but not statistically significant (ES = 0.44; p = .12), whereas estradiol had a large effect on strength normalized to muscle size (ES = 0.66; p = .03).
Conclusion
Overall, estrogen-based treatments were found to beneficially affect strength.
Keywords: Hormone replacement therapy, Estradiol, Postmenopausal
THERE is a loss of skeletal muscle mass and strength, that is, sarcopenia, with age, and this loss contributes to increased risks for comorbidities related to falls, diminished ability to carry out activities of daily living, and an overall decreased quality of life (1). In addition to the age-induced loss of muscle strength, in women, there are reports of accelerated rates of strength loss associated with menopause (2–4). Therefore, it is probable that changes in ovarian hormones due to menopause contribute to muscle weakness. To counter the loss of ovarian hormones, women have the option of taking hormone therapy (HT). In this article, we will use the term HT because it was recently recommended that this terminology be used for menopause-related therapies that encompass estrogen therapy and combined estrogen–progestogen therapy (5,6). The primary active ingredients in HT are estrogenic compounds such as estra-1,3,5,(10)-triene-3,17β-estradiol, estrone, and dihydroequilin. Progestogen and testosterones are secondary components of some HTs. The decision to take HT is largely based on known and perceived benefits versus risks on menopausal symptoms, bone loss, cancers of the female reproductive organs, and cardiovascular events.
In contrast to HT’s known effects on bone, reproductive tissues, and the cardiovascular system, the effects of HT on skeletal muscle are not as well understood or considered clinically. Skeletal muscle is the largest tissue containing estrogen receptors, so there is a logical connection between ovarian hormones and skeletal muscle. There have been a number of studies that have reported the effects of HT on the primary function of skeletal muscle, that is, to generate force, which we evaluate as muscle strength. However, the results of these studies have been equivocal and even when analyzed collectively in traditional narrative reviews, there is no clear answer as to whether or not HT for menopausal women is advantageous for improving muscle strength (3,7). In this study, our objective was to clarify this issue by performing a rigorous systematic review and statistical analysis (meta-analysis) of the studies that have compared muscle strength in postmenopausal women who were and were not on HT. We found a substantial amount of variability in the effects of HT on strength among the studies. Thus, a secondary objective was to analyze several experimental factors whose levels varied among the studies and thus could have affected study outcomes. Finally, to specifically assess the effect of estradiol on strength, a systematic search and meta-analysis were also conducted for studies of muscle strength in rodents that were and were not estradiol deficient.
METHODS
Meta-analyses of Studies on Postmenopausal Women
Systematic review.—
We searched for studies in the research literature in which muscle strength was assessed in postmenopausal women who were and were not administered HT. Strength was operationally defined as a maximal force, torque, or power generated by a group of muscles. Our search for studies began on October 2007 and continued through April 2009. PubMed, Cochrane Library, Biological Abstracts, Web of Science, Digital Dissertation Database, and ClinicalTrials.gov databases were searched. The search terms and strategy were as follows: (a) hormone replacement therapy, (b) estrogen, (c) human, (d) muscle, (e) skeletal muscle, (f) strength, (g) muscle function, (h) muscle force, (i) muscle performance, (j) muscle strength, and (k) (hormone replacement therapy or estrogen) and human and (muscle or skeletal muscle or strength or muscle function or muscle force or muscle performance or muscle strength). The reference lists from the 56 fully evaluated publications (described below) and those of relevant review articles (3,7–11) were also examined for studies not found with the online database searches. Searches were completed independently by two of the authors (S.M.G. and K.A.B.) by searching through the same databases with the same search strategies.
Study inclusion and exclusion criteria.—
Studies meeting the following criteria were considered for review: (a) women were postmenopausal at the start of the study, (b) the study contained both HT and control (non-HT) groups, (c) the HT was estrogen based, (d) an objective measurement of muscular strength was performed, (e) there was an explanation of how muscle strength was tested, (f) details on participant’s age were included, (g) explanations of participant’s inclusion and exclusion criteria were given, and (h) the results were published in English. All studies determined to be relevant were checked for inclusion criteria by two authors (S.M.G. and K.A.B.), and the data selected for analysis were based on a consensus of the two. Studies were excluded for the following reasons: (a) there was a concurrent exercise intervention with no group that did not perform exercise either with or without HT, (b) strength data were presented as adjusted means (e.g., data adjusted for age, body mass index, or smoking status), or (c) strength data could not be extracted from the study (i.e., raw or summary data were not provided).
Selection.—
A total of 3,824 relevant publications were originally identified through the database searches. Of those, 3,768 were initially excluded based on the title and/or a brief review of the abstract. Fifty-six publications were identified at this point and fully evaluated via careful review of the full text. Based on the inclusion and exclusion criteria, 32 articles were excluded, leaving a total of 24 articles to be included in the primary meta-analysis. Of the 24 articles, two reported findings from the same participants though the strength analyses differed (12,13). Thus, data from these two publications were combined, giving a total of 23 studies that were included in the analysis.
Data extraction and study quality assessment.—
In most instances, mean muscle strength was extracted for the HT and control groups from the 23 studies along with standard deviations and sample sizes. Data for strength normalized to muscle cross-sectional area were also extracted when available. If mean values were not available, percent changes and p values were extracted. If data were presented only in graphical form, means and standard deviations were extracted from the graphs. All data extractions were completed by one author (S.M.G.) and cross-checked by a second (K.A.B.) author. From the 23 studies, three corresponding authors were contacted via e-mail in attempts to retrieve data on strength normalized to muscle cross-sectional area. Two sets of these data were obtained and included in the meta-analyses of normalized strength.
The 23 studies were assessed for quality based on the Physiotherapy Evidence-based Database (PEDro) Scale independently by two authors (S.M.G. and K.A.B.). This scale yields a total possible score of 11 points with more points corresponding to higher quality (14). We deemed this quality assessment to be useful as the results yielded a range of scores.
Meta-analysis.—
For studies that were cross-sectional in design, the extracted strength data were converted to a standard format by calculating a standardized mean difference using the equation: (HT mean − control mean) per pooled standard deviation (SD) (15). Alternatively, standardized mean difference was determined from percent differences in strength and p values when only those data were available. For studies that reported paired data from pre- and posttrials (i.e., studies that were longitudinal in design), the SD of change from pre- to posttrials was calculated for each group: ((SDpre2 + SDpost2) − (2r × SDpre × SDpost))½, where r is the correlation of pre- and posttrial measurements (15). The within-group SD was calculated by dividing the SD of change by (2 × (1 – r))½ (15). Because correlations of pre- and posttrial measurements were not reported in any study, a range of correlation coefficients (i.e., .7–.9) was tried in calculating the SDs of change from pre- to posttrials. This range was based on test–retest correlations (i.e., .8–1.0) reported for strength measurements in the literature (16). However, these correlations were mostly derived from studies with short intervals between trials, as opposed to the studies in our meta-analysis that averaged about a year between trials. We rationalized that test–retest correlations would decrease as the time between trials increased, and therefore, we tested a slightly lower range of correlation coefficients. The correlation coefficient used in all final calculations was .8.
When a longitudinal study had more than one time point, the standardized mean differences and variances for the midpoint, endpoint, and any points between the midpoint and endpoint were averaged across time points. Likewise, when a study measured strength in more than one muscle group, the standardized mean differences and standard errors for those muscle groups were averaged. Meta-analyses were run using a random effects model that accounts for true variation in effects that vary from study to study and also for random error within each study. Examples of true variation among the 23 studies that could potentially affect study effect sizes (ESs) are experimental factors including characteristics of the participants, details of HT administration, and parameters related to muscle strength testing.
We next sought to determine the role of experimental factors in explaining the large interstudy variation observed in ESs. These experimental factors can be treated as moderator variables in a meta-analysis. Therefore, meta-regressions (conducted using the method of moments model) or meta-analyses comparing subsets of studies were used to probe the roles of the following factors: (a) the participants’ time since menopause (by meta-regression), (b) the participants’ time on HT (by meta-regression), (c) previous use of HT (no previous use vs previous use), (d) study design (randomized controlled trial [RCT] experimental design vs non-RCT design), (e) muscle group type (thumb adductors vs forearm flexors vs hip abductors vs knee extensors vs knee flexors), and (f) type of muscle contraction used in testing (isometric vs isokinetic). Some studies measured strength of multiple muscle groups or tested strength both isometrically and isokinetically. In those cases, data subsets from a study were treated as separate studies in the meta-analysis.
Meta-analyses and meta-regressions were conducted using the software Comprehensive Meta-Analysis Version 2.2 (Biostat, Englewood, NJ). An α-level of .05 was used in all analyses except when a moderator variable with more than two levels was being probed in a meta-analysis. In this situation, a Bonferroni correction was applied to the α-level to correct for multiple post hoc comparisons. ESs of 0.2, 0.5, and 0.8 were considered small, moderate, and large, respectively (17).
Meta-analyses of Studies on Rodents
Systematic review.—
It was difficult to explore some of the potential moderator variables among the studies on women, particularly those associated with the treatment of HT. For example, the type and amount of HT that women took was quite variable among studies and not controlled within many of the studies. To address this shortcoming, meta-analyses were performed using studies in which strength was measured in estrogen-replete versus estrogen-deficient rodents. In these studies, the type of HT, namely 17β-estradiol, was consistent, and dosages were similar among studies. Also, although only a few of the studies on women measured size of the muscles that were tested for strength, the results of those studies suggest that the effect of HT on strength normalized to muscle cross-sectional area is important. We were able to further explore the effect of HT on normalized strength in the rodent studies because muscle size was measured in many of those.
A search for studies on the effects of estrogen on muscle strength in rodents was conducted using the following terms and strategy: (a) estradiol, (b) estrogen, (c) ovariectomy (OVX), (d) rodent, (e) mouse, (f) rat, (g) muscle, (h) skeletal muscle, (i) strength, (j) muscle function, (k) muscle force, (l) muscle strength, (m) muscle performance, and (n) (estradiol or estrogen or ovariectomy) and (rodent or mouse or rat) and (muscle or skeletal muscle or strength or muscle function or muscle force or muscle strength or muscle performance). Searches were completed on PubMed, Cochrane Library, Biological Abstracts, Web of Science, and Digital Dissertation Database by two of the authors (S.M.G. and K.A.B.).
Inclusion and exclusion criteria of rodent studies.—
Studies meeting the following criteria were considered for review: (a) the rodents were young (i.e., no age-induced ovarian failure), (b) there was an estrogen-replete group (i.e., sham OVX or ovariectomized followed by estradiol treatment) and an estrogen-deficient group (i.e., ovariectomized), and (c) skeletal muscle strength was determined in situ, in vitro, and/or in vivo. Studies on rodents were excluded for the following reasons: (a) all groups received voluntary or forced exercise, (b) all groups were hind limb unweighted, (c) no strength data were presented (e.g., articles only provide data on fatigue), (d) estradiol replacement was mixed with another hormonal intervention such as androgen plus estrogen, with no pure estradiol group.
Selection of rodent studies.—
The search strategy used for the rodent studies found a total of 3,674 studies. Examination of the title and/or abstract led to the exclusion of 3,654 studies. The remaining 20 studies were thoroughly evaluated based on the full text. After careful examination, 10 studies were excluded leaving 10 studies that were used to complete the meta-analysis. Two authors (S.M.G. and K.A.B.) independently checked studies for inclusion and exclusion criteria and came to a consensus regarding the final selection of studies to be included in the rodent analyses.
Data extraction from rodent studies.—
Extraction of absolute strength (force or torque) and strength normalized to muscle size were done as described for the studies on women. All data extraction was completed by one author (S.M.G.) and cross-checked by a second (K.A.B.) author. From the 10 selected studies, five corresponding authors were contacted for missing information. Of those, one was for an explanation of group size, one was for absolute muscle strength data because only normalized strength data were reported, one was for absolute and normalized data of a second muscle that was analyzed but not reported, and two were contacted to request data on normalized muscle strength. The rodent studies were not analyzed for quality because of the very similar experimental designs of the studies.
Meta-analysis of rodent studies.—
An ES for each of the 10 studies was calculated from extracted strength data as described for the studies on women. Meta-analyses were completed on ESs determined from absolute and normalized strength measurements of hind limb muscles from rodents with circulating estradiol and those without. Meta-analyses probing the moderator variables of species type (rat vs mice) and estradiol source (intact ovaries vs estradiol supplementation) were also conducted.
RESULTS
Meta-analyses of Studies on Postmenopausal Women
Description of included studies.—
In total, 23 human studies published between 1987 and 2007 were included in the primary meta-analysis, and characteristics of those studies are summarized in Table 1. The mean participant’s age for the individual studies ranged from 51 to 77 years. The mean time since menopause for participants in the individual studies ranged from 0.5 to 30 years, with an average of ∼12 years. Overall, there were 9,956 postmenopausal women included in the analysis, 7,288 in control groups and 2,668 in HT groups. The type of HT used in the 23 studies was not consistent. Women in 15 of the studies were permitted to take various preparations of HT. For the eight studies that stated a specific HT, seven different preparations were used. The most frequent dosage of HT was equivalent to ∼0.6 mg of estrogen per day, with the lowest being ∼0.4 mg and the highest 4.3 mg per day. Nine studies did not provide any dosage information. There were eight studies that were longitudinal in design, and the average duration of those studies was 12.2 months (Table 1). Six of the eight longitudinal studies stipulated no use of HT prior to the study, whereas all cross-sectional studies permitted previous use. Typically, studies that permitted previous use of HT placed those women in the HT group (although six studies allowed previous users to be allocated to the control group if the individual had been off HT for a given period of time). The mean previous HT use for the 12 studies that allowed and reported a length of time for this use was 110 months (∼9 years; Table 1).
Table 1.
Characteristics of the 23 Studies on Postmenopausal Women
| Author, Publication Year* (reference) | Mean Participant’s Age† (y) | Mean Time Since Menopause‡ (y) | Number of Participants (control/HT) | Type of Estrogen-based HT | HT Dosage of Estrogen Component (mg/d) | Study Design and Duration (mo) | Use of HT Prior to Strength Measurement§ (mo) | PEDro Scale‖ |
| Widrick et al., 2003 (28) | 53 | 2 | 9/8 | Various | ∼0.625 | CS | 24 | 5 |
| Seeley et al., 1995 (29) | 72 | — | 5,616/1,331 | Various | ∼0.625 | CS | 178 | 5 |
| Armstrong et al., 1996 (30) | 61 | 13 | 54/54 | Prempak-C or Premarin (Wyeth, Madison, NJ) | 0.625 | Long (11) | 0 | 9 |
| Bemben and Langdon, 2002 (31) | 59 | 14 | 20/20 | Various | ∼0.65 | CS | 136 | 5 |
| Taaffe et al., 1995 (32) | 69 | 21 | 48/37 | Various | ∼0.625 | CS | 214 | 5 |
| Bassey et al., 1996 (33) | 51 | — | 91/14 | Various | — | CS | — | 5 |
| Maddalozzo et al., 2004 (34) | 51 | 1 | 59/67 | Premarin (Wyeth) | 0.625 | Long (12) | 16 | 4 |
| Krintz-Silverstein et al., 1994 (35) | 77 | 30 | 176/465 | Various | — | CS | — | 4 |
| Maddalozzo et al., 2007 (27) | 52 | 2 | 29/34 | Premarin (Wyeth) | 0.625 | Long (12) | 26 | 5 |
| Baumgartner et al., 1999 (36) | 76 | — | 132/48 | Various | — | CS | — | 5 |
| Taaffe et al., 2005 (37) | 74 | — | 581/259 | Various | — | CS | 214 | 5 |
| Uusi-Rasi et al., 2003 (38) | 62 | 11 | 35/42 | Various | — | CS | 127 | 5 |
| Ribom et al., 2002 (39) | 67 | — | 17/17 | Menorest (Novartis, Basel, Switzerland) | 4.3 | Long (6) | 0 | 9 |
| Greeves et al., 1999 (40) | 51 | 1–3¶ | 10/11 | Various | — | Long (9) | 0 | 5 |
| Preisinger et al., 1995 (41) | 60 | 10 | 22/21 | Various | — | CS | 47 | 5 |
| Cauley et al., 1987 (42) | 57 | 9 | 255/55 | Various | ∼0.37 | CS | 91 | 5 |
| Heikkinen et al., 1997 (43) | 53 | 0.5–3¶ | 26/52 | Divina or Divitren (Orion Pharma, Espoo, Finland) | 2.0 | Long (24) | 0 | 7 |
| Sipila and Taaffe, 2001 and 2003# (12,13) | 50–57¶ | 0.5–5¶ | 17/17 | Kilogest (Novo Nordisk, Bagsvaerd, Denmark) | 2.0 | Long (12) | 0 | 10 |
| Carville et al., 2006 (44) | 69 | — | 14/15 | Various | — | CS | 158 | 5 |
| Onambele et al., 2006 (45) | 63 | 16 | 8/14 | Various | ∼0.3 | CS | — | 5 |
| Brooke-Wavell et al., 2001 (46) | 65 | — | 14/22 | Estraderm TTS 50 (Ciba-Geigy, Basel, Switzerland) | 2.0 | CS | — | 4 |
| Skelton et al., 1999 (47) | 61 | 10 | 50/44 | Premak-C (Wyeth) | 0.625 | Long (12) | 0 | 7 |
| Phillips et al., 1993 (2) | 42–72¶ | 5–17¶ | 35/21 | Various | — | CS | 94 | 4 |
Notes: — = information not provided; CS = cross-sectional; HT = hormone therapy; Long = longitudinal.
Studies arranged from low to high effect size to correspond with Figure 1.
Mean age for all participants in a study regardless of treatment group.
Mean time postmenopausal for all participants regardless of treatment group.
Initial strength measurement for the longitudinal studies.
Quality assessment by the Physiotherapy Evidence-based Database (PEDro) Scale (highest quality = 11, lowest = 1).
Range.
Combined study data for Sipila et al. (2001) and Taaffe et al. (2005).
The quality of the studies ranged from 4 to 10 based on the PEDro Scale (Table 1). Studies with the higher quality rankings, between 7 and 10, were those that encompassed an RCT design. Studies that had a cross-sectional design scored 4 or 5.
Meta-analysis results.—
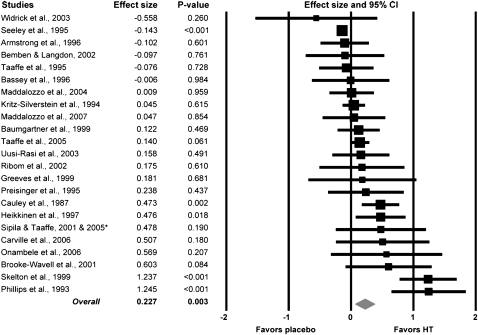
A large variation was observed among studies in the effect of HT on strength, with ESs ranging from −0.56 to 1.25 (Figure 1). Only 6 of the 23 studies showed negative effects of HT on strength (i.e., the first 6 studies in Figure 1). Conversely, 17 of the 23 studies showed positive effects of HT on strength. Overall, the meta-analysis on the 23 human studies indicates a small beneficial effect of HT on skeletal muscle strength that was statistically significant (overall ES = 0.23; p = .003; Figure 1). This ES equates approximately to a 5% greater strength for the postmenopausal women on HT compared with those not on HT.
Figure 1.
Forest plot of effect sizes (ESs) from the 23 studies that assessed muscle strength in postmenopausal women who were and were not on hormone therapy (HT). A square represents the ES for a given study with the size of the square being proportional to the weighting of that study in the meta-analysis. A horizontal line indicates the ES’s 95% confidence interval (CI). Studies are arranged from lowest to highest ESs. The diamond at the bottom represents the overall ES for HT on muscle strength. The width of the diamond represents the 95% CI for the overall ES. *Combined study data for Sipila et al. (2001) and Taaffe et al. (2005).
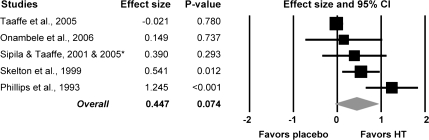
Five of the 23 studies reported strength normalized to muscle size, which provides a measure of muscle quality. In those studies, muscle size was measured by computed tomography or an equivalent method. Imaging was conducted at the times of strength testing such that strength could be normalized to the cross-sectional area of the tested muscles. Results from those studies indicate that HT may have a moderate effect on normalized strength, but the ES (0.45) was not statistically significant due to the low number of studies (Figure 2).
Figure 2.
Forest plot of effect sizes from the meta-analysis conducted on the five studies that reported muscle strength normalized to muscle size in postmenopausal women who were and were not on hormone therapy (HT). *Combined study data for Sipila et al. (2001) and Taaffe et al. (2005).
Analyses of potential moderator variables.—
In an attempt to explain the large variability observed in HT’s effect on absolute strength among the 23 studies, experimental factors that varied among studies were explored as potential contributors to the variability. The factors that might affect muscle strength were grouped into three general categories, that is, participant characteristics, HT administration, and muscle testing.
Participant characteristics.—
Twelve studies reported an average for the participant’s mean time since menopause (see Table 1), and ESs calculated from strength data in those studies were used in a meta-regression. The relationship between mean time since menopause and a study’s strength ES was not significant (p = .49). There was also a large range across the 23 studies for the length of time that participants were on HT. This can be seen in Table 1 within the columns “Use of HT prior to strength measurement” and “Study duration” for the longitudinal studies. To determine if the participant’s length of time on HT influenced the study’s ES, we conducted a meta-regression but did not find a significant relationship between these variables (p = .23).
HT administration.—
Only 9 of the 23 studies required women to take a specific preparation of HT, and even among those 9 studies, the type and dosage of HT varied. Therefore, it was not possible to assess many potential moderator variables related to the type of hormone treatment. There was sufficient information available, however, to probe whether or not two aspects of HT treatment contributed to variability in ESs. First, an analysis of prior HT use was undertaken. Women in 6 of the studies were not permitted to have had prior HT, whereas the other 17 studies accepted women with prior or current use of HT. There was no statistical difference in ESs between studies that did and did not allow previous HT use, but a trend existed toward HT being more favorable when there was no prior use (p = .12; Table 2).
Table 2.
Summary of Analyses of Potential Moderator Variables that Theoretically Could Influence the Effect Sizes of HT on Strength in Postmenopausal Women
| Moderator Variable | Comparison | Between-Group p value |
| Previous HT use | Yes (n = 17; ES = 0.161) | .124 |
| No (n = 6; ES = 0.430) | ||
| Study design | Non-RCT studies (n = 18; ES = 0.161) | .104 |
| RCT studies (n = 5; ES = 0.455) | ||
| Type of muscle contraction | Isokinetic (n = 8; ES = 0.077) | .245 |
| Isometric (n = 20; ES = 0.265) |
Note: ES = effect size; HT = hormone therapy; RCT = randomized controlled trial.
Five of the 24 studies rigorously controlled the HT treatment. Characteristics of these studies were that: (a) HT and placebo treatments were randomly assigned to the women, (b) women who were in the HT group received a specific type and dosage of HT, (c) the length of time each participant received HT was controlled, and (d) women were not permitted to have had prior HT use. As such, each of these studies was an RCT, which is considered the strongest type of clinical study design. The combined ES of those 5 tightly controlled studies was nearly triple that of the 18 studies that were less controlled (ES 0.46 vs 0.16), though this difference was not quite statistically significant (p = .10; Table 2).
Muscle testing.—
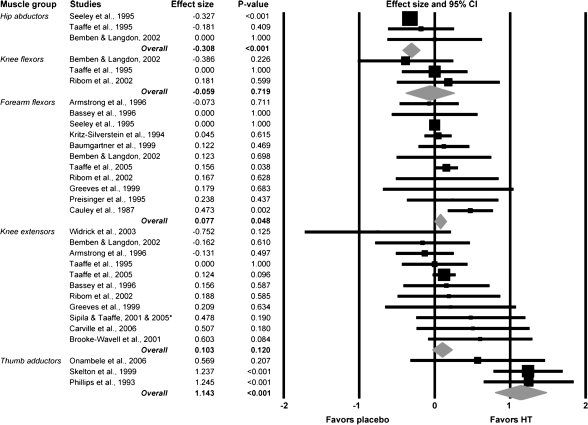
There was a variety of muscle groups tested in the 23 studies. If a muscle group was tested in three or more studies, it was included in assessment of how the moderator variable, muscle group type, influenced variability of the ESs among studies. We found that studies measuring strength of the thumb adductor muscles reported a markedly higher ES compared with studies assessing other muscle groups (p < .001; Figure 3). The overall ES in studies testing the thumb adductor muscles equates to a ∼17% greater strength for women on HT versus those not on HT. There was a negative overall ES for studies testing hip abductors, and this ES was significantly less than those for the forearm flexors and knee extensors (p < .001; Figure 3).
Figure 3.
Forest plot depicting analysis of the moderator variable, muscle group type. Effect sizes (ESs) were compared between studies that measured strength of five different muscle groups. A diamond represents the combined ES for that level of the moderator variable. *Combined study data for Sipila et al. (2001) and Taaffe et al. (2005).
To determine if the type of muscle contraction used during strength assessments contributed to the variability in ESs observed among studies, studies that evaluated strength via isometric contractions were compared with those that used isokinetic contractions. There was no difference in ES between these two groups of studies (p = .25; Table 2).
Meta-analyses of Studies on Rodents
Description of included studies.—
Our literature search yielded 10 studies on rodents that investigated the effects of estradiol on muscle strength. Characteristics of those studies are presented in Table 3. All studies were of cross-sectional design and included Wistar or Sprague Dawley rats, or ICR or C57BL/6 mice. Surgical removal of the ovaries (OVX) occurred between the ages of 3 weeks and 7 months. Thus, hormone manipulation occurred in animals that are considered to be young or young adult, and as such, all rodents had similar hormone histories (e.g., they were virgin rodents, and age-induced ovarian failure had not occurred). Data from a total of 250 rodents were included in the meta-analysis. Of that number, 91 were ovary-intact or sham-operated rodents (i.e., controls), 109 were estrogen deficient due to OVX, and 50 OVX rodents were replaced specifically with 17β-estradiol. Estradiol treatment was accomplished by either subcutaneous injections or time release devices. Duration of estrogen deficiency or replacement ranged from 2.5 to 14 weeks.
Table 3.
Characteristics of the 10 Studies on Rodents
| Author, Publication Year* (reference) | Species | Age at OVX | Number of Animals (intact/OVX/OVX+estradiol) | Estradiol Treatment | Study Duration (wk) |
| Suzuki and Yamamuro, 1985 (48) | Wistar rats | 3 wk | 6/6/6 | 20 mg, 2×/wk, sc injection | 7 |
| McCormick et al., 2004 (49) | Sprague Dawley rats | 7 wk | 8/8/9 | sc implant of silastic tube | 4 |
| Fisher et al., 1998 (50) | Sprague Dawley rats | 6–7 mo | 8/10/— | — | 4 |
| Wohlers et al., 2009 (51) | C57BL/6 mice | 8 wk | 5/5/— | — | 8 |
| Hubal et al., 2005 (52) | ICR mice | 13 wk | 12/9/— | — | 8 |
| Moran et al., 2007 (53) | C57BL/6 mice | 4 mo | 20/31/18 | 0.18 mg, 60-d release pellets | 8 |
| Schneider et al., 2004 (54) | C57BL/6 mice | <8 wk | 13/13/9 | 0.05 mg, 21-d release pellets | 2.5 |
| Warren et al., 1996 (55) | ICR mice | 6 wk | —/8/8 | 40 mg/kg/d, sc injection | 3 |
| Moran et al., 2006 (25) | C57BL/6 mice | 6 mo | 10/13/— | — | 8 |
| Wattanapermpool and Reiser, 1999 (56) | Sprague Dawley rats | 8–10 wk | 3/3/— | — | 14 |
Notes: OVX = ovariectomy; sc = subcutaneous.
Studies are arranged corresponding to Figure 4.
Meta-analysis results.—
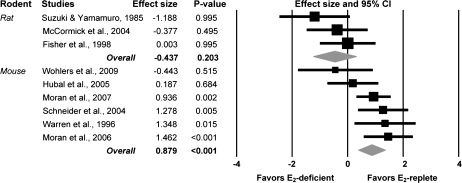
Absolute muscle strength was reported in 9 of the 10 studies on rodents. It appears that circulating estradiol may have a moderate effect on absolute strength, but the effect did not quite reach statistical significance (ES = 0.44; p = .12). These findings were inconclusive because of a significant difference in ESs between studies that used mice versus those that used rats (p < .01). There was a large effect of estradiol on absolute muscle strength in mice (ES = 0.88), whereas there was no beneficial effect of estradiol on absolute strength in rats (Figure 4).
Figure 4.
Forest plot depicting analysis of the moderator variable, species type, in the meta-analysis of estradiol’s effect on absolute strength in rodents. E2 deficient represents rodents that were ovariectomized. E2 replete represents control rodents (e.g., sham-operated rodents) or rodents that were ovariectomized and treated with estradiol.
We also explored whether or not estradiol was the specific ovarian hormone imparting strength benefits. All nine studies that reported absolute strength data included a group of estrogen-deficient rodents via OVX; within those studies, the deficient rodents were compared with those with circulating estrogen either due to intact ovaries (n = 8; ES = 0.38) or specifically from estradiol replacement following OVX (n = 5; ES = 0.39). These resulting ESs were not different (p = .99) indicating that estradiol is the key ovarian hormone.
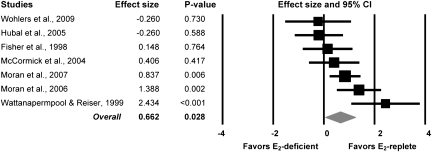
Seven of the 10 studies reported muscle strength normalized to an indicator of muscle size (muscle mass, muscle cross-sectional, fiber cross-sectional area, or contractile protein content). Those data show that estradiol has a large significant effect on normalized strength (ES = 0.66; Figure 5). This effect equates to rodents with circulating estradiol having 7% greater normalized strength than those that were depleted of estradiol. There was no difference in ESs between studies that assessed normalized strength in mice versus those in rats (p = .60).
Figure 5.
Forest plot of effect sizes from the meta-analysis conducted on the seven studies that reported muscle strength normalized to muscle size in rodents that were estradiol deficient and estradiol replete.
DISCUSSION
The results of our meta-analyses show that estrogen-based HT is beneficial to skeletal muscle strength in postmenopausal women. Overall, we found 23 studies in which strength was compared under conditions of HT and non-HT in postmenopausal women. Statistical synthesis of the data from these studies revealed that women on HT were ∼5% stronger than those not on HT, which is a small but statistically significant finding. This relatively small effect could be quite clinically meaningful given that women lose strength at the rate of ∼1% per year after menopause and at a higher rate after the age of 70 years and that these losses lead to frailty (18). Thus, for postmenopausal women who choose to be on HT, it is possible that they may reduce the effects of sarcopenia and the loss of independence.
A potential limitation of a systematic review and meta-analysis is the possibility of publication bias (i.e., unpublished studies with nonsignificant findings and/or small or negative ESs). To address this, we qualitatively examined a funnel plot of the 23 studies on postmenopausal women and found it to be symmetric. Quantitative confirmation was done by calculating Duval and Tweedie’s trim and fill correction (19), which showed that the adjusted and unadjusted ESs were equal. Thus, it is very unlikely that publication bias influenced our findings. Another limitation of our meta-analysis is that the correlation between pre- and postmeasurements was not reported in any study and that we were forced to estimate a value in order to calculate a standardized mean difference for each longitudinal study. We tested a range of correlations in order to determine the most appropriate one to use and found that ESs were altered minimally. For example, using a correlation of .7 resulted in an overall ES of 0.24 (p = .003), whereas using a correlation of .9 resulted in an overall ES of 0.21 (p = .005). Thus, in all our final analyses, a correlation of .8 was used.
Despite the overall result that HT was beneficial to strength of postmenopausal women, there was a large variability in ESs among the 23 individual studies. Study-to-study variability has also been noted in traditional narrative reviews on the effects of HT on muscle strength (3,7). In an attempt to determine what might be contributing to this variability, we considered three categories of possible moderator variables. The first set of possible moderator variables that we examined was participant characteristics. This is important to consider because there is currently an effort to “individualize” HT prescription focusing on early treatment, preferably beginning in peri- or early menopause (5,20). HT treatment beyond 5 years is not considered appropriate due to increased risks of cardiovascular events and breast cancer (5). In many of the studies included in the main meta-analysis, women were beyond this ideal time point to start HT treatment and many had been treated well past the recommended 5-year duration. If time since menopause and treatment duration are important for HT’s effects on strength, we hypothesized that studies in which women started HT soon after menopause would have greater ESs. Likewise, we thought that we would find greater ESs in studies where the duration of HT was not too long. These types of correlations have been speculated previously (12,21), but our meta-regression analyses revealed no significant relationships that would support the contention.
A second set of possible moderator variables considered was related to administration of hormone treatment. Variables that we would have liked to have explored included type of estrogen in the HT, delivery mode of the HT, and dosage of the estrogen component in the HT, but we were not able to explore these variables due to insufficient information. We were able to investigate whether or not previous use of HT influenced a study’s ES but only found a trend for HT to have a more beneficial effect on strength when the women had no previous use. There was also a trend for larger ESs in studies that were RCT and thus ranked higher on the PEDro quality scale. In the five RCT studies, women tended to be younger, had no previous use of HT, received a given type and dosage of HT (or placebo), and took that specific HT for a given duration, typically 1 year.
The third set of possible moderator variables that we examined included aspects of the muscle strength testing. We found that the type of muscle contraction used, isometric or isokinetic, did not explain any of the variability of ESs among studies. However, the muscle group that was tested was important. HT had a small negative effect in studies testing hip abductor strength and a large positive effect on strength in studies testing the thumb adductors. The primary adductor of the thumb is the adductor pollicis muscle, and it functions to adduct and extend the thumb, allowing for movements such as grasping and gripping. A distinguishing feature of this muscle compared with other muscle groups that were tested is its fiber type composition, which is ∼80% Type I fibers. The forearm flexors, hip abductors, and knee extensors are very mixed muscles containing a more equal distribution of Type I and II fibers (22). This suggests the possibility that HT might affect force production of Type I fibers more than Type II fibers. This is intriguing because muscle composed of Type I fibers has been reported to have more α-estrogen receptor messenger RNA relative to muscle composed of Type II fibers (23) and thus may be more responsive to estrogen. However, it appears that this is not the only explanation. Rat soleus muscle is composed predominately of Type I muscle fibers (24), and the three studies assessing that muscle reported no beneficial ESs of estradiol on absolute strength. Additionally, the beneficial effects of estradiol on strength were equivalent in mouse extensor digitorum longus and soleus muscle (25), which are predominated by Type II and I fibers, respectively (26). Another distinguishing feature related to the studies of the adductor pollicis is that all three were conducted in the same laboratory using an apparatus specifically designed for precise measurements of this small muscle group. Therefore, in addition to possible physiological explanations for the differing ESs observed among studies testing different muscles, there exists the possibility that nonphysiological factors (e.g., experimental procedures and instrumentation) may have also contributed to the ES differences.
An interesting observation was the effect of HT on strength normalized to muscle size. Among the studies on women, HT tended to have a greater effect on strength when normalized to muscle size (ES = 0.45), but the result was not significant (p = .074). Although only a small subset of the studies on postmenopausal women reported those data, several studies on rodents did. Those collective results show that rodents with circulating estradiol had an ∼7% greater normalized strength than estradiol-deficient rodents (ES = 0.66). Normalized muscle strength is an indication of muscle quality. Thus, these results suggest that estrogens may influence muscle strength due to a qualitative effect, as opposed to a quantitative or muscle size effect.
A second important finding from the analyses of rodent studies was that it appears that mice mimic women in their response to estrogen more so than do rats. This result should influence one’s choice of a rodent model in future studies of estrogen’s effect on skeletal muscle. Finally, the combined results of the rodent studies confirm that the specific ovarian hormone important for strength is estradiol.
A limitation in translating the results from the rodent studies to the studies on postmenopausal women is that in all rodent studies, the animals were young adults. The OVX model is advantageous for specifically investigating ovarian hormones independent of age but falls short in that hormonal affects on muscle in young rodents and women may be different than those that occur in aged rodents and women who have traversed a natural failure of the ovaries and estrogen production. We are not aware of any published research reporting the effects of HT or estradiol on muscle strength in aged rodents.
In conclusion, the results of our meta-analysis that pooled data from 23 studies show that when estrogen-based HT is given to postmenopausal women there is a small beneficial effect on muscle strength. This finding is supported by analyses of rodent studies in which estradiol was found to beneficially affect normalized muscle strength. It should be noted that the increases in strength with HT are modest (∼5%), especially when compared with other means for improving strength, for example, resistance exercise training, where increases in strength can be at least 8%–14% in 1 year (13,27). Our intent is not to suggest that HT be prescribed for age-related sarcopenia but that skeletal muscle should be recognized clinically as a target tissue when HT is prescribed for other reasons. For example, if HT can benefit muscle in addition to bone, then this argues more for considering the use of HT despite its potential adverse effects of cancer and cardiovascular events. Additionally, future research should further investigate the mechanisms of estrogen’s actions on muscle as this may lead to new strategies for combating sarcopenia, particularly in the menopausal woman.
FUNDING
National Institute of Health (AG25861 to D.A.L. and G.L.W.; T32AR07612 to K.A.B.).
References
- 1.Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001;56(10):B443, B–448. doi: 10.1093/gerona/56.10.b443. [DOI] [PubMed] [Google Scholar]
- 2.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993;84(1):95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 3.Meeuwsen IB, Samson MM, Verhaar HJ. Evaluation of the applicability of HRT as a preservative of muscle strength in women. Maturitas. 2000;36(1):49–61. doi: 10.1016/s0378-5122(00)00132-8. [DOI] [PubMed] [Google Scholar]
- 4.Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000;29(3):235–242. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
- 5.American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocr Pract. 2006;12(3):315–337. doi: 10.4158/EP.12.3.315. [DOI] [PubMed] [Google Scholar]
- 6.Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of The North American Menopause Society. Menopause. 2007;14(2):168–182. doi: 10.1097/gme.0b013e31803167ab. [DOI] [PubMed] [Google Scholar]
- 7.Sipila S, Poutamo J. Muscle performance, sex hormones and training in peri-menopausal and post-menopausal women. Scand J Med Sci Sports. 2003;13(1):19–25. doi: 10.1034/j.1600-0838.2003.20210.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen DE, Samson MM, Kezic S, Verhaar HJJ. Postmenopausal HRT and tibolone in relation to muscle strength and body composition. Maturitas. 2007;58:7–18. doi: 10.1016/j.maturitas.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Sipila S. Body composition and muscle performance during menopause and hormone replacement therapy. J Endocrinol Invest. 2003;26:893–901. doi: 10.1007/BF03345241. [DOI] [PubMed] [Google Scholar]
- 10.Sirola J, Rikkonen T. Muscle performance after the menopause. J Br Menopause Soc. 2005;11(2):45–50. doi: 10.1258/136218005775544561. [DOI] [PubMed] [Google Scholar]
- 11.Bassey EJ. Oestrogen: no evidence that it increases muscle strength. Med Sci Res. 1997;25:795–800. [Google Scholar]
- 12.Sipila S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 2001;101(2):147–157. [PubMed] [Google Scholar]
- 13.Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging. 2005;25(5):297–304. doi: 10.1111/j.1475-097X.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 14.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro) Aust J Physiother. 2002;48:43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 15.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis (Statistics in Practice) Chichester, UK: Wiley; 2009. [Google Scholar]
- 16.Spijkerman DC, Snijders CJ, Stijnen T, Lankhorst GJ. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand J Rehabil Med. 1991;23(4):203–206. [PubMed] [Google Scholar]
- 17.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 18.Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008;32(2):120–126. doi: 10.1152/advan.90111.2008. [DOI] [PubMed] [Google Scholar]
- 19.Duval S. The trim and fill method. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chichester, UK: John Wiley & Sons, Ltd; 2005. pp. 127–144. [Google Scholar]
- 20.Ettinger B, Barrett-Connor E, Hoq LA, Vader JP, Dubois RW. When is it appropriate to prescribe postmenopausal hormone therapy? Menopause. 2006;13(3):404–410. doi: 10.1097/01.gme.0000188735.61994.5b. [DOI] [PubMed] [Google Scholar]
- 21.Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26(5):524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18(1):111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 23.Lemoine S, Granier P, Tiffoche C, et al. Effect of endurance training on oestrogen receptor alpha expression in different rat skeletal muscle type. Acta Physiol Scand. 2002;175(3):211–217. doi: 10.1046/j.1365-201X.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171(3):259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- 25.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol. 2006;100(2):548–559. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 26.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221(2):177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- 27.Maddalozzo GF, Widrick JJ, Cardinal BJ, Winters-Stone KM, Hoffman MA, Snow CM. The effects of hormone replacement therapy and resistance training on spine bone mineral density in early postmenopausal women. Bone. 2007;40(5):1244–1251. doi: 10.1016/j.bone.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 28.Widrick JJ, Maddalozzo GF, Lewis D, et al. Morphological and functional characteristics of skeletal muscle fibers from hormone-replaced and nonreplaced postmenopausal women. J Gerontol A Biol Sci Med Sci. 2003;58A(1):3–10. doi: 10.1093/gerona/58.1.b3. [DOI] [PubMed] [Google Scholar]
- 29.Seeley DG, Cauley JA, Grady D, Browner WS, Nevitt MC, Cummings SR. Is postmenopausal estrogen therapy associated with neuromuscular function or falling in elderly women? Arch Intern Med. 1995;155:293–299. [PubMed] [Google Scholar]
- 30.Armstrong AL, Oborone J, Coupland CAC, Macpherson MB, Bassey EJ, Wallace A. Effects of hormone replacement therapy on muscle performance and balance in post-menopausal women. Clin Sci (Lond) 1996;91:685–690. doi: 10.1042/cs0910685. [DOI] [PubMed] [Google Scholar]
- 31.Bemben DA, Langdon DB. Relationship between estrogen use and musculoskeletal function in postmenopausal women. Maturitas. 2002;42:119–127. doi: 10.1016/s0378-5122(02)00033-6. [DOI] [PubMed] [Google Scholar]
- 32.Taaffe DR, Luz Villa M, Delay R, Marcus R. Maximal muscle strength of elderly women is not influenced by oestrogen status. Age Ageing. 1995;24:329–333. doi: 10.1093/ageing/24.4.329. [DOI] [PubMed] [Google Scholar]
- 33.Bassey EJ, Mockett SP, Fentem PH. Lack of variation in muscle strength with menstrual status in healthy women aged 45–54 years: data from a national survery. Eur J Appl Physiol. 1996;73:328–386. doi: 10.1007/BF02425503. [DOI] [PubMed] [Google Scholar]
- 34.Maddalozzo GF, Cardinal BJ, Fuzhong L, Snow CM. The association between hormone therapy use and changes in strength and body composition in early postmenopausal women. Menopause. 2004;11(4):438–446. doi: 10.1097/01.gme.0000113847.74835.fe. [DOI] [PubMed] [Google Scholar]
- 35.Kritz-Silverstein D, Barrett-Connor E. Grip strength and bone mineral density in older women. J Bone Miner Res. 1994;9(1):45–51. doi: 10.1002/jbmr.5650090107. [DOI] [PubMed] [Google Scholar]
- 36.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly mean and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 37.Taaffe DR, Newman AB, Haggerty CL, et al. Estrogen replacement, muscle composition, and physical function: The health ABC study. Med Sci Sports Exerc. 2005;37(10):1741–1747. doi: 10.1249/01.mss.0000181678.28092.31. [DOI] [PubMed] [Google Scholar]
- 38.Uusi-Rasi K, Beck TJ, Sievanen H, Heinonen A, Vuori I. Associations of hormone replacement therapy with bone structure and physical performance among postmenopausal women. Bone. 2003;32:704–710. doi: 10.1016/s8756-3282(03)00098-x. [DOI] [PubMed] [Google Scholar]
- 39.Ribom EL, Piehl-Aulin K, Ljunghall S, Ljunghall O, Naessen T. Six months of hormone replacement therapy does not influence muscle strength in postmenopausal women. Maturitas. 2002;42:225–231. doi: 10.1016/s0378-5122(02)00079-8. [DOI] [PubMed] [Google Scholar]
- 40.Greeves JP, Cable NT, Reily T, Kingsland C. Changes in muscle strength in women following the menopause: a longitudinal assessment of the efficacy of hormone replacement therapy. Clin Sci (Lond) 1999;97:79–84. [PubMed] [Google Scholar]
- 41.Preisinger E, Alacmlioglu Y, Saradeth T, Resch KL, Holzer G, Metka M. Forearm bone density and grip strength in women after menopause, with and without estrogen replacement therapy. Maturitas. 1995;21:57–63. doi: 10.1016/0378-5122(94)00857-4. [DOI] [PubMed] [Google Scholar]
- 42.Cauley JA, Petrini AM, LaPorte RE, et al. The decline of grip strength in the menopause: relationship to physical activity, estrogen use and anthropometric factors. J Chronic Dis. 1987;40(2):115–120. doi: 10.1016/0021-9681(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 43.Heikkinen J, Kyllonen E, Kurttila-Matero E, et al. HRT and exercise: effects on bone density, muscle strength and lipid metabolism. A placebo controlled 2-year prospective trial on two estrogen-progestin regimens in healthy postmenopausal women. Maturitas. 1997;26:139–149. doi: 10.1016/s0378-5122(96)01098-5. [DOI] [PubMed] [Google Scholar]
- 44.Carville SF, Rutherford OM, Newham DJ. Power output, isometric strength and steadiness in the leg muscles of pre- and postmenopausal women; the effects of hormone replacement therapy. Eur J Appl Physiol. 2006;96:292–298. doi: 10.1007/s00421-005-0078-4. [DOI] [PubMed] [Google Scholar]
- 45.Onambele GNL, Bruce SA, Woledge RC. Oestrogen status in relation to the early training responses in human thumb adductor muscles. Acta Physiol (Oxf) 2006;188:41–52. doi: 10.1111/j.1748-1716.2006.01597.x. [DOI] [PubMed] [Google Scholar]
- 46.Brookr-Wavell K, Prelevic GM, Bakridan C, Ginsburg J. Effects of physical activity and menopausal hormone replacement therapy on postural stability in postmenopausal women—a cross-sectional study. Maturitas. 2001;37:167–172. doi: 10.1016/s0378-5122(00)00182-1. [DOI] [PubMed] [Google Scholar]
- 47.Skelton DA, Phillips SK, Bruce SA, Naylor CH, Woledge RC. Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin Sci (Lond) 1999;96(4):357–364. [PubMed] [Google Scholar]
- 48.Suzuki S, Yamamuro T. Long-term effects of estrogen on rat skeletal muscle. Exp Neurol. 1985;87:291–299. doi: 10.1016/0014-4886(85)90219-5. [DOI] [PubMed] [Google Scholar]
- 49.McCormick KM, Burns KL, Piccone CM, Gosselin LE, Brazeau GA. Effects of ovariectomy and estrogen on skeletal muscle function in growing rats. J Muscle Res Cell Motil. 2004;25:21–27. doi: 10.1023/b:jure.0000021398.78327.39. [DOI] [PubMed] [Google Scholar]
- 50.Fisher JS, Hasser EM, Brown M. Effects of ovariectomy and hindlimb unloading on skeletal muscle. J Appl Physiol. 1998;85(4):1316–1321. doi: 10.1152/jappl.1998.85.4.1316. [DOI] [PubMed] [Google Scholar]
- 51.Wohlers LM, Sweeney SM, Ward CW, Lovering RM, Spangenburg EE. Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activated protein kinases in skeletal muscle after ovariectomy. J Cell Biochem. 2009;107(1):171–178. doi: 10.1002/jcb.22113. [DOI] [PubMed] [Google Scholar]
- 52.Hubal MJ, Ingalls CP, Allen MR, Wenke JC, Hogan HA, Bloomfield SA. Effects of eccentric exercise training on cortical bone and muscle strength in the estrogen-deficient mouse. J Appl Physiol. 2005;98:1674–1681. doi: 10.1152/japplphysiol.00275.2004. [DOI] [PubMed] [Google Scholar]
- 53.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol. 2007;102(4):1387–1393. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 54.Schneider BS, Fine JP, Nadolski T, Tiidus PM. The effects of estradiol and progesterone on plantarflexor muscle fatigue in ovariectomized mice. Biol Res Nurs. 2004;5(4):265–275. doi: 10.1177/1099800403262258. [DOI] [PubMed] [Google Scholar]
- 55.Warren GL, Lowe DA, Inman CL, et al. Estradiol effect on anterior crural muscles-tibial bone relationship and susceptibility to injury. J Appl Physiol. 1996;80(5):1660–1665. doi: 10.1152/jappl.1996.80.5.1660. [DOI] [PubMed] [Google Scholar]
- 56.Wattanapermpool J, Reiser PJ. Differential effects of ovariectomy on calcium activation of cardiac and soleus myofilaments. Am J Physiol Heart Circ Physiol. 1999;277(46):H467–H473. doi: 10.1152/ajpheart.1999.277.2.H467. [DOI] [PubMed] [Google Scholar]