Abstract
We report that KCC2 activity, monitored with wide–field fluorescence, is inhibited by intracellular Zn2+, a key component of neuronal injury. Zn2+–mediated KCC2 inhibition produced a depolarizing shift of GABAA reversal potentials in rat neurons. Moreover, oxygen–glucose deprivation attenuated KCC2 activity in a Zn2+–dependent manner. The link between Zn2+ and KCC2 activity provides a novel target for neuroprotection and may be important in activity–dependent regulation of inhibitory synaptic transmission.
Keywords: Zinc, potassium–chloride co–transporter, ischemia
The K+/Cl− co-transporter-2 (KCC2) is the major Cl− outward transporter in neurons, creating a Cl− equilibrium potential negative to the resting membrane voltage and rendering GABA inhibitory. However, following acute and chronic neuronal injury, KCC2 activity is decreased and GABA becomes excitatory1,2,3, a process that has been tightly linked to neurodegeneration4. Another important contributor to neuronal injury and death is an increase of cytosolic Zn2+ concentrations ([Zn2+]i)5. Although both rise in [Zn2+]i and decrease in KCC2 have been associated with neuronal injury, whether Zn2+ itself can influence KCC2 activity is unknown.
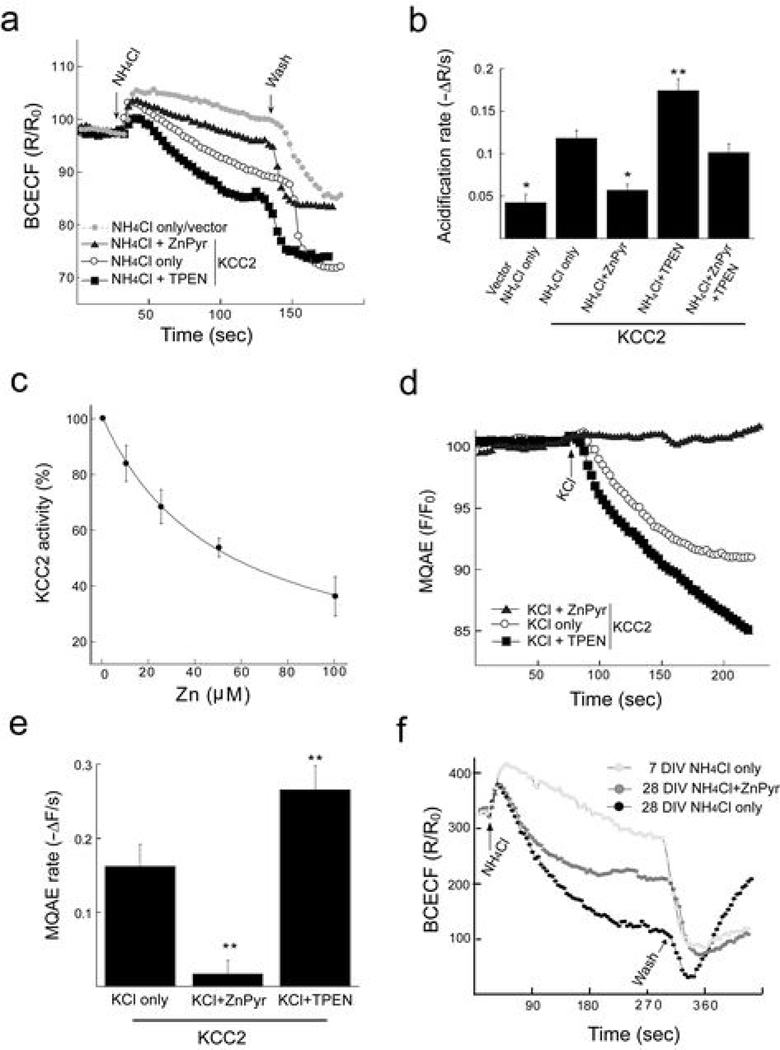
To test whether [Zn2+]i modulates KCC2 activity we first monitored KCC2-mediated ion transport in HEK 293T cells expressing the co-transporter6. NH4+ was used as a surrogate ion for K+ and changes in intracellular pH were monitored using the fluorescent dye BCECF (see Supplemental methods online). KCC2-expressing cells showed a 3–fold faster NH4+-induced acidification rate than cells transfected with empty vector (Fig. 1a,b). Increasing [Zn2+]i by a 2 min application of Zn2+ with the Zn2+ ionophore pyrithione (ZnPyr) was immediately followed by a decrease of KCC2–mediated acidification rate, with an IC50 of ~50 µM Zn2+ (Fig. 1a–c). Application of Zn2+ without pyrithione did not change KCC2 activity (not shown), indicating that KCC2 is inhibited by intracellular Zn2+. Chelating [Zn2+]i with N,N,N’,N’–tetrakis–(2–pyridylmethyl)– ethylenediamine (TPEN) reversed the effects of ZnPyr (Fig. 1b). Moreover, TPEN alone increased KCC2 activity (Fig. 1a,b), indicating that endogenous levels of [Zn2+]i tonically inhibit KCC2.
Figure 1. Increase in [Zn2+]i inhibits KCC2 activity.
(a) KCC2 activity was monitored with the pH-sensitive dye BCECF in HEK 293T cells tranfected with a KCC2 or empty vector. Cells were incubated for 2 min with vehicle, Zn2+ (100 µM) with pyrithione (5 µM) (ZnPyr), or TPEN (10 µM) and the rate of intracellular pH change following application of NH4Cl (10 mM) was monitored (see Supplemental Methods online). (b) NH4+-mediated acidification rates (mean ± SEM) in control and KCC2-expressing HEK 293T cells treated with vehicle (n=17), ZnPyr (n=14), TPEN (n=14), or ZnPyr followed by TPEN (n=10); *p<0.05, **p<0.005 ANOVA/Tukey, compared to KCC2-expressing NH4Cl only group. (c) Concentration-inhibition curve of KCC2 activity by Zn2+ (n=11 for each concentration, mean ± SEM). (d) KCC2 activity monitored with the Cl− sensitive dye MQAE. KCl (10 mM) was applied to KCC2-expressing HEK 293T cells and the rate of Cl− dependent quenching of the signal was determined. (e) Summary of Cl− transport rates; ZnPyr, or TPEN, compared to KCl alone (n=6 for each treatment; mean ± SEM); **p<0.005 ANOVA/Tukey. (f) Endogenous KCC2 activity monitored with BCECF in immature and mature cortical neurons.
Next, we determined changes in intracellular chloride [Cl−]i directly with the Cl− sensitive dye N-(ethoxycarbonylmethyl)6–methoxyquinolinium bromide (MQAE; see Supplemental Methods online). Application of 5 mM KCl reverses KCC2 transport leading to accumulation of intracellular Cl− (Fig. 1d). Increasing [Zn2+]i with ZnPyr blocked the Cl− influx (Fig 1d,e), while TPEN enhanced it (Fig. 1d,e), similar to the results observed with NH4+-induced acidification.
We then tested whether KCC2 inhibition by Zn2+ is also observed in cortical neurons, which endogenously express the co-transporter. In BCECF-loaded immature neurons (7 days in vitro, DIV), expressing low levels of KCC2, NH4+ had little effect on intracellular acidification, similar to empty vector-transfected HEK 293T cells (Fig. 1f). In contrast, in mature neurons (>25 DIV), which express high levels of KCC2, NH4+–induced acidification was pronounced. Importantly, in mature neurons ZnPyr also effectively attenuated KCC2 activity (Fig. 1f).
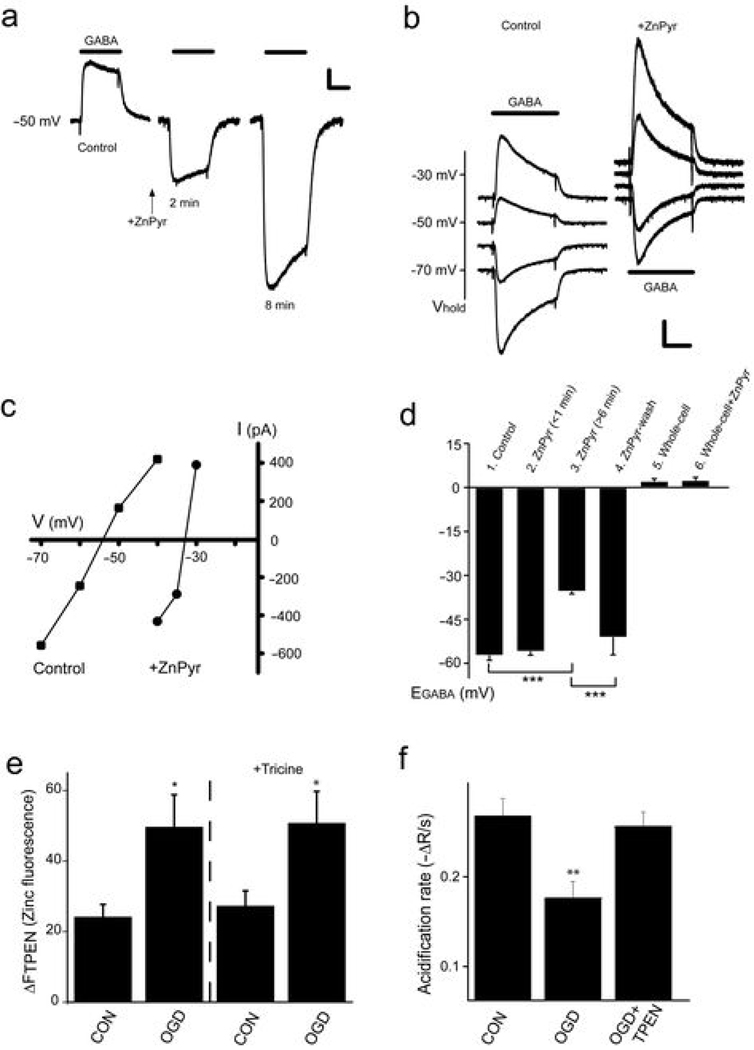
The Zn2+-dependent changes in KCC2 activity should affect the Cl− gradient and thereby the reversal potential of GABAA receptor–mediated currents (EGABA). We measured EGABA using the gramicidin perforated patch technique, which leaves intracellular Cl− undisturbed. A 2 min ZnPyr treatment produced a gradual positive shift in EGABA as, presumably, the intracellular Cl− concentration decreased with KCC2 inhibition (Fig. 2a). EGABA stabilized within 6–8 minutes after the ZnPyr application to a level ~20 mV positive to control (Fig. 2b,c,d). ZnPyr did not affect EGABA under whole-cell recording conditions with equal extracellular and intracellular chloride (Fig. 2d). The Zn2+-induced shift in EGABA reversed within 30 minutes after removing ZnPyr (Fig. 2d), indicating that neurons can tightly regulate [Zn2+]i under non-injurious conditions.
Figure 2. Effects of [Zn2+]i on EGABA, and KCC2 inhibition by OGD in neurons.
(a) Representative gramicidin-perforated whole-cell currents (Vhold −50 mV) in response to 10 µM GABA before and after a 2 min treatment with ZnPyr (100 µM/5 µM). Scale bars: 300 pA, 1 s. (b) Gramicidin-perforated whole-cell currents before and after inhibition of KCC2 by ZnPyr. Scale bars: 300 pA, 1 s. (c) Current-voltage relationships of peak currents shown in (b). ZnPyr shifted EGABA from −54 to −33 mV. (d) EGABA measured with gramicidin-patch in control neurons (n=6), at <1, and 6–10 min after ZnPyr (n=10), and after 30 min rinse (n=3). EGABA measured in whole-cell (equal extracellular and intracellular chloride conditions) under control conditions (n=10), and after ZnPyr (n=5; mean ± SEM ***p<0.001; ANOVA/Tukey) (e) OGD-induced increase in neuronal [Zn2+]i inhibits KCC2. Neurons were exposed to OGD for 90 min and loaded with the Zn2+-sensitive dye FluoZin3. A significant Zn2+ rise, sensitive to TPEN (20 µM), was monitored at 1 hour following OGD. This increase in cytoplasmic Zn2+, was insensitive to the extracellular Zn2+ chelator tricine (1 mM), suggesting the source of the metal was intracellular (n=7–9; mean ± SEM ; *p<0.05; ANOVA/Bonferroni). (f) One hour following OGD treatment, neuronal KCC2 activity was monitored with NH4+/BCECF. TPEN treatment during OGD abolished KCC2 inhibition (n=5–14; mean ± SEM ; **p<0.005; ANOVA/Tukey).
Cerebral ischemia induces acute Zn2+ dysregulation in neurons7. Therefore, we investigated if KCC2 is inhibited by the sustained increases in neuronal [Zn2+]i following oxygen-glucose deprivation in mature neurons (OGD; Fig. 2e). Application of an extracellular Zn2+ chelator did not attenuate the [Zn2+]i rise following OGD (Fig. 2e), strongly suggesting that it is liberated from intracellular binding sites8. Importantly, in ischemic neurons NH4+-induced acidification rates were markedly decreased (Fig. 2f). Moreover, neurons exposed to TPEN during OGD had normal rates of acidification (Fig. 2f), indicating that the rise in [Zn2+]i following ischemic insult led to the inhibition of KCC2.
Our results reveal a novel link between [Zn2+]i and KCC2 activity. KCC2 inhibition by Zn2+ was rapid, suggesting that Zn2+ may directly interact with intracellular cysteine or histidine residues of KCC2. Alternatively, Zn2+ could contribute to the known regulation of KCC2 activity by phosphorylation9 leading to changes in its expression9,10, or by oligomerization11. Zn2+, however, did not induce changes in KCC2 transcription, surface expression, or oligomeric organization (see Supplemental Figs. 1 and 2 online), some of which have been observed in other neuronal injury models2,3,4,9,10. Changes in KCC2 activity without changes in expression have also been reported in a deafness model12.
The downregulation of KCC2 activity following OGD-induced Zn2+ rise may account for acute seizure activity following ischemia13. A major factor in neuronal injury following OGD is intracellular release of Zn2+ and it was recently shown that it is also critical for spreading depression triggered by OGD14. Interestingly, a decrease in GABA inhibition has been proposed to be a mechanism contributing to neuronal death following cerebral ischemia4. Our results indicate that Zn2+-dependent inhibition of KCC2 may be an important contributor to neuronal injury. We also suggest that influx of synaptically-released Zn2+ or its intracellular release, particularly during periods of intense synaptic activity15, may dynamically regulate inhibition by modulating KCC2 activity.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the US–Israel Binational Science Foundation (BSF2007121, MH & EA), and the US National Institutes of Health (NS043277, EA and DC004199, KK). We thank Karen Hartnett and Mia Jefferson for expert technical assistance.
REFERENCES
- 1.Woo NS, et al. Hippocampus. 2002;12:258–268. doi: 10.1002/hipo.10014. [DOI] [PubMed] [Google Scholar]
- 2.Galeffi F, et al. J. Neurosci. 2004;24:4478–4488. doi: 10.1523/JNEUROSCI.0755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonislawski DP, Schwarzbach EP, Cohen AS. Neurobiol. Dis. 2007;25:163–169. doi: 10.1016/j.nbd.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papp E, et al. Neuroscience. 2009;154:677–689. doi: 10.1016/j.neuroscience.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JH, Sensi SL, Koh JY. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, et al. Eur. J. Neurosci. 2005;21:2593–2599. doi: 10.1111/j.1460-9568.2005.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galasso SL, Dyck RH. Mol. Med. 2007;13:380–387. doi: 10.2119/2007-00044.Galasso. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aizenman E, et al. J. Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 9.Wake H, et al. J. Neurosci. 2007;27:1642–1650. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera C, et al. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaesse P, et al. J. Neurosci. 2006;26:10407–10419. doi: 10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vale C, Schoorlemmer J, Sanes DH. J Neurosci. 2003;23:7516–7524. doi: 10.1523/JNEUROSCI.23-20-07516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzsaki G, et al. Exp. Brain Res. 1989;78:268–278. doi: 10.1007/BF00228898. [DOI] [PubMed] [Google Scholar]
- 14.Dietz RM, Weiss JH, Shuttleworth CW. J. Neurosci. 2008;28:8014–8024. doi: 10.1523/JNEUROSCI.0765-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YZ, et al. Neuron. 2008;57:546–558. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.