Abstract
Porphyromonas gingivalis, a gram-negative bacterium that causes periodontitis, activates the kinin system via the cysteine protease R-gingipain. Using a model of buccal infection based on P. gingivalis inoculation in the anterior mandibular vestibule, here we studied whether kinins released by gingipain may link mucosal inflammation to T cell-dependent immunity through the activation of bradykinin B2 receptors (B2R). Our data show that P. gingivalis W83 (WT), but not gingipain deficient mutant or WT bacteria pretreated with gingipain inhibitors, elicited buccal edema and gingivitis in Balb/C or C57BL/6 mice. Studies in TLR2−/−, B2R−/− and neutrophil-depleted C57Bl/6 mice revealed that P. gingivalis induced edema through the sequential activation of TLR2/neutrophils, with the initial plasma leakage being amplified by gingipain-dependent release of vasoactive kinins from plasma-borne kininogens. We then used fimbriae (Fim) Ag as a read-out to verify if activation of the TLR2>PMN>B2R axis at early-stages of mucosal infection had impact on adaptive immunity. Analyzes of T cell recall responses indicated that gingipain drives B2R-dependent generation of IFN-γ-producing Fim T cells in submandibular draining LNs of Balb/C and C57BL/6 mice while IL-17-producing Fim T cells were generated only in Balb/C mice. In summary, our studies suggest that two virulence factors, LPS (an atypical TLR2 ligand) and gingipain, forges a trans-cellular cross-talk between TLR2/B2R, thus forming an innate axis that guides the development of Fim-specific T cells in mice challenged intrabuccally by P. gingivalis. Ongoing research may clarify if kinin-driven modulation of T cell responses may also influence the severity of chronic periodontitis.
INTRODUCTION
Periodontal disease is initiated by a chronic subgingival bacterial infection that destroys the underlying alveolar bone as well as the connective tissue that attaches the gingiva to the tooth root (1). During the progression of periodontitis, composition of the subgingival biofilm is changed: there is an enrichment of anaerobic Gram-negative bacteria belonging to the red complex group of periodontal pathogens, such as Porphyromonas gingivalis, while the normal flora is dominated by facultative Gram-positive bacteria (1). Studies in laboratory animals infected with P. gingivalis (1) suggests that bacteria are required for the initiation of inflammation, while bone erosion is more likely caused by aberrant immune responses to bacterial antigens persistent in the periodontal tissues. The latter hypothesis received indirect support from pathogenesis studies in animal models of autoimmune arthritis, showing that activated T and B cells upregulate RANKL, thus being capable of driving the differentiation of bone-erosive osteoclasts (2, 3). Efforts to characterize osteoclastogenic T cell subsets have recently converged to TH17 (4), a proinflammatory helper T cell subset whose differentiation depends on the cooperative action of IL-6, TGF-β and IL-23 (5–9). Although severity of chronic human periodontal disease is positively correlated with IL-17 levels (10–12), the hypothesis that osteoclastogenic human TH17 cells has a causal role in alveolar bone erosion remains to be confirmed.
As a recently recognized a risk factor in the development of atherosclerosis, both in humans and animal models (13, 14), P. gingivalis relies on multiple virulence factors, such as fimbriae (Fim), LPS, cysteine proteases and hemaglutinin, to colonize and survive in oral mucosal tissues (15). Molecular studies led to the characterization of two major TLR2 ligands of P. gingivalis: an atypical LPS (16) and fimbriae, a multi-unit complex composed by polymerized fimbrillin (FimA) and accessory proteins (FimCDE) (17). More recently, analysis of macrophage responses to P. gingivalis or to their purified wall components (fimbriae and LPS) revealed that NFκB-responsive genes were activated via TLR2- or TLR7-MyD88-p38 MAPK pathways, respectively (18). In another in vitro study, it was shown that fimbriae provide adhesive filamentous appendages for gingival epithelial cells without triggering TLR2-mediated pro-inflammatory responses, since these particular host cell types do not express the CD14 co-factor required for TLR2 signaling (19). Furthermore, recent studies showed that P. gingivalis relies on native fimbriae to persist in mice infected intraperitoneally, the immune subversion mechanism being ascribed to the TLR2/PI3K-dependent upregulation of CR3, a surface receptor that down-modulates intracellular killing by macrophages (20, 21).
Cysteine proteases (CPs) from the gingipain family are responsible for >85% of the general proteolytic activity generated by P. gingivalis (22). Biochemical studies (23) characterize arginine-specific gingipains (RgpA and RgpB) of 95 kDa and 50 kDa, both of which cleaving Arg-Xaa peptide bonds (24) and a lysine-specific gingipain (Kgp) of 105 kDa that specifically hydrolyzes Lys-Xaa peptide bonds (25). Gingipains promote bacterial virulence through a wide range of molecular mechanisms, such as disruption of plasma clotting (26–28), alterations of complement system function (29, 30), modification of neutrophil functions (31) conversion of profimbrilin to mature fimbrilin (32) and degradation of antibacterial peptides and host cell surface receptors (33–36).
More recently, studies with human monocytic cell lines revealed that gingipains, acting synergistically with microbial-borne ligands of TLR and NOD2 receptors, may stimulate innate immunity through the induction of proinflammatory chemokines via signaling of protease-activated receptors (37). The dichotomous effects of gingipains on host-pathogen balance are further highlighted by evidence that these cysteine proteases increase vascular permeability through the activation of the kinin system (38, 39). Kinins are nona/decapeptide hormones liberated proteolytically from an internal segment of high or low molecular weight kininogens. Whether released by plasma, tissue kallikreins or by microbial cysteine proteases, such as gingipains, kinins exert their homeostatic or pro-inflammatory activities through the signaling of B2R and B1R, i.e., pharmacologically distinct subtypes of G-protein coupled kinin receptor that are respectively triggered by bradykinin (BK)/lysyl-BK (LBK) and by Des-ArgBK/LBK, a processed metabolite generated by carboxypeptidase M/N (40, 41). While B2R is constitutively expressed by a broad range of cells, including endothelial, smooth muscle cells, pain-sensitive neurons (42) and dendritic cells (DCs) (43), B1R expression is largely confined to inflamed tissues. Of note, the long-range ligand activity of BK/LBK (B2R agonists) is prevented by the terminating action of host metallopeptidases, such as ACE (44) and TAFI (45).
In the past few years, immunological studies conducted in mice infected by Trypanosoma cruzi, a parasitic protozoan equipped with a kinin-releasing cysteine protease (cruzipain), demonstrated that conventional (immature) DCs sense the presence of this pathogen in peripheral or lymphoid tissues via triggering of B2R (46–49). Once activated by kinins, immature CD11c+ DCs develop into the terminally diffentiating APCs that emigrate to secondary lymphoid tissues where they prime naive T cells while steering TH1 polarization via the IL-12-dependent pathway (43, 46, 47). Analysis of host resistance mechanisms in mice acutely infected with T. cruzi (intraperitoneal route) demonstrated that splenic DCs activated via the kinin/B2R pathway play a critical role in the generation of immunoprotective IFN-γ-producing T cells (47).
Here, we studied the impact of kinin system activation on T cell development in mice challenged by a low intrabuccal dose of P. gingivalis. Analysis of the dynamics of the inflammatory response evoked by this periodontal pathogen revealed that plasma leakage, a vascular response orchestrated by TLR2/neutrophil activation at very early stages of infection, is a limiting step governing the extravascular levels of immunostimulatory kinins released by gingipain. Our study provides the first demonstration that activation of the kinin proteolytic network by a periodontal pathogen may shape the decision-making process underlying effector T cell commitment in intramucosally infected mice.
MATERIALS AND METHODS
All animal experiments were in accordance with NIH Guidelines for animal use in research
Mice and bacterial strains
Experiments were done with mouse strains BALB/c, C57BL/6 WT (B2R+/+ and TLR2+/+), C57BL/6 B2R−/−, and C57BL/6 TLR2−/−. The P. gingivalis W83 strain and its Rgps-deficient mutant (strain Δ Rgps; obtained by isogenic deletion of rgpA and rgpB genes) were grown anaerobically at 37°C in brain heart infusion broth (BHI) with 0.5% (w:v) yeast extract, supplemented with cysteine (0.5 g/L), hemin (5 mg/L) and menadione (1 mg/L) (Sigma-Aldrich). The Δ Rgps mutant was created by truncating RgpB in a rgpA-deficient strain of P. gingivalis W83 by deletional mutagenesis as described previously (50). Briefly, a primer set (5'- ATGAGAGTATCGCTGATTAATTCACACTGCAATTCTCTAATAAGG, 5'- TAATTCACACTGCAATTCTCTAATAAGGGC, 5'- CAGCGATACTCTCATTTAATTTGATGATAGCCTTACCG and 5'- TAATTTGATGATAGCCTTACCGTCTTTCACG) was used to truncate RgpB at residue 410 in the master pURgpB-E plasmid by the SLIM method of mutagenesis (50). Homologous recombination of the modified plasmid into the rgpA-deficient strain resulted in the total loss of RgpB as determined by enzymatic assay and Western blot analysis.
Bacterial molecules
Protein-free LPS from P. gingivalis W83 was extracted with phenol-water and purified by cesium chloride density gradient ultracentrifugation followed by repurification (51). The purified P. gingivalis LPS preparation was free of contamination with proteins, DNA and RNA which were degraded during extensive incubation of LPS-containing fraction with proteinase K, DNase and RNase. The final preparation of LPS stimulated transcription of IL-1β and TNF-α in dose-dependent manner in human monocyte-derived macrophages and this effect was abrogated by polimyxin B, thus excluding the presence of TLR ligands other than LPS in our sample. Fimbriae (Fim) from P. gingivalis 332277 were purified according to a method previously described (52). Briefly, P. gingivalis strains harvested from early stationary cultures by centrifugation were subjected to ultrasonication in an ice bath. Following centrifugation, fimbriae were purified from the supernatant by ammonium sulfate precipitation and chromatography on a DEAE-Sepharose CL-6B column. SDS-PAGE analysis of the final preparation showed a minor and major protein band of 67 kDa (Mfa1) and 41 kDa (FimA) (data not shown), respectively, indicating that a mixture of major (FimA) and minor (Mfa1) fimbriae was obtained. The fimbrial preparations were tested negative for endotoxin (<6 EU/mg protein), according to quantitative Limulus amebocyte lysate assay (BioWhittaker). Arginine-specific (RgpB) was obtained from the P. gingivalis HG66 strain culture fluid as previously described (51). The purity of the enzyme was checked by SDS-PAGE. The amount of active enzyme in purified RgpB was determined by active site titration using Phe-Pro-Arg-chloromethyl ketone (Bachem). The final preparation of gingipain was assayed for possible contamination with LPS using Limulus test and found to be negative (<6 EU). As a cysteine proteinase, RgpB requires pretreatment with a reducing agent to become an active enzyme. Therefore, stock solution of RgpB was 2-fold diluted in 0.2 M HEPES and 5 mM CaCl2 (pH 8.0) supplemented with 20 mM cysteine and incubated at 37°C for 15 min. The activated RgpB was then further diluted to the working concentrations with appropriate buffer.
Edema Assays
Male mice (Balb/C, C57BL/6, C57BL/6.TLR2−/− or C57BL/6.B2R−/−) were injected intrabuccally (bottom of anterior mandibular vestibule) with 30 µl of 1 × 104 P. gingivalis W83 or with the Δ Rgps mutant strain. Where indicated, the mice were injected with P.gingivalis W83 pretreated (20 min at RT) with 10 µM of D-Phe-Pro-Arg-chloromethyl ketone (Bachem) or with PBS, as control. The buccal-lingual edema (volume changes) was measured 3 h after bacteria inoculation, using a paquimeter. The involvement of the kinin/B2R pathway in the development of mucosal edema was investigated by pretreating the mice 1 h earlier with 100 µg/kg (s.c.) of HOE-140 (Aventis). Depletion of polymorphonuclear neutrophils (PMN) was sought by pretreating (− 18 h) the intrabuccally infected mice with 0.45 ml i.p. of a 1:10 dilution in PBS of rabbit antiserum to PMN (Accurate Chemical Corporation). As control groups, mice infected intrabuccally were pretreated with the same volume of normal rabbit serum (46, 53). Paw edema induced by purified P. gingivalis molecules was determined 3 h after s.c injection of 25 µl of a PBS solution containing 7.5 ng of LPS, alone or combined to 10 nM (final concentration) of RgpB. Where indicated, we injected purified LPS combined to gingipain previously inactivated by 100 nM of D-Phe-Pro-Arg-chloromethyl ketone. The paw edema induced by LPS/gingipain or by living bacteria (controls) was expressed as volume differences between injected and contralateral paws, measured with the aid of a plethysmometer (46).
Kinins instruct type-1 cytokine production in P. gingivalis-infected mice
Balb/C, C57BL/6 WT, C57BL/6 TLR2−/− and C57BL/6 B2R−/− mice were inoculated intrabuccally with 1 × 104 of (i) P. gingivalis W83, (ii) W83 pretreated with the gingipain inhibitor D-Phe-Pro-Arg-chloromethyl ketone, as described above, or (iii) Δ Rgps mutant strain. After 10 d, the infected mice were boosted intrabuccally with boiled Fim Ag (1 µg/mouse). Seven days later, the submandibular LN T cells were stimulated in vitro with boiled Fim Ag (100 ng/ml). As controls, Fim Ag was added to culture of pooled LN T cells from normal mice. Culture supernatants were collected after 72 h and IFN-γ and IL-17 were quantified by ELISA utilizing purified and biotinylated Abs (R&D Systems). The effect of purified P. gingivalis LPS and gingipain on the immune profile of OVA-specific T cells was studied by immunizing Balb/C mice (pre-treated or not with HOE-140 as described above) intrabuccally with 75 µl of a formulation containing: (i) OVA antigen (50 µg/mouse) pre-absorbed to aluminum hydroxide (alum) at 5 mg/mouse (ii) RgpB (10 nM) and LPS (7.5 ng). Ten days later, the immunized mice were boosted with OVA (25 µg/ml). Recall assays were performed by adding OVA (2.5 µg/ml) to the cultures of LN T cells isolated 7 d after booster. Values are presented as pg or ng cytokine/ml (mean ± SD). Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test.
Kininogen-dependent rescue of T cell responses in infected TLR2−/− and PMN-depleted Balb/C mice
TLR2−/− or PMN-depleted Balb/C mice infected intrabuccally with a suspension of 1 × 104 P. gingivalis containing 10 µg/mouse of purified human kininogen (HK) (Calbiochem). As indicated, the TLR2−/− or PMN-depleted Balb/C mice challenged as described above were pretreated with HOE-140. As additional controls, the TLR2−/− or PMN-depleted Balb/C were inoculated with a suspension of Δ Rgps strain supplemented with purified HK. After 10 d, mice were boosted with boiled Fim Ag (1 µg/mouse). Seven days later, submandibular LN T cells were stimulated in vitro with boiled Fim Ag (100 ng/ml). Culture supernatants were collected after 72 h and IFN-γ and IL-17 were quantified by ELISA utilizing purified and biotinylated Abs (R&D Systems). Values are presented as pg or ng cytokine/ml (mean ± SD). Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test.
Histology
Maxillary tissues were harvested 48 h after P. gingivalis infection or after injection of bacterial molecules (LPS and/or gingipain) into the mandibular anterior vestibule of Balb/c, TLR2+/+ (C57BL/6 WT), TLR2−/−, B2R+/+ (C57BL/6 WT), B2R−/− mice. Tissues were fixed overnight in 10% neutral buffered formalin, decalcified in CalExII (Fisher Scientific) for 72 h, and dehydrated in 20% ethanol for 1 h, 50% ethanol for 1 h, and 70% ethanol for 1 h. Samples were embedded in paraffin and serially sliced into 0.2-µm sagittal sections and stained with hematoxylin and eosin. Sections were analyzed under oil immersion at 1000× magnification. Positive identification of tissue neutrophils was determined by matching nuclear morphology and cytoplasmic color. Total neutrophils present in gingival epithelium and connective tissue proximal to inferior incisors were determined in 40 sequential sections/mouse. Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test.
Intravital digital microscopy of the hamster cheek pouch
Hamsters were anesthetized by i.p. injection of sodium pentobarbital supplemented with i.v. α-chloralose (2.5 % w/v, solution in saline) through a femoral vein catheter. A tracheal canula (PE 190) was inserted to facilitate spontaneous breathing and the body temperature was maintained at 37°C by a heating pad monitored with a rectal thermistor. The hamster cheek pouch (HCP) was prepared and used for intravital microscopy as recently described (54). Briefly, the cheek pouch was mounted on a microscope stage and an area of about 1 cm2 was prepared for intravital microscopy observations of the microcirculation. Thirty min after the preparation was completed, fluorescein-labeled dextran 100 mg/kg b.w. (FITC-dextran, MW = 150 kDa, TdB Consultancy, Uppsala, Sweden) was injected intravenously as a macromolecular tracer. The cheek pouch (HCP) was continuously superfused with a HEPES-bicarbonate-buffered saline solution pH 7.4, at a constant rate of 5 ml/min. The microcirculation of the HCP was observed using an Axioskop 40 microscope, objective 4 × and oculars 10 × (Carl Zeiss, Germany) equipped with appropriate filters (490/520 nm, FITC-dextran) for observations of fluorescence in epiluminescence. A digital camera, AxioCam HRc, and a computer with the AxioVision 4.4 Software program (Carl Zeiss, Germany) were used for image analysis. Arteriolar diameters and total fluorescence were recorded in a representative rectangular area (5 mm2) of the prepared HCP (∼1 cm2). The recorded fluorescence at 30 minute after FITC-dextran injection in each experiment was adjusted to 2000 fluorescent units for statistical reasons and arteriolar diameter (range 30 – 80 µm) at the same point in time was defined as 100%. Images were recorded at every 5 min interval during the entire experiment and fluorescence of the observed area (5 mm2) was used as a measure of plasma leakage. In two series of experiments the bacteria suspensions (500 µl in PBS) were applied twice with 35 to 40 minutes interval during superfusion arrest for 9 min. The first series of experiments consisted of two groups of hamsters: (i) a control group (n = 4) involving HCP exposed twice to W83 P. gingivalis (ii) a test group involving bacteria application as above (positive control) followed by a second challenge in HCP pretreated with 0.5 µM of HOE-140, added to the superfusion solution 5 min prior to the second bacteria application. Once fluorescence after the second application had returned to pre-application levels a continuous application of BK (520) nM was made during 5 min, to make sure that the effects of HOE-140 were prolonged. In the second series of experiments the HCP (n=5) was challenged with W83 P. gingivalis pretreated with Rgps inhibitor for 10 min. A second application was made with the same inoculum of bacteria pretreated with the PBS diluent. Statistical evaluation was made by a paired t-test comparing the responses of first bacteria application with those of the second. A p-value < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Interstitial edema in infected mucosal tissues depends on functional interplay between TLR2 and the kinin/B2R pathway
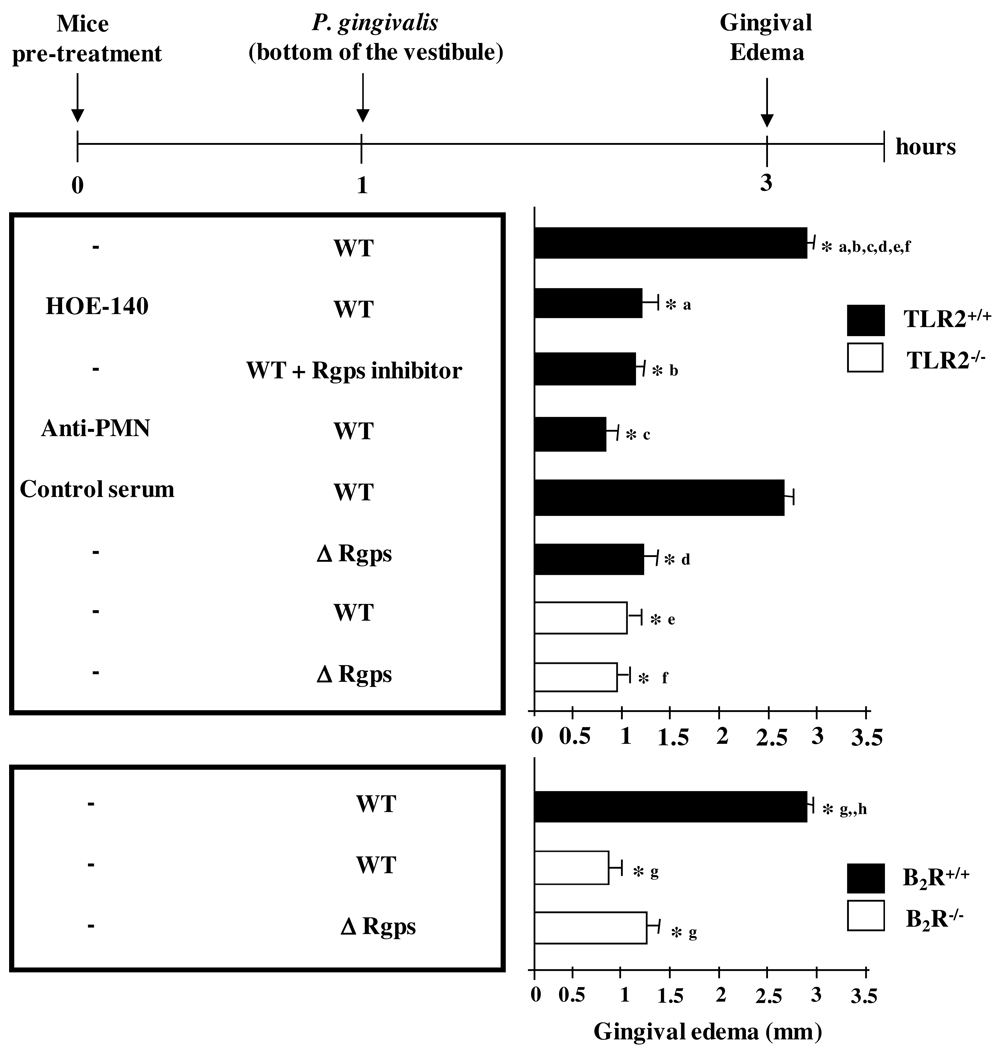
Given that the oral mucosa is a natural niche for P. gingivalis colonization in humans, we first sought to determine if the presence of this periodontal pathogen in mucosal tissues leads to the activation of the kinin system. This was approached by measuring mucosal swelling in mice (Balb/C or C57BL/6) infected intrabuccally with a low dose (104 cells) of WT P. gingivalis (W83). Measurements made at 3 h p.i. indicated that P. gingivalis W83 evokes a potent gingival edema both in C57BL/6 (Fig. 1) and Balb/C mice (data not shown). Notably, the mucosal swelling evoked by P. gingivalis W83 was inhibited in mice pretreated with the specific B2R antagonist, HOE-140 (Fig. 1). Consonant with the pharmacological results, the WT bacteria did not induce significant mucosal swelling in C57BL/6.B2R−/− mice (Fig. 1). Next, we asked if the WT bacteria relied on gingipain to generate kinins in mucosal tissues. First, we found that P. gingivalis W83 pretreated with the Rgps specific inhibitor was unable to induce significant mucosal swelling (Fig. 1). Second, we found that intrabuccal injection of the mutant strain Δ Rgps (a double knockout mutant strain with deleted RgpA and RgpB genes) did not induce significant mucosal swelling in C57BL/6 (Fig.1) or Balb/C mice (data not shown). Collectively, these results suggested that bacterial competence to induce mucosal swelling via activation of the kinin/B2R pathway depends on the enzymatic activity of gingipain.
Figure 1. Mucosal edema induced by P. gingivalis depends on the functional interplay between TR2, neutrophils and the gingipain/B2R pathway.
The graphs depicts the intensity of mucosal edema (buccal-lingual) in C57BL/6 WT (TLR2+/+) versus C57BL/6.TLR2−/− and C57BL/6 WT (B2R+/+) versus C57BL/6.B2R−/− mice that were inoculated 3 h earlier (mandibular anterior vestibule) with 30 µl of either P. gingivalis W83 wild-type (WT) strain, Rgps-deficient mutant (Δ Rgps) or the WT W83 bacteria pretreated with the Rgps inhibitor. Where indicated, the mice were pre-treated with HOE-140, prior to injection of the bacteria. A separate group of mice (C57BL/6 WT) were pretreated (18 h before bacteria injection) with 0.45 ml i.p. of a 1:10 dilution in PBS of rabbit antiserum to PMN or an equivalent volume of normal rabbit serum (control). The results (means ± SD) are representative of three independent experiments (n = 5 mice/group). Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test (*, p <0.01).
Given indications that activation of TLR2 is required for kinin system activation in the mouse model of T. cruzi infection (46), we then asked if mucosal swelling evoked by P. gingivalis is likewise subordinated to TLR2 signaling by microbial “signatures”. Consistent with this premise, our results revealed that P. gingivalis W83 did not induce appreciable edema in C57.BL/6.TLR2−/− mice (Fig. 1). Of note, these animals developed a prominent edema upon injection of synthetic BK, thus confirming that B2R function is intact in the TLR2−/− deficient mice (46).
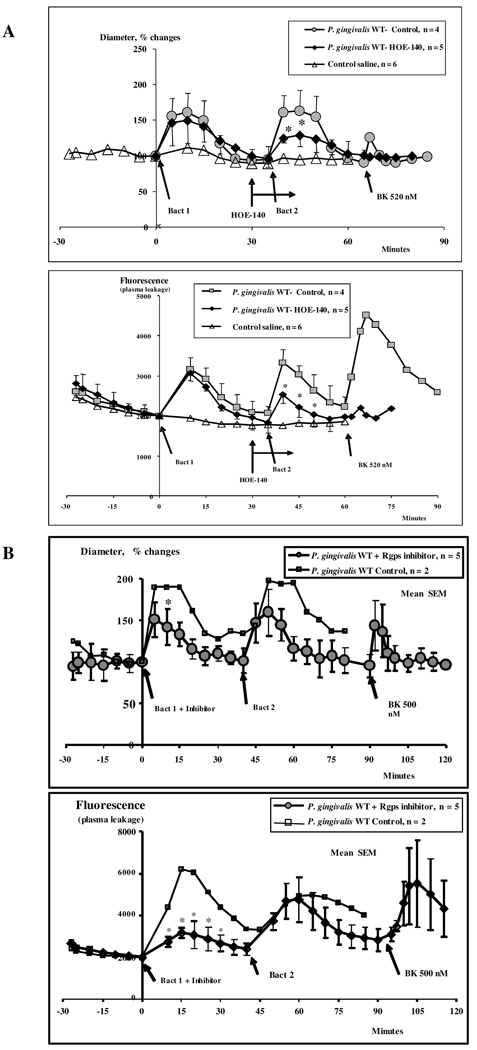
Considering that swelling measurements in the buccal mucosa are made at relatively late time-points (3 h), we resorted to intravital microscopy in the hamster cheek pouch (HCP) to investigate the early-phase microvascular responses evoked by the periodontal pathogen. Our results showed that topically applied P.gingivalis W83 induced an immediate arteriolar dilation (Fig. 2, upper panel) accompanied by increased plasma leakage (lower panel) peaking at approximately 5 min, both of which were resolved after 35–40 minutes. A second challenge with the wild type W83 strain yielded vascular responses of similar magnitude. Pretreatment of HCP with HOE-140 resulted in a significant smaller dilation at 10 and 15 min (p < 0.05) (Fig. 2). Interestingly, HOE-140 did not block the early leakage response evoked by W83 strain. Of note, the application of exogenous BK as an internal control indicated that B2R expression on the HCP vascular beds is preserved at the end of these assays (Fig. 2). Combined, these results suggested that the initial increase in vascular permeability evoked by W83 is driven by kinin/B2R-independent mechanisms. Noteworthy, however, we found that the plasma leakage response (10–20 min) evoked by the second bacteria challenge is significantly reduced the B2R antagonist (p < 0.05). Given indications that the kinin/B2R pathway was activated upon second bacterial challenge, we then asked if gingipain was involved in this process. Indeed, we found that plasma leakage evoked by the second topical application of W83 (peaking at 10–30 minutes) was substantially reduced when we instead exposed the HCP to W83 pretreated with the Rgps inhibitor (p < 0.05) (Fig. 2). Intriguingly, however, the latter treatment did not cancel the vasodilatory responses (Fig. 2, upper panel), thus suggesting that gingipain enzyme activity is not essential for induction of vasodilatation. Collectively, the intravital microscopy studies in the HCP suggest that early vascular permeability increases evoked by P. gingivalis W83 are coordinated by kinin/B2R-independent mechanisms. However, following the onset of inflammation, the initial plasma leakage is rapidly intensified through gingipain-dependent generation of vasoactive kinins (B2R agonists).
Figure 2. Intravital microscopy analysis of early-phase vascular permeability increases elicited by topically applied P. gingivalis.
(A) Upper panel: Diameter changes (means ± SEM) in arterioles in two groups of HCP (n=5) topically exposed to W83 P. gingivalis. Control experiments were performed by adding HOE-140 to one group of hamsters. Note that there was a significant smaller diameter increase as compared with the untreated control group at 10 and 15 minutes after bacteria application, * = p < 0.05. (A), Lower Panel. The graph describes plasma leakage (FITC-dextran) responses (means ± SEM) for two groups of hamsters. After local application of HOE-140 to one group (see second bacteria application) there was significantly less plasma leakage at 10, 15 and 20 min as compared with the untreated control group, * = p < 0.05. (B) Upper Panel. Diameter changes (means ± SEM) in hamster cheek pouch arterioles in one group of hamsters (n = 5) receiving bacteria applications with 40 min interval. The first application was made with the P.gingivalis W83 (Bact 1) pretreated in vitro with Rgps inhibitor. There was only a significant difference (p < 0.05) in arteriolar diameter at 10 min after application of W83 P. gingivalis (Bact 1) + inhibitor as compared with 10 min after application of W83 without Rgps inhibitor (Bact 2). Lower Panel. Vascular permeability changes (means ± SEM) recorded in two groups of hamsters. There were significant differences in plasma leakage (Fluorescence) induced by the first and the second application of bacteria at 10, 15, 20, 25 and 30 min (* = p < 0.05). Plasma leakage increase after application of W83 (Bact 1) + Rgps inhibitor was 64 % of the control response induced by the untreated W83 (Bact 2). Statistical evaluation was made by a paired t-test comparing the responses of first bacteria application with those of the second.
Activation of the TLR2/B2R axis by P. gingivalis promotes neutrophil recruitment to the inflamed mucosal tissues
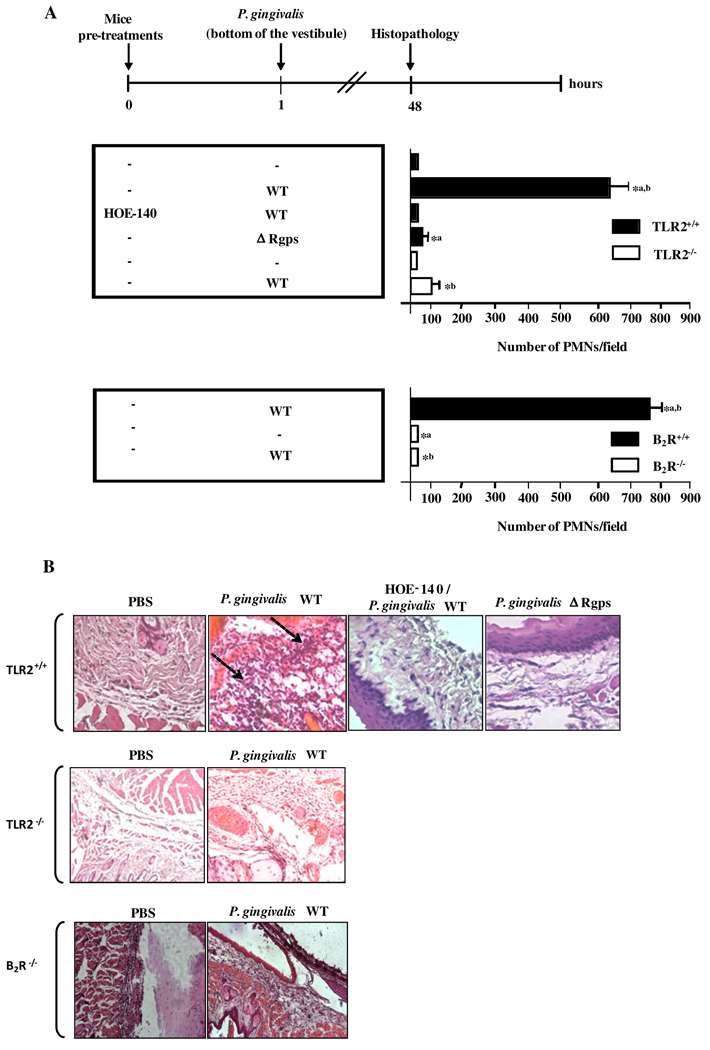
Previous analysis of the dynamics of inflammation elicited by T. cruzi suggested that neutrophils link TLR2-dependent recognition of microbial signatures to the kinin proteolytic cascade. Here we asked if similar mechanisms may operate in W83-infected mucosal tissues. To this end, we injected P. gingivalis W83 intrabuccally in C57Bl/6 mice that were previously (18 h earlier) immunodepleted of neutrophils. Analysis of gingival swelling (3 h p.i.) revealed that the inflammatory edema was nullified in neutrophil-depleted mice (data not shown), in contrast to the intense swelling reactions observed in control animals (pre-treated with non-immune serum). We then sought to characterize the inflammatory infiltrates recruited to the gingival tissues (Fig. 3). Morphometric analysis revealed the presence of prominent neutrophil infiltrates in mucosal tissues of infected C57Bl/6 TLR2+/+mice, but not in TLR2−/− mice or in C5BL/6.B2R−/− mice (Fig. 3A and B). Moreover, there was no significant neutrophil infiltration in mucosal tissues of C57Bl/6 mice injected intrabuccally with either Δ Rgps bacterial strain (Fig. 3A, upper panel) or with wild-type bacteria pretreated with the gingipain inhibitor (data not shown). Altogether, these results strongly suggested that the W83-induced infiltration of neutrophils to mucosal tissues depends on the interplay between TLR2 and the gingipain>kinin/B2R pathway.
Figure 3. TLR2/B2R “cross-talk” drives bacteria-induced gingivitis.
C57BL/6 WT mice pre-treated, or not, with HOE-140 (as indicated) were orally infected (buccal vestibule) either with wild-type P. gingivalis W83, the Δ Rgps strain, or with P. gingivalis W83 pre-treated with the Rgps inhibitor. In a second scheme, C57BL/6 WT (TLR2+/+ and B2R+/+), TLR2−/− and B2R−/− mice were infected as described above with wild-type P. gingivalis W83. Mice were killed 48 h after the inoculation and histological section of gingival tissues were obtained and stained with H&E. (A) Histogram analysis showing the numbers of neutrophils in representative gingival sections. At least 80 sections per condition were examined for neutrophils by oil immersion microscopy at ×1000 magnification. Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test (p<0.05). Arrows indicate the neutrophils infiltrates. (B) Corresponding images acquired with a Zeiss Axoimager microscope system.
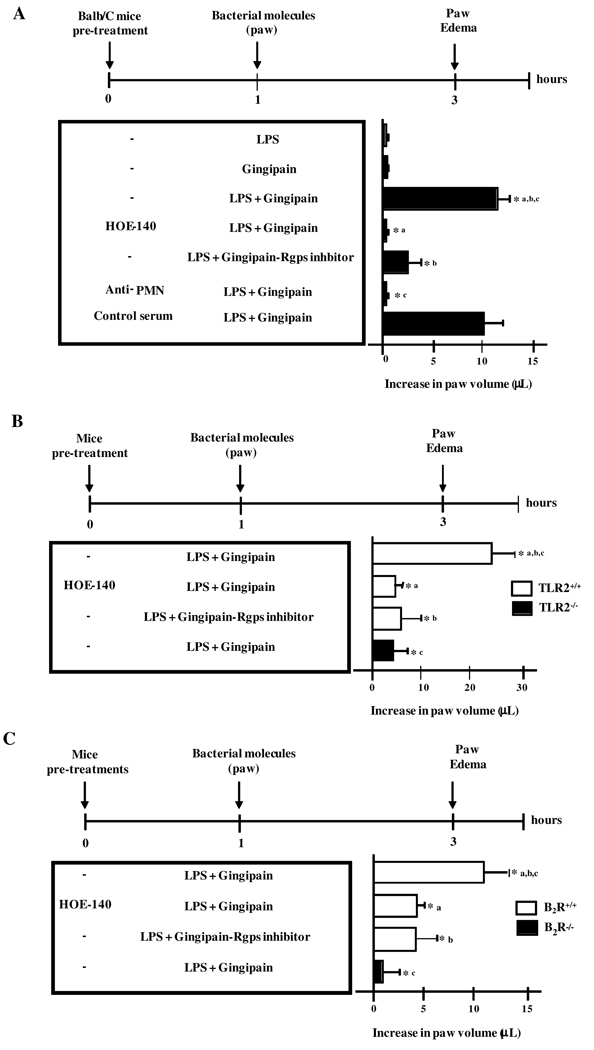
LPS and gingipain, acting synergistically, drive neutrophil-dependent edema via the TLR2/B2R pathway
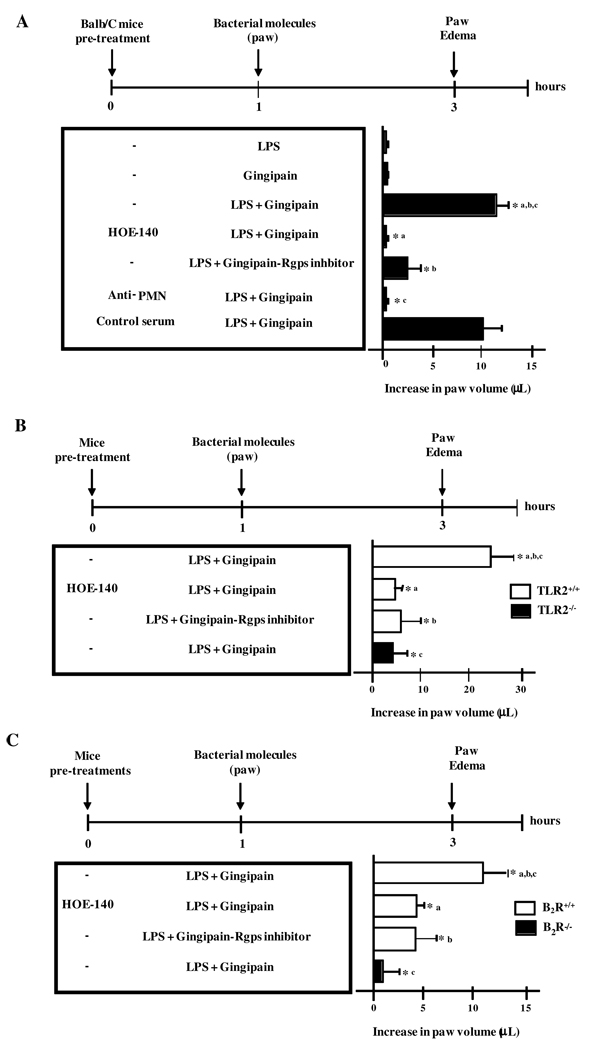
Seeking for proof of concept in favor of our working hypothesis, we then asked if the proinflammatory phenotype of P. gingivalis W83 could be recapitulated by injecting mice with LPS, an atypical TLR2 ligand (16), along with the kinin-releasing protease, i.e., gingipain. Rather than injecting LPS/gingipain in the anterior mandibular vestibule, the purified bacterial molecules were inoculated in the more sensitive paw tissue (Fig. 4). Control experiments performed in Balb/C mice confirmed that the living W83 bacteria potently induced paw edema responses via the gingipain/B2R pathway (Fig. 4). In contrast, there was no detectable footpad swelling in Balb/C mice injected with either LPS or activated gingipain alone (Fig.4). Strikingly, however, mice injected with the combination of LPS and activated gingipain developed a potent paw edema (Fig. 4A). Of note, these proinflammatory effects were blocked either by pre-treating Balb/C mice with HOE-140 or by pre-incubating purified RgpB with the specific inhibitor before mixing it to the LPS sample (Fig. 4A). Consistent with a role for neutrophils in this process, neutrophil-depleted Balb/C mice failed to develop significant paw edema upon injection of LPS/gingipain, whereas control mice pre-treated with normal serum showed overt swelling under the same condition (Fig. 4A). Similarly to data shown for P. gingivalis W83, the injection of LPS/gingipain evoked potent paw edema in C57Bl/6 mice, but not in TLR2−/−or in B2R−/− mutant mice (Fig. 4B and C). Collectively, these results indicate that purified LPS and gingipain (RgpB), respectively acting as the microbial-derived TLR2 ligand and kinin-releasing protease, are able to recapitulate the pro-inflammatory phenotype of P. gingivalis. Noteworthy, the intrabuccal injection of LPS/gingipain also elicited potent neutrophil infiltration in mucosal tissues of C57BL/6 mice, but not in TLR2−/− deficient mice (Fig. S1A and B). Altogether, these results demonstrate that LPS and gingipain, acting synergistically, can elicit edema and gingivatis through mechanisms involving cooperative activation of TLR2/neutrophils and B2R.
Figure 4. Purified P. gingivalis LPS and gingipain induce paw edema in TLR2/B2R-dependent manner.
Edema induced by injection of 25 µl of PBS containing 7.5 ng of purified P. gingivalis LPS alone or combined to 10 nM (final concentration) of RgpB, preactivated with 10 mM cysteine, in the paw of Balb/C (A), C57BL/6 (TLR2+/+) versus C57BL/6.TLR2−/−(B), and C57BL/6 (B2R+/+) versus C57BL/6.B2R−/−(C) mice. PBS was injected in the contralateral paw. Controls are done with gingipain irreversibly inactivated by the Rgps inhibitor. Mice were pre-treated or not with HOE-140, 1 h before the injection. Volume differences were determined after 3 h p.i. The results (means ± SD) are representative of three independent experiments (n = 5 mice/group). Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test (*, p <0.01).
Balb/C mice orally infected by P. gingivalis develop IFN-γ and IL-17 producing anti-Fim T cells in gingipain→kinin/B2R-dependent manner
In the next series of experiments we asked if kinins generated in W83-infected mucosal tissues coordinate adaptive immunity via activation of the B2R pathway. To this end, we inoculated Balb/C mice intrabuccally with P. gingivalis W83 or with the mutant bacterial strain Δ Rgps. Ten days after infection, both groups of Balb/C mice were boosted with fimbriae Ag (Fim Ag) and the T cells were isolated 7 d later from submandibular draining LNs. The results from recall assays performed with Fim Ag showed that experienced T cells from W83-infected Balb/C mice produced high amounts of IFN-γ as well as the TH17 cytokine, IL-17 (Fig. 5A and B). In contrast, Fim-specific T cells from infected mice pretreated with a single-dose of HOE-140 showed drastically reduced IFN-γ and IL-17 responses (Fig. 5A and B). In line with the concept that induction of adaptive immunity was critically dependent on gingipain-mediated generation of kinins in mucosal tissues, we found that Balb/C mice infected with the Δ Rgps or with W83 bacteria pretreated with the gingipain inhibitor failed to produce IFN-γ and IL-17 (Fig. 5A and B). Of note, control experiments indicated there was no significant secretion of IFN-γ and IL-17 cytokines when we added Fim Ag to T cells derived from LNs of non-infected Balb/C mice (data not shown). Collectively, these results suggested that gingipain-mediated generation of kinins in the inflamed mucosa of W83-infected Balb/C mice drives production of IFN-γ and IL-17-producing Fim-experienced T cells in B2R-dependent manner.
Figure 5. Functional profile of fimbriae-experienced T cells isolated from submandibular draining lymph nodes of Balb/C mice infected intrabuccally with P. gingivalis.
Balb/C mice were pre-treated or not with HOE-140, 1 h before being challenged in the mandibular anterior vestibule with P. gingivalis WT, WT pre-treated with D-Phe-Pro-Arg-chloromethyl ketone (gingipain inhibitor) or the Δ Rgps strain. Ten days later, mice were boosted with Fim Ag (1 µg/mouse). Submandibular LN T cells were harvested 7 d after the booster and were stimulated with media alone or with Fim Ag (100 ng/ml) for 72h at 37°C before titers of IFN-γ (A) and IL-17 (B) in the culture supernatants were determined by ELISA. Results are representative of three independent experiments with similar results (n = 5 mice/group). Differences were analysed by ANOVA and pair-wise comparisons were done by the Tukey test (*, p <0.05).
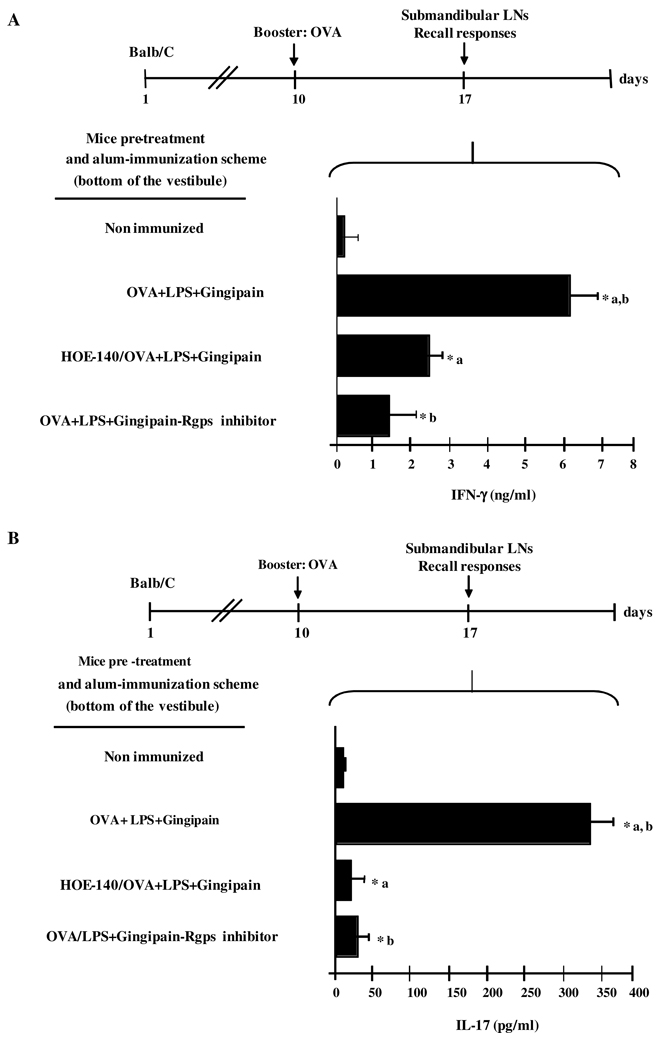
Purified LPS and gingipain, acting synergistically, induce IFN-γ and IL-17-producing T cells in kinin/B2R-dependent manner
Earlier in this work, we presented evidence that purified LPS combined to active gingipain could recapitulate the proinflammatory phenotype (induction of mucosal edema and neutrophil infiltration) of P. gingivalis W83. Here we checked if mice immunized with a conventional immunogen (OVA) in the presence of purified LPS and active gingipain could generate IFN-γ and IL-17-producing T cells in kinin/B2R-dependent manner, hence reproducing effects ascribed to P. gingivalis W83 infection. To this end, we immunized Balb/C mice intrabuccally with alum-based emulsions containing OVA immunogen supplemented (or not) with purified LPS/gingipain (RgpB). At day 10, the mice were boosted with soluble OVA (same immunization site). Seven days later, the submandibular draining LNs were recovered and the OVA-specific recall responses were analyzed (Fig. 6A and B). The results showed that IFN-γ and IL-17 were vigorously secreted by OVA-specific T cells from mice immunized with alum-based OVA combined with both LPS and gingipain. Importantly, OVA-specific T cells were no longer able to secrete IFN-γ and IL-17 if the mice immunized as described above were pretreated with HOE-140 (Fig. 6A and B). Along similar lines, the production of IFN-γ and IL-17 by OVA-experienced T cells were significantly diminished when the enzymatically active gingipain RgbB present in the alum-based OVA/LPS formulation was replaced by gingipain inactivated by the Rgps inhibitor (Fig. 6A and B). Of note, control experiments showed that addition of the Rgps inhibitor to the Alum/Ova immunogen (injected s.c. Balb/C mice) did not interfere with the magnitude of the prototypical TH2 polarized anti-OVA responses observed in this inbred strain (data not shown).
Figure 6. Mice immunized intramucosally with OVA/alum supplemented with LPS and gingipain generate TH1 responses via the B2R pathway.
Balb/C mice were immunized intrabucally with OVA Ag (25 µg/mouse) preabsorbed to alum, with or without supplementation with W83-derived LPS (7.5 ng) and RgpB preactivated with 10 mM cysteine. Separate groups of mice were pre-treated or not with a s.c. injection of HOE-140, 1 h before immunization. One week later, mice were boosted with OVA Ag (10 µg/mice) at the same site. T cells were harvested from submandibular LNs 7 d after the booster and were stimulated with media alone or OVA (1 µg/mice) for 72h at 37°C. Titers of IFN-γ (A) or IL-17 (B) in the culture supernatants were determined by ELISA. Results are representative of three independent experiments with similar results. Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test (*, p <0.01).
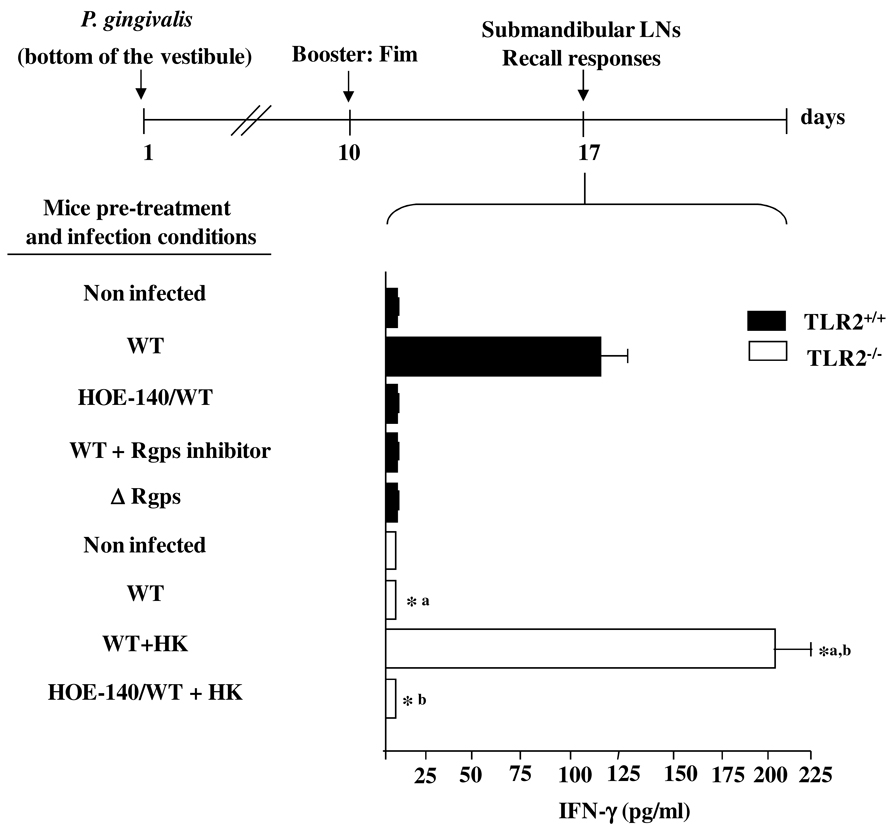
Purified kininogen reconstitutes the type-1 deficient responses of TLR2−/− mice
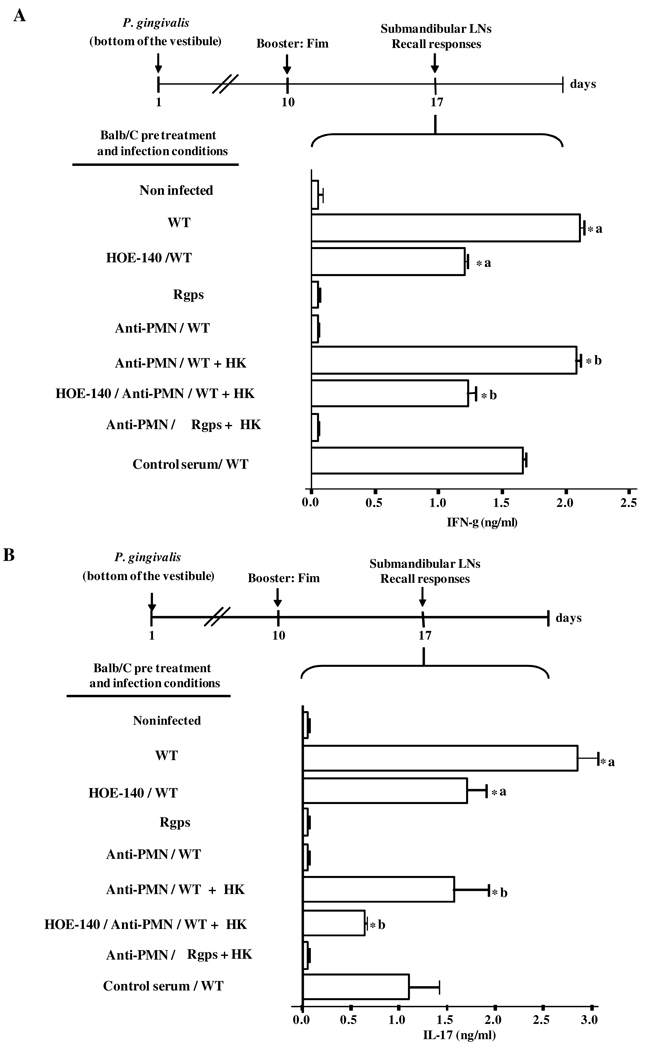
Here we tested the hypothesis that availability of high levels of kininogens (i.e., gingipain substrate) in peripheral tissues is a limiting factor governing the cytokine profile of Fim-specific effector T cells. Recall assays performed with Fim Ag indicate that submandibular T cells from W83-infected TLR2+/+ mice (positive controls) vigorously upregulated IFN-γ, while HOE-140, as predicted, cancelled the type-1 response (Fig. 7). Similarly, TLR2+/+ mice did not generate IFN-γ-producing anti-Fim T cells following injection of P. gingivalis W83 pretreated with the Rgps inhibitor (Fig. 7). In contrast, we found a marked reduction of IFN-γ production by Fim-experienced T cells from W83-infected TLR2−/− mice (Fig. 7). We then checked if the combined injection of P. gingivalis W83 and HK into the mandibular anterior vestibule of TLR2−/− animals would rescue the otherwise deficient type-1 responses of these transgenic mice. Indeed, anti-Fim T cells isolated from TLR2−/− mice that received HK at the time of bacterial challenge robustly secreted IFN-γ, and these effects were cancelled by HOE-140 (Fig.7). Collectively, these results indicate that exogenous HK bypassed the requirement for TLR2-driven influx of plasma borne kininogens. In other words, exogenous HK underwent proteolytic processing in gingipain-dependent manner, liberating the B2R agonist which then acted as a type-1-directing adjuvant, thus restoring the immune dysfunction of TLR2-deficient mice. Next, we verified if the exogenous provision of HK to PMN-depleted Balb/C mice would also bypass the requirement for neutrophils for kinin/B2R-dependent type-1-induction. Infected Balb/C-mice pretreated with non-specific rabbit serum displayed a slightly reduced IFN-γ and IL-17 production by anti-Fim T cells. In contrast with these controls, Fim-stimulated T cells isolated from PMN-depleted infected Balb/C mice or from Balb/c mice that were infected with the Δ Rgps strain did not secrete detectable levels of IFN-γ or IL-17 (Fig. 8A and B). Moreover, HOE-140 brought about a partial, albeit significant reduction of IFN-γ and IL-17 production by anti-Fim T cells from W83-infected Balb/C mice (Fig. 8A and B). Notably, the deficient adaptive responses of PMN-depleted Balb/C mice were fully reconstituted when these animals were injected with P. gingivalis W83 combined to exogenous HK (Fig. 8A and B). Importantly, the combined injection of purified HK and the P. gingivalis Δ Rgps mutant strain failed to induce detectable IFN-γ or IL-17 levels by Fim-stimulated T cells. Taken together, these results suggest that proteolytic generation of the kinin danger signal in infected mucosal tissues is preceded by the extravascular accumulation of plasma-borne kininogens, a response driven by bacterial-induced activation of TLR2/neutrophils at early stages of intrabuccal infection. Once released in high-levels, kinins induce IFN-γ -producing Fim-specific T cells (in C7BL/6 and Balb/C mice) and IL-17-producing Fim-specific T cells (in Balb/C mice) in B2R-dependent manner.
Figure 7. Purified HK corrects the adaptive immune dysfunction of Fim-specific T cells from TLR2−/− mice infected intramucosally by P. gingivalis.
C57BL/6 WT (TLR2+/+) mice were pretreated, or not, with HOE-140, as indicated, before being infected (bottom of mandibular anterior vestibule) with wild-type P. gingivalis W83 or the Δ Rgps strain. Where indicated, mice were infected with the WT bacteria pre-treated with the gingipain inhibitor. In a second scheme, TLR2−/− mice pre-treated or not with HOE-140, as indicated, were orally infected with suspensions of P. gingivalis W83 (WT or Δ Rgps strain) supplemented with human purified kininogen (HK), After 10 d, mice were boosted with Fim Ag (1 µg/mouse). Submandibular LN T cells were recovered and stimulated with Fim Ag (100 ng/ml) in vitro. Culture supernatants were collected after 72 h and IFN-γ were quantified by ELISA. Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test (*, p <0.05).
Figure 8. Purified HK reconstitute the deficient generation of IFN-γ-producing and IL-17 producing Fim-specific T cells in neutrophil-depleted Balb/C mice infected intrabuccally by P. gingivalis.
Levels of IFN-γ (A) and IL-17 (B) secreted by submandibular LN Ag-specific T cells isolated from Balb/C mice orally infected (bottom of the mandibular anterior vestibule) either with wild-type P. gingivalis W83 or the Δ Rgps strain. Where indicated, mice were pre-treated with HOE-140, 1 h before the injection. A separate group of mice were depleted of circulating neutrophils 18 h before infection with 0.45 ml i.p. of a 1:10 dilution in PBS of rabbit antiserum to PMN or an equivalent volume of normal rabbit serum (control). PMN-depleted mice were either challenged with P. gingivalis W83 or Δ Rgps strain. In a second scheme, PMN-depleted mice were infected with P. gingivalis (wild-type and Δ Rgps strains) combined to human purified kininogen (HK), pre-treated or not with HOE-140, as indicated. After 10 d, the infected mice were boosted with Fim Ag (1 µg/mouse). Submandibular LN T cells were recovered and stimulated with Fim Ag (100 ng/ml) in vitro. Culture supernatants were collected after 72 h and IFN-γ and IL-17 were quantified by ELISA. Statistical differences between mean values were evaluated by ANOVA, and pair-wise comparisons were done by the Tukey test (*, p <0.01).
DISCUSSION
Confronted with a highly diversified microbial flora in the oral and digestive mucosae, the immune system is able to discriminate commensal microbes from pathogens. While the repeated exposure of the innate immune system to the commensal flora usually leads to immunological tolerance, the detection of danger cues displayed by pathogens is translated into inflammation and adaptive immunity (56). In the present work, we demonstrate that the functional profile of Fim-specific effector T cells in mice infected intramucosally by P. gingivalis W83 is coordinated by TLR2/B2R, an innate pathway forged by the cooperative signaling of two different types of danger signals: LPS, an atypical TLR2 ligand expressed by P. gingivalis, and bradykinin, a pro-inflammatory endogenous peptide released from plasma-borne kininogens through the proteolytic activity of gingipain.
By developing an infection model based on low dose intrabuccal injection of the periodontal bacteria, we were able to demonstrate that blockade of the kinin/B2R pathway at the onset of inflammation modifies the cytokine profiles of Fim-experienced T cells isolated (17 d p.i.) from the submandibular draining LNs. While admitting that other Ag specificities may likely contribute to the pool of anti-bacterial effector T cells isolated from submandibular draining LNs, we chose to use fimbriae Ag as read-out in recall assays because fimbriated strains of P. gingivalis (rather than non-fimbriated strains) are frequently detected in deep periodontal pockets and in sites of severe periodontal attachment loss (57). Experiments performed in Balb/C mice infected with P. gingivalis W83 (WT) revealed that the bacteria evoked a strong buccal-lingual edema via the B2R pathway (i.e., blocked by HOE-140 or absent in B2R-deficient mice) and provided evidences that B2R-driven mucosal inflammation leads to the accumulation of IFN-γ-producing Fim-specific T cells and to IL-17-producing Fim T cells in submandibular draining LNs. We also showed that mice challenged with W83 pretreated with an irreversible gingipain inhibitor, or inoculated with gingipain-deficient P. gingivalis W83 mutant (Δ Rgps) not only failed to develop B2R-dependent gingivitis but the Fim-experienced T cells present in draining LNs did not secrete IL-17 (Balb/C) or IFN-γ (Balb/C and B6 mice). Combined, these data linked activation of the gingipain→kinin/B2R pathway at the onset of mucosal infection to the shaping of the T cell branch of (adaptive) immunity. Complimentary experiments performed with W83-infected B6.TLR2−/− and with neutrophil-depleted B6 mice indicated that their phenotypes were reminiscent of that displayed by B2R−/− mice, i.e., these mice did not develop significant gingivitis and the DLN T cells from these mice failed to secrete IFN-γ upon ex-vivo stimulation with fimbriae. Remarkably, the immune dysfunction of B6.TLR2−/− and neutrophil-depleted B6 mice was corrected when these animals were injected intramucosally a suspension of P. gingivalis W83 supplemented purified HK (Fig. 7 and Fig. 8). In contrast, the addition of purified HK to a suspension of gingipain-deficient (Δ Rgps) strain failed to rescue the Fim-specific type-1 responses either in TLR2−/− or in neutrophil-depleted B6 mice (Fig. 7 and Fig. 8). These experiments indicated that the direct delivery of exogenous kininogens (gingipain substrate) into the peripheral sites of infection bypassed the requirement for plasma influx via the TLR2/PMN-dependent pathway. In other words, we may infer that plasma leakage, a vascular reaction orchestrated through bacteria-induced activation of TLR2/neutrophils, is a limiting step controlling the extent of gingipain-mediated release of kinin “danger” signals in mucosal al tissues.
Although we cannot rule out the possibility that innate sentinel cells might sense P. gingivalis via TLR2/fimbriae (20), here we presented evidences that the proinflammatory phenotype of P. gingivalis W83 is recapitulated by injecting mice with only two purified virulence factors, i.e., LPS and gingipain (enzymatically active). This conclusion was based on evidences that (i) LPS/gingipain induces a potent buccal-lingual edema in B6 mice via the TLR2/B2R pathway and (ii) Balb/C mice injected (intra-buccally) with OVA/alum formulations containing purified LPS and gingipain developed IFN-γ-producing anti-OVA T cells and IL-17-producing anti-OVA T cells in B2R-dependent manner.
A large body of experimental evidences presented here supported the hypothesis that this gram-negative bacteria induces edema by sequentially activating the TLR2/B2R axis, via LPS (TLR2 ligand) and kinins (B2R agonists) released by gingipain. In general terms, the mechanistic principles outlined here are reminiscent of those described in studies in mice subcutaneously infected with T. cruzi (46). According to our working hypothesis (Fig. 9), P. gingivalis initiates mucosal inflammation through the activation of TLR2 expressed by resident cells (epithelial, macrophages, mast cells). Following upregulated secretion of chemokines, neutrophils adhere to activated endothelium, and drive initial influx of plasma (including kininogens) into extravascular tissues. Acting further downstream, P. gingivalis may generate high-levels of vasoactive kinins through the activity of gingipain, thus further amplifying plasma leakage. This sequential model is consistent with data presented in the current work, showing that TLR2-deficient mice and neutrophil-depleted B6 mice failed to develop the kinin/B2R-dependent gingivitis (Fig. 1). This result, combined to evidence from intravital microscopy studies showing that topically applied P.gingivalis W83 does not immediately activate the kinin/B2R pathway in the HCP microvascular beds, but does so after a second consecutive application of the pathogen, supports the view that activation of TLR2/neutrophils at the upstream end of the inflammatory process might allow for the accumulation of plasma-borne kininogens (i.e., gingipain substrate) into extravascular tissues. In view of complex structural requirements for TLR4 versus TLR2 signaling by P.gingivalis LPS (16), additional studies in TLR4-deficient mice will be required to verify if TLR4 may likewise propel the activation of the kinin proteolytic system. Whatever the structural basis for LPS triggering of TLR4/TLR2, our study showed that the intrabuccal injection of purified HK along with P. gingivalis W83 (but not with the gingipain-deficient Δ Rgps strain) rescued the otherwise deficient type-1 responses of TLR2−/− or neutrophil-depleted mice. These data are in agreement with the concept that plasma-borne kininogens undergo proteolytic processing by gingipain at the downstream end of the inflammatory cascade initiated by TLR2 ligands. Once liberated in mucosal tissues, kinins induce TH1 and/or TH17-polaring cytokines by APCs in B2R-dependent manner.
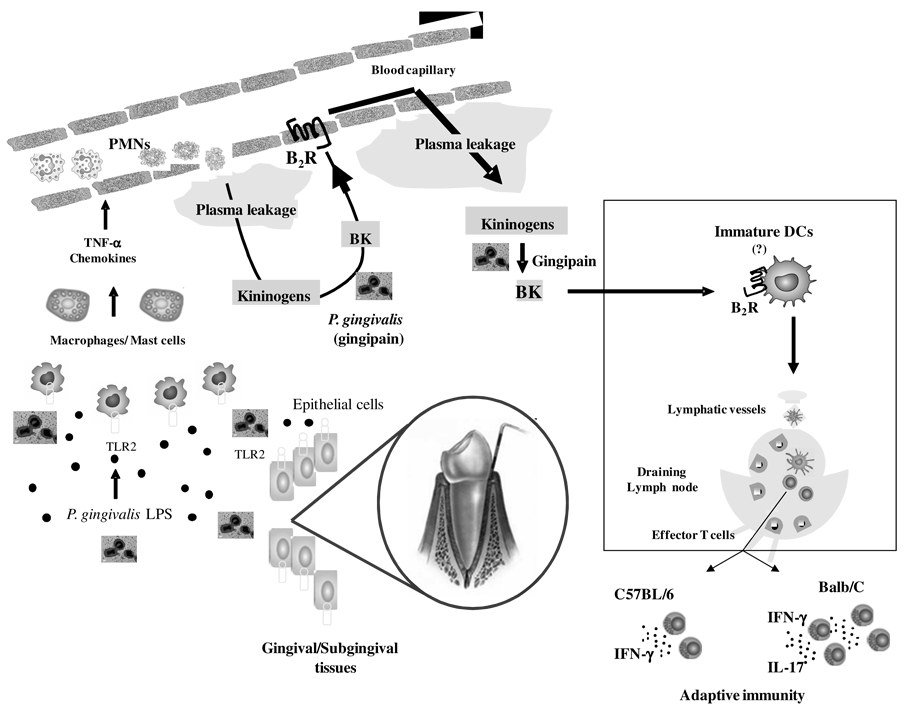
Figure 9. Trans-cellular TLR2/B2R “cross-talk” drives proteolytic generation of immunostimulatory bradykinin in extravascular mucosal tissues.
At the left side of the panel, P. gingivalis W83 (injected in the bottom of mandibular anterior vestibule) stimulate TLR2 expressed by hitherto uncharacterized resident sentinel cells (e.g., gingival epithelial cells, macrophages, mast cells). This innate recognition response translates into secretion of chemokines/cytokines, which then rapidly amplify inflammation through the activation of neutrophils/endothelium. Owing to increased vascular permeability, plasma proteins extravasate, leading to the accumulation of blood-borne kininogens in extravascular tissues (center of panel). Further downstream, the cysteine protease gingipain proteolytically liberates the vasoactive kinin moiety from kininogens. Plasma leakage is fueled through reiterative cycles of B2R-dependent triggering of the endothelium by the vasoactive kinins. Based on analysis of the immunological roles of kinins released by T. cruzi (46, 48, 49), we hypothesize that immature (myeloid) DCs patrolling the inflamed mucosa (see rectangle) are activated via the kinin/B2R pathway. After migrating to submandibular draining lymph nodes, the fimbriae-bearing DCs prime naïve T cells, while at the same time instructing their development into IFN-γ-producing effector T cells (in Balb/C and C57BL/6 mice) and IL-17-producing T cells (Balb/C mice).
An unresolved issue in the present study concerns the identity of the APCs that sense kinins released by gingipain in the inflamed mucosal tissues (58, 59). For example, it remains to be determined if B2R-dependent induction of TH1 polarizing cytokines (IL-12p70) (43, 47) versus TH17–instructive signals (IL-6, TGF-β and IL-23) (5–9) is differentially regulated in Balb/C and C57Bl/6 mice, perhaps reflecting in this context differences in the activation threshold of B2R expressed by DCs residing/recruited to inflamed mucosal tissues (58, 59). Whether involving specialized tissue DC subsets, such as Langerhans cells and dermal interstitial cells, the sampling and processing of fimbriae antigens by intramucosal APCs may involve direct endocytic uptake of W83 P.gingivalis and/or may depend on APC-mediated internalization of apoptotic (bacterial-loaded) neutrophils (60). Noteworthy in this context, it has been reported that the internalization of apoptotic neutrophils by macrophages and DCs leads to decreased secretion of IL-23, a cytokine controlling IL-17 production by different subsets of T cells (60). In view of these findings, it is conceivable that IL-23/IL-17-driven granulopoiesis might be differentially regulated in Balb/C and C57Bl/6 mice infected intrabuccally by P.gingivalis. (61). Although speculative, another possible explanation for the discrepant immune profiles observed in W83-infected Balb/C and C57Bl/6 mice may relate to the fact that bacteria-derived muramyldipeptide are sensed by nucleotide oligomerization domain 2 (NOD2), an intracellular pattern recognition receptor of human DCs whose activation leads to induction of the TH17 polarizing cytokines IL-23 and IL-1 (62). Adding another level of complexity to this problem, it was recently reported that gingipain, acting synergistically with ligands for TLR2 and NOD1/2 ligands, upregulates the production of IL-6 (a cytokine implicated in TH17-development) by macrophages through the signaling of protease activated receptors (37). Admittedly, additional studies may clarify if activation of NOD1/2 might shape the cytokine profile of Fim-specific T cells in mice infected intrabuccally with P. gingivalis.
In spite of variety of animal models and assay systems described in the literature, several studies suggest that TLR2 activation by P. gingivalis might serve the needs of the periodontal pathogen, either because TLR2 signaling reduces bacterial uptake by phagocytes (18, 19) and/or because TLR2 upregulates CR3, a surface receptor that down-modulates microbicidal responses of mononuclear phagocytes (20, 21). On the other hand, there is evidence that TLR2 signaling might benefit the host immune response; for example it was reported that P. gingivalis induced TLR2-dependent secretion of proinflammatory cytokines (e.g., IL-1β, TNF-α, IFN-γ) in subcutaneously inserted mouse chambers (63). Somewhat surprisingly, these authors also noted that alveolar bone loss was attenuated in TLR2-deficient mice (infected for 6 weeks) (63), thus implying that excessive activation of TLR2 may somehow stimulate the activity of bone-resorbing osteoclasts. In another report, Toll-like receptors 2 of human gingival epithelial cells were shown to co-operate with T-cell cytokine interleukin-17 (64). Although these findings raise the possibility that mucosal inflammation elicited by P. gingivalis LPS and/or by alternative TLR2 ligands might upregulate TH17-dependent bone resorption via the kinin/B2R pathway, the infection conditions used in the present study did not lead to persistent infection (data not shown), thus precluding an assessment of the role of IL-17-producing Fim-specific effector T cells in chronic periodontitis. Modified versions of our infection model may help to clarify if sustained activation of the TLR2/B2R axis in W83-infected-Balb/C and other mice strains may promote recruitment of Fim-specific effector/memory TH17 cells and/or of TH1 (65) to periodontal tissues.
In summary, our study revealed that P. gingivalis LPS and gingipain, respectively acting as TLR2 ligand and kinin-releasing protease, forge the activation of TLR2/B2R, an innate axis that amplifies inflammation while generating danger signals (i.e., kinins) that guide the development of fimbriae-specific effector T cells.
Supplementary Material
Acknowledgements
The authors based on Rio de Janeiro wish to thank Leila Faustino, Daniela O. Faustino and Alda F. Alves for the skillful technical assistance.
This work was supported by grants from Instituto Nacional de Pesquisa em Biologia Estrutural e Bio-Imagem (INBEB) do CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPERJ (Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro/Programa Pensa Rio), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), National Institutes of Health, USA (DE 09761 and 1642/B/P01/2008/35) and Department of Scientific Research, Polish Ministry of Science and Education.
Abbreviations used in this work
- Ag
antigen
- APC
antigen-presenting cell
- BK
bradykinin
- BR
bradykinin receptor
- CPs
Cysteine proteases
- DC
dendritic cell
- Fim
fimbriae
- HCP
hamster cheek pouch
- HK
high molecular weight kininogen
- LN
lymph node
- LPS
lipopolysaccharide
- NOD
nucleotide-binding oligomerization domain containing
- OVA
ovalbumin
- P. gingivalis
Porphyromonas gingivalis
- PMN
polymorphonuclear
- Rgps
arginine-specific gingipains
- TAFI
Thrombin-activatable fibrinolysis inhibitor
- TH1
IFN-γ-producing CD4+ T cells TH17, IL-17-producing CD4+T cells
- TLR
Toll-like receptor
- WT
wild type
REFERENCES
- 1.Socransky SS, Haffajee AD, Ximenez-Fyvie LA, Feres M, Mager D. Ecological considerations in the treatment of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis periodontal infections. Periodontology 2000. 1999;20:341–362. doi: 10.1111/j.1600-0757.1999.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. The American journal of pathology. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annual review of immunology. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 4.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. The Journal of experimental medicine. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nature reviews. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 6.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 7.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. Journal of periodontology. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. Journal of clinical periodontology. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 12.Lester SR, Bain JL, Johnson RB, Serio FG. Gingival Concentrations of Interleukin-23 and -17 at Healthy Sites and at Sites of Clinical Attachment Loss. Journal of periodontology. 2007;78:1545–1550. doi: 10.1902/jop.2007.060458. [DOI] [PubMed] [Google Scholar]
- 13.Davé S, TE Van Dyke. The link between periodontal disease and cardiovascular disease is probably inflammation. Oral diseases. 2008;14:95–101. doi: 10.1111/j.1601-0825.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 14.Gibson FC, 3rd, Ukai T, Genco CA. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis. Front Biosci. 2008;13:2041–2059. doi: 10.2741/2822. [DOI] [PubMed] [Google Scholar]
- 15.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darveau RP, Pham T-TTT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Porphyromonas gingivalis Lipopolysaccharide Contains Multiple Lipid A Species That Functionally Interact with Both Toll-Like Receptors 2 and 4. Infection and immunity. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. The Journal of biological chemistry. 2005;280:38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Q, Amar S. Identification of signaling pathways in macrophage exposed to Porphyromonas gingivalis or to its purified cell wall components. J Immunol. 2007;179:7777–7790. doi: 10.4049/jimmunol.179.11.7777. [DOI] [PubMed] [Google Scholar]
- 19.Eskan MA, Hajishengallis G, Kinane DF. Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae. Infection and immunity. 2007;75:892–898. doi: 10.1128/IAI.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Shakhatreh MA, James D, Liang S, Nishiyama S, Yoshimura F, Demuth DR, Hajishengallis G. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 21.Hajishengallis G, Shakhatreh MA, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-Mediated Clearance of Porphyromonas gingivalis and Negates Its Virulence in vivo. J Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- 22.Potempa JPR, Travis J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biological chemistry. 1997;378:223–230. doi: 10.1515/bchm.1997.378.3-4.223. [DOI] [PubMed] [Google Scholar]
- 23.Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infection and immunity. 1995;63:1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Potempa J, Polanowski A, Wikstrom M, Travis J. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. The Journal of biological chemistry. 1992;267:18896–18901. [PubMed] [Google Scholar]
- 25.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. The Journal of biological chemistry. 1994;269:406–411. [PubMed] [Google Scholar]
- 26.Scott CF, Whitaker EJ, Hammond BF, Colman RW. Purification and characterization of a potent 70-kDa thiol lysyl- proteinase (Lys-gingivain) from Porphyromonas gingivalis that cleaves kininogens and fibrinogen. The Journal of biological chemistry. 1993;268:7935–7942. [PubMed] [Google Scholar]
- 27.Imamura T, Potempa J, Pike RN, Moore JN, Barton MH, Travis J. Effect of free and vesicle-bound cysteine proteinases of Porphyromonas gingivalis on plasma clot formation: implications for bleeding tendency at periodontitis sites. Infection and immunity. 1995;63:4877–4882. doi: 10.1128/iai.63.12.4877-4882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura T, Potempa J, Tanase S, Travis J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. The Journal of biological chemistry. 1997;272:16062–16067. doi: 10.1074/jbc.272.25.16062. [DOI] [PubMed] [Google Scholar]
- 29.Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. The Journal of biological chemistry. 1992;267:18902–18907. [PubMed] [Google Scholar]
- 30.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J. Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 31.Jagels MA, Travis J, Potempa J, Pike R, Hugli TE. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infection and immunity. 1996;64:1984–1991. doi: 10.1128/iai.64.6.1984-1991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadowaki T, Nakayama K, Yoshimura F, Okamoto K, Abe N, Yamamoto K. Arg-gingipain Acts as a Major Processing Enzyme for Various Cell Surface Proteins in Porphyromonas gingivalis. The Journal of biological chemistry. 1998;273:29072–29076. doi: 10.1074/jbc.273.44.29072. [DOI] [PubMed] [Google Scholar]
- 33.Amano A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front Biosci. 2007;12:3965–3974. doi: 10.2741/2363. [DOI] [PubMed] [Google Scholar]
- 34.Carlisle MD, Srikantha RN, Brogden KA. Degradation of Human α-and β-Defensins by Culture Supernatants of Porphyromonas gingivalis Strain 381. J. Innate Immunity. 2009;1:118–122. doi: 10.1159/000181015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tada H, Sugawara S, Nemoto E, Takahashi N, Imamura T, Potempa J, Travis J, Shimauchi H, Takada H. Proteolysis of CD14 on human gingival fibroblasts by arginine-specific cysteine proteinases from Porphyromonas gingivalis leading to down-regulation of lipopolysaccharide-induced interleukin-8 production. Infection and immunity. 2002;70:3304–3307. doi: 10.1128/IAI.70.6.3304-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tada H, Sugawara S, Nemoto E, Imamura T, Potempa J, Travis J, Shimauchi H, Takada H. Proteolysis of ICAM-1 on human oral epithelial cells by gingipains. Journal of dental research. 2003;82:796–801. doi: 10.1177/154405910308201007. [DOI] [PubMed] [Google Scholar]
- 37.Uehara A, Imamura T, Potempa J, Travis J, Takada H. Gingipains from Porphyromonas gingivalis synergistically induce the production of proinflammatory cytokines through protease-activated receptors with Toll-like receptor and NOD1/2 ligands in human monocytic cells. Cell Microbiology. 2008;10:1181–1189. doi: 10.1111/j.1462-5822.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- 38.Imamura T, Pike RN, Potempa J, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J Clin Invest. 1994;94:361–367. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imamura T, Potempa J, Pike RN, Travis J. Dependence of vascular permeability enhancement on cysteine proteinases in vesicles of Porphyromonas gingivalis. Infection and immunity. 1995;63:1999–2003. doi: 10.1128/iai.63.5.1999-2003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacological reviews. 1992;44:1–80. [PubMed] [Google Scholar]
- 41.Marceau F, Bachvarov DR. Kinin receptors. Clinical reviews in allergy & immunology. 1998;16:385–401. doi: 10.1007/BF02737658. [DOI] [PubMed] [Google Scholar]
- 42.Leeb-Lundberg LMF, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International Union of Pharmacology. XLV. Classification of the Kinin Receptor Family: from Molecular Mechanisms to Pathophysiological Consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 43.Aliberti J, Viola JPB, Vieira-de-Abreu A, Bozza PT, Sher A, Scharfstein J. Cutting Edge: Bradykinin Induces IL-12 Production by Dendritic Cells: A Danger Signal That Drives Th1 Polarization. J Immunol. 2003;170:5349–5353. doi: 10.4049/jimmunol.170.11.5349. [DOI] [PubMed] [Google Scholar]
- 44.Skidgel RA, Erdos EG. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: a brief history, the beginning and follow-ups to early studies. Peptides. 2004;25:521–525. doi: 10.1016/j.peptides.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Bengtson SH, Sandén C, Mörgelin M, Marx PF, Olin AI, Leeb-Lundberg LMF, Meijers JCM, Herwald H. Activation of TAFI on the Surface of Streptococcus pyogenes Evokes Inflammatory Reactions by Modulating the Kallikrein/Kinin System. Journal of Innate Immunity. 2009;1:18–28. doi: 10.1159/000145543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monteiro AC, Schmitz V, Svensjö E, Gazzinelli RT, Almeida IC, Todorov A, de Arruda LB, Torrecilhas ACT, Pesquero JB, Morrot A, Bouskela E, Bonomo A, Lima APCA, Müller-Esterl W, Scharfstein J. Cooperative Activation of TLR2 and Bradykinin B2 Receptor Is Required for Induction of Type 1 Immunity in a Mouse Model of Subcutaneous Infection by Trypanosoma cruzi. J Immunol. 2006;177:6325–6335. doi: 10.4049/jimmunol.177.9.6325. [DOI] [PubMed] [Google Scholar]
- 47.Monteiro AC, Schmitz V, Morrot A, de Arruda LB, Nagajyothi F, Granato A, Pesquero JB, Müller-Esterl W, Tanowitz HB, Scharfstein J. Bradykinin B2 Receptors of dendritic cells, acting as sensors of kinins proteolytically released by Trypanosoma cruzi, are critical for the development of protective type-1 responses. PLoS Pathog. 2007;3:185e. doi: 10.1371/journal.ppat.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scharfstein J, Schmitz V, Svensjö E, Granato A, Monteiro AC. Kininogens Coordinate Adaptive Immunity through the Proteolytic Release of Bradykinin, an Endogenous Danger Signal Driving Dendritic Cell Maturation. Scandinavian Journal of Immunology. 2007;66:128–136. doi: 10.1111/j.1365-3083.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 49.Scharfstein J, Monteiro AC, Schmitz V, Svensjö E. Angiotensin-converting enzyme limits inflammation elicited by Trypanosoma cruzi cysteine proteases: a peripheral mechanism regulating adaptive immunity via the innate kinin pathway. Biological chemistry. 2008;389:1015–1024. doi: 10.1515/BC.2008.126. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen K-A, Travis J, Potempa J. Does the Importance of the C-Terminal Residues in the Maturation of RgpB from Porphyromonas gingivalis Reveal a Novel Mechanism for Protein Export in a Subgroup of Gram-Negative Bacteria? J. Bacteriol. 2007;189:833–843. doi: 10.1128/JB.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen K-A, DeCarlo AA, Paramaesvaran M, Collyer CA, Langley DB, Hunter N. Humoral Responses to Porphyromonas gingivalis Gingipain Adhesin Domains in Subjects with Chronic Periodontitis. Infect. Immun. 2004;72:1374–1382. doi: 10.1128/IAI.72.3.1374-1382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamada N, Sojar HT, Cho MI, Genco RJ. Isolation and characterization of a minor fimbria from Porphyromonas gingivalis. Infection and immunity. 1996;64:4788–4794. doi: 10.1128/iai.64.11.4788-4794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 2004;18:370–372. doi: 10.1096/fj.03-0708fje. [DOI] [PubMed] [Google Scholar]
- 54.Svensjö E, Saraiva EM, Bozza MT, Oliveira SMP, Lerner EA, Scharfstein J. Salivary Gland Homogenates of Lutzomyia longipalpis and Its Vasodilatory Peptide Maxadilan Cause Plasma Leakage via PAC1 Receptor Activation. Journal of Vascular Research. 2009;46:435–446. doi: 10.1159/000197866. [DOI] [PubMed] [Google Scholar]
- 55.Schmitz V, Svensjö E, Serra RR, Teixeira MM, Scharfstein J. Proteolytic generation of kinins in tissues infected by Trypanosoma cruzi depends on CXC-chemokine secretion by macrophages activated via Toll-like 2 receptors. Journal of Leukocyte biology. 2009;85:1005–1014. doi: 10.1189/jlb.1108693. [DOI] [PubMed] [Google Scholar]
- 56.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 57.Noiri Y, Li L, Yoshimura F, Ebisu S. Localization of Porphyromonas gingivalis-carrying fimbriae in situ in human periodontal pockets. Journal of dental research. 2004;83:941–945. doi: 10.1177/154405910408301210. [DOI] [PubMed] [Google Scholar]
- 58.Cutler CW, Jotwani R. Dendritic Cells at the Oral Mucosal Interface. Journal of Dental Research. 2006;85:678–689. doi: 10.1177/154405910608500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Aït-Yahia S, Vicari A, Kaiserlian D, Dubois B. Dendritic Cells Rapidly Recruited into Epithelial Tissues via CCR6/CCL20 Are Responsible for CD8+ T Cell Crosspriming In Vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol. 2007;179:8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- 62.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SAJ, Kapsenberg ML, de Jong EC. Stimulation of the Intracellular Bacterial Sensor NOD2 Programs Dendritic Cells to Promote Interleukin-17 Production in Human Memory T Cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 Is Required for the Innate Response to Porphyromonas gingivalis: Activation Leads to Bacterial Persistence and TLR2 Deficiency Attenuates Induced Alveolar Bone Resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 64.Beklen A, Sorsa T, Konttinen YT. Toll-like receptors 2 and 5 in human gingival epithelial cells co-operate with T-cell cytokine interleukin-17. Oral Microbiol Immunol. 2009;24:38–42. doi: 10.1111/j.1399-302X.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- 65.Stashenko P, Gonçalves RB, Lipkin B, Ficarelli A, Sasaki H, Campos-Neto A. Th1 immune response promotes severe bone resorption caused by Porphyromonas gingivalis. Am. J. Pathol. 2007;170:203–213. doi: 10.2353/ajpath.2007.060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.