Abstract
Recent studies suggested that various cell lineages exist within the subepicardium and we supposed that this area could host cardiac stem cell niches (CSCNs). Using transmission electron microscopy, we have found at least 10 types of cells coexisting in the subepicardium of normal adult mice: adipocytes, fibroblasts, Schwann cells and nerve fibres, isolated smooth muscle cells, mast cells, macrophages, lymphocytes, interstitial Cajal-like cells (ICLCs) and cardiomyocytes progenitors (CMPs). The latter cells, sited in the area of origin of coronary arteries and aorta, showed typical features of either very immature or developing cardiomyocytes. Some of these cells were connected to each other to form columns surrounded by a basal lamina and embedded in a cellular network made by ICLCs. Complex intercellular communication occurs between the ICLCs and CMPs through electron-dense nanostructures or through shed vesicles. We provide here for the first time the ultrastructural description of CSCN in the adult mice myocardium, mainly containing ICLCs and CMPs. The existence of resident CMPs in different developmental stages proves that cardiac renewing is a continuous process. We suggest that ICLCs might act as supporting nurse cells of the cardiac niches and may be responsible for activation, commitment and migration of the stem cells out of the niches. Briefly, not only resident cardiac stem cells but also ICLCs regulate myocyte turnover and contribute to both cardiac cellular homeostasis and endogenous repair/remodelling after injuries.
Keywords: epicardium, subepicardium, interstitial Cajal-like cells, cardiomyocytes progenitors, cardiac repair, cardiac regeneration, myocardial remodelling, cardiac stem cells niches, shed microvesicle, epicardium-derived progenitor cells (EPDCs)
Introduction
The epicardium appears to be more than a simple serosal ‘umbrella’ over myocardium and this finding could bring out the ultimate gift left in Pandora's box – the hope – the possibility that epicardial progenitor cells could be persuaded to efficiently regenerate the damaged heart.
It is supposed, based on colourful, spectacular (but low resolving power) images using immunohistochemistry and/or confocal microscopy that epicardium is a source of both myocardial and supporting cells capable to replace cardiomyocytes and vessels in the damaged heart [1–17]. It has also been reported that allogeneic stimulated mesenchymal stem cells improve the ejection fraction [18], therefore stressing the importance of the specific supporting connective tissue.
Cellular therapies propose exogenous adult or embryonic stem cells [19–35] and endogenous cardiac stem cells [36–47] for repairing or replacing the damaged cardiac working cells but they were partially effective mainly due to the lack of a structural and functional integration with the pre-existing ‘working’ myocardium [48–52].
We have shown previously the existence of interstitial Cajal-like cells (ICLCs) as a distinct type of cell in human and mammalian myocardium and suggested that they play a role in supporting the cardiomyocytes three-dimensional organization and could influence (control) their microenvironment, e.g. paracrine secretion and/or shedding microvesicles [53–59]. Moreover, very recently, using cell culture and frozen sections, we highlighted ICLCs by fluorescence microscopy in the adult mice epicardium [60]. Although, canonical intestinal interstitial Cajal cells (ICC) are very well known [61–66], Faussone-Pellegrini's group identified ICLCs in human intestine beyond the classical ICC and suggested a supporting role for these cells in the three-dimensional organization and microenvironment of the enteric muscle coat [67].
Noteworthy, recent studies [68–69] described selected areas of the heart as functional units supposed to be cardiac stem cell niches (CSCNs) and now is (generally) accepted that adult stem cells reside in specialized niches that coordinate tissue renewal [69–73]. The cells of the niches seem to be connected to the pre-existing cardiomyocytes and interstitial supporting cells [68, 69] but the identity of the last (but not the least) cells is still unknown.
We report here for the first time the ultrastructural identification and description of the cardiomyocyte progenitors (CMPs) and their structural connections with the ICLCs as supporting nurse cells in epicardial stem cell niches in adult mice. Thus, we show unequivocally the existence of CSCN in adult epicardium and that ICLCs are not simple bystanders, but key actors in adult myocardial renewing. These findings would have important clinical implications for the management of cardiac diseases.
Materials and methods
Hearts from four 16-week-old normal mice (two females and two males; B6129PF2/J strain) purchased from Jackson Laboratories (Bar Harbor ME, USA) were examined by transmission electron microscopy (TEM). The institutional ethical committee approved the study. Small fragments from atrial and ventricular myocardium with epicardium were processed according to routine procedures, as we previously described [55]. Light microscopy was performed on 1 μm semi-thin section stained with 1% toluidine blue and digital images were recorded using a CCD Axiocam HRc Zeiss camera with AxioVision software (Carl Zeiss Imaging solution GmbH, Germany), on Nikon Eclipse E600 microscope (Nikon Instruments, Inc., Tokyo, Japan). Ultrathin sections stained with uranyl acetate and Reynolds's lead citrate solutions were examined using a Morgagni 286 TEM (FEI Company, Eindhoven, The Nederlands) at 60 kV. Digital electron micrographs were recorded with a MegaView III CCD using iTEM-SIS software (Olympus, Soft Imaging System GmbH, Germany). Digital coloured TEM images were processed using Adobe Photoshop software (Adobe Systems, Inc., San Jose, CA, USA). The colour codes used to emphasize different types of cells are blue for ICLCs, brown for cardiomyocyte and progenitors and green for nerve fibres.
Results
Light microscopy
Semi-thin toluidine blue stained sections of the atrium and ventricle of adult mouse heart allowed to identify several cell populations in the subepicardium (i.e.the connective tissue in between the epicardial epithelium and the myocardial tissue). The subepicardium covering the atrium and ventricle appears as thin collagen tissue with scattered cellularity (Fig. 1A, B). Some of these cells were easily recognizable, such as mast cells, adipocytes, macrophages, lymphocytes and nerves, and were present in small numbers everywhere beneath the epicardium (Fig. 1). Cells with long processes are constantly present under the atrial and ventricular epicardium (Fig. 1A, B); however, they are not certainly recognizable as ICLCs and fibroblasts.
1.
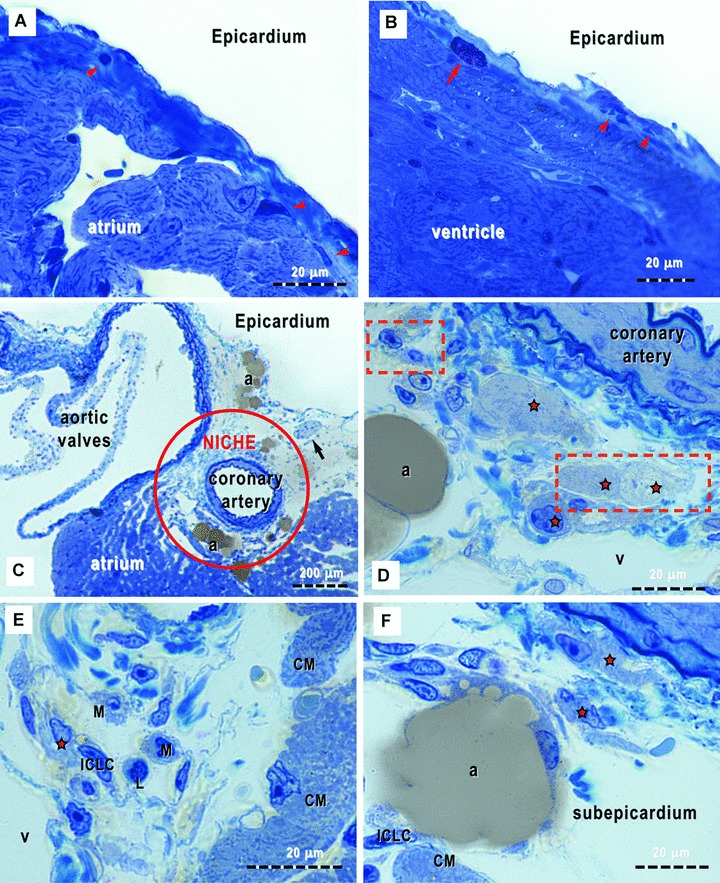
(A)-(F). Light microscopy of toluidine-blue stained semi-thin sections (∼1 μm, or less) of Epon-embedded epicardium from 16-week-old mice (supposed to be the equivalent of 25 human years). The loose connective tissue with very few cells (arrowheads) may be observed immediately below the epicardial mesothelium (the so-called subepicardium), in both cases of atrium (A) and ventricle (B). A metachromatically stained mast cell (red arrow) can be easily recognized. (C) shows the richness of cell population types surrounding the coronary artery and a cross sectioned nerve (arrow). Red circle marks the epicardial stem cell niche. (D)-(F) demonstrate different cellular silhouettes scattered in the loose connective tissue of the cardiac stem cell niche. Note, the presence of ‘young’ cardiomyocytes (red stars); the orange rectangles areas correspond to high-resolution electron microscope images in Fig. 6. ICLC – interstitial Cajal-like cells; CM – cardiomyocytes; L – lymphocyte; M – macrophage; v – venules and a – adipocytes.
In the subepicardial area at the origin of coronary arteries and aorta, the connective tissue is loose and particularly rich in cells (Fig. 1C). All types of cells already mentioned were present in this location, as well as cells we identified as belonging to the myocardial lineage (Fig. 1D–F) and we presumed to be CMPs in a cardiac niche.
Transmission electron microscopy
Under TEM, all the afore-mentioned cells were identified as well as ICLCs (Figs. 2, 3A, inset, 4A, 6–9) and fibroblasts (Fig. 3B). Free smooth muscle cells were occasionally seen, all of them similar to the periarteriolar (data not shown). Some mononuclear cells showed unspecific ultrastructural appearance and they could be stem cells or cells under dormant state (data not shown). Moreover, the presumed CMPs (Figs. 4–7) were unequivocally identified under TEM. Peculiar relationships between the CMPs and the ICLCs were also seen giving rise to structures we identified as epicardial stem cell niche (Figs. 8–14).
2.
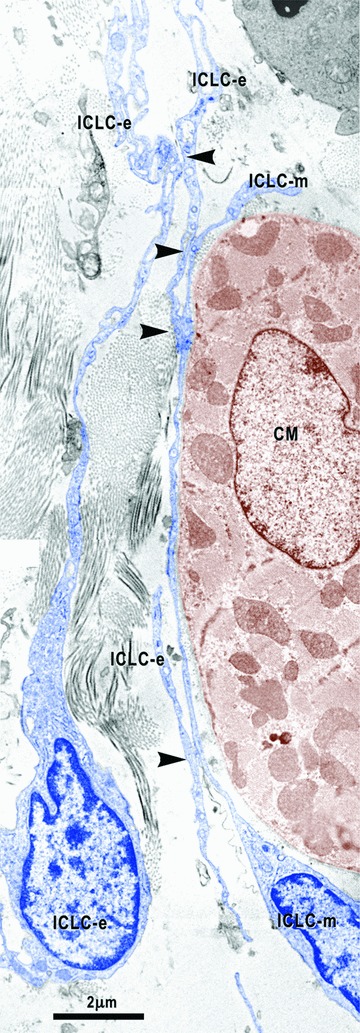
Digitally coloured image shows the close relationships between the epicardial ICLC (ICLC-e) and the ICLC bordering the myocardium (ICLC-m). Arrowheads indicate close contacts between epicardial ICLC and myocardial ICLC. ICLC – interstitial Cajal-like cells are blue coloured and CM – cardiomyocyte coloured in brown.
3.
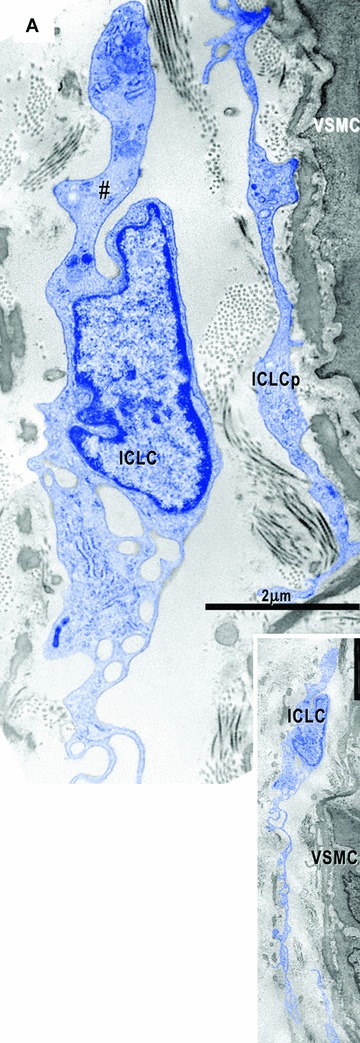
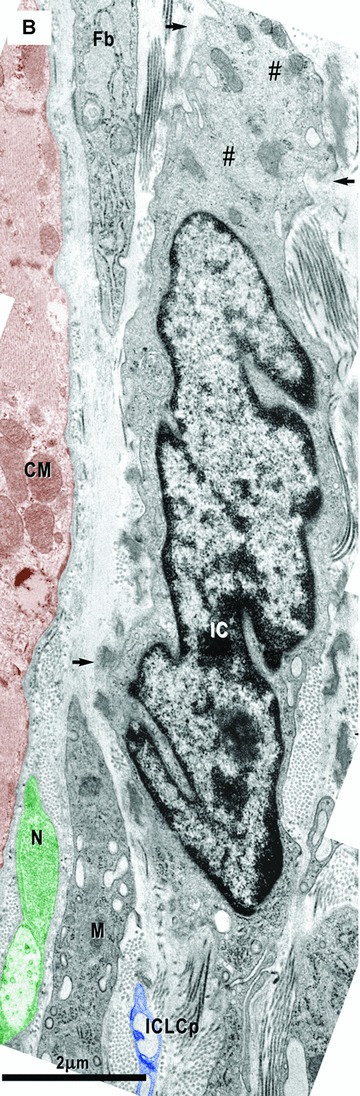
Electron micrographs show interstitial cells in the subepicardial area next to the coronary artery. (A) – Interstitial Cajal-like cells (ICLC, blue) have long and thin processes (inset), a lacunar cytoplasm, few cisternae of the rough endoplasmic reticulum and intermediate filaments (#). No basal lamina appears surrounding these cells. Another ICLC process (ICLCp) is near coronary smooth muscle cells (VSMC). (B) – Next to the atrial myocardium, an interstitial cell (IC) particularly rich in filaments (#) shows focal basal lamina-like material (arrows). N – nerve (green); M – macrophage; Fb – fibroblast; CM – cardiomyocyte (brown).
4.
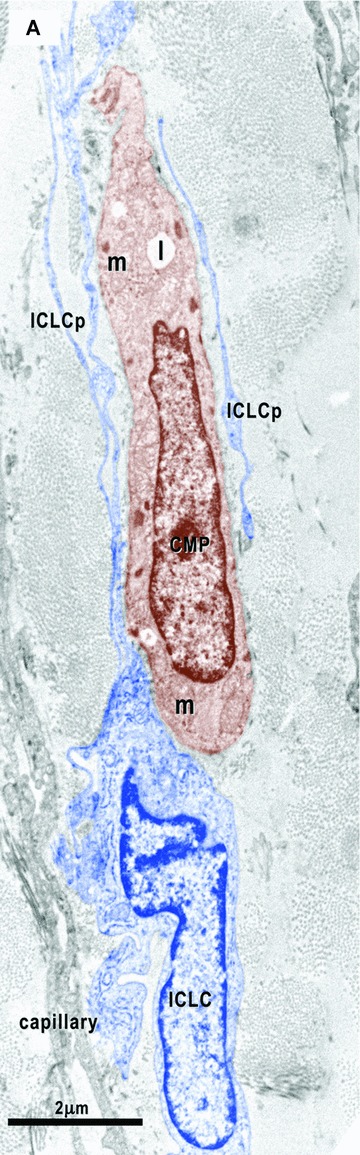
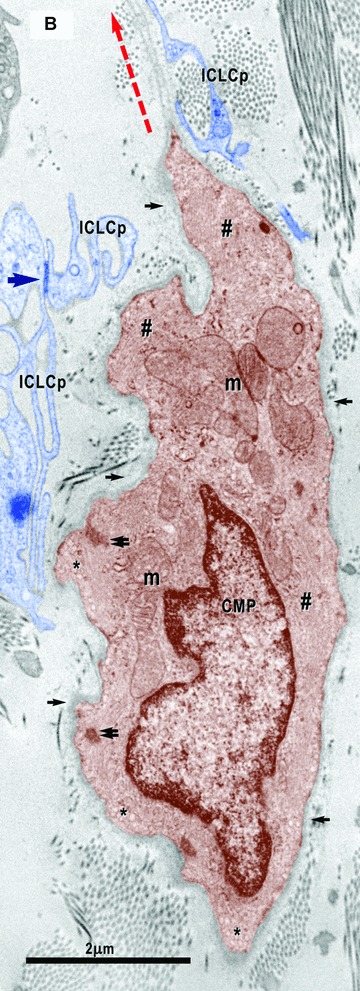
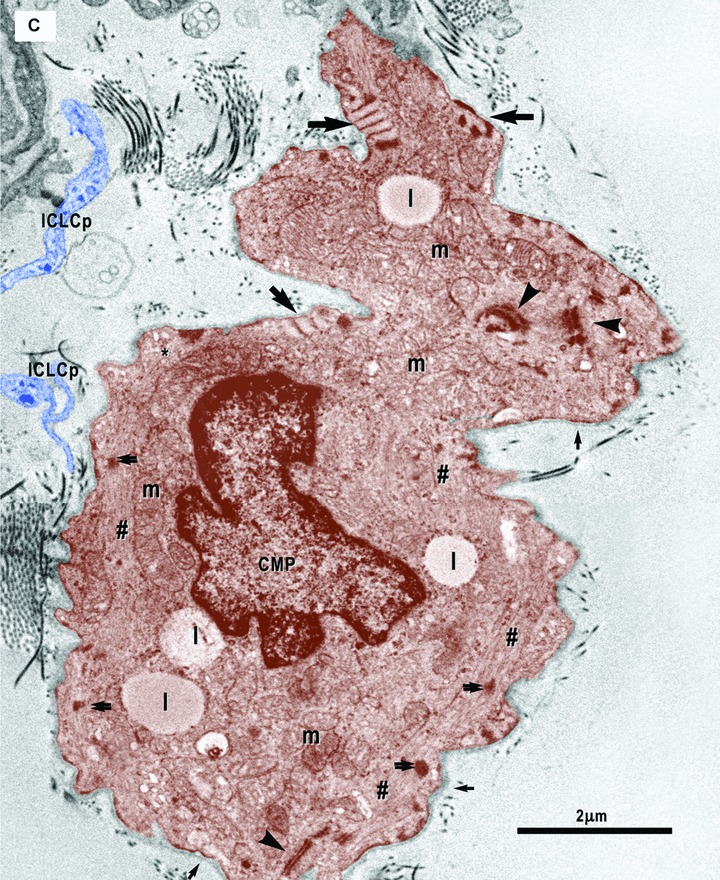
(A)–(C). Digitally coloured electron micrographs show cardiomyocyte progenitors (CMP, brown) in different stages of development and ICLC (blue). CMP have features of immature cardiomyocytes: a high nucleo-cytoplasmatic ratio and a continuous basal lamina (small arrows), numerous mitochondria (m) and ribosomes, few endoplasmic reticulum cisternae, some lipid droplets (l) and caveolae (*). These cells contain unorganized bundles of filaments (#) attached to electron dense structures (double arrows) similar to Z bands. (A) – ICLC processes (ICLCp) closely accompany the immature cardiomyocyte. B– The basal lamina exceeds the cellular profile and delimitates a slender acellular space. An ICLC process seems to support and direct this lamina (red dotted arrow). The large arrow indicates a gap junction in between two ICLCp. (C) – Note the presence of the subplasmalemmal leptofibrils (large arrows) and intracellular desmosome-like structures (arrowheads).
8.
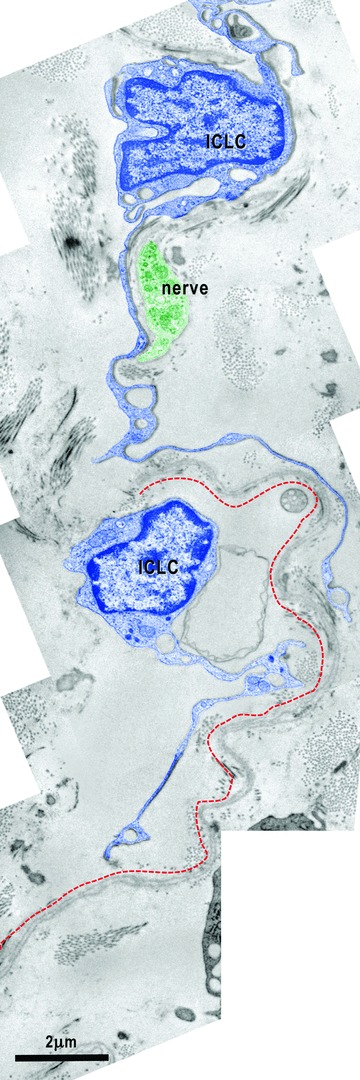
Electron micrograph illustrates the relationships of the ICLC (blue coloured) with a nerve fibre (green) and the basal lamina material that extends over the bodies of the cardiomyocyte progenitors. The processes of two ICLC guide a 30-μm-long bi-layered basal lamina (red dotted line).
Subepicardial interstitial Cajal-like cells
The cells identified as ICLCs fulfil ultrastructural criteria already established for this cell type [54, 55]:
-
1
Characteristic long (several tens of μm), thin and moniliform cell processes (Figs. 2, 3A inset, 4A, 6–9);
-
2
Cell-to-cell junctions (Figs. 2, 4B, 6);
-
3
Caveolae and coated vesicles (Figs. 10, 11);
-
4
Mitochondria, relatively well developed smooth and rough endoplasmic reticulum (Figs. 2, 6–9);
-
5
Intermediate (Fig. 3A) and thin filaments, microtubules and undetectable thick filaments;
-
6
A single cilium (Fig. 13) and
-
7
No basal lamina (Figs. 2–4, 6–11).
10.
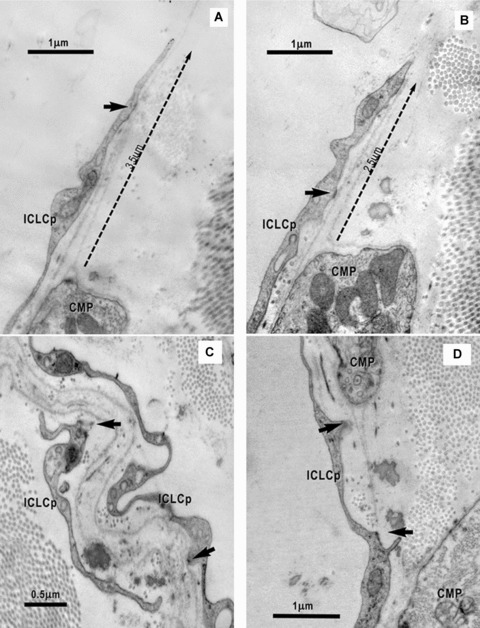
(A) and (B). Electron micrographs on serial ultrathin sections highlight the connections of the ICLC processes (ICLCp) with the basal lamina of the CMP by electron dense nanostructures (arrows). The ICLC process seems to support and guide the basal lamina, which exceeds the cellular profile of the CMP (dotted arrows). (C), (D) – High magnifications of the areas marked in Fig. 6 point out the close relationship of the ICLCp with the basal lamina of the CMP. Arrows indicate adjoining points.
13.
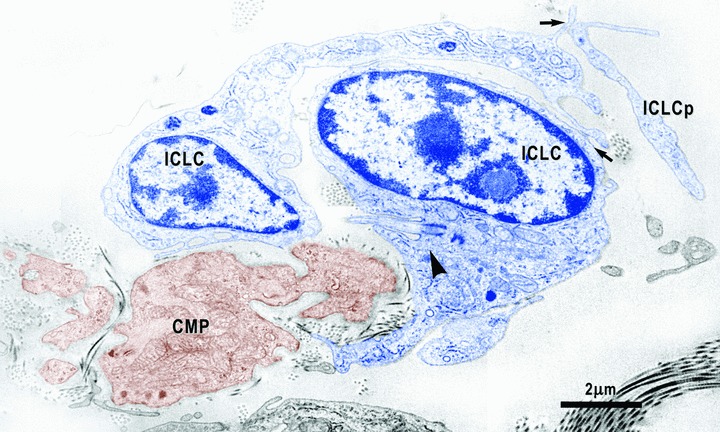
Electron micrograph shows close relationships between cardiomyocyte progenitors (CMP, brown) and ICLC (blue). One of the two ICLC has a cilium (arrowhead) directed towards the CMP. Arrows indicate close contacts between ICLC.
In the entire subepicardium, the ICLCs are located under the epicardial cells and around blood vessels. Similar to the atria, ventricle and myocardial sleeves [54–57], these ICLCs interconnect with each other through point cell-to-cell contacts or gap junctions (Fig. 4B), thus forming a three-dimensional network. Attachment plaques connect the ICLCs to the extracellular matrix and the perivascular elastic material (Fig. 3A) or to the basal lamina of the CMP (Fig. 10). Moreover, close contacts exist between the subepicardial ICLCs and the ICLCs bordering the myocardium (Fig. 2). The subepicardial ICLCs accompany all the blood vessels forming an incomplete sheath around them and the ICLC processes surround the cross-sectioned coronary artery wall (data not shown). In addition, the ICLCs showed a close vicinity with nerves (Figs. 6B, 8), blood capillaries (Figs. 4A, 7) and macrophages (Figs. 3B, 7).
6.
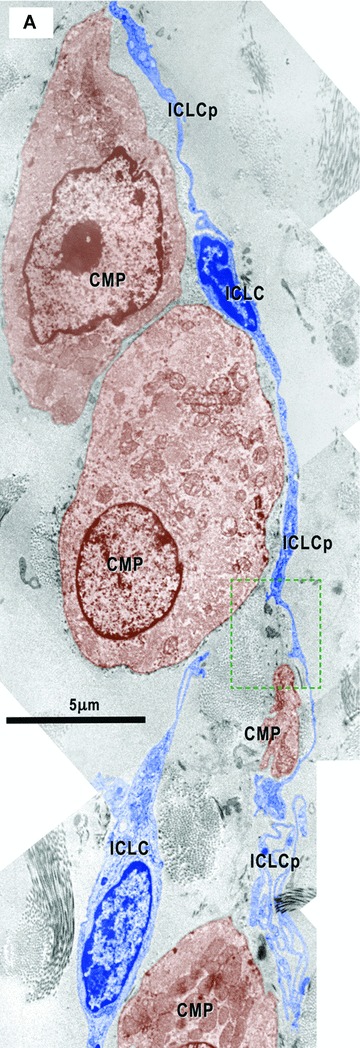
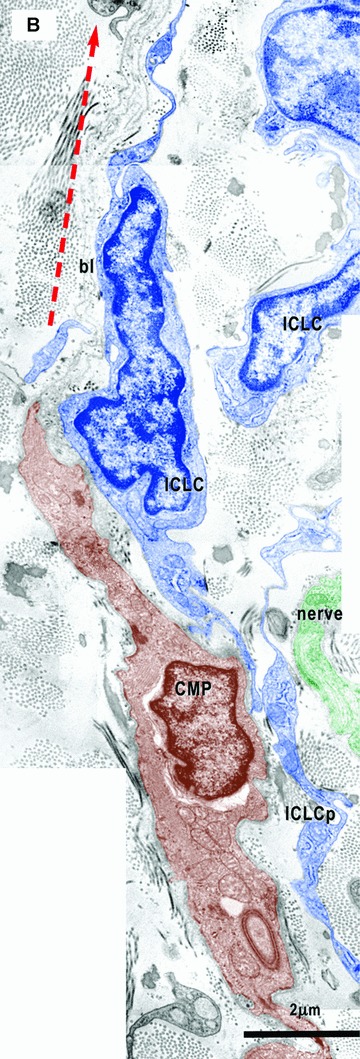
(A) and (B) Electron micrographs (from the areas marked in Fig. 1D) illustrate the relationships of the ICLC (blue coloured) with cardiomyocyte progenitors (CMP, brown). The ICLC processes (ICLCp) run parallel with the main axis of the CMP and seem to establish their direction of development. In (B) it is clear that the ICLC sustain a direction (red dotted arrow) for the basal lamina (bl) which goes beyond the CMP. Details from the upper area in Fig. 10C. The square mark area from A is enlarged in Fig. 10D.
Subepicardial cardiomyocyte progenitors
All the cells identifiable as belonging to the myocardial lineage and reasonably considered to be CMPs have an oval or elongated shape with an irregular contour. CMPs are extremely variable in size (2–10 μm diameter and 15–30 μm length) but smaller than adult cardiomyocytes. The CMPs seem to be randomly oriented, without a preferential localization with the respect to the myocardium. Some slender CMPs were located around the coronary artery in between the ICLCs, a few CMPs with high degree of maturation were located in between coronary artery and the epicardium and some CMPs were located nearby the terminal myocardium but with very few connections with adult tissue (Fig. 14). All the CMPs presented immature structural features but they were in different stages of development: low (Figs. 3B, 4A, 5A), medium (Figs. 4B, C, 5B–D, 6B, 14) and high (Figs. 5E, 6A, 7).
14.
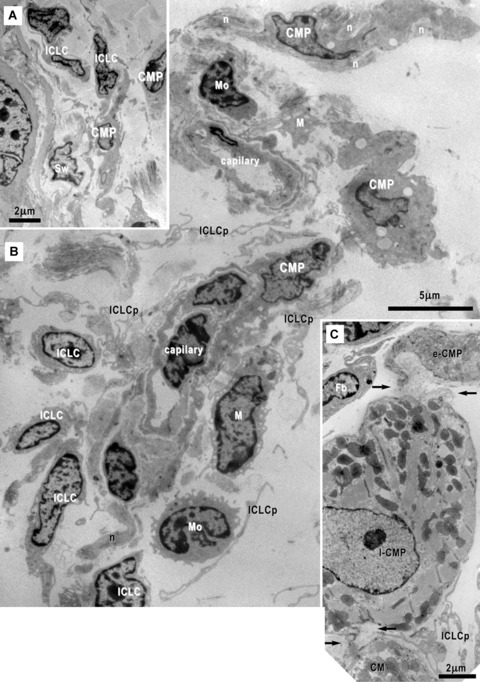
Electron micrographs of the cardiac stem cells niche in the subepicardium surrounding the coronary artery (A, B) and next to the peripheral adult cardiomyocytes (C). (B) Cardiomyocyte progenitors (CMP), interstitial Cajal-like cells (ICLC) and their inconspicuous processes (ICLCp), macrophages (M), mononuclear cells (Mo) and nerves (n) are clustered around capillaries. (C) Early (e-CMP) and late (l-CMP) cardiomyocyte progenitors (CMP) could be seen nearby an adult cardiomyocyte (CM). A basal lamina envelops these cells (arrows) closely assisted by ICLCp. Fb- fibroblast.
5.
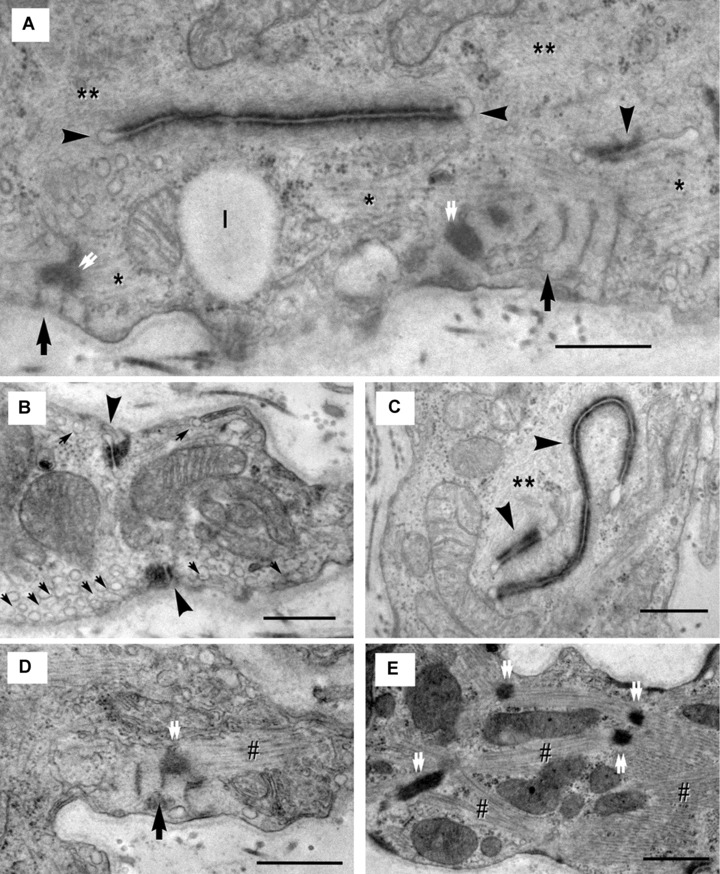
(A)-(E). Electron micrographs show ultrastructural features of the cardiomyocyte progenitors. (A) – Desmosome-like structures (arrowheads) are entirely located inside the cell and seem to organize thin filaments (**). Thick filaments (*) appear to be organized by leptofibrils (arrows). Z-like dense structures (double arrows) are visible at the end of leptofibrils. Image in (B) shows numerous caveolae (small arrows) and confluent intracytoplasmatic vesicles (primordial T tubules) and suggests that the desmosome-like structures (arrowheads) start from periphery and extend towards the centre of the cell. In (C), it is obvious that thin filaments (**) are organized by desmosome-like structures (arrowheads). (D) – Myofibrils (#) appear to be directed by leptofibrils (arrow) that anchor them to the plasma membrane throughout Z-like dense structures (double arrow). In (E), the nascent sarcomeres are visible: Z-like dense structures (double arrows) organize myofibrils (#) in an immature sarcomeric configuration. l – lipid droplet. Scale bar = 0.5 μm.
The most immature CMPs were mainly free. Their cytoplasm showed a dual aspect with mesenchymal features, such as free ribosomes and few cisternae of rough endoplasmic reticulum (Fig. 3B), a lacunar aspect very similar to that of the ICLC (Fig. 3A), and cardiac features such as typical cardiac mitochondria (large, with a clear matrix and numerous and long christae and filaments) (Figs. 4, 5). None of them has a complete basal lamina (Fig. 3B).
The lesser immature CMPs were both free (Fig. 4) and grouped in columns of two to three cells (Figs. 6A, 7) and showed many features characteristic for the myocardial lineage cells and identical to those reported for the immature cardiomyocytes of mouse embryos [74]:
-
1
Bundles of filaments often attached to plasmalemmal dense bands (Fig. 4B, C);
-
2
Intracytoplasmatic dense bodies that give origin to primordial sarcomeres and primordial Z lines (Figs. 4C, 5E);
-
3
Numerous caveolae (Fig. 5B);
-
4
Intracytoplasmatic vesicles often aligned and confluent (to give origin to primordial T tubules) (Fig. 5B);
-
5
Intracytoplasmatic (Figs. 4C, 5A, C, 6B) and peripheral (Fig. 5B) desmosome-like structures (primordial intercalated discs) sometimes forming a complete intercalated disc (Fig. 7);
-
6
Subsarcolemmal leptomeric fibrils (Figs. 4C, 5A, D, 7) and
-
7
A continuous basal lamina (Figs. 4, 6, 7).
7.
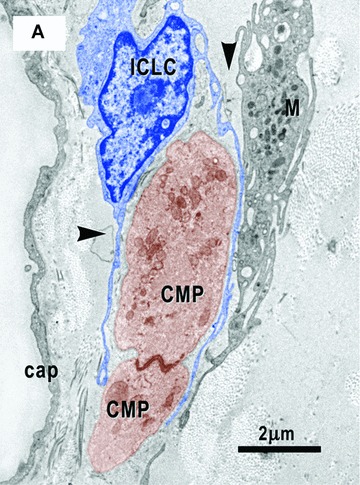
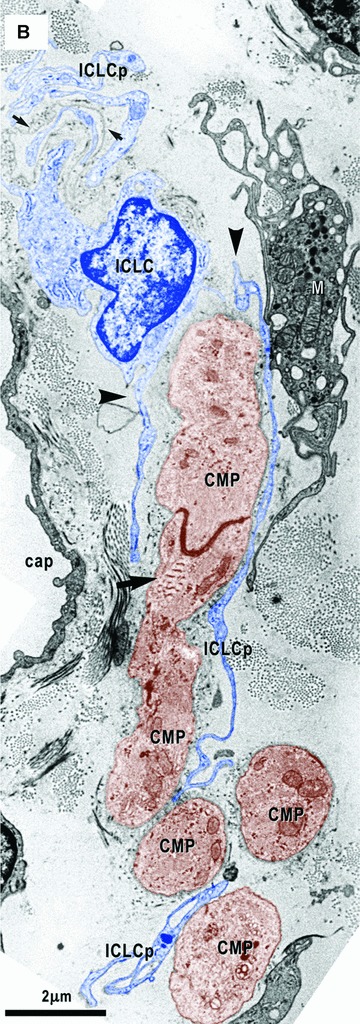
(A) and (B) Electron micrographs on serial sections show an ICLC (blue coloured) with two processes (arrowheads) which hold two cardiomyocyte progenitors (CMP, brown). Note close contacts of one ICLC process (ICLCp) with a macrophage (M). (B) – A basal lamina – like material surrounds an ICLC process (small arrows in the upper left corner). Leptomeric fibrils (arrow) connect the primitive intercalated disk with the plasma membrane. Cap – capillary.
The desmosome-like structures often seem to organize thin filaments (Fig. 5A, C) while thick filaments appear to be organized by leptofibrils (Fig. 5A, D). Leptofibrils in CMP showed filamentous bundles periodically crossed (periodicity: 140–160 nm) by three to nine electron dense small bands (about 30 nm wide). The length of leptofibrils varied between 1.03 and 2.21 μm (mean = 1.21 μm) and their thickness between 0.16 and 0.83 μm (mean = 0.41 μm). Z-like dense structures are visible at the end of leptofibrils (Figs. 4C, 5A, D).
Nerve fibres were frequently seen in the proximity of the CMP columns in contact with either the ICLCs (Figs. 6, 8) or the CMPs (Fig. 12).
12.
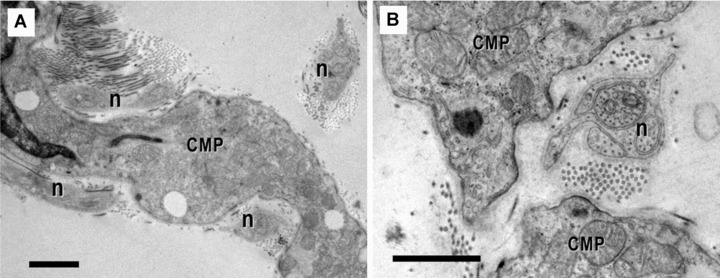
(A) and (B). Electron micrographs show the close vicinity of nerve fibres (n) with cardiomyocytes progenitors (CMP). Scale bar = 1 μm.
Relationships between ICLCs and CMPs
The ICLCs accompany all the CMPs, strictly apposed to them, independently of their degree of differentiation and mainly oriented parallel to their main axis (Figs. 4A, 6, 7). The mean distance between ICLCs and CMPs was 0.10 ± 0.05 μm (minimum = 0.04 μm; maximum = 0.30 μm). Sometimes the ICLCs are directly anchored to the CMPs (Figs. 10, 11) or to their basal lamina (Figs. 6, 8, 10) by means of plasmalemmal areas reinforced by an electron-dense material (Fig. 10). The basal lamina completely surrounds the more mature CMPs (Figs. 4, 6) and when these cells are grouped to form a column this lamina, always lined by the ICLCs, surrounds the entire column often exceeding it at its poles (Figs. 4B, 6, 8, 10). Interestingly, the exceeding basal lamina might continue as a discrete tract, always bordered by the ICLCs (Figs. 4B, 6, 8, 10) and in some images seems to contain an ICLC process (Figs. 7B, 9). The final architecture results in a three-dimensional tunnel-like structure with the CMPs inside, a basal lamina surrounding the column and the ICLCs on the external side of the basal lamina. Primitive cilium, directed to the main axis of the cardiomyoblasts column (Fig. 13), is often present in the ICLCs.
Often, shed microvesicles or exocytotic multi-vesicular bodies (Fig. 11) could be seen in between ICLC and CMP. The microvesicles (80–150 nm diameter) appeared to be generated by ICLC through an exocytotic process as single units or enclosed multi-vesicular bodies (0.5–1 μm wide).
11.
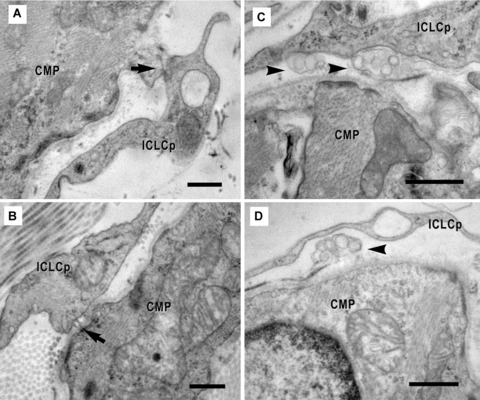
(A)-(D). Electron micrographs show the physical contacts (arrows) of the ICLC processes (ICLCp) with cardiomyocyte progenitors. The connections between the two types of cells could be: direct (A), through dense structures (B) or mediated by shed vesicles (arrowheads) (C, D). Scale bar = 0.5 μm.
Cardiac stem cell niches
The CSCNs were located in the subepicardial area adjacent to the coronary arteries at the emerging point from aorta (Figs. 1C, 14) and showed no separation limit from the surrounding interstitium. The CMPs were noticeable in the inner part of the subepicardial space next to the myocardium and they were not seen immediately beneath the mesothelial layer. The extracellular matrix is loose and contains spare bundles of collagen and few elastic fibres.
The cells present in the niche, CMPs, ICLCs, macrophages, mononuclear cells with stem appearance and nerves, showed a tendency to form clusters around capillaries (Fig. 14). Infrequently, CMPs in different stages of development have been seen nearby adult cardiomyocyte and a basal lamina seems to connect each other (Fig. 14C). TEM images showed no cellular connections of the CMPs with other cells except the ICLCs (Figs. 2–4, 6, 7, 13), nerves (Figs. 12, 14) and other CMPs (Figs. 6A, 14C).
Discussion
This study allowed us to identify different types of cells in the subepicardium of adult mouse. Some of them were present everywhere beneath epicardium, while others were exclusively located nearby the origin of the coronary arteries and aorta in a CSCN. At this location, the cell types identified were cardiomyocytes with immature features (named CMPs), ICLCs, fibroblasts, adipocytes, macrophages, lymphocytes, mononuclear cells, mast cells, free smooth muscle cells, Schwann cells and nerve fibres. The CMPs showed different degrees of immaturity, some of them resembling mesenchymal cells (MSCs) or ICLCs and others mature cardiomyocytes. Interestingly, all of the immature cardiomyocytes have peculiar spatial relationships with the ICLCs.
The CMPs were clearly recognizable as belonging to the cardiomyocyte lineage due to their specific ultrastructural features. Indeed, all of them possess mitochondria with features identical to those of the mature cells and filaments arranged as in the developing cardiomyoblasts [74]. The lesser differentiated CMPs have few cisternae of rough endoplasmic reticulum, a discontinuous basal lamina and bundles of filaments free in the cytoplasm or attached to dense bodies (primordial Z lines). These cells resemble those described in the mouse heart at E9 [74]. The more differentiated cells having a continuous basal lamina, incomplete or complete intercalated discs and primordial sarcomeres resemble the cardiomyoblasts described in the mouse heart at E15 [74]. Moreover, in the cytoplasm of the more mature cells leptomeric fibrils are present [75]. In the adult heart, these structures have been observed associated with structural remodelling, myofibrillar genesis and in the surviving rat cardiomyocytes located in the perinecrotic zone of the infarct [76].
The presence of CMPs in the adult heart represents an important finding and confirms recent reports about the presence of a small pool of cardiac stem and progenitor cells in the adult mammalian myocardium, including mouse [69] and human beings [17]. We have found immature cardiac cells as well as ICLCs in a subepicardial location, this appearing not surprising since it is well known that during embryonic life cardiomyocytes directly origin from epicardium [74], interstitial stromal cells origin from mesenchymal epicardium-derived cells and, indeed, epicardial stem cells isolated and cultured can generate new cardiomyocytes [15, 17]. What we consider of particular importance is that adult mice CMPs are located at a specific site under the epicardium and close to the origin of the coronary arteries and aorta. This finding might help when searching for these cells in order to cultivate or to activate them for heart regeneration.
Recently, very important new insights strongly support the concept that the heart contains the stem cells grouped in niches, i.e.interstitial structures containing either cardio-stem cells or lineage-committed cells connected to supporting cells represented by mature cardiomyocytes and fibroblasts [18, 69]. Therefore, a CSCN requires the co-existence of two different types of cells, stem cells and supporting cells, both of which are necessary to heart renewing and, possibly, repair [68–71]. By junctional and adhesion proteins the cells from these niches are connected structurally and functionally to the pre-existing cardiomyocytes and fibroblasts [69], and the surrounding microenvironment and physical contacts define a niche and the diffusible factors involved in stem cell regulation are crucial for its integrity [70–73, 77].
Since the present study stresses the intercellular connections existing between CMPs and ICLCs, our hypothesis is that taken together these two cell types might be considered forming an actually CSCN. Indeed, the ICLCs accompany the immature cardiac cells all along their length, strictly apposed to them, and are anchored to the basal lamina of the more differentiated cells. When the CMPs are grouped to form a column, the basal lamina, always bordered by the ICLCs, envelops the column and continues at its two poles for a discrete tract. This organization results in a three-dimensional tunnel-like structure made by immature cells inside, a basal lamina around them and ICLCs on the external side. The resulting architecture has an impressive similarity to that found in the adult myocardium [53–59] and gut muscle wall [67]. This architecture is suggestive for an important influence played by the ICLCs on the CMP three-dimensional organization and interconnections and, possibly, also on cardiac repair. In this regard, implantation of MSCs seeded on small intestinal submucosa showed to improve the differentiation of MSCs in cardiomyocytes and smooth muscle cells and the cardiac repair in a rabbit chronic myocardial infarction model [78] thus stressing the importance of MSCs, we presume to be ICLCs in this case [67], in regenerative process. Besides it has been showed that adult serosal mesothelium retains much of its potential of differentiation, not only in the epicardium [79], but also in the gut [80–81], where we suppose that ICLCs [82] are involved.
CMPs are spread out around coronary artery, rather than forming a distinct structure that could be pointed as a niche in the subepicardial area – the cardiac stem cells niche is more likely an open area than an enclosed anatomical structure. This remark points towards the importance of the existence of intercellular communications. As an example, one special intercellular communication between ICLCs and CMPs occurs through shed microvesicles or exosomes (Fig. 11). Recent studies [71, 83–87] reported that microvesicles transfer tissue specific mRNA and proteins and by genetic modulation could determine differentiation and commitment of stem cells. This newly recognized system of intercellular communications seems to have a critical role in physiological and pathological processes and offers a new research direction for stem cell therapy [86, 87]. For all the above reasons we consider the ICLCs network a prerequisite to define an ‘informational space’ proper for cardiac stem cells survival and to control informational ‘packages’ delivered at a distance through microvesicles. Another interesting aspect suggests that ICLCs might be a source of the regenerative cardiomyocytes since (see Fig. 9) ICLC processes might be clearly seen enclosed in the basal lamina of a column. The existence of stromal cells with possible influence on the cardiomyocytes growth and differentiation was already proposed [15, 18, 26, 69, 77] but not yet clarified. A recent study showed that activated CMPs migrate from atrial niches not through coronary circulation but through the interstitium and it was supposed that this migration occurs within tunnels defined by fibronectin [88]. In our opinion, this influence in the adult heart should be the same as that followed during the embryological steps that succeed starting by the trabecular up to the com-pactation of the myocardium. Briefly, we suggest that the ICLCs network is the scaffold capable of guiding the differentiating stem cells whatever their origins are [89–103] and ICLCs are responsible for the secretion of the extracellular matrix molecules functioning as signalling routes necessary to maintain or to repair the myocardial architecture (Figs. 15, 16).
9.
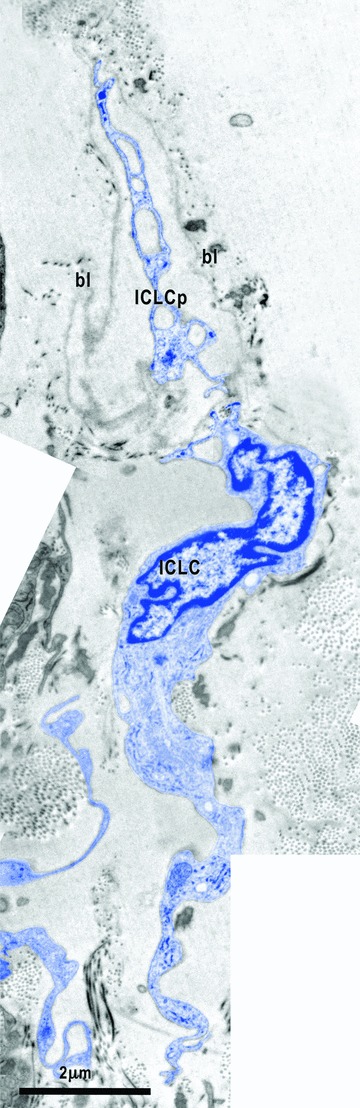
Electron micrograph illustrates the relationships of one ICLC (blue) with the basal lamina (bl). In this micrograph, one ICLC process (ICLCp) with a lacunar cytoplasm seems to be embedded in the space delimited by a continuous basal lamina.
15.
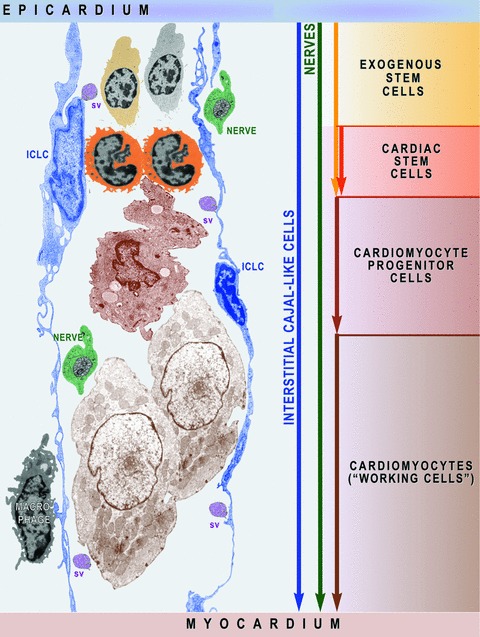
Schematic representation (based on electron micrographs from Figs. 3A inset, 4C, 6A, 12B, 14B) shows that ICLC network is a prerequisite for myocardial cellular homeostasis shaping the scaffold required to activate exogenous and endogenous stem cells and to guide cardiomyocyte progenitors’ migration. Myocardial ICLCs communicate at distance with nurse ICLCs from epicardial niches and deliver information ‘packages’ via shed vesicles (sv). Macrophages and nerves assist the ICLCs to monitor the cardiac renewing.
16.
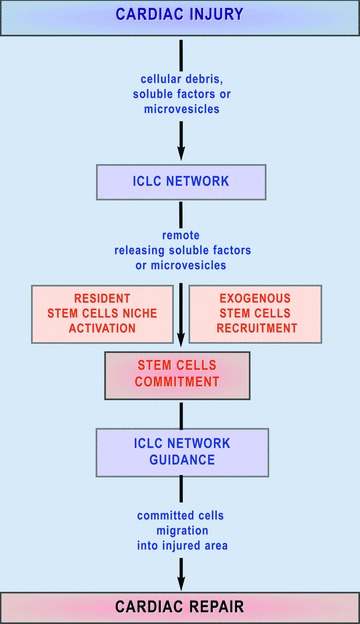
Mechanism proposed for the ICLC involvement in myocardial repair. A cardiac injury could determine a remote cellular reaction in cardiac stem cell niches or could initiate the recruitment of the exogenous adult stem cells via the ICLC wide network. Committed stem cells are guided to migrate into the injured tissue under paracrine signals released by the ICLC.
In summary, we provide here for the first time the ultra-structural description of the CSCNs containing resident CMPs and ICLCs in the adult mice myocardium and prove out of doubt their reality. More than that, the existence in the adult of CMPs in different stages of development proves that cardiac renewing is a continuous process. Here we provide a detailed description of the complex hetero-cellular interactions between CMPs and ICLCs. We can also confirm that the ICLCs make a three-dimensional network in the interstitium of atria, ventricles and epicardium of the adult heart. The CSCNs, that are rare and probably located in strategic areas of the heart, might be integrated in the heart architecture through this wide ICLCs network. A possible reason for an ineffective regeneration of the myocardium in the injuries affecting large areas is that the ICLCs supporting network is broken or the ICLCs have changed their phenotype.
In conclusion, the ICLCs could be not only genuine nurse cells essential for control of stem cells maintenance and lineage commitment, but also be responsible for regulatory mechanisms that control the endogenous or exogenous stem cell activation and a support for the progenitors migration towards a damaged region of the heart. The hope now is to have a successful cell therapy leading the CSCNs to restore the myocardium, and the ICLC, being a key local player, must be taken into consideration in the regenerative cardiology.
References
- 1.Zhou B, Pu WT. More than a cover: epicardium as a novel source of cardiac progenitor cells. Regen Med. 2008;3:633–5. doi: 10.2217/17460751.3.5.633. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Puig S, Wang Z, Chien KR. Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell. 2008;2:320–31. doi: 10.1016/j.stem.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Van Tuyn J, Atsma DE, Winter EM, Van Der Velde-van Dijke I, Pijnappels DA, Bax NA, Knaän-Shanzer S, Gittenberger-de Groot AC, Poelmann RE, Van Der Laarse A, Van Der Wall EE, Schalij MJ, De Vries AA. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells. 2007;25:271–8. doi: 10.1634/stemcells.2006-0366. [DOI] [PubMed] [Google Scholar]
- 4.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages. Development. 2008;135:193–205. doi: 10.1242/dev.001883. [DOI] [PubMed] [Google Scholar]
- 6.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M, Loiaconi V, Pompilio G, Germani A, Capogrossi MC. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–65. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 8.Riley PR. The adult epicardium: realizing the potential for neovascular therapy. Arterioscler Thromb Vasc Biol. 2008;28:803–4. doi: 10.1161/ATVBAHA.108.165191. [DOI] [PubMed] [Google Scholar]
- 9.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–82. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, Von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smart N, Riley PR. Derivation of epicardium-derived progenitor cells (EPDCs) from adult epicardium. Curr Protoc Stem Cell Biol. 2009 doi: 10.1002/9780470151808.sc02c02s8. doi: DOI: 10.1002/9780470151808.sc02c02s8. [DOI] [PubMed] [Google Scholar]
- 12.Sucov HM, Gu Y, Thomas S, Li P, Pashmforoush M. Epicardial control of myocardial proliferation and morphogenesis. Pediatr Cardiol. 2009 doi: 10.1007/s00246-009-9391-8. doi: DOI: 10.1007/s00246-009-9391-8. [DOI] [PubMed] [Google Scholar]
- 13.Wessels A, PÈrez-Pomares JM. The epi-cardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 14.Winter EM, Gittenberger-de Groot AC. Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci. 2007;64:692–703. doi: 10.1007/s00018-007-6522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lie-Venema H, Van Den Akker NM, Bax NA, Winter EM, Maas S, Kekarainen T, Hoeben RC, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. Sci World J. 2007;7:1777–98. doi: 10.1100/tsw.2007.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–81. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 17.Winter EM, Grauss RW, Hogers B, Van Tuyn J, Van Der Geest R, Lie-Venema H, Steijn RV, Maas S, DeRuiter MC, DeVries AA, Steendijk P, Doevendans PA, Van Der Laarse A, Poelmann RE, Schalij MJ, Atsma DE, Gittenberger-de Groot AC. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse. Circulation. 2007;116:917–27. doi: 10.1161/CIRCULATIONAHA.106.668178. [DOI] [PubMed] [Google Scholar]
- 18.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Cli Pract Cardiovasc Med. 2007;4:S21–6. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 19.Kumar D, Kamp TJ, LeWinter MM. Embryonic stem cells: differentiation into cardiomyocytes and potential for heart repair and regeneration. Coron Artery Dis. 2005;16:111–6. doi: 10.1097/00019501-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Synnergren J, Akesson K, Dahlenborg K, Vidarsson H, Améen C, Steel D, Lindahl A, Olsson B, Sartipy P. Molecular signature of cardiomyocyte clusters derived from human embryonic stem cells. Stem Cells. 2008;26:1831–40. doi: 10.1634/stemcells.2007-1033. [DOI] [PubMed] [Google Scholar]
- 21.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–63. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 22.Habib M, Caspi O, Gepstein L. Human embryonic stem cells for cardiomyogene-sis. J Mol Cell Cardiol. 2008;45:462–74. doi: 10.1016/j.yjmcc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marín-García J, Goldenthal MJ. Application of stem cells in cardiology: where we are and where we are going. Curr Stem Cell Res Ther. 2006;1:1–11. doi: 10.2174/157488806775269052. [DOI] [PubMed] [Google Scholar]
- 25.Perino MG, Yamanaka S, Li J, Wobus AM, Boheler KR. Cardiomyogenic stem and progenitor cell plasticity and the dissection of cardiopoiesis. J Mol Cell Cardiol. 2008;45:475–94. doi: 10.1016/j.yjmcc.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, Van Den Brink S, Hassink R, Van Der Heyden M, Opthof T, Pera M, De La Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 27.Leri A, Hosoda T, Rota M, Kajstura J, Anversa P. Myocardial regeneration by exogenous and endogenous progenitor cells. Drug Discov Today Dis Mech. 2007;4:197–203. doi: 10.1016/j.ddmec.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubart R, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 29.Dowell JD, Rubart M, Pasumarthi KBS, Soonpaa MH, Field LJ. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336–50. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 30.Formigli L, Perna AM, Meacci E, Cinci L, Margheri M, Nistri S, Tani A, Silvertown J, Orlandini G, Porciani C, Zecchi-Orlandini S, Medin J, Bani D. Paracrine effects of transplanted myoblasts and relaxin on post-infarction heart remodeling. J Cell Mol Med. 2007;11:1087–110. doi: 10.1111/j.1582-4934.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelacho B, Aranguren XL, Mazo M, Abizanda G, Gavira JJ, Clavel C, Gutierrez-Perez M, Luttun A, Verfaillie CM, Prósper F. Plasticity and cardiovascular applications of multipotent adult progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4:S15–20. doi: 10.1038/ncpcardio0735. [DOI] [PubMed] [Google Scholar]
- 32.Wojakowski W, Kucia M, KaŸmierski M, Ratajczak MZ, Tendera M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: relevant reparatory mechanism. Heart. 2008;94:27–33. doi: 10.1136/hrt.2006.103358. [DOI] [PubMed] [Google Scholar]
- 33.Clavel C, Verfaillie CM. Bone-marrow-derived cells and heart repair. Curr Opin Organ Transplant. 2008;13:36–43. doi: 10.1097/MOT.0b013e3282f428d1. [DOI] [PubMed] [Google Scholar]
- 34.Liao R, Pfister O, Jain M, Mouquet F. The bone marrow–cardiac axis of myocardial regeneration. Prog Cardiovasc Dis. 2007;50:18–30. doi: 10.1016/j.pcad.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartunek J, Vanderheyden M, Wijns W, Timmermans F, Vandekerkhove B, Villa A, Sánchez PL, Arnold R, San Román JA, Heyndrickx G, Fernandez-Aviles F. Bone-marrow-derived cells for cardiac stem cell therapy: safe or still under scrutiny? Nat Clin Pract Cardiovasc Med. 2007;4:S100–5. doi: 10.1038/ncpcardio0744. [DOI] [PubMed] [Google Scholar]
- 36.Barile L, Messina E, Giacomello A, Marbán E. Endogenous cardiac stem cells. Prog Cardiovasc Dis. 2007;50:31–48. doi: 10.1016/j.pcad.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 38.Ott HC, Matthiesen TS, Brechtken J, Grindle S, Goh SK, Nelson W, Taylor DA. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4:S27–39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 39.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang F, Pasumarthi KB. Ultrastructural and immunocharacterization of undifferentiated myocardial cells in the developing mouse heart. J Cell Mol Med. 2007;11:552–60. doi: 10.1111/j.1582-4934.2007.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–63. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 42.Torella D, Ellison GM, Méndez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3:S8–13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 43.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miiller P, Beltrami AP, Cesselli D, Pfeiffer P, Kazakov A, Böhm M. Myocardial regeneration by endogenous adult progenitor cells. J Mol Cell Cardiol. 2005;39:377–87. doi: 10.1016/j.yjmcc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 47.Scobioala S, Klocke R, Kuhlmann M, Tian W, Hasib L, Milting H, Koenig S, Stelljes M, El-Banayosy A, Tenderich G, Michel G, Breithardt G, Nikol S. Up-regulation of nestin in the infarcted myocardium potentially indicates differentiation of resident cardiac stem cells into various lineages including cardiomyocytes. FASEB J. 2008;22:1021–31. doi: 10.1096/fj.07-8252com. [DOI] [PubMed] [Google Scholar]
- 48.Menasche P. Cell-based therapy for heart disease: a clinically oriented perspective. Mol Ther. 2009 doi: 10.1038/mt.2009.40. doi:DOI: 10.1038/mt.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perin EC, Silva GV. Autologous cell-based therapy for ischemic heart disease: clinical evidence, proposed mechanisms of action, and current limitations. Catheter Cardiovasc Interv. 2009;73:281–8. doi: 10.1002/ccd.21807. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg JR, Pearl J, Lee A, Fischbein M, Contag CH, Robbins RC, Wu JC. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–9. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor DA, Zenovich AG. Cardiovascular cell therapy and endogenous repair. Diabetes Obes Metab. 2008;10:5–15. doi: 10.1111/j.1463-1326.2008.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hare JM, Chaparro SV. Cardiac regeneration and stem cell therapy. Curr Opin Organ Transplant. 2008;13:536–42. doi: 10.1097/MOT.0b013e32830fdfc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hinescu ME, Popescu LM. Interstitial Cajallike cells (ICLC) in human atrial myocardium. J Cell Mol Med. 2005;9:972–5. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinescu ME, Gherghiceanu M, Mandache E, Ciontea SM, Popescu LM. Interstitial Cajal-like cells (ICLC) in atrial myocardium: ultrastructural and immuno-histochemical characterization. J Cell Mol Med. 2006;10:243–57. doi: 10.1111/j.1582-4934.2006.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popescu LM, Gherghiceanu M, Hinescu ME, Cretoiu D, Ceafalan L, Regalia T, Popescu AC, Ardeleanu C, Mandache E. Insights into the interstitium of ventricular myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–58. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandache E, Popescu LM, Gherghiceanu M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: shed vesicles as intermediates. J Cell Mol Med. 2007;11:1175–84. doi: 10.1111/j.1582-4934.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gherghiceanu M, Hinescu ME, Andrei F, Mandache E, Macarie CE, Faussone-Pellegrini MS, Popescu LM. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. 2008;12:1777–81. doi: 10.1111/j.1582-4934.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gherghiceanu M, Hinescu ME, Popescu LM. Myocardial interstitial Cajal-like cells (ICLC) in caveolin-1 KO mice. J Cell Mol Med. 2009;13:202–6. doi: 10.1111/j.1582-4934.2008.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial cajal-like cells (ICLC) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suciu L, Popescu LM, Regalia T, Ardelean A, Manole CG. Epicardium: Interstitial Cajal-like cells (ICLC) highlighted by immunofluorescence. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00756.x. doi: DOI: 10.1111/j.1582-4934.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faussone-Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Microsc Res Tech. 1999;47:248–66. doi: 10.1002/(SICI)1097-0029(19991115)47:4<248::AID-JEMT4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 62.Rumessen JJ, Vanderwinden JM. Interstitial cells in the musculature of the gastrointestinal tract: Cajal and beyond. Int Rev Cytol. 2003;229:115–208. doi: 10.1016/s0074-7696(03)29004-5. [DOI] [PubMed] [Google Scholar]
- 63.Faussone-Pellegrini MS. Interstitial cells of Cajal: once negligible players, now blazing protagonists. Ital J Anat Embryol. 2005;110:11–31. [PubMed] [Google Scholar]
- 64.Sanders KM, Ward SM. Interstitial cells of Cajal: a new perspective on smooth muscle function. J Physiol. 2006;576:721–6. doi: 10.1113/jphysiol.2006.115279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 66.Sarna SK. Are interstitial cells of Cajal plurifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol. 2008;294:G372–90. doi: 10.1152/ajpgi.00344.2007. [DOI] [PubMed] [Google Scholar]
- 67.Pieri L, Vannucchi MG, Faussone-Pellegrini MS. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J Cell Mol Med. 2008;12:1944–55. doi: 10.1111/j.1582-4934.2008.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 69.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2009;217:169–80. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 71.Quesenberry PJ, Aliotta JM. The paradoxical dynamism of marrow stem cells: considerations of stem cells, niches, and microvesicles. Stem Cell Rev. 2008;4:137–47. doi: 10.1007/s12015-008-9036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drummond-Barbosa D. Stem cells, their niches and the systemic environment: an aging network. Genetics. 2008;180:1787–97. doi: 10.1534/genetics.108.098244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol. 2008;19:534–40. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Challice CE, Viragh S. The embryologic development of the mammalian heart. In: Dalton AJ, Haguenau F, editors. Ultrastructure of mammalian heart. Vol. 6. New York: Acad. Press; 1973. pp. 91–126. [Google Scholar]
- 75.Arbustini E, Ferrans V, David H, Ghadially FN. Leptofibrils in cardiac myocytes. Ultrastruct Pathol. 1988;12:251–4. doi: 10.3109/01913128809058224. [DOI] [PubMed] [Google Scholar]
- 76.Dedkov EI, Stadnikov AA, Russell MW, Borisov AB. Formation of leptofibrils is associated with remodeling of muscle cells and myofibrillogenesis in the border zone of myocardial infarction. Micron. 2007;38:659–67. doi: 10.1016/j.micron.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 77.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:15529–34. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Tan MY, Zhi W, Wei RQ, Huang YC, Zhou KP, Tan B, Deng L, Luo JC, Li XQ, Xie HQ, Yang ZM. Repair of infarcted myocardium using mesenchymal stem cell seeded small intestinal submucosa in rabbits. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.02.013. doi:DOI: 10.1016/j.biomaterials.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Nesselmann C, Ma N, Bieback K, Wagner W, Ho A, Konttinen YT, Zhang H, Hinescu ME, Steinhoff G. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008;12:1795–810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawaguchi M, Bader DM, Wilm B. Serosal mesothelium retains vasculogenic potential. Dev Dyn. 2007;236:2973–9. doi: 10.1002/dvdy.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–28. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 82.Hinescu ME, Popescu LM, Gherghiceanu M, Faussone-Pellegrini MS. Interstitial Cajal-like cells in rat mesentery: an ultrastructural and immunohistochemical approach. J Cell Mol Med. 2008;12:260–70. doi: 10.1111/j.1582-4934.2008.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, Farber DB. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11:303–11. doi: 10.1038/ncb1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skog J, Würdinger T, Van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stastna M, Abraham MR, Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.03.026. doi:DOI: 10.1016/j.febslet.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Müller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 89.Smits AM, Van Vliet P, Hassink RJ, Goumans MJ, Doevendans PA. The role of stem cells in cardiac regeneration. J Cell Mol Med. 2005;9:25–36. doi: 10.1111/j.1582-4934.2005.tb00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith TK, Bader DM. Signals from both sides: Control of cardiac development by the endocardium and epicardium. Semin Cell Dev Biol. 2007;18:84–9. doi: 10.1016/j.semcdb.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lyon A, Harding S. The potential of cardiac stem cell therapy for heart failure. Curr Opin Pharmacol. 2007;7:164–70. doi: 10.1016/j.coph.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Ballard VL, Edelberg JM. Stem cells and the regeneration of the aging cardiovascular system. Circ Res. 2007;100:1116–27. doi: 10.1161/01.RES.0000261964.19115.e3. [DOI] [PubMed] [Google Scholar]
- 93.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, Kajstura J, Bolli R. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 94.Penn MS. Cell-based gene therapy for the prevention and treatment of cardiac dysfunction. Nat Clin Pract Cardiovasc Med. 2007;4:S83–8. doi: 10.1038/ncpcardio0733. [DOI] [PubMed] [Google Scholar]
- 95.Evans SM, Mummery C, Doevendans PA. Progenitor cells for cardiac repair. Semin Cell Dev Biol. 2007;18:153–60. doi: 10.1016/j.semcdb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Leri A, Kajstura J, Anversa P, Frishman WH. Myocardial regeneration and stem cell repair. Curr Probl Cardiol. 2008;33:91–153. doi: 10.1016/j.cpcardiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 97.Kajstura J, Urbanek K, Rota M, Bearzi C, Hosoda T, Bolli R, Anversa P, Leri A. Cardiac stem cells and myocardial disease. J Mol Cell Cardiol. 2008;45:505–13. doi: 10.1016/j.yjmcc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 98.Roccio M, Goumans MJ, Sluijter JP, Doevendans PA. Stem cell sources for cardiac regeneration. Panminerva Med. 2008;50:19–30. [PubMed] [Google Scholar]
- 99.Smart N, Riley PR. The stem cell movement. Circ Res. 2008;102:1155–68. doi: 10.1161/CIRCRESAHA.108.175158. [DOI] [PubMed] [Google Scholar]
- 100.Ugurlucan M, Yerebakan C, Furlani D, Ma N, Steinhoff G. Cell sources for cardiovascular tissue regeneration and engineering. Thorac Cardiovasc Surg. 2009;57:63–73. doi: 10.1055/s-2008-1039235. [DOI] [PubMed] [Google Scholar]
- 101.Noort WA, Sluijter JP, Goumans MJ, Chamuleau SA, Doevendans PA. Stem cells from in- or outside of the heart: isolation, characterization, and potential for myocardial tissue regeneration. Pediatr Cardiol. 2009 doi: 10.1007/s00246-008-9370-5. doi: DOI: 10.1007/s00246-008-9370-5. [DOI] [PubMed] [Google Scholar]
- 102.Lyngbaek S, Schneider M, Hansen JL, Sheikh SP. Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Res Cardiol. 2007;102:101–14. doi: 10.1007/s00395-007-0638-3. [DOI] [PubMed] [Google Scholar]
- 103.Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, Wu JC. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53:1229–40. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


