Abstract
Nausea and vomiting are among the most common symptoms encountered in medicine as either symptoms of disease or side effects of treatments. Developing novel anti-emetics and identifying emetic liability in novel chemical entities rely on models that can recreate the complexity of these multi-system reflexes. Animal models (especially the ferret and dog) are the current gold standard; however, the selection of appropriate models is still a matter of debate, especially when studying the subjective human sensation of nausea. Furthermore, these studies are associated with animal suffering. Here, following a recent workshop held to review the utility of animal models in nausea and vomiting research, we discuss the limitations of some of the current models in the context of basic research, anti-emetic development and emetic liability detection. We provide suggestions for how these limitations may be overcome using non-animal alternatives, including greater use of human volunteers, in silico and in vitro techniques and lower organisms.
Keywords: C. elegans, conditioned taste aversion, rat, ferret, nausea, pica, vomiting, replacement, 3Rs, NC3Rs
Introduction
Nausea (an unpleasant sensation often associated with the urge to vomit) and vomiting (the forceful oral expulsion of upper gastrointestinal tract contents) are commonly encountered either separately or together as symptoms of diverse diseases (e.g. advanced cancer, cyclic vomiting syndrome, epilepsy, functional dyspepsia, gastroparesis, migraine, raised intra-cranial pressure), systemic (e.g. meningitis) and gastrointestinal (e.g. rotavirus, norovirus, Bacillus cereus) infections, pregnancy (both pregnancy sickness and hyperemesis gravidarum) and exposure to some forms of motion (e.g. parabolic flights) and vection (e.g. illusory self-motion) (Andrews and Horn, 2006). Nausea and vomiting can also be undesirable effects of treatments such as radiotherapy, procedures such as anaesthesia and surgery [post-operative nausea and vomiting (PONV)], and drug treatments. One of the most marked examples of treatment-induced nausea and vomiting is that induced by cytotoxic agents (e.g. cisplatin, cyclophosphamide) used in the treatment of cancer which begins within a few hours of administration (acute phase) and can persist for many days (delayed or protracted phase) after drug administration. This can lead some individuals to become averse to, and avoid further treatment by the induction of anticipatory nausea and vomiting (ANV) whereby patients develop symptoms in anticipation of subsequent cycles of chemotherapy (Rudd and Andrews, 2004).
The clinical need to develop efficacious anti-emetic strategies to deal with the nausea and vomiting associated with chemo- and radiotherapy was one of the major drivers of the resurgence of interest in the basic and applied neuropharmacology of nausea and vomiting in the early 1980s following from the ‘classical’ studies of the emetic reflex in the 1950s by Borison, Wang, McCarthy and Brizzee (Davis, 1995 for historical review). When it was first used the cytotoxic agent cisplatin, while being highly effective against many tumours was associated with particularly intense vomiting which was resistant to anti-emetics available at that time. In patients not receiving anti-emetics the incidence of emesis with high dose cisplatin was 98% in the acute phase and between 44% and 89% in the delayed phase (Gandara et al., 1993; Kris et al., 1985; 1996). Attempts to understand the neuropharmacology of cisplatin-induced vomiting using the ferret model (see below) played a key role in the identification of selective 5-hydroxytryptamine3 (5-HT3) receptor antagonists and arguably tachykinin neurokinin1 (NK1) receptor antagonists (Christie and Tansey, 2007), examples of which are in clinical use. While both 5-HT3 and NK1 receptor antagonists have had a major impact upon patients experience of chemotherapy they do not completely block nausea and vomiting in all patients and nausea is less well treated than vomiting (Hesketh, 2008). Similarly, treatment of PONV has improved but is not optimal (Ho and Gan, 2006). The lack of an anti-emetic that would be efficacious in all clinical (e.g. cyclic vomiting syndrome) and other (e.g. motion sickness) settings against both nausea and vomiting argues that further research is required in this area and raises the issue of which model(s) (animal or otherwise) is most appropriate for such studies.
Nausea and vomiting are also frequently encountered during the identification and development of drugs for a range of diseases. A few examples in the public domain illustrate the problem: PDE4 inhibitors are promising agents for the treatment of asthma but nausea is a dose limiting side effect which remains poorly understood (Spina, 2008); type 2 diabetes can be treated with metformin but it is associated with a high incidence (∼30%) of gastrointestinal side effects, including nausea (Hoffmann et al., 2003); GLP-1 receptor agonists are a promising treatment for type 2 diabetes but can induce nausea and more rarely vomiting (Nauck and Meier, 2005); rimonabant, a cannabinoid receptor antagonist for the treatment of obesity, induces dose-dependent nausea in clinical studies (Pi-Sunyer et al., 2006) and a different CB1 receptor antagonist (AM251) has been shown to enhance the emetic response in the ferret (Van Sickle et al., 2001); and nicotinic receptor agonists are being developed for the treatment of pain and cognitive dysfunction, but nicotinic receptor agonists have a potential to induce nausea and vomiting (Chin et al., 2006) but it may be possible to reduce this by increased selectivity at the α4β2nicotinic receptor (Ji et al., 2007).
Emetic liability can play a major role in delaying or even preventing clinical development (see below). Even for marketed drugs nausea and vomiting are common side effects with the electronic Medicines Compendium identifying that >50% of drugs in current use have nausea as a side effect and >33% have both nausea and vomiting (cited in Lee, 2007) and this may affect patient compliance with treatment. Despite this however, nausea and vomiting are still not automatically considered when assessing preclinical gastrointestinal tract models or techniques for use in safety pharmacology (Harrison et al., 2004).
There is a continuing requirement to develop novel anti-emetic agents for the treatment of nausea and vomiting in diverse clinical settings and to understand the mechanism(s) by which current and potential drugs [novel chemical entities (NCE)] induce nausea and vomiting, so that such effects can be reduced or preferably avoided. If nausea and vomiting cannot be prevented as the mechanism of action is intrinsically emetic, then this may lead to discontinuation of the development of the drug or necessitate concomitant administration of anti-emetic agents.
Developments in understanding the neurophysiology and pharmacology of vomiting and the identification of novel anti-emetic agents have come from experiments using animals and demonstrate that the animal models used have predictive value. However, these studies by their very nature are associated with animal suffering involving protracted episodes of retching and vomiting, for example over several days in delayed cisplatin-induced vomiting studies which mimic the pattern in patients. Protracted vomiting can lead to reduced food intake, weight loss, dehydration and metabolic disturbances. It is appropriate that we should explore application of the ‘3Rs’[replacement, reduction and refinement (see Box 1); Russell and Burch, 1959] to research in this area and in particular whether replacement is a realistic option in any area of research involving a multi-system reflex.
Box 1 The 3Rs
Replacement – methods which avoid or replace the use of animals in research that has the potential to cause them harm.
Reduction – methods which minimize animal use and enable researchers to obtain comparable levels of information from fewer animals or to obtain more information from the same number of animals.
Refinement – improvements to husbandry and procedures which minimize pain, suffering, distress or lasting harm and/or improve animal welfare in situations where the use of animals is unavoidable.
The UK's National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) held a workshop in July 2007 to discuss with experts from academia, industry and regulatory bodies the issues relating to the use of animals in nausea and vomiting research and to identify opportunities for the implementation of the 3Rs. Although the focus of the workshop was nausea and vomiting, there is considerable overlap with other multi-system reflexes (e.g. cough, gastroesophageal reflux, belching and cardiovascular reflexes) and it may be possible to apply the recommendations and outcomes from the meeting to these. A similar approach has been used successfully in a previous NC3Rs initiative examining the use of non-human primates in the development of monoclonal antibodies (Chapman et al., 2007).
This paper outlines some of the issues discussed at the workshop by reviewing aspects of the physiology of vomiting and some of the current animal models used to develop anti-emetics and to identify emetic liability. The final section proposes some potential approaches to replacing animals including studies in humans.
Understanding and modelling the anatomical and physiological complexity of nausea and vomiting
To identify opportunities for the replacement of the use of animals it is necessary to outline the basic physiology and pharmacology of vomiting in mammals. The three components which would need to be modelled are (i) the motor output events, (ii) the input signals and (iii) how these signals and the corresponding outputs are integrated. Detailed reviews of the neurophysiology and mechanics of the emetic reflex have been discussed by others (Hornby, 2001; Fukuda et al., 2003).
Motor outputs
The major motor events in retching and vomiting in mammals involve the anterior abdominal muscles, the diaphragm and the gastrointestinal tract (Figure 1). Prior to the onset of retching the proximal stomach relaxes primarily under the influence of vagal efferent neurones, the gastric antrum is presumed to become quiescent and a retrograde giant contraction, originating in the small intestine and mediated by vagal efferents, runs to the stomach taking some contents with it. These events are preparatory and do not in themselves lead directly to expulsion of material from the stomach. Retching and vomiting both involve the coordinated contraction of the diaphragm, but during vomiting the crural (peri-oesophageal) diaphragm becomes quiescent. The diaphragm is under the control of the phrenic nerve and the abdominal muscles are controlled by spinal motor neurones.
Figure 1.
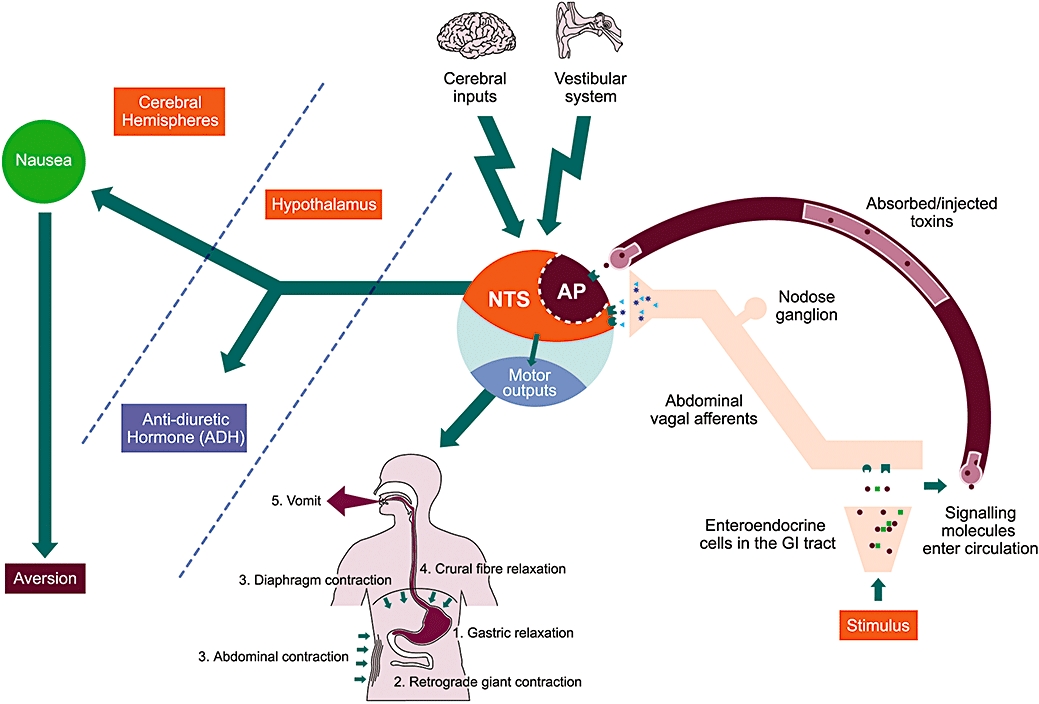
Remodelling the complexity of nausea and vomiting. Vomiting is a consequence of the integration of a number of input signals and the coordination of a number of motor outputs within the brainstem. Inputs from the gastrointestinal tract via abdominal vagal afferents, the circulation via the area postrema (AP), the vestibular system (motion sickness) and the higher cerebral regions (fear-induced and anticipatory vomiting) are all integrated by the nucleus tractus solitarius (NTS) within the dorsal brainstem. The subsequent autonomic and somatic motor outputs arise from nuclei in both the dorsal (e.g. dorsal vagal motor nucleus) and ventral (e.g. nucleus ambiguous, pre-sympathetic, Botzinger complex) brainstem and occur in sequential order. (1) Gastric relaxation and (2) retrograde giant contraction via the vagus; (3 and 4) contraction of the anterior abdominal muscles via spinal motorneurones and the diaphragm via the phrenic nerve; and finally (5) opening of the mouth to allow oral expulsion. A major difference between retching and vomiting is the relaxation of the crural diaphragm during the latter to facilitate evacuation of gastric contents. Further outputs include increased anti-diuretic hormone (ADH, vasopressin) release from the posterior pituitary and the induction of the sensation of nausea, presumably via the cerebral hemispheres.
Inputs
Vomiting is a reflex motor response that can be induced by activation of four main inputs to the brainstem regions which integrate the inputs [primarily the nucleus tractus solitarius (NTS)] and coordinate the motor outputs (Figure 1).
Abdominal vagal afferents supplying the stomach and upper part of the small intestine are the ones most implicated in vomiting with both mechanoreceptors (primarily distension) and mucosal chemoreceptors (e.g. hypertonic solutions) capable of inducing vomiting (Andrews et al., 1990; 1996). The mechanism by which the mucosal receptors transduce the signal is a topic of particular interest as the anatomical substrate is a mucosal enteroendocrine cell which releases a signal molecule to activate a receptor on the vagal afferent terminating in close proximity; and which subsequently causes the release of different signal molecules centrally. The best characterized example is the 5-hydroxytryptamine (5-HT) containing enterochromaffin cell which has been implicated in the mechanism by which chemotherapeutic agents induce vomiting (see Andrews and Rudd, 2004 for review). Any orally administered medication could in theory interact with these mucosal enteroendocrine cells and induce nausea and vomiting prior to absorption into the blood. In general, there is a relative paucity of information about the regulation of signal molecule release from the enteroendocrine cells and this currently limits the potential to use such cells as an in vitro assay of emetic liability.
The area postrema is often called ‘the chemoreceptor trigger zone for vomiting’ although it is present in species which do not vomit (Leslie, 1986). In this region both the blood-brain and CSF-brain barriers are relatively permeable making it an ideal brain site from which to detect chemicals in the blood and CSF (ibid.). It is likely that even drugs described as ‘non-brain penetrant’ can access the area postrema. There is consistent evidence across species that systemic apomorphine and probably morphine acts at the area postrema to induce vomiting although the structural elements (e.g. neurones, glia) upon which these agonists act to induce emesis is still unclear. Clearly, binding of an NCE to the area postrema would indicate a potential emetic liability and magnetic resonance imaging (MRI) techniques are now sufficiently advanced to allow distinction of the area postrema and NTS activation by apomorphine and a nicotinic receptor agonist in awake rats (Chin et al., 2006).
The vestibular system is essential for the induction of motion sickness (Yates et al., 1998; Golding and Gresty, 2005). The vestibular nuclei send projections to all the major brainstem nuclei which have been implicated in coordinating the visceral and somatic components of the vomiting reflex. Although the vestibular system itself is not considered to be a major target at which drugs are likely to act to induce vomiting, agents acting to modulate transmission in the vestibular nuclei could have such a liability.
Higher brain inputs are very poorly characterized in contrast to the other inputs but are responsible for phenomena such as the ANV associated with anti-cancer chemotherapy, as well as the immediate vomiting which may be induced by an horrific sight or repulsive smell and the vomiting which occurs as an icteral sign of temporal lobe epilepsy. ANV associated with anti-cancer chemotherapy is considered to be an example of ‘classical (Pavlovian) conditioning’ with the emetic stimulus being the unconditioned stimulus. Krylov, working in Pavlov's laboratory, recognized that the emetic reflex was particularly sensitive to conditioning in dogs (Pavlov, 1960) and although the ferret does not appear to share this sensitivity (Andrews et al., 1990) conditioned emetic-related behaviours have been reported in the rat and Suncus (Parker and Limebeer, 2006). Context aversion conditioning has been used in the rat to investigate potential behavioural interventions to treat anticipatory nausea (Hall and Symonds, 2006). However, understanding these ‘higher’ inputs would appear to be more appropriately performed in humans than animals as both invasive (deep brain stimulation) and non-invasive (magnetic induction) techniques are available for use in humans and brain imaging techniques can also be applied in both experimental and clinical settings (see below).
Integration
Two aspects need to be considered under this heading: integration of the inputs and coordination of the motor outputs. The key structure involved in integration of the emetic inputs is the medial NTS in the dorsal brainstem (Figure 1); the complexity of processing in the NTS is illustrated by the estimation that the rat NTS has 1 million synapses (Andresen and Kunze, 1994). In addition to being involved in the emetic reflex, the NTS is critically involved in a number of respiratory, cardiovascular and gastrointestinal system reflexes and it is also the primary site for processing gustatory information (Loewy, 1990). Overall it is considered to be the major integrative nucleus for visceral information.
The integrative processes within the NTS are not well understood for vomiting and the detailed pathways and processes involved in coordinating the output motor pathways are still under investigation (see Fukuda et al., 2003; Onishi et al., 2007). Much of what is known is from fictive vomiting in decerebrate-paralysed dogs and cats but recently similar studies in the ferret have led to the conclusion that these circuits are common to all three species (Onishi et al., 2007). It is likely that these brainstem pathways are conserved in humans but it is not known whether the neurotransmitters/co-transmitters and receptor types and distribution are the same in the animal species as in humans at each site in the pathway. The pivotal nature of the NTS and the proposed ‘central pattern generator for vomiting’ mean that these structures are also potential sites at which agents that penetrate the blood-brain barrier could act to induce vomiting (Sanger and Andrews, 2006). There is some evidence that the blood-brain barrier may be ‘leaky’ in the region of the NTS (Gross et al., 1990) and also that dendrites from NTS neurones extend into the area postrema (Morest, 1960) providing further routes by which systemic agents could influence this structure to induce emesis.
Nausea
Knowledge of the physiology and pharmacology of vomiting is relatively detailed but the same cannot be said of nausea. There are a number of reasons for this:
There is considerable debate over what sensory experience an animal may have in comparison to humans when exposed to the same stimulus.
If it is accepted that at least the ‘higher’ mammals do experience an analogous sensation with the same function as in humans (i.e. aversion and avoidance) then it raises the question of how can it be measured effectively and accurately? There are no agreed criteria for identification of nausea in animals (cf. pain, Mayer, 2007), and this may be an unidentified welfare issue (Smith and Jennings, 2004). In the context of discussing analogies between humans and animals Russell and Burch (1959) comment ‘Now nausea is a thoroughly distressing state in man, and by human analogy we might well suppose it to be so in pigeons’.
Relatively little is known about the mechanism of nausea in humans with which to compare any animal data. An elevation of plasma vasopressin (AVP, ADH) and changes in the frequency and rhythm of the electrical activity of the stomach have both been shown to be associated with the presence of nausea in humans but their mechanistic relationship is not clear (Koch et al., 1990a,b; Andrews and Horn, 2006). However, both biomarkers can be utilized in studies of experimental animals (see below). The prevailing view is that nausea can be induced by lower levels of activation of the same inputs as were described above for vomiting, although paradoxically it is harder to treat than vomiting (Sanger and Andrews, 2006). Genesis of the sensation of nausea is presumed to require rostral projection of information from the brainstem to the ‘higher’ areas of the brain involved in conscious sensations and learned aversions; but this has not been the subject of detailed study in humans. This is in contrast to studies of central pain pathways including those involved in visceral pain (Borsook and Becerra, 2006; Van Oudenhove et al., 2007). The only imaging study of experimentally induced (ingested syrup of ipecac and vestibular stimulation) nausea of which the authors are aware used magnetic source imaging and demonstrated activation of the inferior frontal gyrus (Miller et al., 1996). Further brain imaging studies of pathways involved in nausea (and vomiting) are needed so that comparisons can be made with animal studies in which Fos immunohistochemistry has been used to investigate brain regions activated by emetic stimuli (e.g. Horn et al., 2007). This will allow assessment of the relevance of such studies to understanding central pathways for nausea and vomiting in humans.
As humans are the only species in which it is possible to have some degree of certainty about the sensory component of nausea it could be argued that humans are the most relevant species in which to investigate nausea and hence in which to investigate the anti-nausea potential of NCEs. However, such studies not only have their own inherent problems (see below) but also come relatively late in the drug development process so alternative methods are still needed for early identification/prediction of nausea and vomiting liability.
Current animal models
Which species is most predictive of vomiting in humans?
Vomiting is present in many mammalian and non-mammalian species (see Box 2), although not all species have a similar range of sensitivities to emetic stimuli to humans. Opportunities for replacing studies in mammals by the use of arguably ‘less sentient’ species are limited by differences in the mechanics of vomiting related to the absence (fish)/completeness (frogs) of the diaphragm (Pickering and Jones, 2002) and the morphology of the gastrointestinal tract (birds and reptiles).
Box 2 Examples of species with an emetic reflex
Non-mammals
Representative fish (e.g. dogfish, skate, trout, tuna), amphibia (e.g. frog, bullfrog, salamander), reptiles (e.g. salt water crocodile), birds (e.g. pigeon, petrel).
Mammals
Representative insectivores (e.g. house musk shrew), artiodactyls (e.g. pig, goat), carnivores (e.g. cat, dog, ferret, seal), cetaceans (e.g. sperm whale), non-human primates (e.g. marmoset, macaque monkey, baboon).
‘Which animal species is most predictive of humans?’ is a question frequently asked in the context of drug discovery and development and while this is a simple question a few examples will serve to illustrate why it is difficult to answer by naming a single species. The question can only be addressed by asking a supplementary question ‘In response to which stimulus?’ To illustrate this, Table 1 highlights the differences in sensitivity of various species to a ‘simple agonist’ (apomorphine) acting at a well-defined central site (area postrema). Species differences also exist in the sensitivity to motion stimuli and the nature of the most effective type of motion to induce sickness. For example, the squirrel monkey (Saimiri sciureus) is motion sensitive but macaques are reported to be motion insensitive. Furthermore, while the squirrel monkey is only slightly susceptible to vertical oscillation it is highly susceptible to sickness induced by rotation, a stimulus to which the dog is only slightly susceptible (Corcoran et al., 1990; Daunton, 1990). In addition, there are species differences in the ability of ionizing radiation to induce emesis with the ferret, dog and human being relatively sensitive with ED50 values of 100–230 cGy in contrast to the cat requiring >2000 cGy (Harding, 1995). A retrospective investigation of the relative value of studies in the monkey (mainly Maccaca mulatta) and dog (mainly beagle) for assessing the gastrointestinal toxicity of 25 anti-cancer compounds in humans revealed a corrected false negative index of 68% for the monkey compared with 14% in the dog (Schein et al., 1970). This highlights that it should not be assumed that a non-human primate is necessarily the best predictor for emetic liability in humans. The Schein et al. (1970) study compared data from 383 dogs and 153 monkeys with that from >3700 patients receiving the same compounds. Similar published studies are rare but it is likely that information required to make similar comparisons for other classes of compounds is available but scattered in the literature as well as within pharmaceutical company archives (see below). It is clear from the few examples given that to make a more realistic assessment of the relevance of data from various animal species it will be necessary to undertake a more systematic review of the available data. However, this is likely to be of limited value unless data are available from human studies of compounds which were found to have an unacceptable emetic liability.
Table 1.
Species differences in the emetic sensitivity to the dopamine receptor agonist apomorphine
| Species | Emetic sensitivity to apomorphine | Dose of apomorphine | Selected references |
|---|---|---|---|
| Human | Very sensitive | 10–66 µg·kg−1, i.v. 0.05–1 mg·kg−1, s.c. | i.v.: Klein et al. (1968); Shields et al. (1971) s.c.: Isaacs and Macarthur (1954); Isaacs (1956); Proctor et al. (1978) |
| Monkey (Macca cynomologus/mulatta) | Insensitive | Doses up to 25 mg·kg−1, i.v. and 100 mg·kg−1, s.c. tested | Brizzee et al. (1955) |
| Marmoset | Weakly sensitive | 0.5 mg·kg−1, s.c. | Costall et al. (1986) |
| Pig | Very sensitive | 25 µg kg−1, i.v. | Parrott et al. (1991) |
| Dog | Very sensitive | 2.5–20 µg·kg−1, i.v. 0.01–0.15 mg·kg−1, s.c. | i.v.: Niemegeers (1971; 1982; Harding et al. (1987) s.c.: Borison and Hebertson (1959); Share et al. (1965) |
| Cat | Relatively insensitive | 25 mg·kg−1, s.c. | Laffan and Borison (1957); Borison (1959) |
| Ferret | Relatively sensitive | ∼0.025–0.25 mg·kg−1, s.c. | Costall et al. (1989); Andrews et al. (1990) |
| House musk shrew (Suncus) | No response | Up to 100 mg·kg−1, s.c. tested | Ueno et al. (1987) |
| Least shrew | Sensitive | ∼2 mg·kg−1, s.c. | Darmani et al. (1999) |
| Rat | CTA | 1 mg·kg−1, i.p. | Wang et al. (1997) |
| Rat | Pica | 10 mg·kg−1, i.p. | Takeda et al. (1993) |
Note that some studies document that the ferret is not very sensitive to apomorphine, or produces inconsistent responses (Gylys and Gidda, 1986; King, 1988; Tuor et al., 1988). Other species that are responsive include: chicken (Osuide and Adejoh, 1973) and pigeon (Saxena et al., 1977) both requiring 20 mg·kg−1, i.v.; and rainbow trout 120 mg·kg−1, i.p. (Tiersch and Griffith, 1988).
CTA, conditioned taste aversion.
An important component of the ‘Which species?’ question is whether the species selected utilize the same pathway as humans to induce vomiting and even if it does whether it utilises the same transduction processes, hormones, neurotransmitters and receptors in the same location as humans; and that even if the transmitter is present does it play an equally significant role? This is even more difficult to comment upon primarily because of the paucity of data in humans.
What information can be obtained from species in which vomiting is absent?
The above discussion of species has notably made no mention of rats, mice, rabbits or guinea pigs. This is because although there are scattered reports of ‘retching’ or ‘regurgitation’ in some of these species (e.g. for mouse, see Furukawa and Yamada, 1980), there are no consistent reports of vomiting, and studies in which vomiting would have been expected to have been seen did not report it. The rat has been most studied and delayed gastric emptying and increased chewing and swallowing are the main responses to substances which would induce vomiting in other species such as the ferret (Andrews and Horn, 2006). A delay in gastric emptying in the rat and mouse is also observed when these species are given cytotoxic chemotherapy drugs and this has been argued to be a surrogate marker for vomiting in these non-emetic species (Bradner and Schurig, 1981). Rats display two other behaviours which have been argued to be surrogate markers for vomiting, nausea or activation of the emetic reflex afferent pathways and both have been used to investigate emetic liability and anti-emetic activity.
Pica is the consumption of non-nutritive substances and in the laboratory is measured by the consumption of kaolin (clay). Pica increases in a dose-related manner to a range of stimuli such as motion, cytotoxic drugs, apomorphine and intra-gastric copper sulphate, all of which would cause vomiting in species with the emetic reflex (Takeda et al., 1993). While pica is relatively robust in the rat it appears less so or even absent in the mouse (Liu et al., 2005).
Conditioned taste aversion/food avoidance (CTA/CFA) studies involve pairing a novel food or fluid with the administration of the potential emetic and another novel food with administration of vehicle. The animal is subsequently presented with both foods or solutions and the consumption of each is measured, with avoidance of the food/fluid previously paired with the potential emetic being taken as an indication that it induced an unpleasant ‘sensation’ on prior exposure. CTA/CFA is present in species with and without an emetic reflex (Andrews and Horn, 2006) and has been used to investigate novel anti-emetic drugs although with variable success (ibid.).
A number of compounds with either emetic or anti-emetic potential have been investigated in rat pica and CTA models and subsequently investigated in species with an emetic reflex. This provides an opportunity for a systematic review to provide evidence of whether such rodent models could replace some of the studies in the larger species used in emesis studies.
Until the anatomy and physiological and pharmacological mechanisms underlying the absence of the emetic reflex are understood, results from rodents in this area should be treated with caution.
Lessons from chemotherapy-induced vomiting in the ferret: identifying novel anti-emetics
The complexity of replacing animals in studies to identify novel anti-emetics is illustrated by the chemotherapy-induced nausea and vomiting model in the ferret. The ferret has been widely adopted by the pharmaceutical industry globally for investigating both the emetic and the anti-emetic potential of NCEs with studies published among others by Abbott (Ji et al., 2007), Astellas Pharma Inc (Nagakura et al., 2007), Astra Hassle AB (Lehmann and Karrberg, 1996), Dainippon Sumitomo Pharmaceuticals (Isobe et al., 2006), GlaxoSmithKline (Minthorn et al., 2008), Merck Frosst (Cote et al., 2003), Merck Sharp and Dohme (Robichaud et al., 2001), Mitsubishi Tanabe Pharma Corp (Watanabe et al., 2008) and Pfizer (Shishido et al., 2008). In man, cisplatin induces a biphasic pattern of vomiting which is characterized by an acute short latency phase and a delayed phase (Martin, 1996). Improved clinical evaluation of anti-emetic drugs in man showed that 5-HT3 receptor antagonists (e.g. ondansetron, granisetron) were highly effective in preventing vomiting on the first day of chemotherapy treatment (acute vomiting) (Fauser et al., 1999), as is the case in the ferret (Rudd and Andrews, 2004), but were less effective or even ineffective in reducing the vomiting on subsequent days (delayed vomiting) (Rizk and Hesketh, 1999). This differential effect of 5-HT3 antagonists in man could not have been predicted from the initial ferret studies as the observation times to assess anti-emetic potential did not usually extend beyond 6 h (Rudd and Andrews, 2004) and hence did not cover the ‘delayed’ phase.
The realization that delayed vomiting remained a problem in the clinic even after the introduction of 5-HT3 receptor antagonists prompted a review of the original animal models. It was clear that the initial cisplatin (∼10 mg·kg−1)-induced emetic response occurring within the 4–6 h model was mediated primarily by 5-HT acting upon 5-HT3 receptors, but this was not the case with delayed vomiting. Reducing the dose of cisplatin to 5 mg·kg−1 (Rudd et al., 1994) not only enabled animals to tolerate cisplatin for 72 h but also mimicked the biphasic profile of cisplatin-induced vomiting in humans. Acute and delayed cisplatin-induced vomiting models have also been developed in the piglet (Milano et al., 1995), pigeon (Tanihata et al., 2000) and dog (Fukui and Yamamoto, 1999; Yamakuni et al., 2000). Each model has its drawbacks, but they have provided important data to support the hypothesis that the NK1 receptor antagonists could have utility to prevent both acute and delayed vomiting in man (for review see Andrews and Rudd, 2004). Although the NK1 receptor antagonists appeared to have an excellent control of vomiting in animals (for review see Andrews and Rudd, 2004), they were surprisingly less able to prevent nausea and vomiting occurring during the acute phase of vomiting in patients, where the 5-HT3 receptor antagonists have their major activity (Rudd and Andrews, 2004). Clearly, even if a drug prevents vomiting in animal models, we are still not entirely confident that nausea (and vomiting) will be similarly affected until the compound is tested in man under appropriate conditions. Direct comparison of anti-emetic efficacy of compounds between animal models and patients is further complicated by the nature of the data collected: in animals it is possible to count every retch and vomit and to obtain a precise temporal distribution whereas in humans the emetic episodes are often reported by the patients retrospectively in a daily diary. Thus, it may be difficult to assess how well animal data have translated unless a particular compound blocks emesis completely in both the animal and human.
Nausea and vomiting as a hurdle to drug development
Nausea and vomiting are some of the most important side effects of drug treatments in humans, potentially leading to poor quality of life and, as nausea is a highly aversive sensation (it has been argued to be more aversive than pain) (Pelchat and Rozin, 1982), patient compliance with drug treatment may be affected. In addition, if the medicine is not fully absorbed due to it being expelled from the body, or absorption is delayed due to slowing of gastric motility, exposure to the drug may not be adequate to exert its effects. It has been estimated that in healthy volunteer studies the incidence of nausea and vomiting induced by NCEs can be as high as 30% (R Walliset al., unpublished; Pfizer Global Research and Development, Sandwich, UK) and may halt development of a valuable new drug. Figure 2 uses data compiled by Pfizer to highlight the magnitude of this problem. Cancer chemotherapeutic drugs, until the development of the 5-HT3 and NK1 receptor antagonists may have been considered in this category. However, the nature of the condition being treated also needs to be taken into account when assessing the acceptability of nausea and vomiting as side effects. It is important to be able to detect the propensity of a drug to cause nausea and vomiting as early in drug development as possible.
Figure 2.
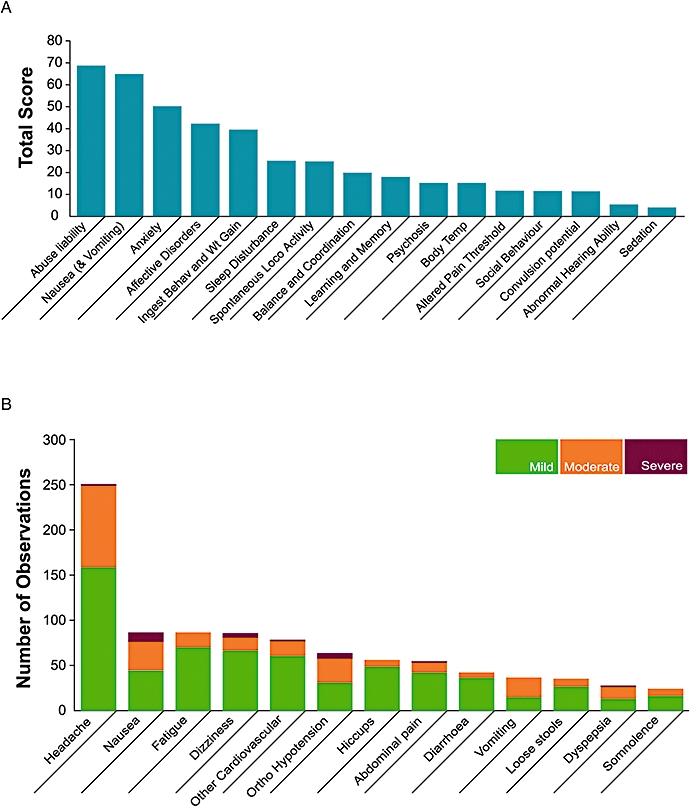
The impact of nausea and vomiting on the development of novel chemical entities. (A) An analysis by Pfizer assessing how various side effects encountered in preclinical safety studies impacted on the development of a medicine for humans. More than 70 novel therapeutics, including antivirals, agents to promote tissue repair, neurology, sex health, allergy and respiratory, cardiovascular, gastrointestinal and urogenital disease targets were used in this analysis. The targets included were agents with both peripheral and central sites of action and were assessed during the period 1998–2000. The total score represents the number of compounds progressing to clinical development that interact with targets that have known or suspected safety liability based on experimental data or from literature reports. This analysis took into account not only the impact to the patients but also to the drug development programme through the need for additional studies to investigate the extent and seriousness of the safety issue and also to support regulatory acceptance by bodies such as the European Medicines Agency and the US Food and Drug Administration. Whereas factors such as sedation, convulsion potential and changes in body temperature were important, they could be easily examined both clinically and preclinically. In contrast nausea and vomiting were considered second only to abuse liability as having an impact on the development of the drug. (B) A further analysis of side effects encountered in 16 phase 1 clinical studies conducted by Pfizer between 2003 and 2005. While the most commonly encountered side effect was headache, with approximately 250 instances, the next most encountered was nausea, which accounted for over 80 instances, nearly half of which were rated as either moderate or severe. There were also a similar number of observations of moderate vomiting. The 16 trials averaged approximately 35 individuals each.
Use of rodents in the assessment of nausea and emetic liability
Ferrets have assumed increasing importance in anti-emetic research and the investigation of emetic liability of novel agents. However, emetic liability is investigated during toxicology studies in rodents to satisfy regulatory requirements for new drugs. Although rodents do not have an emetic response (see above), they have been used to assess emetic liability in two ways: (i) observational behavioural studies or (ii) in functional experiments when the mechanism(s) of the induction of nausea and vomiting are believed to be known.
In behavioural studies, animals are observed for clinical signs which may correlate with nausea and vomiting, including pica, CTA/CFA, chromodacryorrhea, vocalization, hunched body posture, lack of grooming and excessive urination, increased defaecation and salivation (often accompanied by increased swallowing), reduction in food intake and loss of body weight. However, these effects can be induced by a wide variety of compounds and are not necessarily specific to agents known to induce nausea and vomiting in humans. Therefore, the validity of these studies for predicting nausea and emetic liability in humans is questionable.
Studies in anaesthetized or decerebrate animals
Emetic liability can be studied without the use of conscious animals. Electrophysiological techniques have been used extensively to investigate activation of brain regions that are involved in the control of vomiting in dogs, cats and ferrets (see reviews by Leslie and Reynolds, 1993; Fukuda et al., 1998) and have also been used to record from abdominal vagal afferents in response to emetic agents (e.g. Horn et al., 2004). Other anaesthetized or decerebrate in vivo studies have been used to test for anti-emetic efficacy and emetic liability. For example, abdominal vagal afferent nerve stimulation can induce vomiting in the ferret (Watson et al., 1995) and a working heart/brainstem preparation has also been used in Suncus (Smith et al., 2002). These studies are complex to perform and time consuming to conduct on a regular basis with many compounds.
Alternatives to the use of laboratory mammals in the in vivo study of nausea and vomiting
Animal models have provided valuable insight into the development of efficacious anti-emetics. Their use for establishing emetic liability however is an issue for discussion as robust data on their predictive value (especially for nausea) are lacking. Dogs are the commonly used non-rodent species because they are perceived as having a ‘high-sensitivity to emetics’. A positive response in a dog may lead to testing in another species (e.g. a non-human primate) but as was discussed above the outcome may depend upon the nature of the emetic challenge. Similarly, while the lack of an emetic response in the dog may (correctly) be taken as evidence that the substance is unlikely to have a significant emetic liability in humans the data to support such an assumption for a diverse range of compounds is lacking. There are thus both scientific and ethical drivers for applying the 3Rs to this area.
The complexity of multi-system reflexes has meant that alternatives to traditional animal models of nausea and vomiting have not been fully explored. Bringing together experts to examine opportunities for replacement and to inspire the development and adoption of new approaches which can replace, reduce or refine animal use is critical if this is to be addressed. Here we consider how the 3Rs can be implemented in basic research and drug development to reduce animals and accelerate the development of new therapeutics.
Nausea: the neglected symptom
Relying on animal models to study the subjective human sensation of nausea has meant the symptom remains largely untreated. During the 1990s, there was an increase in the number of studies using human volunteers to examine the efficacy of anti-emetics and anti-nauseogenics in an attempt to address this. The majority of these studies used ingested ipecacuanha and this model has been used to test the anti-emetic activity of several different 5-HT3 receptor antagonists with the doses that were effective against ipecac correlating well with clinically effective doses (Minton et al., 1993). Studies of emesis in humans have been conducted for agonists at dopamine receptors (Proctor et al., 1978; Axelsson et al., 2006) and opioid receptors (Rudd and Naylor, 1995). Laboratory-based models of motion sickness have been developed in humans for characterization of the spectrum of anti-emetic effects of 5-HT3, NK1 and muscarinic receptor antagonists (Golding and Stott, 1997; Reid et al., 2000). While using the target species negates the need to extrapolate findings and provides reliable efficacy and dose data it has its own inherent problems (see below).
Nausea and vomiting involve neuronal populations that do not function in isolation. Rather, they interact with other such elements through their afferent and efferent connections in an orchestrated manner. It is clear that in order to fully understand the development and progression of nausea and vomiting in humans, it is essential to tease apart these interactions. Functional imaging technologies offer the best opportunities in this area and rapid advances are being made in the development of techniques such as MRI and positron emission topography which offer a non-invasive alternative approach to study neuronal processes in humans.
The need for human data to address the paucity of knowledge regarding the neural pathways and mechanisms involved in nausea was highlighted at the workshop. When asked what single research question delegates would address if applying for funding relating to the 3Rs and nausea and vomiting research the response was overwhelmingly in favour of conducting brain imaging studies in human volunteers in which nausea has been induced under controlled conditions.
In the last decade, imaging techniques have proven to be effective tools in the study of a number of pathologies including nausea and vomiting. Functional MRI offers the greatest potential for determining where activity occurs in the brain as a result of various experiences and pharmacological challenges. A prerequisite of studying the brain processing of nausea is the ability to induce nausea without vomiting. Recently a number of human models have been described which demonstrate that the two symptoms can be separated. These models rely on various nauseogenic stimuli, including vagal nerve stimulation (Narayanan et al., 2002; Kraus et al., 2007), pharmacologically induced nausea (Miller et al., 1996) and vection-induced nausea (Faas et al., 2001; Kowalski et al., 2006). Kowalski et al. (2006) have described a theoretical experimental approach using fMRI to study cortical activity in humans experiencing circular vection-induced nausea; however, data using this approach have yet to be collected. Combining this with electrogastrogram (EGG) recordings and measurement of plasma vasopressin levels would provide objective biomarkers of nausea with which to correlate fMRI readings.
Brain imaging studies in humans offer the best opportunity to study the neural correlates of nausea and vomiting and could be used to test the efficacy of existing and novel treatment strategies for the management of nausea. Greater uptake of brain imaging could also replace the use of animal models with the most relevant of species: the human. However, encouraging volunteers to participate in a study where nausea and possibly vomiting will be induced can be difficult. Previous studies of human volunteers where anti-emetics have not been available have resulted in protracted bouts of retching and vomiting (Minton et al., 1993; (Hammas et al., 1998b). This problem can be reduced using vection where the subject can stop the stimulus by closing their eyes. However, this will not be the case with systemically administered agents.
Why there have been few recent studies of nausea and vomiting in human volunteers remains unclear. Recruiting volunteers for such studies can be difficult, but not impossible (Minton et al., 1993; Minton, 1994; Hammas et al., 1998a,b; Axelsson et al., 2004; 2006) and some of the issues are similar to those involved in recruiting humans for studies of pain (see Langley et al., 2008). Given the obvious advantages of using human subjects, particularly for studies of nausea it would be sagacious to investigate why the number of studies has been limited and how more work in this area could be encouraged.
Replacement of animals in emetic liability detection
Invertebrates and other lower organisms have been used successfully in many research areas and given the many advantages of these systems for studying human health and disease, their potential as a model for the detection of substances with a potential to induce nausea and vomiting should be explored. Although vomiting in response to food containing toxins has been reported in the sea anemone (Aiptasia pallida; Lindquist and Hay, 1995) and a gastropod mollusc (Pleurobranchea; McClellan, 1983) the potential use of lower organisms to assess emetic liability is illustrated by reference to studies in Caenorhabditis elegans and the social amoeba Dictyostelium discoideum.
Olfactory chemotaxis towards food-associated odours is one of the nematode C. elegans' most robust behaviours (Bargmann et al., 1993) and it has been demonstrated that this behaviour can be altered by a number of factors, including prolonged exposure to aversive odours (Bargmann et al., 1993; Colbert and Bargmann, 1995; Nuttley et al., 2002; Pradel et al., 2007). Zhang et al. (2005) demonstrated that C. elegans uses olfactory aversion as a learned protective mechanism to avoid ingesting pathogenic bacteria which can kill the nematode, much in the same way that animals without an emetic reflex learn to avoid foods associated with visceral malaise (CTA/CFA). This behaviour is mediated through a 5-HT-gated ion channel (MOD-1) in the C. elegans (Zhang et al., 2005). Similarly, 5-HT-mediated activation of 5-HT3 receptors is one of the pathways by which mammals signal intestinal malaise such as nausea. Taken together, the learned olfactory aversion and 5-HT-mediated identification of intestinal pathogens suggest that C. elegans possess a number a molecular parallels with vomiting in mammals and could potentially represent an alternative for nausea and vomiting studies, for example, providing a screen with which to identify emetic liability. Examining whether C. elegans responds selectively to known emetics in a reproducible fashion is an interesting research question.
Bitter taste has evolved as a warning signal against the ingestion of potentially toxic substances and many naturally poisonous substances taste bitter to humans. Recent studies of how the human gastrointestinal tract can detect nutritive and beneficial compounds and absorb them as well as harmful or toxic substances and reject them, have identified a large family of bitter taste receptors (T2Rs) similar to those found within the taste buds of the tongue (Wu et al., 2002; Chen et al., 2006). Furthermore, it has been demonstrated in enteroendocrine STC-1 cells that bitter tastants bind to T2Rs resulting in release of cholecystokinin (CCK) which is capable of inducing nausea and vomiting in man and the ferret (Billig et al., 2001; Castillo et al., 2004; Chen et al., 2006). Cycloheximide, an inhibitor of protein synthesis in eukaryotes, is a bitter tastant that has been shown to elicit an aversive response in humans and some animal species and is emetic in the ferret (Andrews et al., 1990). Interestingly however, C. elegans is strongly attracted to this compound (Tajima et al., 2001; 2003). What the mechanism(s) behind this response is (i.e. 5-HT-mediated) or whether or not this is a universal response of C. elegans to substances that are aversive to humans remains to be examined. It can be assumed that G-protein-coupled receptors (GPCRs) are involved as more than 700 genes encoding putative GPCRs exists in the C. elegans genome (Bargmann, 1998); and two G-protein subunits have been shown to be necessary for detecting other tastants, such as quinine, in the nematode (Hilliard et al., 2004). There are distinct parallels between the C. elegans and mammalian GPCR signalling pathways and researchers have taken advantage of this to develop a C. elegans-based expression system to express functional mammalian GPCRs of medical importance (Teng et al., 2006) and of the T2R family (Conte et al., 2006). These studies demonstrate the potential utility of C. elegans as a heterologous expression system for mammalian GPCRs for screening agonists and antagonists for potential emetic liability, and for carrying out structure-function studies on GPCRs and their ligands. A similar approach could be examined in the social amoeba D. discoideum which has been demonstrated to respond to cisplatin, providing a useful model with which to examine the mechanism of cisplatin resistance (Williams et al., 2006), and which migrates directionally in response to external chemoattractant gradients. Furthermore, this chemotaxis is mediated by a signalling cascade initiated when chemoattractants bind to transmembrane receptors that couple to G-proteins (Kimmel and Parent, 2003). Exposing D. discoideum to known emetogens and assessing its response will quickly determine if this model is worth pursuing as a novel means to potentially detect emetic liability of NCEs.
The adoption of lower organisms to predict NCEs likely to induce nausea and vomiting is worthy of attention and the need to identify emetic liability early in the drug development process should be enough to merit examination of these tools. Assessing the utility of these models is potentially quick and inexpensive and would involve exposing these organisms to known emetogens in rank order of emetic potential and examining their response. Validating these models against existing preclinical and clinical data is essential if they are to ever be considered viable as a screening tool by industry; potentially improving lead compound identification and reducing both the number of compounds going forward to preclinical studies and the rate of attrition of NCEs due to emetic liability.
Pharmacogenomics
Important lessons for nausea and vomiting can be learnt from the use of gene analysis in drug discovery for treating depression. The challenges of treating depression are similar to those of treating nausea and vomiting. The complexity of both pathologies has meant that drug activity can only be tested in vivo at the present time and the development of novel anti-emetics/nauseogenics and psychoactive drugs has often been limited to compounds aimed at known therapeutic targets or with activities similar to existing drugs. Microarray-based gene expression profiling is a high-throughput, automated technology platform that offers researchers the opportunity to identify therapeutic efficacy and secondary drug targets in vitro. Using gene expression profiles induced in primary human neurons by various psychoactive drugs, Gunther et al. were able to derive general efficacy profiles of biomarker gene expression that correlate with antidepressant, antipsychotic and opioid drug action in vitro (Gunther et al., 2003). A similar approach could be taken for known emetics using neurons from the NTS, area postrema and enteroendocrine cells from the gut. This could be used as a template to identify possible emetic liability of novel compounds.
In vitro approaches
Very few studies examining molecular changes induced by known anti-emetics and emetics have been carried out at the cell/tissue level. Tissue models of enteroendocrine cells from the gastrointestinal (GI) tract have been developed in many studies investigating the release of neurotransmitters in response to mechanical forces (Bertrand, 2006) and dietary components (Chen et al., 2006; Cummings and Overduin, 2007; Sternini, 2007) and there is potential to apply these to nausea and vomiting. Tissue engineering models incorporating enteroendocrine cells and vagal afferents could be used to assess if NCEs elicit the release of neurotransmitters associated with nausea and vomiting. Other in vitro techniques including the isolated abdominal vagal grease gap preparation (Nemoto et al., 2001) and the nodose ganglion (the location of the vagal afferent cell bodies) preparations from both rats and humans (Burdyga et al., 2006; de Lartigue et al., 2007) could be used to assess a compounds emetic liability and perhaps to identify novel targets for anti-emetic agents intended to target peripheral emetic inputs. Additionally, tissue slices of the area postrema/brainstem have been used to examine the effect of angiotensin II in the NTS (Butcher et al., 1999; Kasparov and Paton, 1999) and could be modified to study nausea and vomiting.
Cell cultures of enteroendocrine STC-1 cells have been used to demonstrate that CCK is released in the presence of aversive substances such as bitter tastants (Chen et al., 2006). Once again, this is mediated by activation of luminal GPCRs, most likely T2Rs in this case and could result in the release of CCK which may enter the circulation and activate targets such as the area postrema potentially inducing nausea and vomiting. The functional implications of chemosensing components within the GI tract and their relationship with neural pathways responsible for the generation of specific responses to luminal contents have been summarized elsewhere (Sternini, 2007).
Other cell types have also been examined for their utility in studies of nausea and vomiting. Mantovani and colleagues have demonstrated that cisplatin induces 5-HT release from human peripheral blood mononuclear cells, highlighting an additional mechanism through which cisplatin could induce emesis (Mantovani et al., 1996). In addition, this effect is reduced by methylprednisolone which has anti-emetic effects in patients undergoing cisplatin-based chemotherapy. These models offer an opportunity to screen NCEs for their potential to release neurotransmitters associated with nausea and vomiting in relevant human tissues. However, while some techniques are currently available (e.g. abdominal vagal grease gap, nodose ganglia), others need further development (e.g. tissue engineering).
Emetic database
A database containing information from the public domain and pharmaceutical industry regarding (i) species' responses to various chemical classes with known anti-emetic efficacy or emetic liability; (ii) human data from clinical trials of successful drugs and those that have failed on the basis of emetic liability and (iii) pharmacogenomic data, would be an invaluable tool for improving drug development and reducing animal use. Including data from studies that are never likely to be repeated such as early studies using non-human primates (Light and Bysshe, 1933) or intra-cerebroventricular administration in humans (Cushing, 1931) would ensure best use of this data.
Nausea and vomiting is a relatively circumscribed and manageable subject area and conducting systematic reviews and/or meta-analyses of the literature would be the most appropriate way to compile the information required for such a database. Percie Du Sert et al. have conducted such a review of the ferret model of cisplatin-induced emesis which identifies ways to refine the experimental protocol by reducing the observation time required to identify anti-emetic effects in the acute phase and to reduce the number of animals required to draw valid conclusions (Percie Du Sert and Andrews, 2007). Furthermore, quantitative systematic analysis of the literature provides the basis for a predictive algorithm of emetic liability and anti-emetic efficacy of new drugs which could potentially lead to a reduction and perhaps the eventual replacement of animal-based studies in this area. The algorithm would provide (i) a method to identify whether a substance in a particular class has been tested before and (ii) an evidence-based assessment of the probability of emetic liability of NCEs in humans. Additionally, information about species sensitivity or resistance to specific classes of compound could aid species selection, for example, dogs are particularly sensitive to apomorphine whereas the house musk shrew is resistant.
Predictive modelling
Predictive modelling using functional in vitro assays or in silico methods (e.g. quantitative structure–activity relationships) not only provide information on whether a drug interacts with its intended targets, but also on its interaction with secondary unintended targets and can therefore identify possible side effects including nausea and vomiting. Using ligand profiling in vitro it is possible to assess a NCEs potential for affinity at particular receptors/sites known to lead to vomiting in animals or humans. This allows assessment at early stages of development and rejection of compounds with undesirable binding. This is an iterative process allowing additional assays to be conducted as novel sites are identified that have liability for the induction of vomiting or indeed any other undesirable activity. Xie et al. used a similar protein-ligand binding approach, incorporating additional small molecule screening and functional protein site similarity searches to identify the molecular mechanism that defines the adverse effects of selective oestrogen receptor modulators (Xie et al., 2007). The authors postulate that their strategy could be applied to discover off-target interactions in other commercially available pharmaceuticals. This could potentially include off-target interactions responsible for nausea and vomiting. This strategy would provide the opportunity to remove or modify the drug prior to preclinical and clinical studies.
A caveat to some of these approaches is the possibility of discarding a potentially beneficial drug based on transient emetic liability. For example, several classes of the antidepressant monoamine reuptake inhibitors initially cause nausea and vomiting following the first dose. However, subsequent doses see these side effects decrease over time as the therapeutic action of the drug is established (Kasper et al., 1992). Furthermore, drugs such as cisplatin and methotrexate used in the treatment of cancer and arthritis respectively, where the therapeutic benefit is considered to outweigh the nausea and vomiting side effects could have been removed from development based on emetic liability. Avoiding this requires a degree of flexibility in the screening system. The decision of whether a drug should continue through development should include an analysis of patient benefit so that if a NCE is efficacious a decision could be made to accept a degree of nausea and vomiting as a side effect. Anti-emetics can then be administered concurrently, as is the case with chemotherapy.
Opportunities for reduction and refinement in anti-emetic development
Developing anti-emetics is likely to require the continued use of animals since (i) the mechanism by which emetogens induce nausea and vomiting is complicated and can involve more than one afferent pathway; and (ii) emetogens can act via the release of secondary mediators (e.g. 5-HT and hormones from the gut). Where animals are required, it is important that every effort is made to reduce their numbers and refine procedures to minimize suffering. Biomarkers provide useful tools to support both reduction and refinement. Two physiological markers have been proposed to correlate with malaise in animal models of nausea and vomiting and nausea in humans. These are (i) blood levels of the neurohypophyseal hormone vasopressin (Verbalis et al., 1987; Nussey et al., 1988; Koch et al., 1990b; Billig et al., 2001); and (ii) gastric dysrhythmia measured either from surface recordings (EGG) or implanted electrodes (Koch et al., 1990a; Caras et al., 1997; Lang et al., 1999). Note that marked elevation of vasopressin is only observed in species with a vomiting reflex, in the rat a marked elevation of oxytocin occurs in response to emetic stimuli (for references see Verbalis et al., 1987; Billig et al., 2001). By conducting dose escalation studies and measuring such markers it is possible to identify when to stop an experiment as higher doses could lead to unnecessary suffering through nausea and vomiting.
Further refinement and reduction can be achieved by using an implanted telemetry device to continually capture valuable physiological data (e.g. blood pressure, heart rate, temperature, gastric electrical activity and abdominal pressure) that would otherwise be missed, without unnecessary handling and invasive procedures (Percie Du Sert et al., 2007). Furthermore, telemetry allows physiological and locomotor activity data to be obtained from a freely moving animal once it has been returned to its home cage environment, allowing the more effective study of delayed nausea and vomiting. This would increase understanding of events occurring during vomiting, and reveal early warnings on potential drug candidates, in addition to providing sub-emetic end points and reducing the number of animals in emetic research.
Future steps and considerations
Replacing animals in the study of multi-system reflexes, such as nausea and vomiting, is inherently complicated and this has contributed to the lack of non-animal alternatives in this area of research. However, the gaps in knowledge regarding the basic mechanisms involved in nausea and vomiting in humans, the paucity of efficacious anti-emetics/nauseogenics and the consistent observation of nausea and vomiting as adverse side effects of NCEs in man should be drivers for a change in experimental approach. The workshop has demonstrated that by challenging the status quo and asking experts to think critically about the use of animal models and laterally about the use of non-animal alternatives, it is possible to identify many potential opportunities for replacements.
There was considerable support at the workshop for the development and use of alternative models that could realistically minimize the use of animals, especially using humans for nausea studies and better models for predicting emetic liability. Figure 3 describes a theoretical approach to assessing emetic liability of NCEs using the alternative methods described in this review. Data obtained using the in silico/in vitro approaches may provide the researcher with enough information regarding the emetogenic risk of a NCE to allow the use of only one in vivo study (preferably in the lowest sentient species) prior to deciding if the compound should progress through development. The cost of such approaches is minimal when compared with the cost of end-stage drug failures. Providing funding for research and facilitating collaboration between industry and academia is critical if the use of alternatives is to be explored and exploited in nausea and vomiting research.
Figure 3.
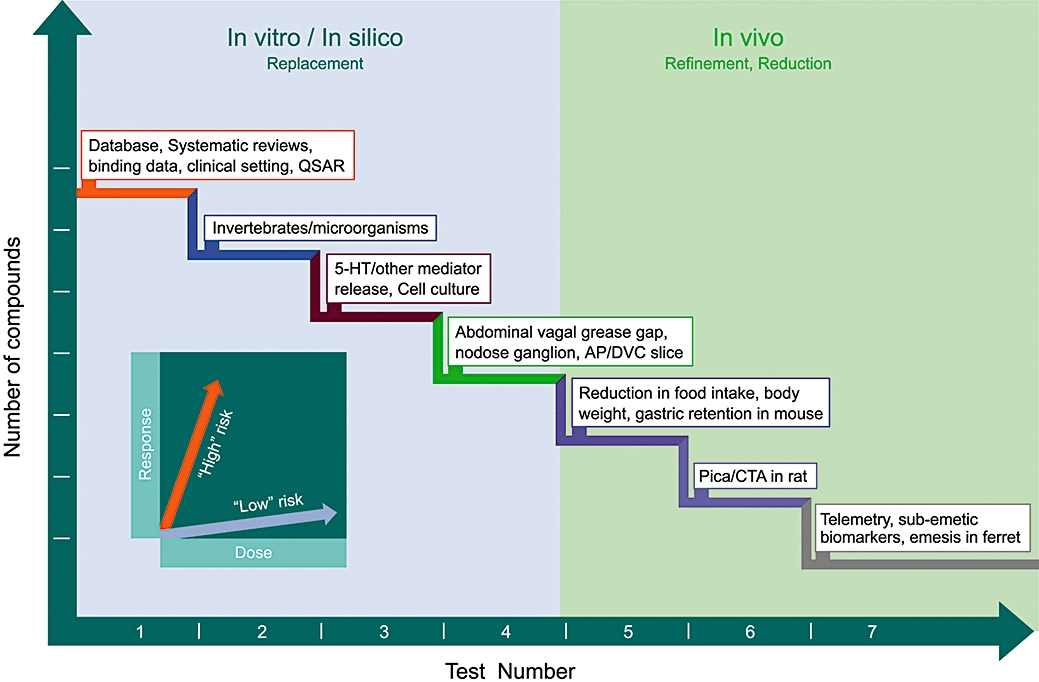
A hypothetical tiered approach to illustrate assessment of potential emetic liability of novel chemical entities (NCEs) that could reduce animal use. This approach consists of a series of tests, starting with in vitro/in silico methods to assess emetic liability prior to progressing to conscious animal studies. Each test investigates the dose-response (e.g. C. elegans chemotaxis, neurotransmitter release, pica) relationships of the NCE (see inset). Such a weight-of-evidence approach would enable researchers to classify the emetic potential of a NCE as either relatively ‘high’ or ‘low’ risk over a series of in vitro/in silico tests. This should provide a more accurate overall indication of a NCEs potential to be emetic prior to undertaking any in vivo studies and may perhaps obviate their necessity. NCEs would be compared against a panel of compounds with known emetic liability in humans. An increasing probability of emetic liability in each test increases the probability of emetic liability being seen in humans. The in vitro/in silico studies would inform the in vivo studies and may enable studies to stop at a lower sentient species and potentially use less animals overall by reducing the number of compounds/doses tested in vivo. 5-HT, 5-hydroxytryptamine; AP, area postrema; CTA, conditioned taste aversion; DVC, dorsal vagal complex.
Acknowledgments
The authors thank James H. Casey and Jon Scatchard (Pfizer Global Research and Development, Sandwich, Kent) who carried out the analyses contained in Figure 2. Particular thanks to Vicky Robinson for help in developing the concepts in this review.
The National Centre for the Replacement, Refinement and Reduction of Animals in Research expresses its sincere thanks to all speakers and delegates at the workshop. Particular thanks to Ian Ragan for chairing the workshop.
Glossary
Abbreviations:
- CTA/CFA
conditioned taste aversion/food avoidance
- EGG
electrogastrogram
- NCE
novel chemical entity
- MRI
magnetic resonance imaging
- NC3Rs
National Centre for the Replacement, Refinement and Reduction of Animals in Research
- NTS
nucleus tractus solitarius
- PONV
post-operative nausea and vomiting
- 3Rs
replacement, reduction, refinement
This Review is the subject of a Commentary in this issue of BJP entitled Less is more: reducing the reliance on animal models for nausea and vomiting research (Robinson, pp. 863–864). To view this article visit http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2009
Conflicts of interest
FD Tattersall is an in vivo scientist working for Pfizer. Other than this the authors state no conflicts of interest.
References
- Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Andrews P, Torii Y, Saito H, Matsuki N. The pharmacology of the emetic response to upper gastrointestinal tract stimulation in Suncus murinus. Eur J Pharmacol. 1996;307:305–313. doi: 10.1016/0014-2999(96)00275-0. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PLR, Rudd JA. The role of tachykinins and the tachykinin NK1 receptor in nausea and emesis. In: Holzer P, editor. Handbook of Experimental Pharmacology. Berlin, Germany: Springer; 2004. pp. 359–440. [Google Scholar]
- Axelsson P, Thorn SE, Lovqvist A, Wattwil L, Wattwil M. Betamethasone does not prevent nausea and vomiting induced by the dopamine-agonist apomorphine. Can J Anaesth. 2006;53:370–374. doi: 10.1007/BF03022501. [DOI] [PubMed] [Google Scholar]
- Axelsson P, Thorn SE, Wattwil M. Betamethasone does not prevent nausea and vomiting induced by ipecacuanha. Acta Anaesthesiol Scand. 2004;48:1283–1286. doi: 10.1111/j.1399-6576.2004.00527.x. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol. 2006;577:689–704. doi: 10.1113/jphysiol.2006.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1243–1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- Borison HL. Effect of ablation of medullary emetic chemoreceptor trigger zone on vomiting responses to cerebral intraventricular injection of adrenaline, apomorphine and pilocarpine in the cat. J Physiol. 1959;147:172–177. doi: 10.1113/jphysiol.1959.sp006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borison HL, Hebertson LM. Role of medullary emetic chemoreceptor trigger zone (CT zone) in postnephrectomy vomiting in dogs. Am J Physiol. 1959;197:850–852. doi: 10.1152/ajplegacy.1959.197.4.850. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra LR. Breaking down the barriers: fMRI applications in pain, analgesia and analgesics. Mol Pain. 2006;2:30. doi: 10.1186/1744-8069-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner WT, Schurig JE. Toxicology screening in small animals. Cancer Treat Rev. 1981;8:93–102. doi: 10.1016/s0305-7372(81)80029-1. [DOI] [PubMed] [Google Scholar]
- Brizzee KR, Neal LM, Williams PM. The chemoreceptor trigger zone for emesis in the monkey. Am J Physiol. 1955;180:659–662. doi: 10.1152/ajplegacy.1955.180.3.659. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- Butcher WJ, Kasparov S, Paton FJ. Differential effects of apamin on neuronal excitability in the nucleus tractus solitarii of rats studied in vitro. J Auton Nerv Syst. 1999;77:90–97. [PubMed] [Google Scholar]
- Caras SD, Soykan I, Beverly V, Lin Z, McCallum RW. The effect of intravenous vasopressin on gastric myoelectrical activity in human subjects. Neurogastroenterol Motil. 1997;9:151–156. doi: 10.1046/j.1365-2982.1997.d01-37.x. [DOI] [PubMed] [Google Scholar]
- Castillo EJ, Delgado-Aros S, Camilleri M, Burton D, Stephens D, O'Connor-Semmes R, Walker A, Shachoy-Clark A, Zinsmeister AR. Effect of oral CCK-1 agonist GI181771X on fasting and postprandial gastric functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2004;287:G363–369. doi: 10.1152/ajpgi.00074.2004. [DOI] [PubMed] [Google Scholar]
- Chapman K, Pullen N, Graham M, Ragan I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nat Rev Drug Discov. 2007;6:120–126. doi: 10.1038/nrd2242. [DOI] [PubMed] [Google Scholar]
- Chen MC, Wu SV, Reeve JR, Jr., Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006;291:C726–739. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- Christie DA, Tansey EM. London: Wellcome Trust; The discovery, use and impact of platinum salts as chemotherapy agents for cancer: The transcript of a Witness Seminar held by the Wellcome Trust Centre for the History of Medicine at UCL, London, on 4 April 2006; p. 148. [Google Scholar]
- Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14:803–812. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- Conte C, Guarin E, Marcuz A, Andres-Barquin PJ. Functional expression of mammalian bitter taste receptors in Caenorhabditis elegans. Biochimie. 2006;88:801–806. doi: 10.1016/j.biochi.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Corcoran ML, Fox RA, Daunton NG. The susceptibility of rhesus monkeys to motion sickness. Aviat Space Environ Med. 1990;61:807–809. [PubMed] [Google Scholar]
- Costall B, Domeney AM, Naylor RJ. A model of nausea and emesis in the common marmoset. Br J Pharmacol. 1986;88:375. [Google Scholar]
- Costall B, Naylor J, Owera-Atepo J, Tattersall F. The responsiveness of the ferret to apomorphine-induced emesis. Br J Pharmacol. 1989;96:329P. [Google Scholar]
- Cote B, Frenette R, Prescott S, Blouin M, Brideau C, Ducharme Y, Friesen RW, Laliberte F, Masson P, Styhler A, Girard Y. Substituted aminopyridines as potent and selective phosphodiesterase-4 inhibitors. Bioorg Med Chem Lett. 2003;13:741–744. doi: 10.1016/s0960-894x(02)01030-2. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing H. I. The Reaction to Posterior Pituitary Extract (Pituitrin) When Introduced into the Cerebral Ventricles. Proc Natl Acad Sci USA. 1931;17:163–170. doi: 10.1073/pnas.17.4.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Zhao W, Ahmad B. The role of D2 and D3 dopamine receptors in the mediation of emesis in Cryptotis parva (the least shrew) J Neural Transm. 1999;106:1045–1061. doi: 10.1007/s007020050222. [DOI] [PubMed] [Google Scholar]
- Daunton NG. Animal Models in Motion Sickness Research. In: Crampton GH, editor. Motion and Space Sickness. Boca Raton, USA: CRC Press; 1990. pp. 87–104. [Google Scholar]
- Davis CJ. Emesis research: a concise history of the critical concepts and experiments. In: Reynolds DJM, Andrews PLR, Davis CJ, editors. Serotonin and the scientific basis of anti-emetic therapy. Oxford: Oxford Clinical Communications; 1995. pp. 9–24. [Google Scholar]
- de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–2882. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas H, Feinle C, Enck P, Grundy D, Boesiger P. Modulation of gastric motor activity by a centrally acting stimulus, circular vection, in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G850–857. doi: 10.1152/ajpgi.2001.280.5.G850. [DOI] [PubMed] [Google Scholar]
- Fauser AA, Fellhauer M, Hoffmann M, Link H, Schlimok G, Gralla RJ. Guidelines for anti-emetic therapy: acute emesis. Eur J Cancer. 1999;35:361–370. doi: 10.1016/s0959-8049(98)00417-1. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T, Furukawa N, Nakamura E, Shiroshita Y. The tachykinin NK1 receptor antagonist GR205171 prevents vagal stimulation-induced retching but not neuronal transmission from emetic vagal afferents to solitary nucleus neurons in dogs. Brain Res. 1998;802:221–231. doi: 10.1016/s0006-8993(98)00630-1. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T, Furukawa N, Nakamura E, Hatano M, Yanagihara M. The site of antiemetic action of NK1 receptor. In: Donner J, editor. Antiemetic Therapy. Basel: Karger; 2003. pp. 33–77. [Google Scholar]
- Fukui H, Yamamoto M. Methotrexate produces delayed emesis in dogs: a potential model of delayed emesis induced by chemotherapy. Eur J Pharmacol. 1999;372:261–267. doi: 10.1016/s0014-2999(99)00219-8. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Yamada K. The alpha-naphthoxyacetic acid-elicited retching involves dopaminergic inhibition in mice. Pharmacol Biochem Behav. 1980;12:735–738. doi: 10.1016/0091-3057(80)90158-6. [DOI] [PubMed] [Google Scholar]
- Gandara DR, Harvey WH, Monaghan GG, Perez EA, Hesketh PJ. Delayed emesis following high-dose cisplatin: a double-blind randomised comparative trial of ondansetron (GR 38032F) versus placebo. Eur J Cancer. 1993;29A(Suppl. 1):S35–38. doi: 10.1016/s0959-8049(05)80259-x. [DOI] [PubMed] [Google Scholar]
- Golding JF, Gresty MA. Motion sickness. Curr Opin Neurol. 2005;18:29–34. doi: 10.1097/00019052-200502000-00007. [DOI] [PubMed] [Google Scholar]
- Golding JF, Stott JR. Comparison of the effects of a selective muscarinic receptor antagonist and hyoscine (scopolamine) on motion sickness, skin conductance and heart rate. J Clin Pharmacol. 1997;43:633–637. doi: 10.1046/j.1365-2125.1997.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Gunther EC, Stone DJ, Gerwien RW, Bento P, Heyes MP. Prediction of clinical drug efficacy by classification of drug-induced genomic expression profiles in vitro. Proc Natl Acad Sci USA. 2003;100:9608–9613. doi: 10.1073/pnas.1632587100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylys JA, Gidda JA. Radiation induced emesis in ferrets: An experimental model of emesis. Gastroenterology. 1986;90:1446. [Google Scholar]
- Hall G, Symonds M. Overshadowing and latent inhibition of context aversion conditioning in the rat. Auton Neurosci. 2006;129:42–49. doi: 10.1016/j.autneu.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Hammas B, Hvarfner A, Thorn SE, Wattwil M. Effects of propofol on ipecacuanha-induced nausea and vomiting. Acta Anaesthesiol Scand. 1998a;42:447–451. doi: 10.1111/j.1399-6576.1998.tb05140.x. [DOI] [PubMed] [Google Scholar]
- Hammas B, Hvarfner A, Thorn SE, Wattwil M. Propofol sedation and gastric emptying in volunteers. Acta Anaesthesiol Scand. 1998b;42:102–105. doi: 10.1111/j.1399-6576.1998.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Harding RK. 5-HT3 receptor antagonists and radiation-induced emesis: preclinical data. In: Reynolds DJM, Andrews PLR, Davis CJ, editors. Serotonin and the scientific basis of anti-emetic therapy. Oxford: Oxfrod Clinical Communications; 1995. pp. 127–133. [Google Scholar]
- Harding RK, Hugenholtz H, Kucharczyk J, Lemoine J. Central mechanisms for apomorphine-induced emesis in the dog. Eur J Pharmacol. 1987;144:61–65. doi: 10.1016/0014-2999(87)90009-4. [DOI] [PubMed] [Google Scholar]
- Harrison AP, Erlwanger KH, Elbrond VS, Andersen NK, Unmack MA. Gastrointestinal-tract models and techniques for use in safety pharmacology. J Pharmacol Toxicol Methods. 2004;49:187–199. doi: 10.1016/j.vascn.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. Embo J. 2004;23:1101–1111. doi: 10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KY, Gan TJ. Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin Anaesthesiol. 2006;19:606–611. doi: 10.1097/01.aco.0000247340.61815.38. [DOI] [PubMed] [Google Scholar]
- Hoffmann IS, Roa M, Torrico F, Cubeddu LX. Ondansetron and metformin-induced gastrointestinal side effects. Am J Ther. 2003;10:447–451. doi: 10.1097/00045391-200311000-00012. [DOI] [PubMed] [Google Scholar]
- Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci. 2007;132:44–51. doi: 10.1016/j.autneu.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci. 2004;115:74–81. doi: 10.1016/j.autneu.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111(Suppl. 8A):106S–112S. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Isaacs B. The influence of hyoscine and atropine on apomorphine-induced vomiting in man. Clin Sci (Lond) 1956;15:177–182. [PubMed] [Google Scholar]
- Isaacs B, Macarthur JG. Influence of chlorpromazine and promethazine on vomiting induced with apomorphine in man. Lancet. 1954;267:570–572. doi: 10.1016/s0140-6736(54)90352-9. [DOI] [PubMed] [Google Scholar]
- Isobe Y, Kurimoto A, Tobe M, Hashimoto K, Nakamura T, Norimura K, Ogita H, Takaku H. Synthesis and biological evaluation of novel 9-substituted-8-hydroxyadenine derivatives as potent interferon inducers. J Med Chem. 2006;49:2088–2095. doi: 10.1021/jm051089s. [DOI] [PubMed] [Google Scholar]
- Ji J, Bunnelle WH, Anderson DJ, Faltynek C, Dyhring T, Ahring PK, Rueter LE, Curzon P, Buckley MJ, Marsh KC, Kempf-Grote A, Meyer MD. A-366833: a novel nicotinonitrile-substituted 3,6-diazabicyclo[3.2.0]-heptane alpha4beta2 nicotinic acetylcholine receptor selective agonist: Synthesis, analgesic efficacy and tolerability profile in animal models. Biochem Pharmacol. 2007;74:1253–1262. doi: 10.1016/j.bcp.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Paton JF. Differential effects of angiotensin II in the nucleus tractus solitarii of the rat–plausible neuronal mechanism. J Physiol. 1999;521Pt1:227–238. doi: 10.1111/j.1469-7793.1999.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Fuger J, Moller HJ. Comparative efficacy of antidepressants. Drugs. 1992;43(Suppl. 2):11–22. doi: 10.2165/00003495-199200432-00004. discussion 22–3. [DOI] [PubMed] [Google Scholar]
- Kimmel AR, Parent CA. The signal to move: D. discoideum go orienteering. Science. 2003;300:1525–1527. doi: 10.1126/science.1085439. [DOI] [PubMed] [Google Scholar]
- King GL. Characterization of radiation-induced emesis in the ferret. Radiat Res. 1988;114:599–612. [PubMed] [Google Scholar]
- Klein RL, Militello TE, Ballinger CM. Antiemetic effect of metoclopramide… evaluation in humans. Anesth Analg. 1968;47:259–264. [PubMed] [Google Scholar]
- Koch KL, Stern RM, Vasey MW, Seaton JF, Demers LM, Harrison TS. Neuroendocrine and gastric myoelectrical responses to illusory self-motion in humans. Am J Physiol. 1990a;258:E304–310. doi: 10.1152/ajpendo.1990.258.2.E304. [DOI] [PubMed] [Google Scholar]
- Koch KL, Summy-Long J, Bingaman S, Sperry N, Stern RM. Vasopressin and oxytocin responses to illusory self-motion and nausea in man. J Clin Endocrinol Metab. 1990b;71:1269–1275. doi: 10.1210/jcem-71-5-1269. [DOI] [PubMed] [Google Scholar]
- Kowalski A, Rapps N, Enck P. Functional cortical imaging of nausea and vomiting: a possible approach. Auton Neurosci. 2006;129:28–35. doi: 10.1016/j.autneu.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Kraus T, Hosl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- Kris MG, Cubeddu LX, Gralla RJ, Cupissol D, Tyson LB, Venkatraman E, Homesley HD. Are more antiemetic trials with a placebo necessary? Report of patient data from randomized trials of placebo antiemetics with cisplatin. Cancer. 1996;78:2193–2198. doi: 10.1002/(sici)1097-0142(19961115)78:10<2193::aid-cncr22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kris MG, Gralla RJ, Clark RA, Tyson LB, O'Connell JP, Wertheim MS, Kelsen DP. Incidence, course, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol. 1985;3:1379–1384. doi: 10.1200/JCO.1985.3.10.1379. [DOI] [PubMed] [Google Scholar]
- Laffan RJ, Borison HL. Emetic action of nicotine and lobeline. J Pharmacol Exp Ther. 1957;121:468–476. [PubMed] [Google Scholar]
- Lang IM, Sarna SK, Shaker R. Gastrointestinal motor and myoelectric correlates of motion sickness. Am J Physiol. 1999;277:G642–652. doi: 10.1152/ajpgi.1999.277.3.G642. [DOI] [PubMed] [Google Scholar]
- Langley CK, Aziz Q, Bountra C, Gordon N, Hawkins P, Jones A, Langley G, Nurmikko T, Tracey I. Volunteer studies in pain research–opportunities and challenges to replace animal experiments: the report and recommendations of a Focus on Alternatives workshop. Neuroimage. 2008;42:467–473. doi: 10.1016/j.neuroimage.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Lee A. Adverse drug reactions. 2nd. London: Pharmaceutical Press; 2007. [Google Scholar]
- Lehmann A, Karrberg L. Effects of N-methyl-D-aspartate receptor antagonists on cisplatin-induced emesis in the ferret. Neuropharmacology. 1996;35:475–481. doi: 10.1016/0028-3908(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Leslie RA. Comparative aspects of the area postrema: fine-structural considerations help to determine its function. Cell Mol Neurobiol. 1986;6:95–120. doi: 10.1007/BF00711065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie RA, Reynolds DJM. Neurotransminers and receptors in the emetic pathway. In: Andrews PLR, Sanger GJ, editors. Emesis in Anti-Cancer Therapy: Mechanisms and Treatment. London: Chapman and Hall Medical; 1993. p. 91. [Google Scholar]
- Light RU, Bysshe SM. The administration of drugs into the cerebral ventricles of monkeys: pituitrin, certain pituitary fractions, pitressin, pitocin, histamine, acetyl scholine, and pilocarpine. J Pharmacol Exp Ther. 1933;47:17–36. [Google Scholar]
- Lindquist N, Hay ME. Can small rare prey be chemically defended? The case for marine larvae. Ecology. 1995;76:1347–1358. [Google Scholar]
- Liu YL, Malik N, Sanger GJ, Friedman MI, Andrews PL. Pica–a model of nausea? Species differences in response to cisplatin. Physiol Behav. 2005;85:271–277. doi: 10.1016/j.physbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Central autonomic pathways. In: Loewy AD, Spyer KM, editors. Central regulations of autonomic functions. New York: Oxford University Press; 1990. pp. 88–103. [Google Scholar]
- Mantovani G, Maccio A, Esu S, Lai P. Evidence that cisplatin induces serotonin release from human peripheral blood mononuclear cells and that methylprednisolone inhibits this effect. Eur J Cancer. 1996;32A:1983–1985. doi: 10.1016/0959-8049(96)00204-3. [DOI] [PubMed] [Google Scholar]
- Martin M. The severity and pattern of emesis following different cytotoxic agents. Oncology. 1996;53(Suppl 1):26–31. doi: 10.1159/000227637. [DOI] [PubMed] [Google Scholar]
- Mayer J. Use of behavior analysis to recognize pain in small mammals. Lab Anim (NY) 2007;36:43–48. doi: 10.1038/laban0607-43. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Higher order neurons in buccal ganglia of Pleurobranchaea elicit vomiting motor activity. J Neurophysiol. 1983;50:658–670. doi: 10.1152/jn.1983.50.3.658. [DOI] [PubMed] [Google Scholar]
- Milano S, Blower P, Romain D, Grelot L. The piglet as a suitable animal model for studying the delayed phase of cisplatin-induced emesis. J Pharmacol Exp Ther. 1995;274:951–961. [PubMed] [Google Scholar]
- Miller AD, Rowley HA, Roberts TP, Kucharczyk J. Human cortical activity during vestibular- and drug-induced nausea detected using MSI. Ann N Y Acad Sci. 1996;781:670–672. doi: 10.1111/j.1749-6632.1996.tb15755.x. [DOI] [PubMed] [Google Scholar]
- Minthorn E, Mencken T, King AG, Shu A, Rominger D, Gontarek RR, Han C, Bambal R, Davis CB. Pharmacokinetics and brain penetration of casopitant, a potent and selective neurokinin-1 receptor antagonist, in the ferret. Drug Metab Dispos. 2008;36:1846–1852. doi: 10.1124/dmd.108.021758. [DOI] [PubMed] [Google Scholar]
- Minton N, Swift R, Lawlor C, Mant T, Henry J. Ipecacuanha-induced emesis: a human model for testing antiemetic drug activity. Clin Pharmacol Ther. 1993;54:53–57. doi: 10.1038/clpt.1993.109. [DOI] [PubMed] [Google Scholar]
- Minton NA. Volunteer models for predicting antiemetic activity of 5-HT3-receptor antagonists. Br J Clin Pharmacol. 1994;37:525–530. doi: 10.1111/j.1365-2125.1994.tb04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morest DK. A study of the structure of the area postrema with Golgi methods. Am J Anat. 1960;107:291–303. doi: 10.1002/aja.1001070307. [DOI] [PubMed] [Google Scholar]
- Nagakura Y, Kakimoto S, Matsuoka N. Purinergic P2X receptor activation induces emetic responses in ferrets and Suncus murinus (house musk shrews) Br J Pharmacol. 2007;152:464–470. doi: 10.1038/sj.bjp.0707418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan JT, Watts R, Haddad N, Labar DR, Li PM, Filippi CG. Cerebral activation during vagus nerve stimulation: a functional MR study. Epilepsia. 2002;43:1509–1514. doi: 10.1046/j.1528-1157.2002.16102.x. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Meier JJ. Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regul Pept. 2005;128:135–148. doi: 10.1016/j.regpep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Nemoto M, Endo T, Minami M, Yoshioka M, Ito H, Saito H. 5-Hydroxytryptamine (5-HT)-induced depolarization in isolated abdominal vagus nerves in the rat: involvement of 5-HT3 and 5-HT4 receptors. Res Commun Mol Pathol Pharmacol. 2001;109:217–230. [PubMed] [Google Scholar]
- Niemegeers CJ. Antiemetic specificity of dopamine antagonists. Psychopharmacology (Berl) 1982;78:210–213. doi: 10.1007/BF00428152. [DOI] [PubMed] [Google Scholar]
- Niemegeers CJ. The apomorphine antagonism test in dogs. Experimental evidence and critical considerations on specific methodological criteria. Pharmacology. 1971;6:353–364. doi: 10.1159/000136263. [DOI] [PubMed] [Google Scholar]
- Nussey SS, Hawthorn J, Page SR, Ang VT, Jenkins JS. Responses of plasma oxytocin and arginine vasopressin to nausea induced by apomorphine and ipecacuanha. Clin Endocrinol (Oxf) 1988;28:297–304. doi: 10.1111/j.1365-2265.1988.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Atkinson-Leadbetter KP, Van Der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi T, Mori T, Yanagihara M, Furukawa N, Fukuda H. Similarities of the neuronal circuit for the induction of fictive vomiting between ferrets and dogs. Auton Neurosci. 2007;136:20–30. doi: 10.1016/j.autneu.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Osuide G, Adejoh PO. Effects of apomorphine and its interaction with other drugs in the domestic fowl. Eur J Pharmacol. 1973;23:56–66. doi: 10.1016/0014-2999(73)90244-6. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL. Conditioned gaping in rats: a selective measure of nausea. Auton Neurosci. 2006;129:36–41. doi: 10.1016/j.autneu.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Parrott RF, Ebenezer IS, Baldwin BA, Forsling ML. Hormonal effects of apomorphine and cholecystokinin in pigs: modification of the response to cholecystokinin by a dopamine antagonist (metoclopramide) and a kappa opioid agonist (PD117302) Acta Endocrinol (Copenh) 1991;125:420–426. doi: 10.1530/acta.0.1250420. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes; an investigation of the physiological activity of the cerebral cortex. New York: Dover Publications; 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchat ML, Rozin P. The special role of nausea in the acquisition of food dislikes by humans. Appetite. 1982;3:341–351. doi: 10.1016/s0195-6663(82)80052-4. [DOI] [PubMed] [Google Scholar]
- Percie Du Sert N, Andrews PLR. 6th World congress on Alternatives and Animal Use in the Life Sciences. Hotel East 21, Tokyo, Japan: Japanese Society for Alternatives to Animal Experiments; 2007. Systematic review of the ferret model of cisplatin-induced emesis. [Google Scholar]
- Percie Du Sert N, Rudd JA, Andrews PLR. Simultaneous telemetric recording of electrogastrogram (EGG) and emesis in the ferret. Neurogastroenterol Motil. 2007;19(Suppl 3):39. [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Jama. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Pickering M, Jones JF. The diaphragm: two physiological muscles in one. J Anat. 2002;201:305–312. doi: 10.1046/j.1469-7580.2002.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel E, Zhang Y, Pujol N, Matsuyama T, Bargmann CI, Ewbank JJ. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:2295–2300. doi: 10.1073/pnas.0610281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor JD, Chremos AN, Evans EF, Wasserman AJ. An apomorphine-induced vomiting model for antiemetic studies in man. J Clin Pharmacol. 1978;18:95–99. doi: 10.1002/j.1552-4604.1978.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Reid K, Palmer JL, Wright RJ, Clemes SA, Troakes C, Somal HS, House F, Stott JR. Comparison of the neurokinin-1 antagonist GR205171, alone and in combination with the 5-HT3 antagonist ondansetron, hyoscine and placebo in the prevention of motion-induced nausea in man. Br J Clin Pharmacol. 2000;50:61–64. doi: 10.1046/j.1365-2125.2000.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk AN, Hesketh PJ. Antiemetics for cancer chemotherapy-induced nausea and vomiting. A review of agents in development. Drugs R D. 1999;2:229–235. doi: 10.2165/00126839-199902040-00001. [DOI] [PubMed] [Google Scholar]
- Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262–269. doi: 10.1016/s0028-3908(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Andrews PL. Mechanisms of acute, delayed and anticipatory vomiting in cancer and cancer treatment. In: Hesketh P, editor. Management of nausea and vomiting in cancer and cancer treatment. New York: Jones and Barlett Publishers Inc.; 2004. pp. 15–66. [Google Scholar]
- Rudd JA, Jordan CC, Naylor RJ. Profiles of emetic action of cisplatin in the ferret: a potential model of acute and delayed emesis. Eur J Pharmacol. 1994;262:R1–2. doi: 10.1016/0014-2999(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Naylor JR. Opioid receptor involvement in emesis and antiemesis. In: Reynolds DJM, Andrews PLR, Davis CJ, editors. Serotonin and the Scientific Basis of Anti-Emetic Therapy. Oxford: Clinical Communications; 1995. pp. 208–221. [Google Scholar]
- Russell WMS, Burch RL. The principles of humane experimental technique. London: Methuen & Co Ltd.; 1959. [Google Scholar]
- Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci. 2006;129:3–16. doi: 10.1016/j.autneu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Saxena PN, Chawla N, Johri MB, Iqbal S. Nature of receptors involved in apomorphine responses in pigeons. Psychopharmacology (Berl) 1977;53:89–95. doi: 10.1007/BF00426699. [DOI] [PubMed] [Google Scholar]
- Schein PS, Davis RD, Carter S, Newman J, Schein DR, Rall DP. The evaluation of anticancer drugs in dogs and monkeys for the prediction of qualitative toxicities in man. Clin Pharmacol Ther. 1970;11:3–40. doi: 10.1002/cpt19701113. [DOI] [PubMed] [Google Scholar]
- Share NN, Chai CY, Wang SC. Emesis Induced by Intracerebroventricular Injections of Apomorphine and Deslanoside in Normal and Chemoreceptive Trigger Zone Ablated Dogs. J Pharmacol Exp Ther. 1965;147:416–421. [PubMed] [Google Scholar]
- Shields KG, Ballinger CM, Hathaway BN. Antiemetic effectiveness of haloperidol in human volunteers challenged with apomorphine. Anesth Analg. 1971;50:1017–1024. doi: 10.1213/00000539-197150060-00023. [DOI] [PubMed] [Google Scholar]
- Shishido Y, Wakabayashi H, Koike H, Ueno N, Nukui S, Yamagishi T, Murata Y, Naganeo F, Mizutani M, Shimada K, Fujiwara Y, Sakakibara A, Suga O, Kusano R, Ueda S, Kanai Y, Tsuchiya M, Satake K. Discovery and stereoselective synthesis of the novel isochroman neurokinin-1 receptor antagonist ‘CJ-17,493. Bioorg Med Chem. 2008;16:7193–7205. doi: 10.1016/j.bmc.2008.06.047. [DOI] [PubMed] [Google Scholar]
- Smith JA, Jennings M. Categorising the severity of scientific procedures on animals: Summary and reports from three round-table discussions http://www.boyd-group.demon.co.uk/
- Smith JE, Paton JF, Andrews PL. An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing. Exp Physiol. 2002;87:563–574. doi: 10.1113/eph8702424. [DOI] [PubMed] [Google Scholar]
- Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457–461. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- Tajima T, Takiguchi N, Kato J, Ikeda T, Kuroda A, Ohtake H. Mutants of the nematode Caenorhabditis elegans that are defective specifically in their attraction to cycloheximide. J Biosci Bioeng. 2003;96:149–153. doi: 10.1016/s1389-1723(03)90117-4. [DOI] [PubMed] [Google Scholar]
- Tajima T, Watanabe N, Kogawa Y, Takiguchi N, Kato J, Ikeda T, Kuroda A, Ohtake H. Chemotaxis of the nematode Caenorhabditis elegans toward cycloheximide and quinine hydrochloride. J Biosci Bioeng. 2001;91:322–324. doi: 10.1263/jbb.91.322. [DOI] [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- Tanihata S, Igarashi H, Suzuki M, Uchiyama T. Cisplatin-induced early and delayed emesis in the pigeon. Br J Pharmacol. 2000;130:132–138. doi: 10.1038/sj.bjp.0703283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MS, Dekkers MP, Ng BL, Rademakers S, Jansen G, Fraser AG, McCafferty J. Expression of mammalian GPCRs in C. elegans generates novel behavioural responses to human ligands. BMC Biol. 2006;4:22. doi: 10.1186/1741-7007-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiersch TR, Griffith JS. Apomorphine-induced vomiting in rainbow trout (Salmo gairdneri) Comp Biochem Physiol A. 1988;91:721–725. doi: 10.1016/0300-9629(88)90956-5. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Kondysar MH, Harding RK. Emesis, radiation exposure, and local cerebral blood flow in the ferret. Radiat Res. 1988;114:537–549. [PubMed] [Google Scholar]
- Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci. 1987;41:513–518. doi: 10.1016/0024-3205(87)90229-3. [DOI] [PubMed] [Google Scholar]
- Van Oudenhove L, Coen SJ, Aziz Q. Functional brain imaging of gastrointestinal sensation in health and disease. World J Gastroenterol. 2007;13:3438–3445. doi: 10.3748/wjg.v13.i25.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, Sharkey KA. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Richardson DW, Stricker EM. Vasopressin release in response to nausea-producing agents and cholecystokinin in monkeys. Am J Physiol. 1987;252:R749–753. doi: 10.1152/ajpregu.1987.252.4.R749. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lavond DG, Chambers KC. The effects of cooling the area postrema of male rats on conditioned taste aversions induced by LiC1 and apomorphine. Behav Brain Res. 1997;82:149–158. doi: 10.1016/s0166-4328(97)80984-9. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Okamoto M, Ishii T, Takatsuka S, Taniguchi H, Nagasaki M, Saito A. Long-lasting anti-emetic effect of T-2328, a novel NK(1) antagonist. J Pharmacol Sci. 2008;107:151–158. doi: 10.1254/jphs.08027fp. [DOI] [PubMed] [Google Scholar]
- Watson JW, Gonsalves SF, Fossa AA, McLean S, Seeger T, Obach S, Andrews PL. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol. 1995;115:84–94. doi: 10.1111/j.1476-5381.1995.tb16324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Boeckeler K, Graf R, Muller-Taubenberger A, Li Z, Isberg RR, Wessels D, Soll DR, Alexander H, Alexander S. Towards a molecular understanding of human diseases using Dictyostelium discoideum. Trends Mol Med. 2006;12:415–424. doi: 10.1016/j.molmed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Wang J, Bourne PE. In silico elucidation of the molecular mechanism defining the adverse effect of selective estrogen receptor modulators. PLoS Comput Biol. 2007;3:e217. doi: 10.1371/journal.pcbi.0030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuni H, Sawai H, Maeda Y, Imazumi K, Sakuma H, Matsuo M, Mutoh S, Seki J. Probable involvement of the 5-hydroxytryptamine(4) receptor in methotrexate-induced delayed emesis in dogs. J Pharmacol Exp Ther. 2000;292:1002–1007. [PubMed] [Google Scholar]
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998;47:395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hang L, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]


