Abstract
Phosphodiesterase (PDE)4, and to a lesser extent, PDE3/4 inhibitors have attracted considerable interest as potential therapeutic agents for diseases including chronic obstructive pulmonary disease. Indeed, ibudilast and theophylline are utilized clinically, and roflumilast is in late-stage clinical development. Unfortunately, however many PDE4 and dual PDE3/4 inhibitors have failed in early development due to low therapeutic ratios. The majority of these compounds are however orally administered and non-selective for either PDE3(A, B) or PDE4(A, B, C, D) subtypes. Developing an inhaled dual PDE3/4 inhibitor with subtype specificity may represent one strategy to improve the therapeutic index. Indeed combined inhibition of PDE3 and PDE4 inhibitor has additive and synergistic anti-inflammatory and bronchodilatory effects versus inhibition of either PDE3 or PDE4 alone. Given that synergy has been seen in terms of efficacy end points, an obvious concern is that synergy may also be observed in side effects. Interestingly, however, no synergy or additive effects with a combination of a PDE3 and PDE4 inhibitor in a cardiomyocyte assay were observed. This review will summarize the rationale for developing an inhaled dual PDE3/4 inhibitor, as a treatment for chronic obstructive pulmonary disease together with recent advances in trying to understand the pathogenesis of PDE inhibitor-induced mesenteric vasculitis (a key potential dose-limiting side effect of these agents), highlighting potential early and sensitive predictive biomarkers.
Keywords: phosphodiesterase, chronic obstructive pulmonary disease, synergistic, anti-inflammatory, bronchodilation
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality and is currently the fourth most common cause of death in the world according to the World Health Organisation (WHO). The WHO estimates that by 2020, COPD will be the third leading cause of death and the fifth leading cause of disability worldwide (Murray and Lopez, 1997). It is estimated that more than one million people in the UK suffer from this disease (Barnes, 1998), with the regional (adult) prevalence in 2000 varying from 0.5% in parts of Africa to 3–4% in North America (reviewed by Lopez et al., 2006). COPD is defined as ‘a preventable and treatable disease with some significant extrapulmonary effects that may contribute to the severity in individual patients. Its pulmonary component is characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases’[Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2006] (Pauwels et al., 2001; Rabe, 2006). Airflow limitation in COPD patients results from mucosal inflammation and oedema, bronchoconstriction, increased secretions in the airways and loss of elastic recoil. Risk factors for the development of COPD include cigarette smoking and occupational exposure to dust and chemicals (Zaher et al., 2004; Blanc et al., 2008). Inhaled corticosteroids and bronchodilator agents are the main therapies used for the treatment of COPD (Gosens et al., 2006) and have modest beneficial effects on health-related quality of life and FEV1 (Mahler et al., 2002). Steroids, are however, relatively ineffective at suppressing the airway wall thickening and luminal occlusion in COPD patients (Hogg et al., 2007) and the consequent disease progression. There is thus a high unmet medical need for novel effective therapies to treat COPD.
Inflammation is a prominent feature of COPD, highlighted by the presence of activated CD8+ T-and B-lymphocytes, neutrophils, macrophages and dendritic cells in the bronchial mucosa, together with increased levels of pro-inflammatory mediators [e.g. IL-6, IL-1β, tumour necrosis factor (TNF)-α, Gro-α, MCP-1 and IL-8] in the lung (Saetta et al., 1993; Riise et al., 1995; Vassallo et al., 2008). These cell types persist even in the absence of overt infection (Barnes, 2003). It has been hypothesized that an increased oxidant burden, both directly as a result of smoking, or indirectly by the release of increased amounts of reactive oxygen species from airspace leukocytes may not be sufficiently counteracted by the lung's antioxidant systems, resulting in oxidant stress. Excessive oxidants may then lead to an increase in pro-inflammatory gene expression and protein release, inactivation of anti-proteases and oxidative tissue injury leading to COPD. Exacerbations of COPD are considered to reflect a worsening of the underlying chronic inflammation in the airways and are characterized by a marked elevation in neutrophils and their markers (e.g. neutrophil elastase) in the airways (Qiu et al., 2003; Drost et al., 2005), together with a significant increase in cytokines such as IL-8 and TNF-α, which are known to act as chemoattractants for neutrophils (Drost et al., 2005). Interestingly, a significant correlation has been observed between the percentage of neutrophils in bronchoalveolar lavage fluid and severity of airways obstruction assessed by FEV1/FVC ratio (Drost et al., 2005). Increases in eosinophils have also been reported in patients undergoing exacerbations of chronic bronchitis (Saetta et al., 1994).
Chronic obstructive pulmonary disease is also characterized by goblet cell hyperplasia and submucosal gland hypertrophy associated with loss of ciliated epithelial cell numbers and function, which leads to reduced mucociliary clearance and mucus plug formation (Maestrelli et al., 2001). Hyper-production of mucus, together with reduced mucociliary clearance is thought to also contribute to the airways obstruction observed in COPD (Rogers, 2005).
Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are second messengers that regulate a number of critical cellular processes such as metabolism, cell proliferation and differentiation, secretion, vascular and airway smooth muscle relaxation and the release of inflammatory mediators (Beavo and Brunton, 2002). The phosphodiesterase (PDE) enzyme family hydrolyses, cAMP and cGMP, to inactive 5′AMP and 5′GMP respectively, and thus inhibition of PDEs represents a potential mechanism by which cellular processes can be modulated (Conti et al., 2003). Eleven major PDE gene families have been identified, denoted PDE1–11, which differ in primary structures, affinities for cAMP and cGMP, responses to specific effectors, sensitivities to specific inhibitors and mechanisms of regulation (Bingham et al., 2006). Each family contains at least one member, and in some cases the members are products of more than one gene.
PDE4 is described as a low km (∼1–10 µM) cAMP-specific PDE with only a weak affinity for cGMP (km > 50 µM). The PDE4 family is comprised of four genes (PDE4A, B, C, D), with each gene having multiple splice variants. PDE4 gene products have a broad tissue distribution; including the brain, gastrointestinal tract, spleen, lung, heart, testis and kidney (Zhang et al., 2005). In addition PDE4 is expressed in almost all inflammatory cell types except blood platelets (Peachell et al., 1992; Giembycz et al., 1996; Ito et al., 1996; Gantner et al., 1998; Wright et al., 1998;Baroja et al., 1999; Fuhrmann et al., 1999; Liu and Maurice, 1999; Wang et al., 1999; Landells et al., 2001; LeJeune et al., 2002; Pryzwansky and Madden, 2003; Barber et al., 2004; Netherton and Maurice, 2005; Tilley and Maurice, 2005; Millen et al., 2006; Netherton et al., 2007; Peter et al., 2007; Billington et al., 2008; Campos-Toimil et al., 2008).
PDE3 hydrolyses both cAMP and cGMP with relatively high affinities (km cAMP < 0.4 µM; km cGMP < 0.3 µM); however, the Vmax for cAMP hydrolysis is nearly 10-fold higher than for cGMP. Two genes have been identified for PDE3 known as PDE3A and PDE3B that have >80% amino acid identity for the catalytic region. Splice variants have only conclusively been demonstrated for the 3A isoform. PDE3A is expressed in platelets, as well as in vascular smooth muscle, cardiac myocytes, oocytes (Shakur et al., 2001) and B-lymphocytes (Gantner et al., 1998). PDE3B is relatively highly expressed in adipocytes, hepatocytes and spermatocytes, but can also be detected in vascular smooth muscle cells, the pancreas, T-lymphocytes and macrophages (Shakur et al., 2001). To date, there have been no inhibitors described that clearly distinguish between PDE3A and PDE3B, although there are two reports of compounds showing approximately 10-fold selectivity (Sudo et al., 2000; Edmondson et al., 2003).
While PDE4 inhibitors are very efficacious at inhibiting pro-inflammatory mediator release from certain cell types (e.g. neutrophils, eosinophils) (Banner et al., 1996; Hatzelmann and Schudt, 2001; Trevethick et al., 2007a), there is evidence to suggest that dual inhibition of PDE3 and PDE4 is additive or synergistic at suppressing the activation/functions of other cell types, which are thought to play a role in COPD (e.g. macrophages, dendritic cells, epithelial cells, lymphocytes, airway smooth muscle cells and endothelial cells) (Schudt et al., 1993; Giembycz et al., 1996; Wright et al., 1998; Blease et al., 1998; Gantner et al., 1999). In addition, PDE4 or PDE3 inhibitors alone are unable to inhibit spasmogen-induced contraction of human airway, but in combination act synergistically (Schmidt et al., 2000). PDE4 inhibitors have also been shown to activate the cystic fibrosis transmembrane conductance regulator (CFTR)-mediated Cl− secretion, suggesting they may be able to stimulate mucociliary clearance (Liu et al., 2005), and a PDE3 inhibitor has been shown to inhibit cough (Ishiura et al., 2005). This diverse spectrum of biological effects has thus implicated PDE4 and PDE3/4 inhibitors as potential therapeutic agents for a range of disease indications including COPD. Orally administered non-isoform-selective PDE4 inhibitors, do, however, have a low therapeutic ratio. It is conceivable that administration of a dual PDE3/4 inhibitor by the inhaled route may offer increased efficacy with a reduced side effect potential versus an orally administered PDE4 inhibitor.
In vitro effects of PDE3 and PDE4 inhibitors along with evidence for synergy
Monocyte and macrophage
Macrophage numbers are significantly increased in bronchial biopsies from patients with COPD (O'Shaughnessy et al., 1997), and subepithelial macrophages increase with severity of disease and the presence of airways obstruction (Di Stefano et al., 1998). Given the capacity of the macrophage to release a range of pro-inflammatory mediators that are increased in COPD patients, this cell type has been implicated in the tissue injury associated with COPD.
All four subtypes of PDE4 have been detected in human lung macrophages and peripheral blood monocytes (Tenor et al., 1995; Barber et al., 2004), and PDE3B has been detected in macrophages (Shakur et al., 2001). Interestingly, PDE4A4 is up-regulated in lung macrophages from smokers with COPD compared with control smokers (Barber et al., 2004), and increased amounts of PDE4A4 and PDE4B2 have been detected in peripheral blood monocytes from smokers versus non-smokers (Barber et al., 2004). Indeed, PDE4B2 appears to be the predominant PDE isoform in human monocytes (Wang et al., 1999) and is selectively induced by lipopolysaccharide (LPS). This induction is inhibited by IL-4 and IL-10. PDE4 inhibitors are capable of completely abolishing LPS-stimulated TNF-α release from peripheral blood monocytes (Molnar-Kimber et al., 1993; Manning et al., 1999) and interestingly, in PDE4B−/− mice (but not PDE4D−/− mice), there is a marked reduction in the ability of LPS to stimulate TNF-α release from peripheral blood leukocytes (Jin and Conti, 2002), suggesting a key role for PDE4B in this response. Further support for PDE4B involvement in LPS-induced TNF-α release from monocytes can be derived from a separate study demonstrating that mean IC50 values for inhibition of LPS-stimulated TNF-α release significantly correlated with compound potency against the catalytic activity of recombinant human PDE4B (and PDE4A), but not the catalytic activity of recombinant human PDE4D (Manning et al., 1999). In contrast to studies with monocytes however, only a partial inhibitory effect of PDE4 inhibitors is observed on LPS-stimulated TNF-α release from human alveolar macrophages (Schudt et al., 1993). This appears to be due to the presence of PDE3B (Shakur et al., 2001), as a dual PDE3/4 inhibitor can completely suppress this effect (Schudt et al., 1993). This clearly suggests that combined inhibition of PDE3 and PDE4 is required for maximal inhibition of pro-inflammatory mediator release from macrophages.
Dendritic cells
A significant increase in dendritic cells has been observed in the small airways of patients with COPD, with a positive correlation between dendritic cell infiltration and disease severity (Demedts et al., 2007).
PDE3 and PDE4 have been identified in monocyte-derived dendritic cells (Gantner et al., 1999) with PDE4A appearing to be the predominant PDE4 isoform (Heystek et al., 2003), although PDE4B and D are also present (Heystek et al., 2003). Interestingly, while the PDE4 inhibitor rolipram only partially inhibited (by 37%) LPS-stimulated TNF-α release from dendritic cells, and the PDE3 inhibitor, motapizone, only had a minimal inhibitory effect, in combination, rolipram and motapizone acted in a synergistic manner to reduce TNF-α production in dendritic cells by 82% (Gantner et al., 1999). Similar to the macrophage, this also suggests that dual inhibition of PDE3 and PDE4 is required to effectively suppress the pro-inflammatory activity of dendritic cells.
Lymphocytes
Increased numbers of CD3+ and CD8+ T-lymphocytes have been found in bronchial biopsies from COPD patients versus normal people (O'Shaughnessy et al., 1997).
PDE4A, B and D (but not PDE4C) and PDE3 has been detected in human T- and B-lymphocytes (Giembycz et al., 1996; Gantner et al., 1998; Baroja et al., 1999; Landells et al., 2001; Barber et al., 2004). In terms of PDE3 isoform expression, PDE3A has been detected in B-lymphocytes (Gantner et al., 1998), and PDE3B has been identified in T-lymphocytes (Shakur et al., 2001).
In a recent study PDE4 subtype-specific siRNAs were used to investigate the functional impact of subtype-specific knockdown on anti-CD3/CD28-stimulated cytokine release from CD4+ T-cells. Knockdown of PDE4B or PDE4D (but not PDE4A) inhibited IL-2 release, whereas knockdown of PDE4D showed the most predominant inhibitory effect on interferon-γ and IL-5 release (Peter et al., 2007). PDE4 inhibitors have also been shown to partially inhibit IL-4 and IL-5 gene expression in TH2 cells (Essayan et al., 1997), together with IL-4 and IL-5 release from human CD4+ T-cells (Tenor et al., 1996). In contrast, a separate study demonstrated that specific inhibition of PDE4 had no significant effect on TH2 cell-mediated IL-4 or IL-13 generation, but preferentially inhibited TH1 cell cytokine generation (IFN-γ) (Claveau et al., 2004). PDE4 inhibitors have also been shown to partially inhibit phytohaemagglutinin and anti-CD3/CD28-stimulated proliferation of CD4+ and CD8+ T-cells (Giembycz et al., 1996; Hatzelmann and Schudt, 2001). In a separate study dual PDE4A/B- and PDE4D-selective inhibitors inhibited antigen-stimulated human T-cell proliferation, with mean IC50 values significantly correlating with compound potency against the catalytic activity of recombinant PDE4A or B, but not with catalytic activity of recombinant PDE4D (Manning et al., 1999). In contrast, a PDE4D siRNA (but not PDE4A or B siRNAs) also significantly inhibited anti-CD3/CD28-stimulated CD4+ proliferation (Peter et al., 2007). The reason for this apparent difference in PDE4 subtype involvement in this proliferative response is unclear, but could be related to the fact that different T-cell populations were used, or the fact that different stimuli were used to elicit proliferation. As with macrophages and dendritic cells, there is evidence for PDE3 and PDE4 inhibitors acting synergistically. Specifically, while the PDE4 inhibitor, rolipram only partially inhibited (by 40–60%) mitogen-stimulated IL-2 release from CD4+ and CD8+ human T-cells (Giembycz et al., 1996; Hatzelmann and Schudt, 2001) and the PDE3 inhibitor, SK&F 95654 had no effect alone, SK&F 95654 potentiated the ability of rolipram to suppress mitogen-stimulated IL-2 release (Giembycz et al., 1996).
Airway epithelial cells
In addition to their role in lung defence, airway epithelial cells are believed to play an integral role in the pathophysiology of airway diseases such as COPD through their ability to release multiple pro-inflammatory and pro-remodelling mediators (Wright et al., 1998). Indeed, the lung epithelium is one of the first targets of cigarette smoke. In addition, CFTR is the primary cAMP-activated chloride channel on the apical membrane of airway epithelia, and thus plays an integral role in controlling the electrolyte/fluid balance and regulating mucociliary clearance (Liu et al., 2005). Indeed activation of CFTR has the potential to enhance mucociliary clearance, which may be of benefit in COPD.
PDE4A, C and D and PDE3 are expressed in human airway epithelial cells (Dent et al., 1998; Wright et al., 1998; Fuhrmann et al., 1999). Interestingly, the PDE4 inhibitor, rolipram, only partially inhibited IL-1β-stimulated GMCSF release from human airway epithelial cells and A549 cells; whereas treatment with a dual PDE3/4 inhibitor (ORG-9935) completely suppressed this effect (Wright et al., 1998). In addition, while the PDE4 inhibitor, rolipram inhibited LPS-stimulated IL-6 release from human airway epithelial cells, relatively high concentrations were required to see an inhibitory effect, with an IC50 of 24 µM, suggesting the effect could have been mediated through other PDE enzymes such as PDE3 (Haddad et al., 2002). It would thus appear that dual inhibition of PDE3 and PDE4 is required to maximally suppress pro-inflammatory mediator release from epithelial cells. In addition, inhibition of PDE4 (in particular the D isoform) and also inhibition of PDE3 has been shown to activate CFTR-mediated chloride secretion in an epithelial cell line (Kelley et al., 1995; Liu et al., 2005) suggesting that PDE3/4 inhibitors may have additional benefit in COPD by being able to enhance mucocilary clearance.
Airway smooth muscle
Vagal tone is increased in airway inflammation associated with COPD resulting from exaggerated acetylcholine release and enhanced expression of downstream signalling components in airway smooth muscle (reviewed by Gosens et al., 2006).
PDE3 together with PDE4B and D are expressed in human airway smooth muscle cells (Rabe et al., 1993; LeJeune et al., 2002; Billington et al., 2008). Some studies have demonstrated that PDE4 inhibitors can relax inherent tone in isolated human bronchial muscle (Naline et al., 1996; Schmidt et al., 2000), while other studies have found that PDE3 or PDE4 inhibitors alone are ineffective, but in combination effectively relax inherent tone (Rabe et al., 1993). In addition, PDE3 or PDE4 inhibition alone had no effect on allergen- or LTC4-induced contraction of human airway smooth muscle, but in combination acted synergistically to inhibit contraction (Schmidt et al., 2000). Interestingly, in a study using siRNA targeted to PDE4D5, this PDE4 splice variant was shown to be the key physiological regulator of β2 adrenoceptor-induced cAMP turnover within human airway smooth muscle (LeJeune et al., 2002; Billington et al., 2008). Further evidence to support a key role for the D isoform in the contractile response of airway smooth muscle can be derived from a study with PDE4D−/− mice, in which a significant disruption in airway smooth muscle contractility was observed in isolated tracheas from PDE4D−/− mice, highlighted by a marked reduction in maximal tension and reduced sensitivity to muscarinic cholinergic agonists (Méhats et al., 2003).
It is likely that airway smooth muscle cells also contribute to airway remodelling observed in COPD, through the release of growth factors, cytokines and extracellular matrix (ECM) proteins (Burgess et al., 2006). Interestingly, the PDE4 inhibitor roflumilast can inhibit both transforming growth factor (TGF)-β-induced fibronectin deposition in human airway smooth muscle cells and also TGF-β-induced CTGF, collagen I and fibronectin expression in human bronchial tissue rings (Burgess et al., 2006).
Airway nerves
Airway smooth muscle receives cholinergic, adrenergic and non-adrenergic, non-cholinergic (NANC) neural input. In guinea-pig airways cholinergic and NANC nerves provide contractile innervation, while adrenergic and NANC nerves provide relaxant pathways. In contrast, the major relaxant innervation in human airways is NANC in nature. PDE3 and to a greater extent PDE4 inhibitors have been shown to inhibit NANC contractions in guinea-pig isolated bronchus while having no effect on electrical field-stimulated cholinergic contractions (Qian et al., 1994; Undem et al., 1994). In addition, PDE4 inhibitors have been shown to potentiate NANC relaxation of human isolated bronchus (Fernandes et al., 1994). This data are reviewed by Fernandes (1996) in detail. Modulation of the neural control of airway smooth muscle may thus represent another mechanism by which PDE3 and PDE4 inhibitors can influence airways function.
Endothelial cells
The endothelium acts as a major permeability barrier of the blood vessel wall and facilitates the transmigration of blood cells to tissue, through expression of adhesion molecules. Inhibition of adhesion molecule expression could thus be one potential strategy to inhibit excessive recruitment of inflammatory cells to the lungs.
Human aortic, umbilical vein and microvascular endothelial cells express PDE4A, B and D (Netherton and Maurice, 2005; Netherton et al., 2007; Campos-Toimil et al., 2008) and PDE3 (Suttorp et al., 1993; Suttorp et al., 1996). While, inhibition of PDE4 in combination with appropriate activation of adenylate cyclase could inhibit TNF-α-induced E-selectin expression on human lung microvascular endothelial cells (Blease et al., 1998), combined inhibition of PDE3 and PDE4 had a synergistic inhibitory effect on vascular cell adhesion molecule-1 expression and eosinophil adhesion to activated human lung microvascular endothelial cells (Blease et al., 1998).
In addition to the role of the endothelium in facilitating transmigration of cells, during inflammation, leukocytes may damage endothelial cells resulting in an increased vascular permeability (Suttorp et al., 1993). The PDE4 inhibitor, rolipram has been shown to potently block H2O2-induced endothelial permeability when combined with PGE1 (Suttorp et al., 1993), thus suggesting this may be an additional beneficial effect of PDE4 inhibition.
Fibroblasts
Pulmonary fibroblast to myofibroblast conversion is a pathophysiological feature of COPD (Dunkern et al., 2007), which results in an increase in the production of ECM degrading enzymes. Matrix metalloproteinases (MMPs) are involved in the proteolytic degradation of the ECM and are thus thought to play an important role in the destruction of the elastin fibres in the lung parenchyma of COPD patients. Therapies that mitigate the fibrotic process may thus be able to slow progressive loss of airways function observed in COPD.
PDE4 is expressed in human fibroblasts, although the subtype(s) present have not yet been defined (Dunkern et al., 2007). The PDE4 inhibitor, piclamilast, has been shown to inhibit lung fibroblast to myofibroblast differentiation (induced by TGF-β) (Dunkern et al., 2007). In addition, PDE4 inhibitors can inhibit TNF-α-stimulated pro-MMP1 and pro-MMP2 release from human lung fibroblasts (Martin-Chouly et al., 2004; Lagente et al., 2005) as well as the chemotaxis of fetal lung fibroblasts towards fibronectin (Kohyama et al., 2002).
Neutrophils
Neutrophils are thought to play a pivotal role in chronic lung inflammation and tissue destruction present in COPD through their ability to release many toxic substances such as proteases and oxygen radicals, which cause tissue injury and remodelling (Watt et al., 2005; Tirouvanziam, 2006).
PDE4A, B and D are expressed in human neutrophils (Wang et al., 1999; Pryzwansky and Madden, 2003; Barber et al., 2004) with evidence that PDE4B2 is the predominant PDE4 isoform (Wang et al., 1999). PDE4A is exclusively located within a subset of myeloperoxidase containing neutrophil granules (Pryzwansky and Madden, 2003). PDE4 inhibitors can inhibit the release of a range of pro-inflammatory mediators from human neutrophils. For example, they inhibit fMLP-stimulated release of LTB4 and reactive oxygen species (Hatzelmann and Schudt, 2001; Trevethick et al., 2007a), together with the fMLP/TNF-α-stimulated release of MMP-9 and neutrophil degranulation products such as neutrophil elastase and myeloperoxidase (Jones et al., 2005). PDE4 inhibitors can also inhibit platelet activating factor (PAF)-induced CD11b expression and L-selectin shedding by ∼50% (Berends et al., 1997) and also TNF-α- and fMLP-mediated neutrophil adhesion to human umbilical vein endothelial cells (Derian et al., 1995; Jones et al., 2005). PDE4 inhibitors have also been shown to delay spontaneous human neutrophil apoptosis (Parkkonen et al., 2008).
Eosinophils
Eosinophils can release a plethora of pro-inflammatory mediators, which cause tissue injury, remodelling and contraction of smooth muscle (Watt et al., 2005), and elevated numbers of eosinophils have been detected in lung secretions and subepithelial regions of central airway wall of individuals undergoing exacerbations of chronic bronchitis (Saetta et al., 1994).
PDE4A, B and D, but not PDE3, have been detected in human eosinophils, with evidence to suggest that PDE4A is exclusively located in all eosinophil granules (Pryzwansky and Madden, 2003). A range of PDE4 inhibitors have been shown to inhibit fMLP-stimulated release of reactive oxygen species from human eosinophil (Hatzelmann and Schudt, 2001) as well as inhibiting C5a and PAF-stimulated LTC4 synthesis (Tenor et al., 1996). PDE4 inhibitors can also inhibit PAF-induced CD11b expression and L-selectin shedding by ∼50% (Berends et al., 1997), P- and E-selectin expression (Sanz et al., 2002) and also C5a and PAF-stimulated eosinophil chemotaxis (Tenor et al., 1996; Cooper et al., 1999). In addition, they have also been shown to delay spontaneous human eosinophil apoptosis (Parkkonen et al., 2008).
Mechanisms underlying synergy
The mechanism(s) underlying the apparent synergistic effects of dual PDE3/4 inhibition are unclear. Interestingly, it has been reported that PDE3 inhibitors alone have little or no effect on total intracellular cAMP levels in T-lymphocytes (Giembycz et al., 1996), and they do not further enhance the cAMP accumulation induced by rolipram in polymorphonuclear cells (Denis and Riendeau, 1999). It has, however, been suggested that PDE3 (which is predominantly localized to the particulate cellular fraction) and PDE4 (which is predominantly cytosolic) may regulate different pools of cAMP (Denis and Riendeau, 1999).
In vivo effects of PDE3 and PDE4 inhibitors and evidence for efficacy via the inhaled route
In acute cigarette smoke exposure studies in mice, oral treatment with the PDE4 inhibitor, cilomilast inhibited neutrophil recruitment to the lung as well as suppressing the increase in MIP-1β in bronchoalveolar lavage fluid (Leclerc et al., 2006), and in a separate study, oral treatment with roflumilast partially inhibited neutrophil influx to the lung (Martorana et al., 2005). In more chronic cigarette smoke exposure studies, roflumilast (oral treatment for 7 months) has been shown to fully prevent emphysema in mice (Martorana et al., 2005), and the PDE4 inhibitor, GPD-1116 (oral treatment for 8 weeks) also markedly attenuated the development of cigarette smoke-induced emphysema in the senescence-accelerated mice P1 strain (Mori et al., 2008). PDE4 inhibitors have also been shown to reduce MMP-9 and TGF-β release during LPS-induced lung injury in mice (Lagente et al., 2005).
Local administration of PDE4 and dual PDE3/4 inhibitors directly to the lung has also been shown to be effective at inhibiting LPS-induced neutrophil recruitment to the lung in a range of species: rats (Kuss et al., 2003; Trevethick et al., 2007b), ferrets (Kuss et al., 2003) and pigs (Kuss et al., 2003). In addition local administration of an inhaled PDE3/4 inhibitor, SDZ ISQ 844 in dogs decreased airways responsiveness to inhaled methacholine at a dose that did not affect base-line respiratory resistance (Horikoshi et al., 1994).
In studies with PDE4 subtype deficient mice, PDE4B and PDE4D (but not PDE4A) appeared to be important in mediating LPS-induced neutrophil recruitment to the lung, as neutrophil migration was inhibited by 31% and 48% in PDE4B−/− and PDE4D−/− mice respectively. These effects were associated with a reduction in the expression of CD18, but not CD11 (Ariga et al., 2004) (Table 1), suggesting that PDE4B and PDE4D inhibition of adhesion molecule expression may contribute to the reduced neutrophil recruitment.
Table 1.
Phenotypes of PDE4A, B and D knockout mice
| PDE4A | PDE4B | PDE4D | |
|---|---|---|---|
| Physiological | |||
| Neonatal growth, survival and fertility | Normal | Normal | Impaired |
| Anti-inflammatory | |||
| LPS-stimulated TNF-α release from circulating leukocytes | Normal | 90% Reduction | Normal |
| LPS-induced neutrophil recruitment to lung | Normal | 31% Reduction | 48% Reduction |
| Airway contractility | |||
| Allergen and cholinergic agonist-induced increase in airway hyper-reactivity | Reduced | Reduced | Absent |
| Cholinergic agonist-induced tracheal contractility | Normal | Normal | 34% Reduction in maximum efficacy; fivefold reduction in sensitivity |
| Cardiovascular | |||
| Myocyte contraction rate mediated by β2 adrenoceptor | Normal | Normal | Increased |
| Progressive age-related cardiomyopathy | Not reported | Not reported | Increased versus wt mice |
| Exercise-induced arrhythmias | Not reported | Not reported | Observed in 100% mice |
| Emesis | |||
| PDE4 inhibitor-induced shortening of α2 adrenoceptor-mediated anaesthesia (behavioural correlate of emesis) | Not reported | Normal | Reduced |
| Depressive behaviour | Not reported | Display depressive behaviour | Not reported |
| Anxiogenic behaviour | Not reported | Display anxiogenic-like behaviour | Normal |
References: Hansen et al. (2000); Jin and Conti (2002); Robichaud et al. (2002); Zhang et al. (2002); Méhats et al. (2003); Ariga et al. (2004); Lehnart et al. (2005); Siuciak et al. (2008); Zhang et al. (2008).
LPS, lipopolysaccharide; PDE, phosphodiesterase; TNF, tumour necrosis factor.
Adverse effects of PDE3 and PDE4 Inhibitors
The therapeutic window of orally administered selective PDE4 inhibitors in clinical trials is limited by gastrointestinal side effects of nausea, vomiting, diarrhoea, abdominal pain and dyspepsia, although some of these appear to resolve with continued treatment. Regulatory agencies are however particularly concerned by the development of mesenteric vasculitis in laboratory animals.
Mesenteric vasculitis has, however, never been seen in man. Indeed mesenteric vasculitis has never been seen in people treated for many years with bronchodilator doses of theophylline, a regime that produces medial necrosis of mesenteric vessels in rats (Collins et al., 1988; Nyska et al., 1998). Rats and dogs may have an increased susceptibility to drug-induced vascular legions as arteriopathies commonly occur in these species (Bishop, 1989; Ruben et al., 1989), and species differences have been shown to exist in terms of both PDE4 expression and the functional effects of PDE4 inhibitors. For example, a recent study demonstrated that levels of PDE4 enzyme activity are much higher in rats than humans in multiple tissues, which could explain why rats are more susceptible to PDE4 inhibitor-induced toxicities (Bian et al., 2004). In addition, the PDE4 inhibitor IC542 (structure not available) markedly enhanced LPS-induced IL-6 release from rat whole blood (Dietsch et al., 2006), but did not potentiate LPS-induced IL-6 release from human or non-human primate blood. In addition while, cilomilast caused medial necrosis of mesenteric arteries in rodents, these findings were not observed in comparable studies in primates (http://www.fda.gov/ohrms/dockets/ac/03/slides/3976S1_01_Glaxo-Ariflo.ppt). There is however, one report of the PDE4 inhibitor, SCH 351591, causing arteriopathy in nonhuman primates (Losco et al., 2004). Vasculitis, thus, clearly requires careful monitoring in man, and indeed current research is focused on identifying potential predictive biomarkers. While the vascular lesions in rats have been well characterized histologically, only very little was known, until recently, about their pathogenesis. Interestingly, tissue inhibitor of metalloproteinase 1 appears to be an early and sensitive predictive biomarker of the inflammatory and tissue remodelling components of PDE4 inhibitor-induced vascular injury in rats (Dagues et al., 2007).
Some insight into the potential subtypes responsible for mediating side effects of PDE4 inhibition can be gleaned from studies with subtype knockout mice, although clearly potential species differences need to be borne in mind. Table 1 summarizes published data on phenotypes of PDE4A−/−, B−/− and D−/− mice. We are not aware of any published reports of PDE4C−/− mice. PDE4A−/− and B−/− mice have normal neonatal growth survival and fertility, whereas this is impaired in PDE4D−/− mice (Lehnart et al., 2005). Specifically, PDE4D−/− mice have been shown to suffer from various cardiovascular complications (Lehnart et al., 2005) (summarized in Table 1). PDE4D−/−, but not PDE4B−/− mice have shortened α2 adrenoceptor-mediated anaesthesia, which is thought to be a behavioural correlate of emesis (Robichaud et al., 2002), and thus it has been suggested that the D isoform is responsible for the emesis that has been observed with orally administered PDE4 inhibitors. In addition, it has recently been suggested that a rat model of conditioned gating can detect the nauseating properties of PDE4 inhibitors (Rock et al., 2009)
PDE3 inhibitors were developed in the 1980s as ‘safer’ alternatives to cardiac glycosides for the treatment of dilated cardiomyopathy, and in the short term, beneficial effects on the force of myocardial contraction and vascular smooth muscle tone were reported. However, chronic treatment resulted in a significant increase in mortality (Movsesian and Alharethi, 2002). The PDE3 inhibitor, milrinone, is however, currently used clinically in the short-term therapy of severe congestive heart failure. In addition, PDE3 inhibitors have been shown to cause arteritis in rats and dogs (Joseph, 2000). The relative contribution of PDE3A and B to these adverse effects is however unclear, as these inhibitors inhibit both PDE3 isoforms. Some insight into the potential roles of PDE3A and 3B can be derived from knockout mouse studies, and the phenotypes of PDE3A−/− and PDE3B−/− mice are summarized in Table 2. PDE3A−/− and PDE3B−/− mice have normal neonatal growth and survival, but PDE3A−/− mice have an increased heart rate and are infertile (Masciarelli et al., 2004). PDE3B−/− mice do not share these characteristics (Table 2). Metabolic dysregulation including systemic insulin resistance has, however, been observed in PDE3B−/− mice (Choi et al., 2006).
Table 2.
Phenotypes of phosphodiesterase (PDE)3A and B knockout mice
| PDE3A | PDE3B | |
|---|---|---|
| Physiological | ||
| Neonatal growth and survival | Normal | Normal |
| Fertility | Infertile | Metabolic dysregulation including systemic insulin-resistant |
| Cardiovascular | Increased heart rate | Normal heart rate |
References: Masciarelli et al. (2004); Choi et al. (2006); Sun et al. (2007).
Given that additive and synergistic effects of dual PDE3/4 inhibition have been observed in terms of efficacy end points, a clear concern is that additive or synergistic effects could also be seen with adverse events. The phenotype of dual PDE3−/− and PDE4−/− mice would clearly be of interest, but we are not aware of such mice being available. It is of interest, however, that while selective inhibition of PDE3 or PDE4 in wild-type cardiomyocytes elevated calcium transients, sarcoplasmic reticulum Ca2+ content and phospholamban phosphorylation (Kerfant et al., 2007), combined PDE3 and PDE4 inhibition caused no further increases in sarcoplasmic reticulum function. The reason for this perhaps unexpected finding is unclear, but could be related to compartmentalization of pools of cAMP. Nevertheless, no preclinical findings were identified in toxicology studies that prevented some dual PDE3/4 inhibitors progressing to early clinical trials.
Published dual PDE3/4 inhibitors
Compounds reaching clinical trials
There are few dual PDE3/4 inhibitors to date that have been published as reaching clinical trials. Two of the more well-known compounds are zardaverine (Nycomed, Zurich, Switzerland) and benafentrine (aka AH-21-132, Sandoz, Novartis, Basel, Switzerland) (Figure 1).
Figure 1.
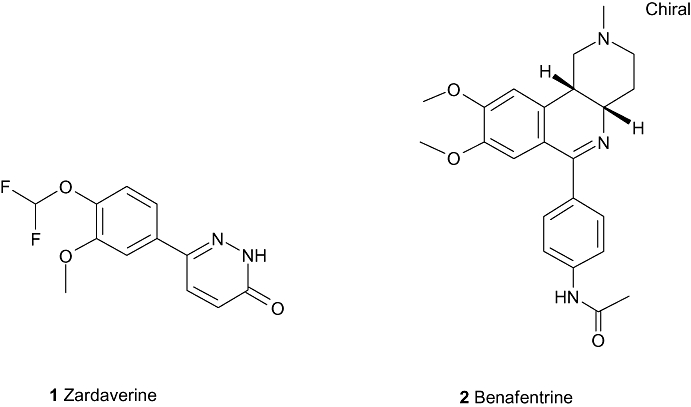
Compounds reaching clinical trials (i).
Zardaverine (1) is a 6-phenyl-2H-pyridazin-3-one from Nycomed, which has reported IC50 values on human PDE3 and PDE4 as 110 nM and 210 nM respectively (Pitts et al., 2004) and has shown bronchodilation (Hoymann et al., 1994; Underwood et al., 1994) and anti-inflammatory (Schudt et al., 1991) activity in animal models. In 1995, results were reported of a PhII clinical trial in which zardaverine was tested in 10 patients with partially reversible chronic airflow obstruction. Zardaverine was administered by metered dose inhaler at single doses of 1.5 mg, 3.0 mg or 6.0 mg, and compared with salbutamol (0.3 mg) and placebo (administered on separate days). In this trial, salbutamol induced a significant bronchodilatation, whereas zardaverine did not improve airway function. Unwanted effects of the study medication were not observed (Ukena et al., 1995). The results are somewhat surprising, given that the compound had been previously shown (Brunnee et al., 1992) to have a modest and short-lasting bronchodilating activity when give by inhalation to 12 patients with reversible bronchial obstruction. In this study, four puffs of either zardaverine (total dose 6 mg) or placebo were inhaled at 15 min intervals. Compared with placebo, specific airway conductance (sGaw) and FEV1 increased significantly during the first hour of repeated inhalations. In seven patients FEV1 increased by >15%, but the duration of action varied considerably between patients. Three patients complained of side effects (headache, drowsiness, vertigo, nausea), and one of these dropped out of the study due to vomiting. At best then, zardaverine appears to have a fairly modest and certainly short-lived effect bronchodilatory effect in man and appears to have been discontinued as a drug candidate.
Benafentrine (2) is a benzonaphthyridine derivative that inhibits PDE3 from guinea-pig platelets with an IC50 of 1.74 µM and PDE4 from guinea-pig neutrophils with an IC50 of 1.76 µM (Hatzelmann et al., 1996). Despite the fact that this compound is a relatively weak inhibitor of the PDE3 and PDE4 enzymes, it has been tested in man. Normal human subjects were dosed with benafentrine at doses up to 90 mg orally, but no bronchodilator activity was seen after methacholine challenge. However, when given by inhalation, benafentrine produced a dose-dependent bronchodilation to methacholine challenge, with an ED50 of approximately 9.2 mg. Interestingly the drug was also given by i.v. infusion (at 20 or 40 mg), in which capacity a short-lived bronchodilatation was also observed without affecting blood pressure or heart rate (Foster et al., 1992). Unfortunately, a detailed analysis of pharmacokinetic data is missing from this report; however, the study does imply that an inhaled approach directly to the airways smooth muscle may give the best therapeutic benefit in obstructive airways disease. Despite these encouraging early results, benafentrine appears to have been discontinued as a drug.
Pumafentrine (3, Figure 2) is a compound from Nycomed, which has entered the clinic more recently and has reported IC50 values for PDE3 and PDE4 of 28 nM and 7 nM respectively (Dony et al., 2008). Also known as BY343, this compound is of the benzonaphthyridine class, and similar in structure to benafentrine – the differences being in the reversed amide, and the ethoxymethoxycatechol. This compound was believed to have been in PhII clinical trials for the treatment of asthma, but was discontinued in 2002 due to a failure to meet expectation regarding duration of action. It has been speculated that the focus shifted to an active metabolite of pumafentrine – hydroxypumafentrine (exact structure unknown) – however there are no published data on this compound (Giembycz, 2005).
Figure 2.
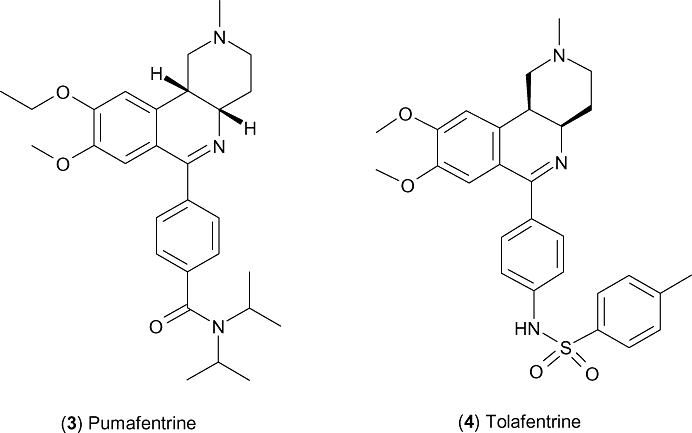
Compounds reaching clinical trials (ii).
Tolafentrine (4, Figure 2) is from Nycomed and is the third compound in this benzonaphthyridine class, in this case replacing the amide with a p-methylphenylsulphonamide. The compound is variously cited as a dual PDE3/4 inhibitor, although there is in fact a dearth of published information regarding the affinity of the drug to each enzyme. The compound has been shown to be effective by inhaled delivery in rodent models of pulmonary hypertension (Schermuly et al., 2004; Pullamsetti et al., 2005) and in 2002 was reported to be in PhI clinical trials for primary pulmonary hypertension (Bayes et al., 2002) (having previously been described as synergistically prolonging the vasodilating properties of prostanoid in secondary pulmonary hypertension) (Ghofrani et al., 2001).
Compounds in preclinical testing
While there is a scarcity of dual inhibitors that have been tested in man, there are some interesting tool compounds available and some preclinical candidates that may be approaching human testing.
The pharmacology of two new PDE3/4 inhibitors (RPL554 and RPL565) has recently been described (Boswell-Smith et al., 2006) (Figure 3). RPL554 is a trequinsin (PDE3 IC50= 40 pM, PDE4 IC50∼ 400 nM) (Liu et al., 2005) analogue, with reported IC50 values at human PDE3 and PDE4 of 400 pM and 1.5 µM respectively (3440× selective for PDE3). Compound RPL565 is similar in structure, but lacks the urea side chain and has an ether link to the diisopropylbenzene moiety; this compound has reported IC50 values of 107 nM and 1.2 µM at human PDE3 and PDE4 respectively (giving a more balanced 11× selectivity for PDE3). Both compounds have been shown to be effective in an in vivo model of inflammation, inhibiting eosinophil recruitment in ovalbumin-sensitized guinea-pigs at 10 mg·kg−1 p.o. (Boswell-Smith et al., 2006).
Figure 3.
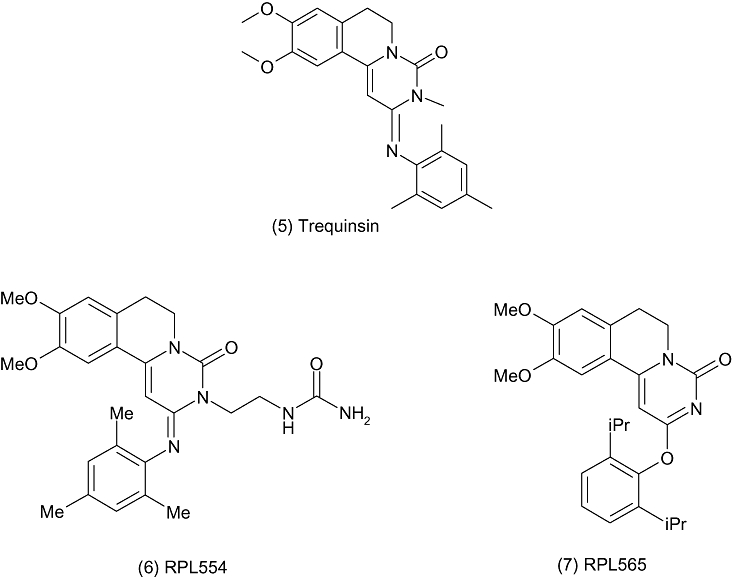
Trequinsin analogues.
These compounds have been tested in preclinical models by dosing directly to the airways, possibly to support inhaled delivery in man. Both compounds were shown to significantly inhibit histamine-induced plasma protein extravasation in the trachea and histamine-induced bronchoconstriction after inhaled administration of dry powder (2.5% RPL554 in lactose, 25% RPL565 in lactose) to the guinea-pig. Although it is difficult to measure the exact dose given in such an experiment, 3–5 mg of the lactose blend was delivered into a ‘volumatic’ chamber nine times in 3 min prior to the i.v. challenge with histamine. Presumably the idea behind local delivery of the drugs would be to reduce systemic side effects. In this experiment, inhalation of RPL554 significantly reduced mean arterial blood pressure over 4.5 h by 60% of control, whereas RPL565 had no significant effect on mean arterial blood pressure compared with controls (Boswell-Smith et al., 2006). While such results are intriguing, they can be difficult to interpret further without a full pharmacokinetic analysis, which is unfortunately not reported.
Of the two compounds, RPL554 appears to be preferred and it has been reported that it has passed safety and toxicology studies, allowing Verona pharma to prepare the submissions in support of regulatory approval for clinical trials with this compound as a long-acting bronchodilator for allergic respiratory diseases (Verona Pharma website, 2008).
Researchers at the Leiden/Amsterdam Center for Drug Research have reported the synthesis and structure activity relationship (SAR) of a new series of potent dual PDE3/4 inhibitors that are cis-tetrahydrophthalazinone/pyridazinone hybrids and are based around hybrid structures combining pharmacophores for PDE3 and PDE4 activity combined by a linker group (Figure 4) (Van der Mey et al., 2003). By following this strategy, the team were able to produce compounds with dual activity. Of these, compound (10) was shown to be effective in an in vivo model of arachidonic acid – induced ear oedema in the mouse, giving 44% inhibition at an oral dose of 16 mg·kg−1 (Van der Mey et al., 2003).
Figure 4.
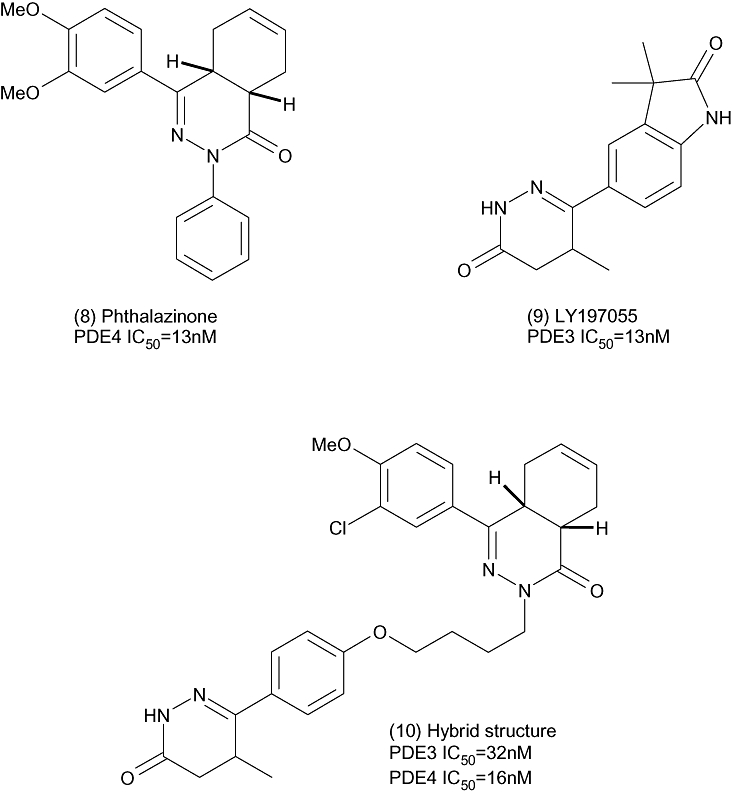
A cis-tetrahydrophthalazinone/pyridazinone hybrid.
Researchers at Altana Pharmaceuticals have filed a number of patent applications for benzonaphthyridine derivatives as dual PDE3/4 inhibitors. The in vitro and in vivo data for these compounds are not published; however, some representative structures are illustrated in Figure 5; the compounds do appear to be continuing the pharmacophore of compounds such as benafentrine and pumafentrine.
Figure 5.
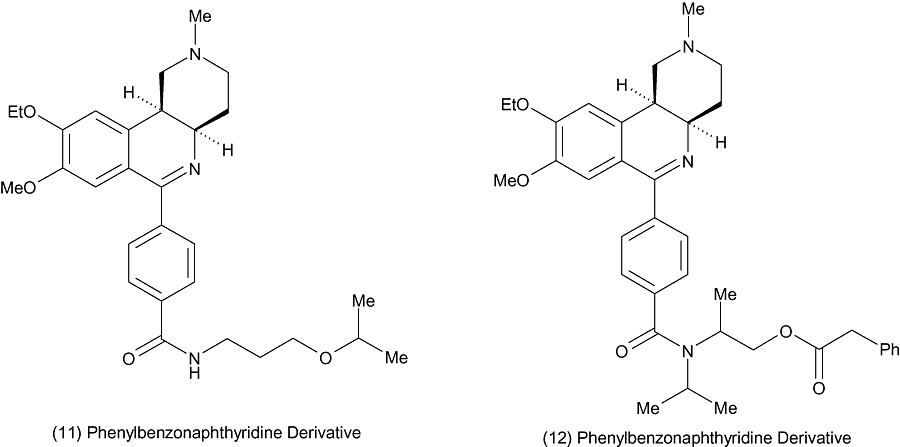
Benzonaphthyridine derivatives from Altana.
Conclusion and future directions
Dual inhibition of PDE3 and PDE4 would appear to be attractive from an efficacy perspective to target key pathological features of COPD, given the broad anti-inflammatory and bronchodilatory activity of these agents, together with their potential to stimulate mucociliary clearance. It is clear that dual inhibition of PDE3 and PDE4 is required to inhibit the activity of certain key cell types involved in the pathogenesis of COPD, a fact that may explain in part why selective PDE4 inhibitors have had limited efficacy in the clinic. The key challenge would be to develop an agent with a sufficient therapeutic ratio given the well-known side effects of these agents. One strategy could be to develop an inhaled agent that is rapidly cleared from the systemic circulation. Designing an agent with subtype selectivity could also offer an advantage. Indeed, it would appear from knockout mouse data together with the expression profile of PDE3B, that a compound that could selectively inhibit PDE3B, rather than PDE3A would be beneficial to potentially mitigate cardiovascular risk, although selective inhibitors of PDE3A and PDE3B would clearly be required to confirm this. It would seem that PDE4A, B and D (the role of 4C is less clear, although its expression is much more restricted than the other PDE4 isoforms) all play important roles in mediating the anti-inflammatory effects of PDE4 inhibitors, and that 4D is involved in the bronchodilator activity, and thus from an efficacy perspective a non-PDE4 isoform-selective inhibitor would appear to be the most attractive. A key question that remains, however, is whether the synergy of PDE3/4 inhibitors could be exploited from an efficacy perspective, without observing synergistic effects on potential adverse effects.
Glossary
Abbreviations:
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- PDE
phosphodiesterase
- COPD
chronic obstructive pulmonary disease
Conflicts of interest
None.
References
- Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, et al. Non-redundant function of phosphodiesterase 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol. 2004;173:7531–7538. doi: 10.4049/jimmunol.173.12.7531. [DOI] [PubMed] [Google Scholar]
- Banner KH, Moriggi E, Da Ros B, Schioppacassi G, Semeraro C, Page CP. The effect of selective phosphodiesterase 3 and 4 isoenzyme inhibitors and established anti-asthma drugs on inflammatory cell activation. Br J Pharmacol. 1996;119:1255–1261. doi: 10.1111/j.1476-5381.1996.tb16030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R, Baillie GS, Bergmann R, Shepherd MC, Sepper R, Houslay MD, et al. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L332–L343. doi: 10.1152/ajplung.00384.2003. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New therapies for chronic obstructive pulmonary disease. Thorax. 1998;53:137–147. doi: 10.1136/thx.53.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med. 2003;54:113–129. doi: 10.1146/annurev.med.54.101601.152209. [DOI] [PubMed] [Google Scholar]
- Baroja ML, Cieslinski LB, Torphy TJ, Wange RL, Madrenas J, Gantner F, et al. Specific CD3e association of a phosphodiesterase 4B isoform determines its selective tyrosine phosphorylation after CD3 ligation. J Immunol. 1999;162:2016–2023. [PubMed] [Google Scholar]
- Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2002;24:159–184. [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Berends C, Dijkhuizen B, de Monchy JG, Dubois AE, Gerritsen J, Kauffman HF. Inhibition of PAF-induced expression of CD11b and shedding of L-selectin on human neutrophils by the type 4 selective PDE inhibitor, rolipram. Eur Respir J. 1997;10:1000–1007. doi: 10.1183/09031936.97.10051000. [DOI] [PubMed] [Google Scholar]
- Bian H, Zhang J, Wu P, Varty LA, Jia Y, Mayhood T, et al. Differential type 4 cAMP-specific phosphodiesterase (PDE4) expression and functional sensitivity to PDE4 inhibitors among rats, monkeys and humans. Biochem Pharmacol. 2004;68:2229–2236. doi: 10.1016/j.bcp.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Billington CK, LeJeune IR, Young KW, Hall IP. A major functional role for phosphodiesterase 4D5 in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:1–7. doi: 10.1165/rcmb.2007-0171OC. [DOI] [PubMed] [Google Scholar]
- Bingham J, Sudarsanam S, Srinivasan S. Profiling human phosphodiesterase genes and splice isoforms. Biochem Biophys Res Commun. 2006;350:25–32. doi: 10.1016/j.bbrc.2006.08.180. [DOI] [PubMed] [Google Scholar]
- Bishop SP. Animal models of vasculitis. Toxicol Pathol. 1989;17:109–117. doi: 10.1177/019262338901700106. [DOI] [PubMed] [Google Scholar]
- Blanc PD, Iribarren C, Trupin L, Earnest G, Katz PP, Balmes J, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 2009;64:6–12. doi: 10.1136/thx.2008.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blease K, Burke-Gaffney A, Hellewell PG. Modulation of cell adhesion molecule expression and function on human lung microvascular endothelial cells by inhibition of phosphodiesterases 3 and 4. Br J Pharmacol. 1998;124:229–237. doi: 10.1038/sj.bjp.0701833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido [6,1-a]isoquinolin-4-one] J Pharmacol Exp Ther. 2006;318:840–848. doi: 10.1124/jpet.105.099192. [DOI] [PubMed] [Google Scholar]
- Brunnee T, Engelstatter R, Steinijans VW, Kunkel G. Bronchodilatory effect of inhaled zardaverine, a phosphodiesterase III and IV inhibitor, in patients with asthma. Eur Respir J. 1992;5:982–985. [PubMed] [Google Scholar]
- Burgess JK, Oliver BG, Poniris MH, Ge Q, Boustany S, Cox N, et al. A phosphodiesterase 4 inhibitor inhibits matrix protein deposition in airways in vitro. J Allergy Clin Immunol. 2006;118:649–657. doi: 10.1016/j.jaci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Campos-Toimil M, Keravis T, Orallo F, Takeda K, Lugnier C. Short-term or long-term treatments with a phosphodiesterase-4 (PDE4) inhibitor result in opposing agonist-induced Ca(2+) responses in endothelial cells. Br J Pharmacol. 2008;154:82–92. doi: 10.1038/bjp.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, Haluzik M, et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116:3240–3251. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claveau D, Chen SL, O'Keefe S, Styhler A, Liu S, Huang Z, et al. Preferential inhibition of T helper 1, but not T helper 2, cytokines in vitro by L-826,141 [4-[2-(3,4-Bisdifluromethoxyphenyl)-2-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-phenyl]-ethyl]3-methylpyridine-1-oxide], a potent and selective phosphodiesterase 4 inhibitor. J Pharmacol Exp Ther. 2004;310:752–760. doi: 10.1124/jpet.103.064691. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Elwell MR, Lamb JC, Manus AG, Heath JE, Makovec GT. Subchronic toxicity of orally administered (gavage and dosed-feed) theophylline in Fischer 344 rats and B6C3F1 mice. Fundam Appl Toxicol. 1988;11:472–484. doi: 10.1016/0272-0590(88)90111-x. [DOI] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park J-Y, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Cooper N, Teixeira MM, Warneck J, Miotla JM, Wills RE, Macari DM, et al. A comparison of the inhibitory activity of PDE4 inhibitors on leukocyte PDE4 activity in vitro and eosinophil trafficking in vivo. Br J Pharmacol. 1999;126:1863–1871. doi: 10.1038/sj.bjp.0702520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagues N, Pawlowski V, Sobry C, Hanton G, Borde F, Soler S, et al. Investigation of the molecular mechanisms preceding PDE4-inhibitor-induced vasculopathy in rats: TIMP-1, a potential predictive biomarker. Toxicol Sci. 2007;100:238–247. doi: 10.1093/toxsci/kfm161. [DOI] [PubMed] [Google Scholar]
- Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- Denis D, Riendeau D. Phosphodiesterase 4-dependent regulation of cyclic AMP levels and leukotriene B4 biosynthesis in human polymorphonuclear leukocytes. Eur J Pharmacol. 1999;367:343–350. doi: 10.1016/s0014-2999(98)00987-x. [DOI] [PubMed] [Google Scholar]
- Dent G, White SR, Tenor H, Bodtke K, Schudt C, Leff AR, et al. Cyclic nucleotide phosphodiesterase in human bronchial epithelial cells: characterization of isoenzymes and functional effects of PDE inhibitors. Pulm Pharmacol Ther. 1998;11:47–56. doi: 10.1006/pupt.1998.0115. [DOI] [PubMed] [Google Scholar]
- Derian CK, Santulli RJ, Rao PE, Solomon HF, Barrett JA. Inhibition of chemotactic peptide-induced neutrophil adhesion to vascular endothelium by cAMP modulators. J Immunol. 1995;154:308–317. [PubMed] [Google Scholar]
- Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 1998;158:1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- Dietsch GN, DiPalma CR, Eyre RJ, Pham TQ, Poole KM, Pefaur NB, et al. Characterization of the inflammatory response to a highly selective PDE4 inhibitor in the rat and the identification of biomarkers that correlate with toxicity. Toxicol Pathol. 2006;34:39–51. doi: 10.1080/01926230500385549. [DOI] [PubMed] [Google Scholar]
- Dony E, Lai YJ, Dumitrascu R, Pullamsetti SS, Savai R, Ghofrani HA, et al. Partial reversal of experimental pulmonary hypertension by phosphodiesterase-3/4 inhibition. Eur Respir J. 2008;31:599–610. doi: 10.1183/09031936.00002007. [DOI] [PubMed] [Google Scholar]
- Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, et al. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkern TR, Feurstein D, Rossi GA, Sabatini F, Hatzelmann A. Inhibition of TGF-beta induced lung fibroblast to myofibroblast conversion by phosphodiesterase inhibiting drugs and activators of soluble guanylyl cyclase. Eur J Pharmacol. 2007;572:12–22. doi: 10.1016/j.ejphar.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Edmondson SD, Mastracchio A, He J, Chung CC, Forrest MJ, Hofsess S, et al. Benzyl vinylogous amide substituted aryldihydropyridazinones and aryldimethylpyrazolones as potent and selective PDE3B inhibitors. Bioorg Med Chem Lett. 2003;13:3983–3987. doi: 10.1016/j.bmcl.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Essayan DM, Kagey-Sobotka A, Lichtenstein LM, Huang SR. Differential regulation of human antigen-specific Th1 and Th2 lymphocyte responses by isozyme selective cyclic nucleotide phosphodiesterase inhibitors. J Pharmacol Exp Ther. 1997;282:505–512. [PubMed] [Google Scholar]
- Fernandes LB. Phosphodiesterase inhibitors and endothelin as modulators of respiratory neurotransmission. Clin Exp Pharmacol Physiol. 1996;23:980–982. doi: 10.1111/j.1440-1681.1996.tb01153.x. [DOI] [PubMed] [Google Scholar]
- Fernandes LB, Ellis JL, Undem BJ. Potentiation of nonadrenergic noncholinergic relaxation of human isolated bronchus by selective inhibitors of phosphodiesterase isozymes. Am J Respir Crit Care Med. 1994;150:1384–1390. doi: 10.1164/ajrccm.150.5.7952568. [DOI] [PubMed] [Google Scholar]
- Foster RW, Rakshi K, Carpenter JR, Small RC. Trials of the bronchodilator activity of the isoenzyme-selective phosphodiesterase inhibitor AH 21-132 in healthy volunteers during a methacholine challenge test. Br J Clin Pharmacol. 1992;34:527–534. doi: 10.1111/j.1365-2125.1992.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann M, Jahn HU, Seybold J, Neurohr C, Barnes PJ, Hippenstiel S, et al. Identification and function of cyclic nucleotide phosphodiesterase isoenzymes in airway epithelial cells. Am J Respir Cell Mol Biol. 1999;20:292–302. doi: 10.1165/ajrcmb.20.2.3140. [DOI] [PubMed] [Google Scholar]
- Gantner F, Gotz C, Gekeler V, Schudt C, Wendel A, Hatzelmann A. Phosphodiesterase profile of human B lymphocytes from normal and atopic donors and the effects of PDE inhibition on B cell proliferation. Br J Pharmacol. 1998;123:1031–1038. doi: 10.1038/sj.bjp.0701688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner F, Schudt C, Wendel A, Hatzelmann A. Characterization of the phosphodiesterase (PDE) pattern of in vitro-generated human dendritic cells (DC) and the influence of PDE inhibitors on DC function. Pulm Pharmacol Ther. 1999;12:377–386. doi: 10.1006/pupt.1999.0220. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Olschewski H, Rose F, Schermuly R, Weissmann N, Schudt C, et al. Prolongation of prostanoid inhalation-induced pulmonary vasodilation by concomitant PDE-3/4 inhibition in patients with sever pulmonary hypertension. Am J Respir Crit Care Med. 2001;163:A540. [Google Scholar]
- Giembycz MA. Phosphodiesterase-4: selective and dual-specificity inhibitors for the therapy of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:326–333. doi: 10.1513/pats.200504-041SR. [DOI] [PubMed] [Google Scholar]
- Giembycz MA, Corrigan CJ, Seybold J, Newton R, Barnes PJ. Identification of cyclic AMP phosphodiesterases 3,4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the biosynthesis of interleukin-2. Br J Pharmacol. 1996;118:1945–1958. doi: 10.1111/j.1476-5381.1996.tb15629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ, Land SC, Tarnow-Mordi WO, Zembala M, Kowalczyk D, Lauterbach R. Immunopharmacological potential of selective phosphodiesterase inhibition. I. Differential regulation of lipopolysaccharide-mediated proinflammatory cytokine (interleukin-6 and tumor necrosis factor-alpha) biosynthesis in alveolar epithelial cells. J Pharmacol Exp Ther. 2002;300:559–566. doi: 10.1124/jpet.300.2.559. [DOI] [PubMed] [Google Scholar]
- Hansen G, Jin S, Umetsu DT, Conti M. Absence of muscarinic cholinergic airway responses in mice deficient in the cyclic nucleotide phosphodiesterase PDE4D. Proc Natl Acad Sci USA. 2000;97:6751–6756. doi: 10.1073/pnas.97.12.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–279. [PubMed] [Google Scholar]
- Hatzelmann A, Engelstaetter R, Morley J, Mazzoni L. Enzymic and functional aspects of dual-selective PDE3/4 inhibitors. In: Schudt C, Dent G, Rabc KF, editors. Phosphodiesterase Inhibitors. London: Academic Press; 1996. pp. 147–160. [Google Scholar]
- Heystek HC, Thierry AC, Soulard P, Moulon C. Phosphodiesterase 4 inhibitors reduce human dendritic cell inflammatory cytokine production and Th1-polarizing capacity. Int Immunol. 2003;15:827–835. doi: 10.1093/intimm/dxg079. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med. 2007;176:454–459. doi: 10.1164/rccm.200612-1772OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi S, Imai T, Idaira K, Adachi M, Okamoto M. Effects of inhaled SDZ ISQ 844, a cyclic nucleotide phosphodiesterase isoenzyme type III/IV inhibitor, on airway responsiveness in beagles. Arerugi. 1994;43:551–556. [PubMed] [Google Scholar]
- Hoymann HG, Heinrich U, Beume R, Kilian U. Comparative investigation of the effects of zardaverine and theophylline on pulmonary function in rats. Exp Lung Res. 1994;20:235–250. doi: 10.3109/01902149409064385. [DOI] [PubMed] [Google Scholar]
- Ishiura Y, Fujimura M, Nobata K, Abo M, Oribe T, Myou S, et al. Phosphodiesterase 3 inhibition and cough in elderly asthmatics. Cough. 2005;1:11. doi: 10.1186/1745-9974-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Nishikawa M, Fujioka M, Miyahara M, Isaka N, Shiku H, et al. Characterization of the isoenzymes of cyclic nucleotide phosphodiesterase in human platelets and the effects of E4021. Cell Signal. 1996;8:575–581. doi: 10.1016/s0898-6568(96)00112-x. [DOI] [PubMed] [Google Scholar]
- Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci USA. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Boswell-Smith V, Lever R, Page CP. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm Pharmacol Ther. 2005;18:93–101. doi: 10.1016/j.pupt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Joseph EC. Arterial lesions induced by phosphodiesterase III (PDE III) inhibitors and D1 agonists. Toxicol Lett. 2000;112:537–546. doi: 10.1016/s0378-4274(99)00221-0. [DOI] [PubMed] [Google Scholar]
- Kelley TJ, al-Nakkash L, Drumm ML. CFTR-mediated chloride permeability is regulated by type III phosphodiesterases in airway epithelial cells. Am J Respir Cell Mol Biol. 1995;13:657–664. doi: 10.1165/ajrcmb.13.6.7576703. [DOI] [PubMed] [Google Scholar]
- Kerfant BG, Zhao D, Lorenzen-Schmidt I, Wilson LS, Cai SW, Chen SR, et al. PI3Kγ is required for PDE4, not PDE3, activity in subcellular microdomains containing the sarcoplasmic reticular calcium ATPase in cardiomyocytes. Circ Res. 2007;101:400–408. doi: 10.1161/CIRCRESAHA.107.156422. [DOI] [PubMed] [Google Scholar]
- Kohyama T, Liu X, Wen FQ, Wang H, Kim HJ, Takizawa H, et al. PDE4 inhibitors attenuate fibroblast chemotaxis and contraction of native collagen gels. Am J Respir Cell Mol Biol. 2002;26:694–701. doi: 10.1165/ajrcmb.26.6.4743. [DOI] [PubMed] [Google Scholar]
- Kuss H, Hoefgen N, Johanssen S, Kronbach T, Rundfeld C. In vivo efficacy in airway disease models of N-(3,5-Dichloro-pyrid-4-yl)-[1-(4-flurobenzyl)-5-hydroxy-indole-3-yl]-glyoxylic acid amide (AWD 12-281), a selective phoshodiesterase 4 inhibitor for inhaled administration. JPET. 2003;307:373–385. doi: 10.1124/jpet.103.053942. [DOI] [PubMed] [Google Scholar]
- Lagente V, Martin-Chouly C, Boichot E, Martins MA, Silva PM. Selective PDE4 inhibitors as potent anti-inflammatory drugs for the treatment of airway diseases. Mem Inst Oswaldo Cruz. 2005;100:131–136. doi: 10.1590/s0074-02762005000900023. [DOI] [PubMed] [Google Scholar]
- Landells LJ, Szilagy CM, Jones NA, Banner KH, Allen JM, Doherty A, et al. Identification and quantification of phosphodiesterase 4 subtypes in CD4 and CD8 lymphocytes from healthy and asthmatic subjects. Br J Pharmacol. 2001;133:722–729. doi: 10.1038/sj.bjp.0704120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc O, Lagente V, Planquois J-M, Berthelier C, Artola M, Eichholtz T, et al. Involvement of MMP-12 and phosphodiesterase type 4 in cigarette smoke-induced inflammation in mice. Eur Respir J. 2006;27:1102–1109. doi: 10.1183/09031936.06.00076905. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XHT, Reiken S, Warrier S, Belevych AE, Harvery RD, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeJeune IR, Shepherd M, Van Heeke G, Houslay MD, 7, Hall IP. Cyclic AMP-dependent transcriptional up-regulation of phosphodiestease 4D5 in human airway smooth muscle cells. J Biol Chem. 2002;277:35980–53989. doi: 10.1074/jbc.M204832200. [DOI] [PubMed] [Google Scholar]
- Liu H, Maurice DH. Phosphorylation-mediated activation and translocation of the cyclic AMP-specific phosphodiesterase PDE4D3 by cyclic AMP–dependent protein kinase and mitogen–activated protein kinases. A potential mechanism allowing for the coordinated regulation of PDE4D activity and targeting. J Biol Chem. 1999;274:10557–10565. doi: 10.1074/jbc.274.15.10557. [DOI] [PubMed] [Google Scholar]
- Liu S, Veilleux A, Young A, Zhang L, Kwok E, Laliberté F, et al. Dynamic activation of cystic fibrosis transmembrane conductance regulator by type 3 and type 4D phosphodiesterase inhibitors. J Pharmacol Exp Ther. 2005;314:846–854. doi: 10.1124/jpet.105.083519. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- Losco PE, Evans EW, Barat SA, Blackshear PE, Reyderman L, Fine JS, et al. The toxicity of SCH 351591, a novel phosphodiesterase-4 inhibitor, in Cynomolgus monkeys. Toxicol Pathol. 2004;32:295–308. doi: 10.1080/01926230490431493. [DOI] [PubMed] [Google Scholar]
- Maestrelli P, Saetta M, Mapp CE, Fabbri LM. Remodelling in response to infection and injury, airway inflammation and hypersecretion of mucus in smoking subjects with COPD. Am J Respir Crit Care Med. 2001;164:S76–S80. doi: 10.1164/ajrccm.164.supplement_2.2106067. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1084–1091. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- Manning CD, Burman M, Christensen SB, Cieslinski LB, Essayan DM, Grous M, et al. Suppression of human inflammatory cell function by subtype-selective PDE4 inhibitors correlates with inhibition of PDE4A and PDE4B. Br J Pharmacol. 1999;128:1393. doi: 10.1038/sj.bjp.0702911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Chouly CA, Astier A, Jacob C, Pruniaux MP, Bertrand C, Lagente V. Modulation of matrix metalloproteinase production from human lung fibroblasts by type 4 phosphodiesterase inhibitors. Life Sci. 2004;75:823–840. doi: 10.1016/j.lfs.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Martorana PA, Beume R, Lucattelli M, Wollin L, Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med. 2005;172:848–853. doi: 10.1164/rccm.200411-1549OC. [DOI] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, et al. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méhats C, Jin SL, Wahlstrom J, Law E, Umetsu DT, Conti M. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003;17:1831–1841. doi: 10.1096/fj.03-0274com. [DOI] [PubMed] [Google Scholar]
- Millen J, MacLean MR, Houslay M. Hypoxia-induced remodelling of PDE4 isoform expression and cAMP handling in human pulmonary artery smooth muscle cells. Eur J Cell Biol. 2006;85:679–691. doi: 10.1016/j.ejcb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Molnar-Kimber K, Yonno L, Heaslip R, et al. Modulation of TNF alpha and IL-1 beta from endotoxin-stimulated monocytes by selective PDE isozyme inhibitors. Agents Actions. 1993;39:C77–C79. doi: 10.1007/BF01972726. [DOI] [PubMed] [Google Scholar]
- Mori H, Nose T, Ishitani K, Souma S, Kasagi S, Akiyoshi T, et al. Phosphodiesterase 4 inhibitor GPD-1116 markedly attenuates the development of cigarette smoke-induced emphysema in senescence-accelerated mice P1 strain. Am J Physiol Lung Cell Mol Physiol. 2008;294:L196–L204. doi: 10.1152/ajplung.00173.2007. [DOI] [PubMed] [Google Scholar]
- Movsesian MA, Alharethi R. Inhibitors of cyclic nucleotide phosphodiesterase PDE3 as adjunct therapy for dilated cardiomyopathy. Expert Opin Investig Drugs. 2002;11:1529–1536. doi: 10.1517/13543784.11.11.1529. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Naline E, Qian Y, Advenier C, Raeburn D, Karlsson JA. Effects of RP 73401, a novel, potent and selective phosphodiesterase type 4 inhibitor, on contractility of human, isolated bronchial muscle. Br J Pharmacol. 1996;118:1939–1944. doi: 10.1111/j.1476-5381.1996.tb15628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherton SJ, Maurice DH. Vascular endothelial cell cyclic nucleotide phosphodiesterases and regulated cell migration: implications in angiogenesis. Mol Pharmacol. 2005;67:263–272. doi: 10.1124/mol.104.004853. [DOI] [PubMed] [Google Scholar]
- Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res. 2007;101:768–776. doi: 10.1161/CIRCRESAHA.106.146159. [DOI] [PubMed] [Google Scholar]
- Nyska A, Herbert RA, Chan PC, Haseman JK, Hailey JR. Theophylline-induced mesenteric periarteritis in F344/N rats. Arch Toxicol. 1998;72:731–737. doi: 10.1007/s002040050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–857. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- Parkkonen J, Hasala H, Moilanen E, Giembycz MA, Kankaanranta H. Phosphodiesterase 4 inhibitors delay human eosinophil and neutrophil apoptosis in the absence and presence of salbutamol. Pulm Pharmacol Ther. 2008;21:499–506. doi: 10.1016/j.pupt.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798–825. [PubMed] [Google Scholar]
- Peachell PT, Undem BJ, Schleimer RP, MacGlashan D, Lichtenstein L, Cieslinski LB, et al. Preliminary identification and role of phosphodiesterase isozymes in human basophils. J Immunol. 1992;148:2503–2510. [PubMed] [Google Scholar]
- Peter D, Jin SL, Conti M, Hatzelmann A, Zitt C. Differential expression and function of phosphodiesterase 4 (PDE4) subtypes in human primary CD4+ T cells: predominant role of PDE4D. J Immunol. 2007;178:4820–4831. doi: 10.4049/jimmunol.178.8.4820. [DOI] [PubMed] [Google Scholar]
- Pitts WJ, Vaccaro W, Huynh T, Leftheris K, Roberge JY, Barbosa J, et al. Identification of purine inhibitors of phosphodiesterase 7 (PDE7) Bioorg Med Chem Lett. 2004;14:2955–2958. doi: 10.1016/j.bmcl.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Pryzwansky KB, Madden VJ. Type 4A cAMP-specific phosphodiesterase is stored in granules of human neutrophils and eosinophils. Cell Tissue Res. 2003;312:301–311. doi: 10.1007/s00441-003-0728-y. [DOI] [PubMed] [Google Scholar]
- Pullamsetti S, Krick S, Yilmaz H, Ghofrani HA, Schudt C, Weissmann N, et al. Inhaled tolafentrine reverses pulmonary vascular remodeling via inhibition of smooth muscle cell migration. Respir Res. 2005;6:128. doi: 10.1186/1465-9921-6-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Girard V, Martin CA, Molimard M, Advenier C. Rolipram, but not siguazodan or zaprinast, inhibits the excitatory noncholinergic neurotransmission in guinea-pig bronchi. Eur Respir J. 1994;7:306–310. doi: 10.1183/09031936.94.07020306. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, et al. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:968–975. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- Rabe KF. Guidelines for chronic obstructive pulmonary disease treatment and issues of implementation. Proc Am Thorac Soc. 2006;3:641–644. doi: 10.1513/pats.200604-099SS. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Tenor H, Dent G, Schudt C, Liebig S, Magnussen H. Phosphodiesterase isoenzymes modulating inherent tone in human airways: identification and characterization. Am J Physiol. 1993;264:L458–L464. doi: 10.1152/ajplung.1993.264.5.L458. [DOI] [PubMed] [Google Scholar]
- Riise GC, Ahlstedt S, Larsson S, Enander I, Jones I, Larsson P, et al. Bronchial inflammation in chronic bronchitis assessed by measurement of cell products in bronchial lavage fluid. Thorax. 1995;50:360–365. doi: 10.1136/thx.50.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberté F, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EM, Benzaquen J, Limebeer CL, Parker LA. Potential of the rat model of conditioned gaping to detect nausea produced by rolipram, a phosphodiesterase-4 (PDE4) inhibitor. Pharmacol Biochem Behav. 2009;91:537–541. doi: 10.1016/j.pbb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Rogers DF. The role of airway secretions in COPD: pathophysiology, epidemiology and pharmacotheraputic options. COPD. 2005;2:341–353. doi: 10.1080/15412550500218098. [DOI] [PubMed] [Google Scholar]
- Ruben Z, Deslex P, Nash G, Redmond NI, Poncet M, Dodd DC. Spontaneous disseminated panarteritis in laboratory beagle dogs in a toxicity study: a possible genetic predilection. Toxicol Pathol. 1989;17:145–152. doi: 10.1177/019262338901700111. [DOI] [PubMed] [Google Scholar]
- Saetta M, Di Stefano A, Maestrelli P, Ferraresso A, Drigo R, Potena A, et al. Activated T-lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. Am Rev Respir Dis. 1993;147:301–306. doi: 10.1164/ajrccm/147.2.301. [DOI] [PubMed] [Google Scholar]
- Saetta M, Di Stefano A, Maestrelli P, Turato G, Ruggieri MP, Roggeri A, et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150:1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- Sanz MJ, Alvarez A, Piqueras L, Cerdá M, Issekutz AC, Lobb RR, et al. Rolipram inhibits leukocyte-endothelial cell interactions in vivo through P- and E-selectin downregulation. Br J Pharmacol. 2002;135:1872–1881. doi: 10.1038/sj.bjp.0704644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly RT, Kreisselmeier KP, Ghofrani HA, Samidurai A, Pullamsetti S, Weissmann N, et al. Antiremodeling effects of iloprost and the dual-selective phosphodiesterase 3/4 inhibitor tolafentrine in chronic experimental pulmonary hypertension. Circ Res. 2004;94:1101–1108. doi: 10.1161/01.RES.0000126050.41296.8E. [DOI] [PubMed] [Google Scholar]
- Schmidt DT, Watson N, Dent G, Rühlmann E, Branscheid D, Magnussen H, et al. The effect of selective and non-selective phosphodiesterase inhibitors on allergen- and leukotriene C(4)-induced contractions in passively sensitized human airways. Br J Pharmacol. 2000;131:1607–1618. doi: 10.1038/sj.bjp.0703725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schudt C, Winder S, Eltze M, Kilian U, Beume R. Zardaverine: a cyclic AMP specific PDE III/IV inhibitor. Agents Actions Suppl. 1991;34:379–402. New Drugs Asthma Ther. [PubMed] [Google Scholar]
- Schudt C, Tenor H, Loos U, Mallmann P, Szamel M, Resch K. Effects of selective PDE inhibitors on activation of human macrophages and lymphocytes. Eur Respir J. 1993;6:367S. [Google Scholar]
- Shakur Y, Holst LS, Landstrom TR, Movsesian M, Degerman E, Manganiello V. Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol. 2001;66:241–277. doi: 10.1016/s0079-6603(00)66031-2. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Martin AN. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology. 2008;197:115–126. doi: 10.1007/s00213-007-1014-6. [DOI] [PubMed] [Google Scholar]
- Sudo T, Tachibana K, Toga K, Tochizawa S, Inoue Y, Kimura Y, et al. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000;59:347–356. doi: 10.1016/s0006-2952(99)00346-9. [DOI] [PubMed] [Google Scholar]
- Sun B, Li H, Shakur Y, Hensley J, Hockman S, Kambayashi J, et al. Role of phosphodiesterase type 3A and 3B in regulating platelet and cardiac function using subtype-selective knockout mice. Cell Signal. 2007;19:1765–1771. doi: 10.1016/j.cellsig.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Suttorp N, Weber U, Welsch T, Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest. 1993;91:1421–1428. doi: 10.1172/JCI116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N, Ehreiser P, Hippenstiel S, Fuhrmann M, Krüll M, Tenor H, et al. Hyperpermeability of pulmonary endothelial monolayer: protective role of phosphodiesterase isoenzymes 3 and 4. Lung. 1996;174:181–194. doi: 10.1007/BF00173310. [DOI] [PubMed] [Google Scholar]
- Tenor H, Hatzelmann A, Kupferschmidt R, Stanciu L, Djukanoviv R, Schudt C, et al. Cyclic nucleotide phosphodiesterases from purified human CD4+ and CD8+ T lymphocytes. Clin Exp Allergy. 1995;25:625–663. doi: 10.1111/j.1365-2222.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Tenor H, Hatzelmann A, Church MK, Schudt C, Shute JK. Effects of theophylline and rolipram on leukotriene C4 (LTC4) synthesis and chemotaxis of human eosinophils from normal and atopic subjects. Br J Pharmacol. 1996;118:1727–1735. doi: 10.1111/j.1476-5381.1996.tb15598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley DG, Maurice DH. Vascular smooth muscle cell phenotype-dependent phosphodiesterase 4D short form expression: role of differential histone acetylation on cAMP-regulated function. Mol Pharmacol. 2005;68:596–605. doi: 10.1124/mol.105.014126. [DOI] [PubMed] [Google Scholar]
- Tirouvanziam R. Neutrophilic inflammation as a major determinant in the progression of cystic fibrosis. Drug News Perspect. 2006;19:609–614. doi: 10.1358/dnp.2006.19.10.1068008. [DOI] [PubMed] [Google Scholar]
- Trevethick M, Banner KH, Ballard S, Barnard A, Lewis A, Browne J, et al. UK-500 001, a potent selective inhibitor of phosphodiesterase type 4 (PDE4) designed for inhaled delivery, inhibits TNF-α, MIP-1β and LTB4 release from native human blood cells. Am J Res Crit Care Med. 2007a;175:A116. [Google Scholar]
- Trevethick M, Philip J, Chaffe P, Sladen L, Cooper C, Nagendra R, et al. In vivo profile of intratracheally delivered phosphodiesterase type 4 (PDE4) inhibitor UK-500 001-inhibits bronchoconstriction and inflammation. Am J Res Crit Care Med. 2007b;175:A927. [Google Scholar]
- Ukena D, Rentz K, Reiber C, Sybrecht GW. Effects of the mixed phosphodiesterase III/IV inhibitor, zardaverine, on airway function in patients with chronic airflow obstruction. Respir Med. 1995;89:441–444. doi: 10.1016/0954-6111(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Meeker SN, Chen J. Inhibition of neurally mediated nonadrenergic, noncholinergic contractions of guinea pig bronchus by isozyme-selective phosphodiesterase inhibitors. J Pharmacol Exp Ther. 1994;271:811–817. [PubMed] [Google Scholar]
- Underwood DC, Kotzer CJ, Bochnowicz S, Osborn RR, Luttmann MA, Hay DWP, et al. Comparison of phosphodiesterase III, IV and dual III/IV inhibitors on bronchospasm and pulmonary eosinophil influx in guinea pigs. J Pharmacol Exp Ther. 1994;270:250–259. [PubMed] [Google Scholar]
- Van der Mey M, Bommele KM, Boss H, Hatzelmann A, Van Slingerland M, Sterk GJ, et al. Synthesis and structure-activity relationships of cis-tetrahydrophthalazinone/pyridazinone hybrids: a novel series of potent dual PDE3/PDE4 inhibitory agents. J Med Chem. 2003;46:2008–2016. doi: 10.1021/jm030776l. [DOI] [PubMed] [Google Scholar]
- Vassallo R, Kroening PR, Parambil J, Kita H. Nicotine and oxidative cigarette smoke constituents induce immune-modulatory and pro-inflammatory dendritic cell responses. Mol Immunol. 2008;45:3321–3329. doi: 10.1016/j.molimm.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona Pharma website. Planned anti-asthma and hay fever treatment passes crucial tests. http://www.veronapharma.com.
- Wang P, Wu P, Ohleth K, Egan R, Billah M. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol Pharmacol. 1999;56:170–174. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- Watt AP, Schock BC, Ennis M. Neutrophils and eosinophils: clinical implications of their appearance, presence and disappearance in asthma and COPD. Curr Drug Targets Inflamm Allergy. 2005;4:415–423. doi: 10.2174/1568010054526313. [DOI] [PubMed] [Google Scholar]
- Wright LC, Seybold J, Robichaud A, Adcock I, Barnes P. Phosphodiesterase expression in human epithelial cells. Am J Physiol. 1998;275:L694–L700. doi: 10.1152/ajplung.1998.275.4.L694. [DOI] [PubMed] [Google Scholar]
- Zaher C, Halbert R, Dubois R, George D, Nonikov D. Smoking-related diseases: the importance of COPD. Int J Tuberc Lung Dis. 2004;8:1423–1428. [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–595. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, et al. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacology. 2008;33:1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KY, Ibrahim PN, Gillette S, Bollag G. Phosphodiesterase 4 as a potential drug target. Expert Opin Ther Targets. 2005;9:1283–1305. doi: 10.1517/14728222.9.6.1283. [DOI] [PubMed] [Google Scholar]


