Abstract
As arginine can serve as precursor to a wide range of compounds, including nitric oxide, creatine, urea, polyamines, proline, glutamate and agmatine, there is considerable interest in elucidating mechanisms underlying regulation of its metabolism. It is now becoming apparent that the two isoforms of arginase in mammals play key roles in regulation of most aspects of arginine metabolism in health and disease. In particular, work over the past several years has focused on the roles and regulation of the arginases in vascular disease, pulmonary disease, infectious disease, immune cell function and cancer. As most of these topics have been considered in recent review articles, this review will focus more closely on results of recent studies on expression of the arginases in endothelial and vascular smooth muscle cells, post-translational modulation of arginase activity and applications of arginase inhibitors in vivo.
Keywords: arginase, arginase inhibitors, arginine, cancer, endothelial, genetic knockout, nitric oxide, polyamines, vascular
Introduction
There is widespread interest in arginine because it is involved in multiple metabolic processes that play important roles in a very wide range of physiological and pathophysiological conditions. Although the roles of arginine as an intermediate in the urea cycle and as a precursor in creatine biosynthesis have been familiar to biochemists for many years, there was an explosion of interest in this amino acid beginning 20 years ago, stemming from the recognition that arginine is the source of the nitrogen atom in the biosynthesis of nitric oxide (NO) (Hibbs et al., 1988; Palmer et al., 1988). As most readers are probably well aware of, this discovery has resulted in the unintended and unwelcome consequence of an ongoing flood of spam emails advertising arginine supplements, primarily involving exaggerated claims for enhancing sexual performance.
The NO-centric view of arginine has been so predominant that the complexities of arginine metabolism can be easily overlooked. Briefly, the free arginine pool is derived from diet, endogenous synthesis and turnover of cellular protein (Figure 1) (Morris, 2004; Wu and Morris, 2004). Endogenous synthesis is comprised of de novo synthesis from the carbon skeleton of glutamine and of the enzymatic recycling of citrulline produced in reactions catalyzed by the NO synthase (NOS) and dimethylarginine dimethylaminohydrolase (DDAH) enzymes. Despite erroneous claims in some biochemistry textbooks, the urea cycle accounts for very little or no net synthesis of arginine. In healthy adults, endogenous synthesis provides sufficient arginine that it is not required in the diet, but it cannot provide enough arginine to meet metabolic requirements in certain conditions, including inflammation, dysfunction of small bowel or kidney, or in neonates and premature infants. Thus, arginine is classified as a semi-essential or conditionally essential amino acid (Abumrad and Barbul, 2004; Flynn et al., 2002).
Figure 1.
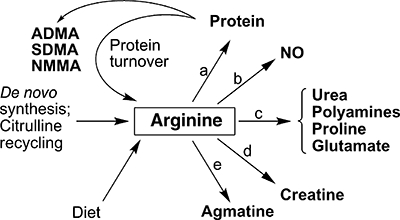
Overview of mammalian arginine metabolism. Sources of the free arginine pool are indicated, as well as the various end products of arginine metabolism. In addition to arginine, the methylated arginine derivatives asymmetric dimethyl-L-arginine (ADMA), symmetric dimethyl-L-arginine (SDMA) and NG-monomethyl-L-arginine (NMMA) are released upon the turnover of post-translationally methylated proteins. Enzymes that use arginine as substrate are: (a) arginyl-tRNA synthetase; (b) NO synthases; (c) arginases; (d) arginine:glycine amidinotransferase; and (e) arginine decarboxylase. Not shown are the various transporters that are required for movement of arginine across plasma and mitochondrial membranes.
Arginine is used as substrate by five sets of enzymes, leading to its incorporation or transformation into the products shown in Figure 1. Interestingly, charged tRNAArg is required not only for protein synthesis but also plays a role in protein degradation. Transfer of the arginine from the charged tRNA to N-terminal Asp, Glu or Cys of proteins by arginyltransferase targets the modified proteins for degradation by the N-end rule pathway (Tasaki and Kwon, 2007). The greatest diversity of end products arises from catabolism of arginine by arginase (Figure 1). The hydrolysis of arginine by arginase produces urea and ornithine, which in turn can serve as precursor for synthesis of polyamines, proline or glutamate. Thus, in some cells it is arginase rather than ornithine decarboxylase that is limiting for polyamine synthesis (Li et al., 2001; 2002; Wei et al., 2001). Moreover, there are significant secondary consequences of reductions in the availability of arginine due to its catabolism by arginase, including reductions in the NO synthesis [and increased superoxide production by NOS when arginine levels are sufficiently low (Xia et al., 1996)] and either increases or decreases in expression of specific proteins [e.g. the cationic amino acid transporter CAT-1 and inducible isoform of NOS (iNOS), respectively] (Figure 2) (Morris, 2006). Consideration of the Km and Vmax values for NOS and the arginases indicates that the arginases can effectively compete with NOS for arginine under physiological conditions (Wu and Morris, 1998; Santhanam et al., 2008). The complexity of the outcomes shown in Figure 2 poses a considerable challenge to understanding the precise roles of the arginases in specific organs and cell types in health and disease.
Figure 2.
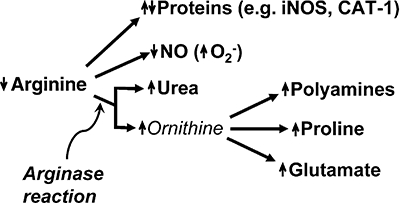
Consequences of increased arginase activity. Up and down arrows indicate increases or decreases in amount or rate of synthesis of the indicated biochemical products. Depending on the specific protein, reduced arginine availability may result in either decreased translational efficiency (e.g. iNOS) or increased translational efficiency (e.g. CAT-1) of the corresponding mRNA (reviewed in Morris, 2007).
Adding to this complexity is the fact that two arginase isoforms are expressed in mammals: arginase I (cytosolic) and arginase II (mitochondrial) (Jenkinson et al., 1996). These isoforms are the products of distinct genes located on different chromosomes. The arginase genes are regulated independently, can be expressed in many different cell types and are inducible by a wide range of agents and in many pathophysiological conditions (Mori and Gotoh, 2000; Morris, 2000; Mori, 2007). In the recent years the arginases have become implicated in many disease processes, including various types of vascular disease, pulmonary disease, infectious disease, immune cell dysfunction and cancer (Bansal and Ochoa, 2003; Kim et al., 2004; Lange et al., 2004; Mori and Gotoh, 2004; Bronte and Zanovello, 2005; Maarsingh et al., 2008; Rodriguez and Ochoa, 2008). As it is not possible to consider all these topics here, this article will focus primarily on selected recent findings, with emphasis on expression of the arginases in endothelial and vascular smooth muscle cells, post-translational modulation of arginase activity and applications of arginase inhibitors in vivo. Various aspects of functional roles of the arginases in vascular biology, including atherosclerosis, have been reviewed recently and thus will not be considered here (Morris, 2005; Huynh and Chin-Dusting, 2006; Yang and Ming, 2006; Durante et al., 2007; Santhanam et al., 2008).
Arginases in vascular smooth muscle cells
As described in the following section, immunohistochemical studies have indicated that most of the arginases in blood vessels are present in the endothelium. However, vascular smooth muscle cells do express low levels of arginase, and arginase expression in these cells can be induced by interleukin-4 (IL-4) and IL-13 (Wei et al., 2000), transforming growth factor-β (Durante et al., 2001), lysophosphatidycholine (Durante et al., 1997) and mechanical strain (Durante et al., 2000). Rather than modulating NO synthesis, arginase in vascular smooth muscle cells appears to function primarily to enhance synthesis of polyamines and proline for cell proliferation and collagen synthesis, respectively (Durante et al., 2007). Thus, elevated arginase expression in vascular smooth muscle cells may be an important factor in development of intimal hyperplasia and vascular stiffness in response to injury or during ageing. In proof-of-principle experiments, simply overexpressing arginase I in cultured vascular smooth muscle cells resulted in increased polyamine synthesis and cell proliferation (Wei et al., 2001), supporting the notion that elevated arginase expression may play a role in the initiation or progression of intimal hyperplasia following vascular injury (Marinova et al., 2008).
Arginase expression in endothelial cells
Arginase is constitutively expressed in endothelial cells, but the expression of the specific isoforms differs among mammalian species (Table 1). Although some arginase I has been detected in human endothelial cells, the predominant isoform is arginase II. There is strong evidence that constitutive levels of arginase activity in endothelial cells limit NO synthesis and NO-dependent vasodilatory function (e.g. Zhang et al., 2001; Berkowitz et al., 2003; Lim et al., 2007). A wide range of agents can induce arginase expression when administered to cultured endothelial cells, including lipopolysaccharide (LPS) (Buga et al., 1996), tumour necrosis factor-α (TNFα) (Gao et al., 2007), LPS + TNFα (Bachetti et al., 2004; Chicoine et al., 2004; Nelin et al., 2005), thrombin (Ming et al., 2004; Topal et al., 2006; Yang et al., 2006a; Lewis et al., 2008), high glucose (Romero et al., 2008), oxidized low-density lipoprotein (LDL) (Ryoo et al., 2006) and H2O2 (Thengchaisri et al., 2006). Conditions that result in elevated endothelial arginase expression in vivo include hypertension (Zhang et al., 2004; Johnson et al., 2005), ischaemia–reperfusion (Hein et al., 2003), intimal hyperplasia (Loyaga-Rendon et al., 2005) and ageing (Berkowitz et al., 2003). Other than effects of plant compounds such as genistein (Nelin et al., 2005) and cocoa flavanols (Schnorr et al., 2008) or simvastatin (Romero et al., 2008), there is little information regarding agents that suppress arginase expression or prevent its induction in endothelial cells. Arginase activity is greatly diminished in endothelial cells of the spontaneously diabetic BB rat, compared with cells from the non-diabetic BB strain (Wu and Meininger, 1995); the arginase isoform(s) in these cells were not identified. Seemingly in contrast, arginase activity and arginase I expression were elevated in aortas of streptozotocin diabetic rats (Romero et al., 2008), but it was not determined whether the increased expression occurred in the endothelium and/or vascular smooth muscle. The latter group also found that incubation of bovine coronary endothelial cells with high (25 mM) glucose for 24 h resulted in increased arginase activity without increasing arginase I protein levels (Romero et al., 2008), indicating an increase in catalytic efficiency of the arginase (see also the following section). Additional studies are required to elucidate the basis for the apparent discrepancies in results of these two studies.
Table 1.
Arginase isoforms detected in endothelial cells of various mammalian species
| Species | Arginase isoform | References |
|---|---|---|
| Human | I and II | (Bachetti et al., 2004; Xu et al., 2004; Marinova et al., 2008) |
| II | (Ryoo et al., 2006; Topal et al., 2006; Yang et al., 2006a) | |
| Rat | I | (Lewis et al., 2008) |
| I and II | (Buga et al., 1996; Berkowitz et al., 2003; Suschek et al., 2003; Johnson et al., 2005) | |
| Mouse | I | (Bivalacqua et al., 2007; Gao et al., 2007) |
| II | (Lim et al., 2007) | |
| Pig | I | (Zhang et al., 2001; 2004; Hein et al., 2003; Thengchaisri et al., 2006) |
| II | (Lee et al., 2007; Zharikov et al., 2008) | |
| Cow | I | (Stanley et al., 2006; Romero et al., 2008) |
| I and II | (Chicoine et al., 2004; Nelin et al., 2005) |
Because of differences in the sensitivity and specificity of the various detection methods used (Western blot, immunohistochemistry, real-time polymerase chain reaction), the report of only one arginase isoform in a particular study does not necessarily indicate that the other isoform is not also expressed. Some differences in patterns of isoform expression listed here also may reflect characteristics of endothelial cells isolated from different vascular beds, disease conditions or variable contamination with vascular smooth muscle cells.
Model studies have demonstrated that simply overexpressing either arginase I or II in endothelial cells may not only reduce NO synthesis but also can enhance polyamine synthesis and cell proliferation as well as proline synthesis (Li et al., 2001; 2002). From the discussion in this section it is apparent that the precise consequences of arginase expression in endothelial cells will depend upon the animal species, the relative activities of enzymes such as NOS and enzymes involved in ornithine metabolism, whose expression in turn will depend on the particular vascular bed in which the endothelial cells are located and the nature of the various stimuli to which these cells are exposed.
Post-translational modulation of arginase activity
Although it has not been rigorously evaluated in most cases, arginase activity usually appears to be proportional to the amount of arginase protein, which, in turn, is determined primarily by transcription of the arginase genes. However, several recent studies have reported distinct mechanisms by which arginase activity can be modulated independently of changes in amount of arginase protein. Santhanam et al. reported that cysteine residues 168 and 303 in arginase I can undergo S-nitrosylation (Santhanam et al., 2007). In particular, S-nitrosylation of Cys303 stabilizes the arginase trimer, resulting in a sixfold decrease in its Km for arginine. Cys168 is conserved in mammalian arginases I and II, but there is no cysteine in mammalian arginase II that corresponds to Cys303 in arginase I, suggesting that S-nitrosylation may not alter the activity of arginase II. Thus, this mechanism of modulating arginase activity may not occur in human endothelial cells, which express arginase II but little or no arginase I (Table 1). These authors also presented evidence for S-nitrosylation of arginase I in blood vessels of ageing rats in vivo, likely as a consequence of the activity of iNOS. They therefore suggested that this increase in arginase I activity may contribute to endothelial dysfunction in ageing. This appears somewhat paradoxical in view of earlier studies showing that NG-hydroxy-L-arginine (NOHA), an intermediate in NO synthesis and a potent endogenous inhibitor of arginases, can accumulate sufficiently in iNOS-expressing cells to inhibit arginase activity (Buga et al., 1996). Moreover, as arginase II also may be expressed in endothelial cells, depending on species, additional studies will be needed to determine the relative contributions of arginase I and II to endothelial dysfunction. It will be important also to characterize conditions that promote or reverse S-nitrosylation of arginase I, as well as to identify additional physiological or pathophysiological conditions in which arginase activity is regulated by this mechanism.
Following up on observations that elevated serum levels of uric acid are often associated with pulmonary hypertension, Zharikov et al. found that uric acid inhibited NO production by activated pulmonary artery endothelial cells and that this inhibition could be overcome by an arginase inhibitor (Zharikov et al., 2008). Further studies demonstrated that uric acid activated arginase by reducing its Km for arginine. This effect was seen for arginase activity in lysates of endothelial cells, rat liver and rat kidney, indicating that uric acid has a similar effect on both arginase I and II. As elevated serum levels of uric acid are associated also with pre-eclampsia (Bainbridge and Roberts, 2008; Kenny et al., 2008), these results raise the possibility that uric acid-dependent activation of arginase may play a role in hypertension in this disorder.
Finally, in vitro experiments using human erythrocyte lysates or arginase preparations from bovine liver have provided evidence that arginase activity at physiological pH can be enhanced by hydroxyl radicals produced by the Fenton reaction; arginase activity in both these samples is due to arginase I (Iyamu et al., 2008). The precise mechanism underlying this effect has not been identified. These authors speculate that this activation of arginase may contribute to impaired NO homeostasis under conditions of oxidative stress. Although additional studies are needed to determine whether there is evidence for this mechanism of arginase activation in intact cells and to determine whether it applies also to arginase II, it is intriguing to note that H2O2, which can be converted to hydroxyl radical, was found to induce arginase I expression in porcine coronary arterioles (Thengchaisri et al., 2006). However, arginase activity was not measured in this study, so it was not possible to ascertain whether there was any increase in arginase activity independent of increases in the amount of arginase protein.
Inhibition of arginase activity or expression in vivo
Several potent arginase inhibitors have been developed and become commercially available within the past few years (Figure 3). Older inhibitors had low potency and thus were more likely to have side effects because of the high concentrations required. For example, norvaline, which has sometimes been used as an arginase inhibitor, is a good substrate for branched chain aminotransferases (Davoodi et al., 1998). NOHA, which is an intermediate in NO synthesis, is a potent endogenous inhibitor of the arginases (Boucher et al., 1994; Daghigh et al., 1994; Buga et al., 1996). However, its utility as an arginase inhibitor is complicated by the fact that it also is a precursor for synthesis of NO not only by NOS enzymes but also by cytochrome P450 enzymes (Boucher et al., 1992). α-Difluoromethylornithine (DFMO) has been used as a non-specific arginase inhibitor for in vitro (e.g. Ming et al., 2004; Santhanam et al., 2007; Lewis et al., 2008) and in vivo experiments (Demougeot et al., 2005), although an effect of DFMO on arginase activity was not directly evaluated in most studies. It is important to note that DFMO is a very poor inhibitor of arginase (Ki= 3.9 ± 1.0 mM) (Selamnia et al., 1998), whereas it is well-established as a potent irreversible inhibitor of ornithine decarboxylase (Metcalf et al., 1978). As concentrations of DFMO required to significantly inhibit arginase activity in intact cells are very high (e.g. 10 mM) (Selamnia et al., 1998), there are concerns about nonspecific effects of such high concentrations, as well as concerns about effects of inhibiting polyamine synthesis. As arginase is subject to inhibition by its product ornithine (Hunter and Downs, 1945; Selamnia et al., 1998), observations that DFMO concentrations in the micromolar range can enhance NO-dependent vascular responses may be secondary to intracellular accumulation of ornithine due to the DFMO inhibition of ornithine decarboxylase. Because of these considerations, use of DFMO as an arginase inhibitor will not be discussed in detail here.
Figure 3.
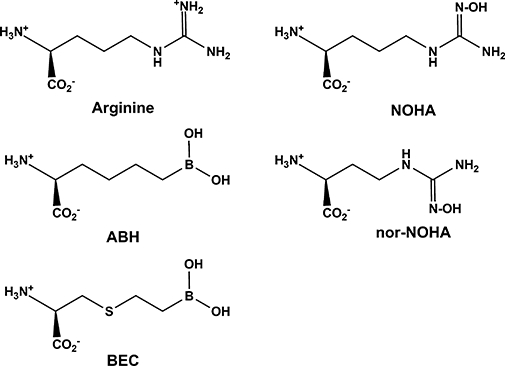
Structures of arginine and arginase inhibitors. Values of Ki for these inhibitors are in the sub-micromolar to micromolar range (Christianson, 2005). ABH, 2(S)-amino-6-boronohexanoic acid; BEC, S-(2-boronoethyl)-L-cysteine; NOHA, NG-hydroxy-L-arginine; nor-NOHA, Nω-hydroxyl-nor-L-arginine.
The arginase inhibitors shown in Figure 3 are competitive inhibitors and do not inhibit the NOS enzymes at concentrations that inhibit the arginases. These inhibitors and their interactions with active site residues of arginase have been reviewed elsewhere (Christianson, 2005). Unfortunately, none of these inhibitors exhibit sufficient differences in affinity for the two arginase isoforms to provide isoform-selective inhibition in cultured cells or in vivo. Comparative studies using intact cells or tissues to systematically evaluate effects of multiple arginase inhibitors have been lacking. The exception is a recent study that used isolated rat aorta and mesenteric arteries to compare vascular effects of several arginase inhibitors (Huynh et al., 2009). These investigators found that only S-(2-boronoethyl)-L-cysteine (BEC) and NOHA – but not Nω-hydroxy-nor-L-arginine (nor-NOHA), L-valine, DFMO or norvaline – significantly reversed vascular tolerance to acetylcholine, suggesting increases in NO bioavailability. Of concern was the finding that BEC and NOHA also elicited endothelium-independent vasorelaxation in rat aorta, implying that these compounds may have cellular targets in addition to the arginases. It should be noted, however, that neither arginase activities nor NO production were directly measured in this study.
In addition to their application in numerous studies using isolated tissues or cultured cells, arginase inhibitors have been applied in vivo to evaluate their effects on allergen-induced airway hyper-responsiveness and inflammation (Ckless et al., 2008; Maarsingh et al., 2008), liver ischaemia/reperfusion injury (Reid et al., 2007; Jeyabalan et al., 2008), experimental autoimmune encephalitis (Xu et al., 2003) and erectile function (Bivalacqua et al., 2007). Dosages and duration of treatment are provided in Table 2. In general, administration of the inhibitors resulted in improvement of pathological features, usually ascribed to increased NO production and activity of NO-dependent processes. However, inflammation was unexpectedly increased in one model of allergic inflammation (Ckless et al., 2008) but decreased in another model (Maarsingh et al., 2008). Additional studies are required to determine whether the different outcomes reflect differences in species, the specific arginase inhibitor used or route of administration. Because there are no assays to evaluate arginase activity in situ, evaluation of arginase inhibition in vivo is indirect. So far, there has been no systematic study to identify possible off-target effects of arginase inhibitors in vivo, nor is there published data on pharmacokinetics of the arginase inhibitors. This information is essential for evaluating the therapeutic potential of these compounds. Cost can become a significant factor in experimental studies of the arginase inhibitors in vivo. Based on October 2008 catalog prices from a company that sells all the arginase inhibitors, 50 mg of the inhibitors would cost $720 (nor-NOHA), $1300 (BEC) or $2400 (ABH).
Table 2.
Studies that have used arginase inhibitors in vivo
| Species (strain) | Inhibitor | Dose and route of administration | Duration | Equivalent dose/day in 70-kg human | References | |
|---|---|---|---|---|---|---|
| Mouse (ApoE−/−) | ABH | A | ∼200 µg/day; in drinking water | 14 days | ∼0.7 g | (Ryoo et al., 2008) |
| Mouse (B6/129) | ABH | B | 3.2 µg/kg; intracavernosal | 20 min | 224 µg | (Bivalacqua et al., 2007) |
| Mouse (C57BL/6) | ABH | B | 0.7 mg/day; i.p. | 23 days | ∼2.4 g | (Xu et al., 2003) |
| Mouse (C57BL/6) | nor-NOHA | B | 2 × 100 mg/kg; i.v. | ≤24 h | 14 g | (Jeyabalan et al., 2008) |
| Mouse (C57BL/6) | nor-NOHA | B | 100 µg/day; i.p. | 14 days | ∼0.35 g | (Bratt et al., 2009) |
| Rat (Lewis) | nor-NOHA | B | 2 × 100 mg/kg; i.v. | ≤24 h | 14 g | (Reid et al., 2007) |
| Rat (SHR) | nor-NOHA | B | 10 or 40 mg/kg/day; i.p. | 21 days | 0.7 or 2.8 g | (Bagnost et al., 2008) |
| Mouse (BALB/c) | BEC | C | 2.3 µg; single aspiration | 48 h | ∼8 mg | (Ckless et al., 2008) |
| Guinea pig (Dunkin Hartley) | ABH | C | 25 mmol·L−1 in aerosol; 1–3 × 15 min inhalation | ≤24 h | unknown | (Maarsingh et al., 2008) |
A, oral; ABH, 2(S)-amino-6-boronohexanoic acid; B, injection; BEC, S-(2-boronoethyl)-L-cysteine; C, inhalation; nor-NOHA, Nω-hydroxy-nor-L-arginine;
Localized effects of arginase inhibitors on vascular function in humans have been carried out using microdialysis. Administration of a combination of BEC and nor-NOHA resulted in augmented reflex vasodilation in skin of hypertensive subjects but not in age-matched normotensive subjects (Holowatz and Kenney, 2007). These authors speculated that arginase activity was elevated in skin of the hypertensive individuals, thus limiting availability of arginine for NO synthesis; it should be noted, however, that arginase activity, urea production or NO production were not measured in this study.
As currently available arginase inhibitors are not isoform-selective, RNA interference has been used to inhibit expression of specific arginase isoforms. Two studies employing this technique to selectively inhibit arginase expression in vivo have been reported. In one study plasmids encoding short hairpin RNA (shRNA) directed against arginase I were administered to mice by intratracheal instillation, followed by similar administration of IL-13 to induce airway hyper-responsiveness (Yang et al., 2006b). Administration of shRNA directed against arginase I resulted in greatly reduced expression arginase I in the lung at the mRNA and protein levels, with no effect on expression of arginase II, and also significantly attenuated IL-13-induced airway hyper-responsiveness. Although the impact of shRNA on total arginase activity was not determined, the results clearly indicated a specific role for arginase I underlying the induction of airway hyper-responsiveness in this model. Another group used adeno-associated virus to deliver antisense RNA to arginase I in order to evaluate the role of this arginase in erectile function of mice (Bivalacqua et al., 2007). This group had shown that arginase I expression was elevated in penis of aged mice, compared with young mice, and correlated with erectile dysfunction and inhibition of NO-dependent processes. Virus was injected directly into the corpus cavernosum, and arginase I expression and erectile function were evaluated 30 days later. Arginase I expression was decreased, cGMP concentration increased and erectile responses improved in mice that received virus encoding antisense sequence to arginase I, whereas a control virus encoding beta-galactosidase had no effect on these parameters.
Mouse strains with genetic ablation of arginase expression
Mouse strains carrying genetic knockouts of each of the arginases have been developed. Mice with homozygous deletion of arginase I expression (Arg I−/−) die in the perinatal period, apparently as a consequence of a nonfunctional urea cycle (Iyer et al., 2002). In contrast, mice with homozygous deletion of arginase II expression (Arg II−/−) are viable and exhibit mild phenotypic changes, such as elevated plasma levels of arginine (Shi et al., 2001). The elevated plasma levels of arginine are consistent with earlier work indicating that arginine catabolism plays a significant role in regulating arginine homeostasis (Castillo et al., 1993). Studies using the arginase deletion strains have documented changes in tissue levels of polyamines, amino acids and guanidino compounds (Deignan et al., 2006; 2007; 2008). The arginase deletion strains also are being utilized to evaluate physiological functions of the arginases. For example, pressurized carotid arteries from Arg II−/− mice exhibited an enhanced vasorelaxation response to acetylcholine and a reduced response to phenylephrine, compared with carotid arteries from wild-type mice, indicating that endothelial arginase II normally limits arginine availability to endothelial NOS (Lim et al., 2007). This same group also found that when the Arg II−/− strain was crossed into the ApoE deletion strain (ApoE−/−), there was a reduction in development of atherosclerotic plaque in the double deletion strain, relative to the ApoE−/− strain when fed a high cholesterol diet (Ryoo et al., 2008). The diet-induced decreases in vascular NO synthesis and increases in endothelial production of reactive oxygen species also were less pronounced in the double deletion strain.
Studies also have been conducted using cells or tissue isolated from the arginase knockout mice. For example, ablation of arginase I expression was found to result in increased proliferation of neural stem cells isolated from embryonic and newborn mice (Becker-Catania et al., 2006). This result was unexpected in view of earlier work supporting the notion that arginase expression can be limiting for polyamine synthesis and cell proliferation (Holtta and Pohjanpelto, 1982; Singh et al., 2000; Li et al., 2001; 2002; Wei et al., 2001). Microarray and real-time polymerase chain reaction analyses of RNA from neural stem cells isolated from the arginase I knockout mice demonstrated altered expression of several genes, some of which may be involved in an alternate pathway of polyamine synthesis (Becker-Catania et al., 2006). Importantly, these changes in expression of other genes illustrate the difficulties in interpreting the metabolic and physiological consequences of ablating expression of specific genes. Without additional experimental data, it is unclear whether the increased proliferation rate of the neural stem cells from the arginase I knockout mice is due to the polyamine synthesis via an alternate biosynthetic pathway, to increased uptake of polyamines from the culture medium, or by the altered expression of other genes involved in cell proliferation. Similarly, ablation of arginase II expression in a mouse model of prostate cancer resulted in more aggressive tumour growth and development, again suggesting that the alternate pathways of polyamine biosynthesis or uptake may have become more active in the cancer cells in the absence of arginase II expression (Mumenthaler et al., 2008).
Given the lack of isoform-specific arginase inhibitors and the limitations of studying mice with global knockout of arginase expression, more precise elucidation of the function of the arginases in vivo will require mouse strains with conditional knockout of arginase isoform expression in specific cell types. This is particularly important in the case of arginase I because mice homozygous for deletion of arginase I die in the perinatal period (Iyer et al., 2002). Thus, mouse strains with ablation of arginase I expression in macrophages have recently been developed to demonstrate the role of this enzyme in modulating host defense against intracellular pathogens (El Kasmi et al., 2008). We anticipate that additional strains will be developed to address the role of arginase I in other cell types.
Conclusions
It is now well-appreciated that expression of the arginases is highly regulated in many tissues and cell types and that changes in their activity can have a significant impact on a variety of critical physiological and pathophysiological processes. The application of potent new arginase inhibitors and recombinant DNA tools to selectively inhibit arginase expression is beginning to yield new insights into the roles of the arginases in isolated cells and tissues and in animal models. As indicated by this review, much attention is being devoted to evaluating the roles and regulation of the arginases in the function of vascular cells. However, elucidation of their roles in human health and disease is complicated by the fact that the patterns of arginase expression in animal cells do not always accurately represent the patterns of expression in corresponding human cell types. As discussed in more detail elsewhere (Morris, 2007), we also should not overlook the fact that a more complete understanding of arginine metabolism and the roles of the arginases will require greater characterization of the roles and regulation of the many other enzymes and transporters involved in determining the metabolic fates of arginine. Thus, there are many important questions yet to be addressed by talented investigators.
Acknowledgments
Work in the author's laboratory has been supported by NIH grants GM57384 and GM64509.
Glossary
Abbreviations:
- ABH
2(S)-amino-6-boronohexanoic acid
- ADMA
asymmetric dimethyl-L-arginine
- BEC
S-(2-boronoethyl)-L-cysteine
- CAT-1
cationic amino acid transporter-1
- DDAH
dimethylarginine dimethylaminohydrolase
- DFMO
α-difluoromethylornithine
- iNOS
inducible isoform of NOS
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide
- NMMA
NG-monomethyl-L-arginine
- NO
nitric oxide
- NOHA
NG-hydroxy-L-arginine
- nor-NOHA
Nω-hydroxy-nor-L-arginine
- NOS
nitric oxide synthase
- SDMA
symmetric dimethyl-L-arginine; shRNA, short hairpin RNA
- TNFα
tumour necrosis factor-α
Conflict of interest
The author has received honoraria for speaking and consulting and financial support for attending meetings from Ajinomoto LLC. No funding has been provided for this article.
References
- Abumrad NN, Barbul A. The use of arginine in clinical practice. In: Cynober LA, editor. Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition. Boca Raton, FL: CRC Press; 2004. pp. 595–611. [Google Scholar]
- Bachetti T, Comini L, Francolini G, Bastianon D, Valetti B, Cadei M, et al. Arginase pathway in human endothelial cells in pathophysiological conditions. J Mol Cell Cardiol. 2004;37:515–523. doi: 10.1016/j.yjmcc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Bagnost T, Berthelot A, Bouhaddi M, Laurant P, Andre C, Guillaume Y, et al. Treatment with the arginase inhibitor N(omega)-hydroxy-nor-L-arginine improves vascular function and lowers blood pressure in adult spontaneously hypertensive rat. J Hypertens. 2008;26:1110–1118. doi: 10.1097/HJH.0b013e3282fcc357. [DOI] [PubMed] [Google Scholar]
- Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29(Suppl. A):S67–S72. doi: 10.1016/j.placenta.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care. 2003;6:223–228. doi: 10.1097/00075197-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Becker-Catania SG, Gregory TL, Yang Y, Gau CL, de Vellis J, Cederbaum SD, et al. Loss of arginase I results in increased proliferation of neural stem cells. J Neurosci Res. 2006;84:735–746. doi: 10.1002/jnr.20964. [DOI] [PubMed] [Google Scholar]
- Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007;292:H1340–H1351. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- Boucher JL, Genet A, Vadon S, Delaforge M, Henry Y, Mansuy D. Cytochrome P450 catalyses the oxidation of Nω-hydroxy-L-arginine by NADPH and O2 to nitric oxide and citrulline. Biochem Biophys Res Commun. 1992;187:880–886. doi: 10.1016/0006-291x(92)91279-y. [DOI] [PubMed] [Google Scholar]
- Boucher JL, Custot J, Vadon S, Delaforge M, Lepoivre M, Tenu JP, et al. Nω-hydroxy-L-arginine, an intermediate in the L-arginine to nitric oxide pathway, is a strong inhibitor of liver and macrophage arginase. Biochem Biophys Res Commun. 1994;203:1614–1621. doi: 10.1006/bbrc.1994.2371. [DOI] [PubMed] [Google Scholar]
- Bratt JM, Franzi LM, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2009;234:273–280. doi: 10.1016/j.taap.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Buga GM, Singh R, Pervin S, Rogers NE, Schmitz DA, Jenkinson CP, et al. Arginase activity in endothelial cells: inhibition by NG-hydroxyarginine during high-output nitric oxide production. Am J Physiol. 1996;271:H1988–H1998. doi: 10.1152/ajpheart.1996.271.5.H1988. [DOI] [PubMed] [Google Scholar]
- Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, et al. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA. 1993;90:7749–7753. doi: 10.1073/pnas.90.16.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L60–L68. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- Christianson DW. Arginase: structure, mechanism, and physiological role in male and female sexual arousal. Acc Chem Res. 2005;38:191–201. doi: 10.1021/ar040183k. [DOI] [PubMed] [Google Scholar]
- Ckless K, Lampert A, Reiss J, Kasahara D, Poynter ME, Irvin CG, et al. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 2008;181:4255–4264. doi: 10.4049/jimmunol.181.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daghigh F, Fukuto JM, Ash DE. Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications for the regulation of nitric oxide biosynthesis by arginase. Biochem Biophys Res Commun. 1994;202:174–180. doi: 10.1006/bbrc.1994.1909. [DOI] [PubMed] [Google Scholar]
- Davoodi J, Drown PM, Bledsoe RK, Wallin R, Reinhart GD, Hutson SM. Overexpression and characterization of the human mitochondrial and cytosolic branched-chain aminotransferases. J Biol Chem. 1998;273:4982–4989. doi: 10.1074/jbc.273.9.4982. [DOI] [PubMed] [Google Scholar]
- Deignan JL, Livesay JC, Yoo PK, Goodman SI, O'Brien WE, Iyer RK, et al. Ornithine deficiency in the arginase double knockout mouse. Mol Genet Metab. 2006;89:87–96. doi: 10.1016/j.ymgme.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Deignan JL, Livesay JC, Shantz LM, Pegg AE, O'Brien WE, Iyer RK, et al. Polyamine homeostasis in arginase knockout mice. Am J Physiol Cell Physiol. 2007;293:C1296–C1301. doi: 10.1152/ajpcell.00393.2006. [DOI] [PubMed] [Google Scholar]
- Deignan JL, Marescau B, Livesay JC, Iyer RK, De Deyn PP, Cederbaum SD, et al. Increased plasma and tissue guanidino compounds in a mouse model of hyperargininemia. Mol Genet Metab. 2008;93:172–178. doi: 10.1016/j.ymgme.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens. 2005;23:971–978. doi: 10.1097/01.hjh.0000166837.78559.93. [DOI] [PubMed] [Google Scholar]
- Durante W, Liao L, Peyton KJ, Schafer AI. Lysophosphatidylcholine regulates cationic amino acid transport and metabolism in vascular smooth muscle cells. Role in polyamine biosynthesis. J Biol Chem. 1997;272:30154–30159. doi: 10.1074/jbc.272.48.30154. [DOI] [PubMed] [Google Scholar]
- Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Physiological cyclic stretch directs L-arginine transport and metabolism to collagen synthesis in vascular smooth muscle. FASEB J. 2000;14:1775–1783. doi: 10.1096/fj.99-0960com. [DOI] [PubMed] [Google Scholar]
- Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Transforming growth factor-beta(1) stimulates L-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation. 2001;103:1121–1127. doi: 10.1161/01.cir.103.8.1121. [DOI] [PubMed] [Google Scholar]
- Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn NE, Meininger CJ, Haynes TE, Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother. 2002;56:427–438. doi: 10.1016/s0753-3322(02)00273-1. [DOI] [PubMed] [Google Scholar]
- Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, et al. TNF-alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2007;27:1269–1275. doi: 10.1161/ATVBAHA.107.142521. [DOI] [PubMed] [Google Scholar]
- Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol. 2007;581:863–872. doi: 10.1113/jphysiol.2007.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtta E, Pohjanpelto P. Polyamine dependence of Chinese hamster ovary cells in serum-free culture is due to deficient arginase activity. Biochim Biophys Acta. 1982;721:321–327. doi: 10.1016/0167-4889(82)90085-4. [DOI] [PubMed] [Google Scholar]
- Hunter A, Downs CE. The inhibition of arginase by amino acids. J Biol Chem. 1945;157:427–446. [Google Scholar]
- Huynh N, Harris E, Chin-Dusting J, Andrews K. The vascular effects of different arginase inhibitors in rat isolated aorta and mesenteric arteries. Br J Pharmacol. 2009;156:84–93. doi: 10.1111/j.1476-5381.2008.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh NN, Chin-Dusting J. Amino acids, arginase and nitric oxide in vascular health. Clin Exp Pharmacol Physiol. 2006;33:1–8. doi: 10.1111/j.1440-1681.2006.04316.x. [DOI] [PubMed] [Google Scholar]
- Iyamu EW, Perdew H, Woods GM. Cysteine-iron promotes arginase activity by driving the Fenton reaction. Biochem Biophys Res Commun. 2008;376:116–120. doi: 10.1016/j.bbrc.2008.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer RK, Yoo PK, Kern RM, Rozengurt N, Tsoa R, O'Brien WE, et al. Mouse model for human arginase deficiency. Mol Cell Biol. 2002;22:4491–4498. doi: 10.1128/MCB.22.13.4491-4498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol. 1996;114B:107–132. doi: 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- Jeyabalan G, Klune JR, Nakao A, Martik N, Wu G, Tsung A, et al. Arginase blockade protects against hepatic damage in warm ischemia-reperfusion. Nitric Oxide. 2008;19:29–35. doi: 10.1016/j.niox.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1057–R1062. doi: 10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Broadhurst D, Brown M, Dunn WB, Redman CW, Kell DB, et al. Detection and identification of novel metabolomic biomarkers in preeclampsia. Reprod Sci. 2008;15:591–597. doi: 10.1177/1933719108316908. [DOI] [PubMed] [Google Scholar]
- Kim NN, Christianson DW, Traish AM. Role of arginase in the male and female sexual arousal response. J Nutr. 2004;134:2873S–2879S. doi: 10.1093/jn/134.10.2873S. discussion 2895S. [DOI] [PubMed] [Google Scholar]
- Lange PS, Langley B, Lu P, Ratan RR. Novel roles for arginase in cell survival, regeneration, and translation in the central nervous system. J Nutr. 2004;134:2812S–2817S. doi: 10.1093/jn/134.10.2812S. discussion 2818S–2819S. [DOI] [PubMed] [Google Scholar]
- Lee MY, Tse HF, Siu CW, Zhu SG, Man RY, Vanhoutte PM. Genomic changes in regenerated porcine coronary arterial endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2443–2449. doi: 10.1161/ATVBAHA.107.141705. [DOI] [PubMed] [Google Scholar]
- Lewis C, Zhu W, Pavkov ML, Kinney CM, Dicorleto PE, Kashyap VS. Arginase blockade lessens endothelial dysfunction after thrombosis. J Vasc Surg. 2008;48:441–446. doi: 10.1016/j.jvs.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, et al. Regulatory role of arginase I and II in syntheses of nitric oxide, polyamines and proline in endothelial cells. Am J Physiol. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- Li H, Meininger CJ, Hawker JR, Jr, Kelly KA, Morris SM, Jr, Wu G. Activities of arginase I and II are limiting for endothelial cell proliferation. Am J Physiol. 2002;282:R64–R69. doi: 10.1152/ajpregu.2002.282.1.R64. [DOI] [PubMed] [Google Scholar]
- Lim HK, Lim HK, Ryoo S, Benjo A, Shuleri K, Miriel V, et al. Mitochondrial arginase II constrains endothelial NOS-3 activity. Am J Physiol Heart Circ Physiol. 2007;293:H3317–H3324. doi: 10.1152/ajpheart.00700.2007. [DOI] [PubMed] [Google Scholar]
- Loyaga-Rendon RY, Sakamoto S, Beppu M, Aso T, Ishizaka M, Takahashi R, et al. Accumulated endogenous nitric oxide synthase inhibitors, enhanced arginase activity, attenuated dimethylarginine dimethylaminohydrolase activity and intimal hyperplasia in premenopausal human uterine arteries. Atherosclerosis. 2005;178:231–239. doi: 10.1016/j.atherosclerosis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Maarsingh H, Zuidhof AB, Bos IS, van Duin M, Boucher JL, Zaagsma J, et al. Arginase inhibition protects against allergen-induced airway obstruction, hyperresponsiveness, and inflammation. Am J Respir Crit Care Med. 2008;178:565–573. doi: 10.1164/rccm.200710-1588OC. [DOI] [PubMed] [Google Scholar]
- Marinova GV, Loyaga-Rendon RY, Obayashi S, Ishibashi T, Kubota T, Imamura M, et al. possible involvement of altered arginase activity, arginase type I and type II expressions, and nitric oxide production in occurrence of intimal hyperplasia in premenopausal human uterine arteries. J Pharmacol Sci. 2008;106:385–393. doi: 10.1254/jphs.fp0072275. [DOI] [PubMed] [Google Scholar]
- Metcalf BW, Bey P, Danzin C, Jung MJ, Casara P, Vevert JP. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C.4.1.1.17) by substrate and product analogs. J Am Chem Soc. 1978;100:2551–2553. [Google Scholar]
- Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- Mori M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J Nutr. 2007;137:1616S–1620S. doi: 10.1093/jn/137.6.1616S. [DOI] [PubMed] [Google Scholar]
- Mori M, Gotoh T. Relationship between arginase activity and nitric oxide production. In: Ignarro LJ, editor. Nitric Oxide. Biology and Pathology. San Diego, CA: Academic Press; 2000. pp. 199–208. [Google Scholar]
- Mori M, Gotoh T. Arginine metabolic enzymes, nitric oxide and infection. J Nutr. 2004;134:2820S–2825S. doi: 10.1093/jn/134.10.2820S. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr . Regulation of arginine availability and its impact on NO synthesis. In: Ignarro LJ, editor. Nitric Oxide. Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 187–197. [Google Scholar]
- Morris SM., Jr Enzymes of arginine metabolism. J Nutr. 2004;134:2743S–2747S. doi: 10.1093/jn/134.10.2743S. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr Arginine metabolism in vascular biology and disease. Vasc Med. 2005;10:S83–S87. doi: 10.1177/1358836X0501000112. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr Arginine: beyond protein. Am J Clin Nutr. 2006;83:508S–512S. doi: 10.1093/ajcn/83.2.508S. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- Mumenthaler SM, Rozengurt N, Livesay JC, Sabaghian A, Cederbaum SD, Grody WW. Disruption of arginase II alters prostate tumor formation in TRAMP mice. Prostate. 2008;68:1561–1569. doi: 10.1002/pros.20816. [DOI] [PubMed] [Google Scholar]
- Nelin LD, Chicoine LG, Reber KM, English BK, Young TL, Liu Y. Cytokine-induced endothelial arginase expression is dependent on epidermal growth factor receptor. Am J Respir Cell Mol Biol. 2005;33:394–401. doi: 10.1165/rcmb.2005-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RMJ, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Reid KM, Tsung A, Kaizu T, Jeyabalan G, Ikeda A, Shao L, et al. Liver I/R injury is improved by the arginase inhibitor, N(omega)-hydroxy-nor-L-arginine (nor-NOHA) Am J Physiol Gastrointest Liver Physiol. 2007;292:G512–G517. doi: 10.1152/ajpgi.00227.2006. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, et al. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, et al. Endothelial arginase II. A novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, et al. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- Santhanam L, Christianson DW, Nyhan D, Berkowitz DE. Arginase and vascular aging. J Appl Physiol. 2008;105:1632–1642. doi: 10.1152/japplphysiol.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr O, Brossette T, Momma TY, Kleinbongard P, Keen CL, Schroeter H, et al. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch Biochem Biophys. 2008;476:211–215. doi: 10.1016/j.abb.2008.02.040. [DOI] [PubMed] [Google Scholar]
- Selamnia M, Mayeur C, Robert V, Blachier F. Alpha-difluoromethylornithine (DFMO) as a potent arginase activity inhibitor in human colon carcinoma cells. Biochem Pharmacol. 1998;55:1241–1245. doi: 10.1016/s0006-2952(97)00572-8. [DOI] [PubMed] [Google Scholar]
- Shi O, Morris SM, Jr, Zoghbi H, Porter CW, O'Brien WE. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol. 2001;21:811–813. doi: 10.1128/MCB.21.3.811-813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Pervin S, Karimi A, Cederbaum S, Chaudhuri G. Arginase activity in human breast cancer cell lines: Nω-hydroxy-L-arginine selectively inhibits cell proliferation and induces apoptosis in MDA-MB-468 cells. Cancer Res. 2000;60:3305–3312. [PubMed] [Google Scholar]
- Stanley KP, Chicoine LG, Young TL, Reber KM, Lyons CR, Liu Y, et al. Gene transfer with inducible nitric oxide synthase decreases production of urea by arginase in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L298–L306. doi: 10.1152/ajplung.00140.2005. [DOI] [PubMed] [Google Scholar]
- Suschek CV, Schnorr O, Hemmrich K, Aust O, Klotz LO, Sies H, et al. Critical role of L-arginine in endothelial cell survival during oxidative stress. Circulation. 2003;107:2607–2614. doi: 10.1161/01.CIR.0000066909.13953.F1. [DOI] [PubMed] [Google Scholar]
- Tasaki T, Kwon YT. The mammalian N-end rule pathway: new insights into its components and physiological roles. Trends Biochem Sci. 2007;32:520–528. doi: 10.1016/j.tibs.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, et al. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2006;26:2035–2042. doi: 10.1161/01.ATV.0000233334.24805.62. [DOI] [PubMed] [Google Scholar]
- Topal G, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial arginase II modulates nitric-oxide synthesis through nonfreely exchangeable L-arginine pools in human endothelial cells. J Pharmacol Exp Ther. 2006;318:1368–1374. doi: 10.1124/jpet.106.103747. [DOI] [PubMed] [Google Scholar]
- Wei LH, Jacobs AT, Morris SM, Jr, Ignarro LJ. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am J Physiol. 2000;279:C248–C256. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- Wei LH, Wu G, Morris SM, Jr, Ignarro LJ. Elevated arginase I expression in rat aortic smooth muscle cells increases cell proliferation. Proc Natl Acad Sci USA. 2001;98:9260–9264. doi: 10.1073/pnas.161294898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Meininger CJ. Impaired arginine metabolism and NO synthesis in coronary endothelial cells of the spontaneously diabetic BB rat. Am J Physiol. 1995;269:H1312–H1318. doi: 10.1152/ajpheart.1995.269.4.H1312. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr . Arginine metabolism in mammals. In: Cynober LA, editor. Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition. Boca Raton, FL: CRC Press; 2004. pp. 153–167. [Google Scholar]
- Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Hilliard B, Carmody RJ, Tsabary G, Shin H, Christianson DW, et al. Arginase and autoimmune inflammation in the central nervous system. Immunology. 2003;110:141–148. doi: 10.1046/j.1365-2567.2003.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- Yang L, Lewis CM, Chandrasekharan UM, Kinney CM, Dicorleto PE, Kashyap VS. Arginase activity is increased by thrombin: a mechanism for endothelial dysfunction in arterial thrombosis. J Am Coll Surg. 2006a;203:817–826. doi: 10.1016/j.jamcollsurg.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Yang M, Rangasamy D, Matthaei KI, Frew AJ, Zimmmermann N, Mahalingam S, et al. Inhibition of arginase I activity by RNA interference attenuates IL-13-induced airways hyperresponsiveness. J Immunol. 2006b;177:5595–5603. doi: 10.4049/jimmunol.177.8.5595. [DOI] [PubMed] [Google Scholar]
- Yang Z, Ming XF. Endothelial arginase: a new target in atherosclerosis. Curr Hypertens Rep. 2006;8:54–59. doi: 10.1007/s11906-006-0041-8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J. 2001;15:1264–1266. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, et al. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–943. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]
- Zharikov SI, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


