Abstract
Background and purpose:
We compared the dose-dependent reductions in cellular superoxide anion (O2−) by catalytic agents: superoxide dismutase (SOD), polyethylene glycol (PEG)-SOD and the nitroxide 4-hydroxy-2,2,6,6,-tetramethylpiperidine-1-oxyl (tempol) with uncharacterized antioxidants: 5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinate iron (III)(Fe-TTPS), (-)-cis-3,3′,4′,5,7-pentahydroxyflavane (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol (-epicatechin), 2-phenyl-1,2-benzisoselenazol-3(2H)-one (ebselen) and N-acetyl-L-cysteine (NAC) with the spin trap nitroblue tetrazolium (NBT) and with the vitamins or their analogues: ascorbate, α-tocopherol and 6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxy acid (trolox).
Experimental approach:
O2− was generated in primary cultures of angiotensin II-stimulated preglomerular vascular smooth muscle cells from spontaneously hypertensive rats and detected by lucigenin-enhanced chemiluminescence.
Key results:
SOD, PEG-SOD, NAC and tempol produced a similar maximum inhibition of O2− of 80–90%. -Epicatechin, NBT, ebselen and Fe-TTPS were significantly (P < 0.0125) less effective (50–70%), whereas trolox, α-tocopherol and ascorbate had little action even over 24 h of incubation (<31%). Effectiveness in disrupted and intact cells was similar for the permeable agents, PEG-SOD and tempol, but was enhanced for SOD. Generation of O2− was increased by NAC and NBT at low concentrations but reduced at high concentrations.
Conclusions and implications:
Maximum effectiveness against cellular production of O2− requires cell membrane permeability and catalytic action as exemplified by PEG-SOD or tempol. NAC and NBT have biphasic effects on O2− production. Vitamins C and E or analogues have low efficacy.
Keywords: hypertension, tempol, superoxide dismutase, vitamin C, vitamin E
Introduction
Oxidative stress results from an imbalance between the production of reactive oxygen species (ROS), such as superoxide anion (O2−) and their metabolism, for example, by superoxide dismutase (SOD). ROS have been implicated in the development and complications of hypertension and cardiovascular and kidney diseases (Wilcox, 2005; Valko et al., 2007). O2− is a free radical formed by the univalent reduction of molecular oxygen either as a by-product of O2 metabolism in mitochondria or by oxidative enzymes. O2− can bio-inactivate nitric oxide (NO) (Wilcox, 2005) and contribute to endothelial dysfunction, vasoconstriction, thrombosis, inflammation, vascular smooth muscle cell (VSMC) proliferation, vascular and cardiac remodelling and atherosclerosis (Wilcox, 2005; Valko et al., 2007). Cellular mechanisms to limit O2− may be enzymatic (e.g. SOD) or non-enzymatic (e.g. thiols, some vitamins, metals, or food components such as isoflavones, polyphenols, catechins and flavonoids) (Yusoff, 2002; Valko et al., 2007). However, these are insufficient to protect blood vessels or tissues against the damaging effects of O2− in many states of enhanced risk or overt disease of the cardiovascular and renal systems. This has promoted an intense search for drugs to enhance the metabolism of O2− which is the primary ROS from which other species are derived.
We have compared the effects of vitamins C and E with other agents that can reduce O2−. A motivation for this study was the disappointing results of clinical trials using these vitamins, which might result from their limited efficacy in reducing O2− within VSMCs.
The aim of this study was to compare the efficacy of a range of compounds representative of catalytic or stoichiometric agents or vitamins. We have used low-dose lucigenin-enhanced chemiluminescence to detect O2− as lucigenin penetrates cell membranes and is a water-soluble probe for cellular O2− (Bhunia et al., 1997; Li et al., 1998). We have selected primary cultures of preglomerular VSMCs (PGVSMCs) as vascular O2− has important physiological effects on contractility and tone of the renal resistance vessels (Wilcox, 2005). We have cultured these cells from the spontaneously hypertensive rat (SHR), as these animals provide a model of oxidative stress and enhanced renal vascular actions of angiotensin (Ang) II (Welch et al., 2003) which generates considerable ROS in VSMCs of SHR (Cruzado et al., 2005). We harvested PGVSMCs from SHR and stimulated them with Ang II as a model system of oxidative stress to examine the potency and efficacy of a series of antioxidant compounds in inhibiting the generation of O2−. We tested Cu/Zn SOD, polyethylene glycol covalently linked SOD (PEG-SOD; a relatively cell membrane permeable form of SOD), the nitroxide 4-hydroxy-2,2,6,6,-tetramethylpiperidine-1-oxyl (tempol), the metal porphyrin, 5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinate iron (III)(Fe-TTPS), a natural antioxidant extracted from green tea, (-)-cis-3,3′,4′,5,7-pentahydroxyflavane (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol (-epicatechin), a drug often used as a peroxynitrite scavenger, 2-phenyl-1,2-benzisoselenazol-3(2H)-one (ebselen), a clinically available thiol antioxidant, N-acetyl-L-cysteine (NAC), a spin trap, redox-cycling agent, nitroblue tetrazolium (NBT) and vitamins, ascorbate, α-tocopherol and the vitamin E derivative, 6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxy acid (trolox). Figure 1 shows the molecular structures of these compounds. We tested the hypothesis that depletion of cellular O2− would be more effective with catalytic than with stoichiometric agents or vitamins.
Figure 1.
The molecular structures of the antioxidant compounds tested. Ebselen, 2-phenyl-1,2-benzisoselenazol-3(2H)-one; -epicatechin, (-)-cis-3,3′,4′,5,7-pentahydroxyflavane (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol; Fe-TTPS, 5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinate iron (III); NAC, N-acetyl-L-cysteine; NBT, nitroblue tetrazolium; tempol, nitroxide 4-hydroxy-2,2,6,6,-tetramethylpiperidine-1-oxyl; trolox, 6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxy acid.
Methods
Cell culture
All animal care and experimental procedures complied with National Institutes of Health guidelines and was approved by Georgetown University Animal Care and Use Committee. PGVSMCs were isolated from 13- to 15-week-old male SHR purchased from Taconic Farms (Germantown, NY, USA) as previously described (Dubey et al., 1992; Andresen et al., 2001). Briefly, rats were anaesthetized [thiobutabarbital sodium (Inactin), 120 mg·kg−1 i.p.; Research Biochemicals, Natick, MA, USA] and 1% Fe2O3 in phosphate buffered saline (PBS) was injected forcefully through the renal artery into the isolated kidney. The iron-loaded kidney was removed from the rat and the cortex was minced and washed in a 1% collagenase IV solution in a 15 mL conical tube. The collagenase digestion was terminated when all visible tissue was absent from the solution. A magnet was applied to the side of the tube to attract the Fe2O3 (both free and within vessels); the solution was removed and the iron particles and attached tissue were re-suspended in PBS. This was repeated three times to remove all the collagenase and microscopic tissue that did not contain Fe2O3. Lastly, the solution was washed with Dulbecco's modified Eagle's medium: Ham's nutrient mixture F-12 (DMEM/F12) supplemented with 10% fetal calf serum (FCS) and 20 U of penicillin–streptomycin. The Fe2O3-containing tissue was plated in a 100 mm dish to culture cells from the microvessels that had contained the magnetic Fe2O3 particles. The particle size was sufficiently small that they lodged in microvessels within the iron-perfused kidney, thereby allowing harvesting of these vessels. After the cells from the isolated vessels reached confluence, the cells were trypsinized and passed into a fresh 100 mm dish. After 20 min, the medium was transferred to a new 100 mm dish. This was repeated two more times. This process selected smooth muscle cells. The third dish was found to contain only PGVSMCs. The phenotype of the PGVSMCs was confirmed as described (Dubey et al., 1992; Jackson et al., 2005) based on characteristic morphology (hill-and-valley pattern), contractions to noradrenaline and Ang II, expression of smooth muscle-specific α-actin and smooth muscle myosin heavy chain, and the absence of mRNA for von Willebrand factor (endothelial cell marker). Experiments were conducted between passage 5 and 15. PGVSMCs were cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 100 U·mL−1 penicillin, 100 µg·mL−1 streptomycin and 200 µg·mL−1 glutamine at 37°C in 5% CO2− 95% air at 98% humidity.
Measurement of superoxide production in PGVSMCs
Lucigenin is an acridinium compound that emits light upon interaction with O2−. It was used to measure O2− production in intact VSMCs as described by Bhunia et al. (1997) with modifications (Li et al., 1998). Briefly, PGVSMCs were seeded into a 96-well plate at densities of 1 × 105 cells per well in 200 µL of DMEM/F-12 medium. After 24 h, the cells were incubated overnight in serum-free medium which was replaced before incubation for 2 h with SOD, PEG-SOD, tempol, -epicatechin, NBT, NAC, ebselen or FeTTPS. As we detected almost no antioxidant activity at 2 h of incubation with α-tocopherol, ascorbic acid or trolox, the cells were pretreated with these compounds for 20 h. Then, the cells were treated with Ang II (10−6 mol·L−1) for 4 h during continued exposure to antioxidant drugs. Thereafter, cells were washed twice with 200 µL per well of balanced salt solution (130 mmol·L−1 NaCl, 5 mmol·L−1 KCl, 1 mmol·L−1 MgCl2, 1 mmol·L−1 CaCl2, 35 mmol·L−1 phosphoric acid and 20 mmol·L−1 4-(2-hydroxyethyl)-L-piperazineethanesulfonic acid (HEPES), pH 7.4). The viability of the cells was >90% as determined by exclusion of trypan blue. To measure O2− production, 100 µL of the balanced salt solution containing dark-adapted lucigenin (10 µmol·L−1) as the electron acceptor and NADPH (100 µmol·L−1) as the electron donor was added to each well. Lucigenin-enhanced chemiluminescence was measured in a 1420 Multilabel Counter (PerkinElmer; Shelton, CT, USA). Final values were expressed as relative light units normalized to protein concentration, as described previously (Kitiyakara et al., 2003). Lucigenin, in low dose, is a well-characterized probe for O2− (Munzel et al., 2002).
In other experiments, PGVSMCs were cultured in a 12-well plate and treated as above. The cells were washed twice with ice-cold PBS, scraped into a 12 mL tube and centrifuged at 2500×g for 5 min. The cell pellets were suspended in 0.5 mL of the balanced salt solution and sonicated on ice with 3–4 sets of 10-s pulses using a Sonifier Sonicator 250 (Branson; Danbury CT, USA; output 3.0, duty cycle 30%). The pellets were returned to the ice between each pulse to cool the sample. Thereafter, the cell homogenate was transferred into a 96-well plate and incubated with SOD, PEG-SOD or tempol. Lucigenin-enhanced chemiluminescence was determined as described above. Comparison of the effectiveness of the drugs in disrupted and intact cells gave insight into the importance of cell permeability in the response to these three antioxidants.
Results are expressed as percentage inhibition of O2− generation by each dose of drug, compared to the relevant vehicle.
Statistical analysis
Results are expressed as mean ± standard error of the mean. An analysis of variance was performed and differences between two experimental groups were compared by Student's t-test. When comparisons were made among three or more groups of compounds, a Bonferroni correction was applied and a value of P < 0.05/3 = 0.0125 was considered as statistically significant.
Materials
Ang II, SOD, PEG-SOD, tempol, NAC, -epicatechin, α-tocopherol, ascorbic acid, NBT, collagenase IV, lucigenin, β-NADPH and Fe2O3 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ebselen was obtained from Alexis Inc. (Portland, OR, USA), trolox from OXIS Inc. (Foster City, CA, USA), Fe-TTPS from EMD Biosciences, Inc. (San Diego, CA, USA), DMEM/F-12 from Gibco (Carlsband, CA, USA) and FBS from American Type Culture Collection (ATCC; Manassas, VA, USA).
Results
Ang II-induced O2− production in intact PGVSMCs
The first set of preliminary studies was undertaken to compare PGVSMCs from Wistar Kyoto (WKY) and SHR after incubation with vehicle or angiotensin II (Ang II, 10−6 mol·L−1) for 8 h. As shown in Figure 2, SHR cells given vehicle had enhanced NADPH oxidase (NOX) activity and expression of p22phox and NOX-1, but not NOX-4. Cells from both strains had increased NOX, p22phox and NOX-1 with Ang II, whereas cells from SHR actually had a decline in NOX-4 expression. These results confirm our previous findings in the rat kidney cortex (Welch et al., 2000; Chabrashvili et al., 2002; Chabrashvili et al., 2003). They indicate that the SHR is a model of increased NOX that can be increased further due to up-regulation of p22phox and NOX-1 by incubation with Ang II, whereas NOX-4 responds differently. We selected SHR cells stimulated with Ang II for this study.
Figure 2.
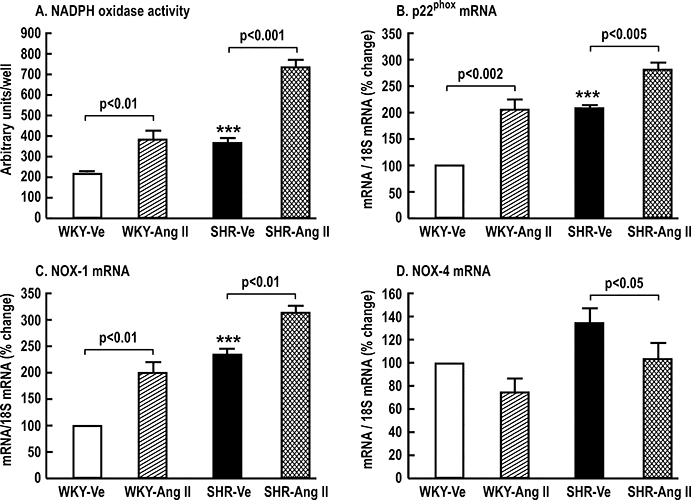
Mean ± standard error of the mean values (n= 3 per group) for NADPH oxidase (NOX) activity (Panel A) and mRNA expression of p22phox (Panel B), NOX-1 (Panel C) and NOX-4 (Panel D), comparing results in preglomerular vascular smooth muscle cells (PGVSMCs) from normotensive WKY and spontaneously hypertensive rats (SHR). The cells were treated for 8 h with a vehicle (Ve) or with 10−6 mol·L−1 angiotensin II (Ang II). Comparing SHR-Ve with WKY-Ve cells; ***P < 0.005.
A second set of preliminary studies showed that incubation with 10−6 mol·L−1 Ang II was a fully effective dose (Figure 3A), as in previous studies (Yoshida et al., 2004; Jackson et al., 2005) and incubation with 10−6 mol·L−1 Ang for 4 h increased O2− levels to 215 ± 7% of control (Figure 3B). This was inhibited by tempol and diphenyliodonium, consistent with NOX as the principal source of O2−. Therefore, we selected a 4 h period of incubation with 10−6 mol·L−1 Ang II for these studies.
Figure 3.
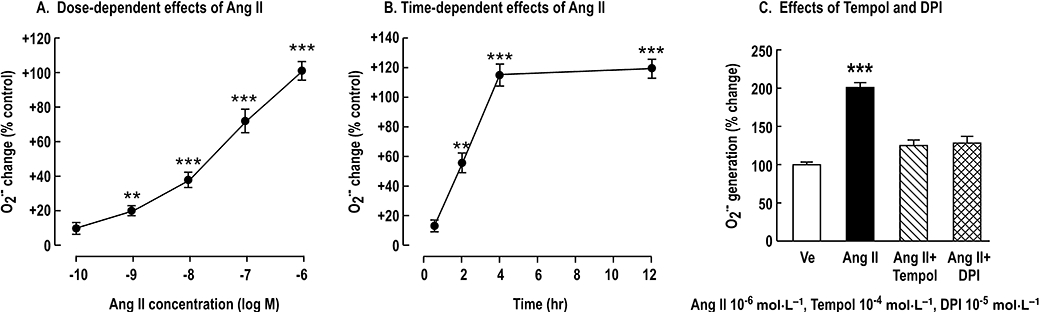
Mean ± standard error of the mean values (n= 3) for angiotensin II (Ang II)-induced O2− production in preglomerular vascular smooth muscle cells (PGVSMCs) from spontaneously hypertensive rats. Panel A: dose-dependent O2− generation; PGVSMCs were treated with vehicle or graded concentrations (10−10 to 10−6 mol·L−1) of Ang II for 4 h before assessing O2− from lucigenin-enhanced chemiluminescence. The values were expressed as percentage change from control. Panel B: effect of 10−6 mol·L−1 Ang II over 12 h of incubation. Panel C: effects of nitroxide 4-hydroxy-2,2,6,6,-tetramethylpiperidine-1-oxyl (tempol) or diphenyliodonium (DPI) on O2− generation. **P < 0.01, ***P < 0.005 vs. control group.
Catalytic antioxidants
Incubation of intact Ang II-stimulated PGVSMCs for 4 h with catalytic antioxidants led to dose-dependent attenuations of O2− generation with a similar maximum effect for SOD, PEG-SOD and tempol (Figure 4; Table 1). Parallel studies in disrupted PGVSMCs showed no difference from intact cells for PEG-SOD and tempol but a significant increase in the maximum attenuation of O2− with SOD in disrupted cells of 90 ± 3 versus 83 ± 1% (P < 0.05). Comparison of PEG-SOD, SOD and tempol in disrupted cells showed no significant differences in maximal effect (90 ± 3; 89 ± 4 and 86 ± 4% respectively). The expected dose to produce a 50% response (ED50) value for PEG-SOD was less than that for SOD in intact cells (Table 1) but similar to that for SOD in disrupted cells (1.6 ± 0.2 × 10−7 mol·L−1 for PEG-SOD and 1.5 ± 0.4 × 10−7 mol·L−1 for SOD). However, the ED50 for tempol in intact (44 ± 12) or disrupted (35 ± 11 × 10−7 mol·L−1) cells was significantly higher than that for SOD (Table 1).
Figure 4.
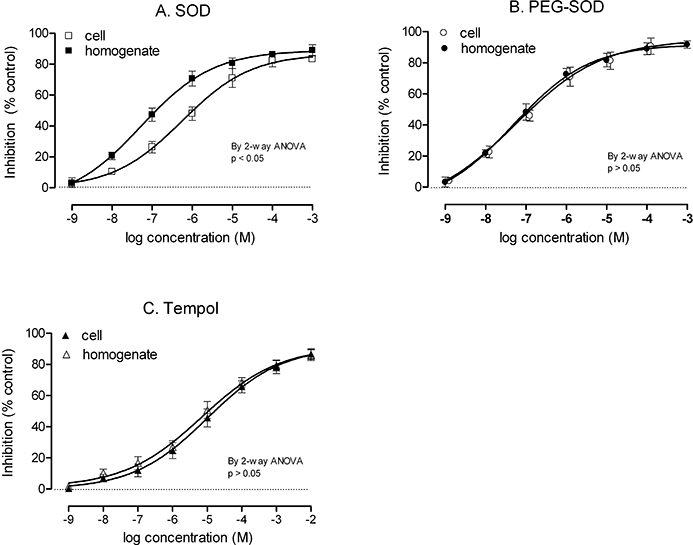
Inhibition of angiotensin II (Ang II)-induced O2− generation in intact or homogenized preglomerular vascular smooth muscle cells by catalytic antioxidants. Cells were pretreated for 2 h with graded concentrations of superoxide dismutase (SOD) (Panel A), polyethylene glycol (PEG)-SOD (Panel B) or nitroxide 4-hydroxy-2,2,6,6,-tetramethylpiperidine-1-oxyl (Panel C), followed by incubation with 10−6 mol·L−1 Ang II for 4 h. Thereafter, O2− was measured by lucigenin-enhanced chemiluminescence. The values are expressed as mean ± standard error of the mean values for percentage inhibition, compared with control (0% inhibition, dotted line). anova, analysis of variance.
Table 1.
Comparison of efficacy and sensitivity of various antioxidants in whole PGVSMCs
| Antioxidants | Duration (h) | Maximal inhibition (%) | ED50 (×10−7mol·L−1) |
|---|---|---|---|
| Catalytic antioxidants | |||
| PEG-SOD | 6 | 93.0 ± 2.5 | 0.6 ± 0.1* |
| SOD | 6 | 83.3 ± 1.2 | 5.7 ± 0.8 |
| Tempol | 6 | 86.7 ± 3.5 | 44.5 ± 12.3* |
| Uncharacterized antioxidants | |||
| NAC | 6 | 84.0 ± 1.9 | 86.7 ± 10.5* |
| -Epicatechin | 6 | 69.3 ± 2.7* | 17.9 ± 7.4* |
| NBT | 6 | 64.7 ± 0.9* | 171.1 ± 12.0* |
| Ebselen | 6 | 60.7 ± 4.7* | 6.7 ± 3.8 |
| Fe-TTPS | 6 | 54.0 ± 3.6* | 16.5 ± 7.6* |
| Vitamins | |||
| Trolox | 24 | 30.7 ± 3.2* | 246.8 ± 161.9* |
| Tocopherol | 24 | 22.0 ± 4.1* | 22.0 ± 5.2* |
| Ascorbic acid | 24 | 17.7 ± 2.2* | 1895.5 ± 2160.5* |
| Ascorbic acid + Trolox | 24 | 52.0 ± 3.5* | 17.0 ± 4.8* |
Mean ± SEM (N = 3); compared to SOD;
P < 0.0125.
Ebselen, 2-phenyl-1,2-benzisoselenazol-3(2H)-one; -epicatechin, (-)-cis-3,3′,4′,5,7-pentahydroxyflavane (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol; Fe-TTPS, 5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinate iron (III); NAC, N-acetyl-L-cysteine; NBT, nitroblue tetrazolium; PEG-SOD, polyethylene glycol covalently linked superoxide dismutase; PGVSMCs, preglomerular vascular smooth muscle cells; SOD, superoxide dismutase; tempol, nitroxide 4-hydroxy-2,2,6,6,-tetramethylpiperidine-1-oxyl; trolox, 6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxy acid.
NAC, -epicatechin, NBT, ebselen and Fe-TTPS
The maximal effectiveness of NAC was similar to that of SOD (Figure 5 and Table 1). However, the efficacy of the other uncharacterized antioxidants was significantly lower than that of SOD. All agents in this group except ebselen had a significantly greater ED50 than SOD (Table 1). Interestingly, NBT (10−7 mol·L−1) and NAC (10−8 mol·L−1) showed paradoxical increases in O2− generation, as indicated by significantly (P < 0.05) negative values in Figure 5. Only doses of ≥10−5 mol·L−1 NBT and >10−6 mol·L−1 NAC inhibited Ang II-induced O2− generation significantly (P < 0.05).
Figure 5.
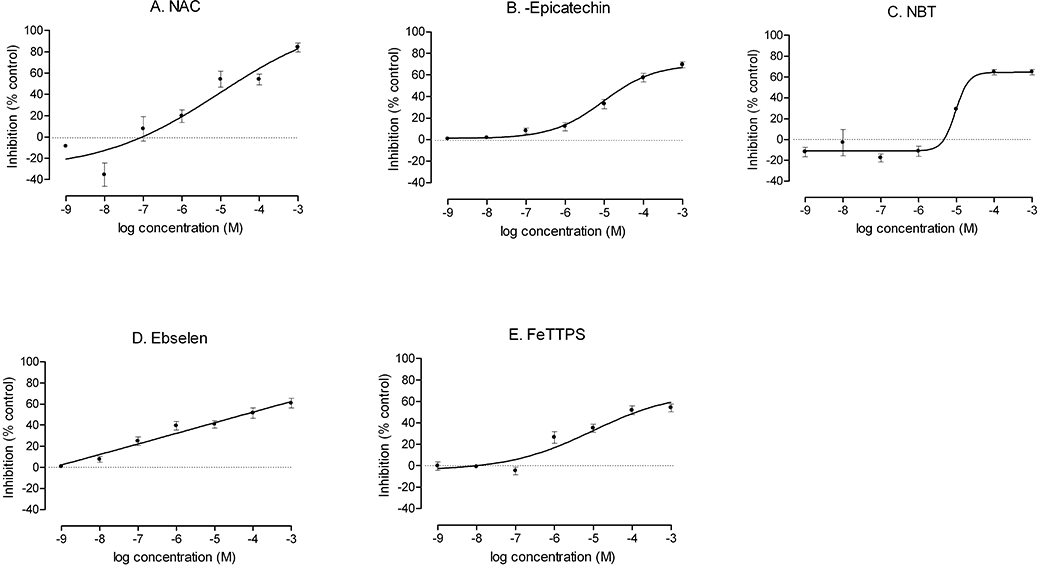
Inhibition of angiotensin II (Ang II)-induced O2− generation in intact PGVSMCs by uncharacterized antioxidants. Cells were pretreated for 2 h with graded concentrations of N-acetyl cysteine (NAC; panel A), (-)-cis-3,3′,4′,5,7-pentahydroxyflavane (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol (epicatechin) (panel B), nitroblue tetrazolium (NBT; panel C), ebselen (panel D) or 5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinate iron (III)(FeTPPS) (panel E), followed by incubation with 10−6 mol·L−1 Ang II for 4 h. Thereafter, O2− was measured by inhibition of lucigenin-enhanced chemiluminescence. The values are expressed as mean ± standard error of the mean inhibition, compared with control (0% inhibition, dotted line).
Vitamins or derivatives
Pretreatment of PGVSMCs for 20 h with ascorbic acid, α-tocopherol, trolox or ascorbic acid + trolox followed by incubation with Ang II for 4 h produced only modest reductions of O2− generation (Figure 6, Table 1). These were significantly less than that produced by SOD.
Figure 6.
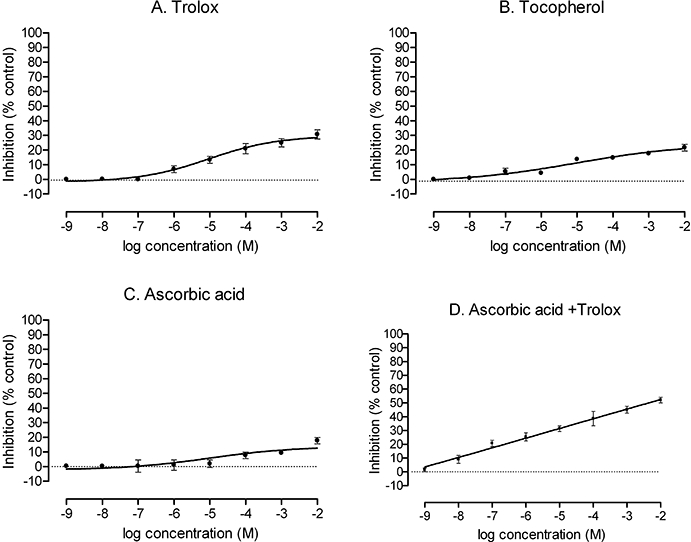
Inhibition of angiotensin II (Ang II)-induced O2− generation in intact preglomerular vascular smooth muscle cells (PGVSMCs) by vitamins. Cells were pretreated for 20 h with graded concentrations of 6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxy acid (trolox) (panel A), tocopherol (panel B), ascorbic acid (panel C) or ascorbic acid plus trolox (panel D), followed by incubation with 10−6 mol·L−1 Ang II for 4 h. Thereafter, O2− was measured by inhibition of lucigenin-enhanced chemiluminescence. The values are expressed as mean ± standard error of the mean inhibition, compared with control (0% inhibition, dotted line).
Discussion and conclusions
This study has four main novel findings. First, when compared under closely regulated conditions, 11 agents used to combat oxidative stress had widely divergent effects in reducing cellular O2−. Second, catalytic agents were more effective than putative stoichiometric agents. Third, NBT and NAC had biphasic effects on cellular O2−. Fourth, vitamins had low efficacy in this model.
The rank order of efficacy for reducing cellular O2− was: PEG-SOD = SOD = tempol = NAC > -epicatechin = NBT = ebselen > Fe-TTPS > trolox > α-tocopherol = ascorbic acid. The order of sensitivity (ED50) was PEG-SOD > SOD = ebselen > Fe-TPPS = -epicatechin =α-tocopherol = tempol >NAC > NBT = trolox > ascorbic acid. Ascorbic acid, α-tocopherol and trolox were minimally effective even over 24 h of pre-incubation. Ascorbic acid and trolox had additive effects.
SOD catalyses the conversion of O2− into molecular oxygen and hydrogen peroxide (Beckman et al., 1988). It is a critical cellular defence against the toxic effects of O2−, for example, during exposure to high levels of oxygen. SOD competes with NO for O2−, thereby preserving NO activity. We selected Cu/Zn SOD (SOD-1) and PEG-SOD (Cu/Zn form), as SOD-1 is the principal isoform in the rat kidney and VSMCs (Welch et al., 2006). PEG-SOD binds to cell membranes and rapidly penetrates into cells, whereas SOD has limited cellular penetration (Beckman et al., 1988). This explains the significantly greater efficacy of SOD, but not PEG-SOD, in the cell-disrupted system in this study. Moreover, the log order of increased sensitivity to PEG-SOD, compared to SOD, that was abolished after cell membrane lysis (Figure 4) indicates that much, but not all, of the O2− was generated within the cells.
Tempol was as effective as SOD in metabolizing O2− in the intact cell system. Tempol is a stable, low molecular weight and amphilite nitroxide that is freely diffusible into cells. It interacts both catalytically and stoichiometrically to oxidize O2− to H2O2 with the production of the reduced hydroxylamine form (tempol-H) (Soule et al., 2007). Catalytic activity is facilitated by a ‘boat and chair’ conformational change that presents the nitroxide catalytic site and accounts for the rapid metabolism of O2− by tempol (Soule et al., 2007). Further electron flux interconverts tempol-H with the oxammonium cation form which itself can be converted to the nitroxide tempol or to tempol-H (Soule et al., 2007). These redox reactions are reversible, accounting for the large range of oxidative and reductive reactions catalysed by tempol and similar nitroxide drugs. Thus, tempol also limits the formation of hydroxyl radicals generated by the Fenton reaction by reducing the intracellular levels of ferrous iron or transition metal (Soule et al., 2007) and by a reversible reaction at the 4-C site of the tempol molecule with hydroxyl radical to form 4-oxo-2,2,6,6-tetramethylpiperidine-N-oxyl (Saito et al., 2003). Tempol inhibits the nitration of phenolic compounds by peroxynitrite and has peroxidase- and catalase-like actions (Soule et al., 2007).
The systemic administration of tempol to hypertensive animal models causes rapid vasodilatation and decrease in blood pressure (BP) (Patel et al., 2006). This rapid effect is likely due to metabolism of O2− by tempol as the antihypertensive effectiveness of nitroxides is predicted by their SOD mimetic activity (Patel et al., 2006). However, the response is rapidly reversible. The reversibility is likely a consequence of the conversion of tempol to the hydroxylamine, which is not effective as a vasodilator (Soule et al., 2007). Our data suggest that the O2− scavenging effect of tempol in SHR PGVSMCs is comparable to that of SOD.
NAC, -epicatechin, NBT, ebselen and Fe-TTPS reduced O2− generation modestly. Fe-TTPS is an iron porphyrinate and a putative catalyst of the decomposition of peroxynitrite (ONOO·). It can protect against a decreased SOD activity and increased lipid peroxidation induced by quinolinic acid in brain synaptic vesicles (Perez-De La Cruz et al., 2005). Metal porphyrin compounds are especially effective in protecting against oxidative stress caused by chelation of metals (MacKenzie and Martin, 1998). However, these are not reliable agents to deplete O2−, as they can potentiate the generation of O2− and cause destruction of NO in the rat aorta (MacKenzie et al., 1999), although this was not apparent in the present study. Ebselen is a synthetic heterocyclic seleno-organic compound with glutathione peroxidase (GPx) mimetic as well as direct radical scavenging activities (Matsushita et al., 2004; Baljinnyam et al., 2006). Ebselen inhibits endothelin I-mediated O2− production by rat aortic tissues (Loomis et al., 2005). -Epicatechin is a member of a group of polyphenolic compounds collectively known as ‘catechins’ that belong to the flavonoid family. -Epicatechin is a constituent of grape seeds and grape skin and tea tannins, cocoa flavonoids and red wine (Alvarez et al., 2006). It can increase glutathione, the activities of SOD, GPx and catalase (Quine and Raghu, 2005) and prevent nitrotyrosine formation (Gorg et al., 2007). It is not as effective as tempol in restoring endothelium-dependent relaxation response of the mesenteric vessels from rats with Ang II-induced oxidative stress (Antonello et al., 2007), consistent with our finding that it is less effective than tempol in reducing O2− in Ang II-stimulated cells. NBT interacts with O2− without forming hydrogen peroxide (Weissmann et al., 1998; Chen et al., 2007). We found that cellular O2− generation was increased at a low concentration of NBT (10−7 mol·L−1) but reduced at higher concentrations at or above 10−5 mol·L−1 (Figure 5C). This biphasic effect of NBT may relate to its redox cycling activity which can confer oxidative and reductive actions. NAC increases cellular synthesis of glutathione and can reduce O2− generation (Puertollano et al., 2003). We found that cellular O2− generation was increased at a low concentration of NAC (10−8 mol·L−1) but was reduced at higher concentrations at or above 10−6 mol·L−1 (Figure 5A). NAC has been considered a poor anti-inflammatory agent in vivo (Puertollano et al., 2003).
The effectiveness of vitamins in protecting against cardiovascular disease has been extensively tested. While an inverse association between the intake of vitamins A and E in food and cardiovascular disease has been reported (Diaz et al., 1997; Frei, 1999; Yusoff, 2002), many experimental (Brasen et al., 2002; Versari et al., 2006) and clinical studies (Diaz et al., 1997; Lonn et al., 2001; Waters et al., 2002; Yusoff, 2002) have failed to demonstrate a protective effect of additional doses of these vitamins on atherosclerotic disease or its complications. We found that these vitamins, or the water-soluble tocopherol analogue, trolox, were very weak in reducing cellular O2− even over 20 h of pre-incubation. While vitamins C and E can scavenge lipid peroxyl, alkoxyl and hydroxyl radicals (Beckman et al., 1988), α-tocopherol is almost ineffective in scavenging O2− produced in permeabilized mitochondrial membranes (Kruglov et al., 2008), as confirmed in the present study in intact cells. Our findings imply that these vitamins or derivatives have little O2−-scavenging activity at the doses used. A recent study in patients with oxidative stress due to dyslipidemia demonstrated that vitamin E must be given for a prolonged time, and in doses that are four- to 16-fold higher than those used in earlier clinical studies, to reduce parameters of lipid peroxidation (Roberts et al., 2007). The poor efficacy of vitamins is not likely due to limited cellular penetration as ascorbate is hydrophilic and tocopherol is lipophilic, yet neither was effective in reducing cellular O2−.
Further in vivo comparison between drugs that metabolize O2− would be very helpful. This cellular study is a first step. It indicates that tempol and NAC may be more effective than vitamins. Indeed, a recent preliminary report has shown that tempol and NAC provided full protection in the rat against intravenous iron-induced endothelial dysfunction, whereas ascorbic acid was not effective (Nouri et al., 2007).
We acknowledge the limitations of this in vitro cell study. We have not included agents that target specific pathways for O2− generation. We elected not to study tiron (4,5-dihydroxy-1,3-benzene disulphonic acid) because recent studies demonstrated that its principal biological effects were due to chelation of calcium rather than scavenging of O2− (Ghosh et al., 2002). While ascorbic acid and trolox were additive, suggesting that they act via distinct mechanisms, we did not examine the interaction between other drugs in this study.
In conclusion, the effectiveness in preventing cellular O2− generation is high for cell permeable catalytic antioxidants (PEG-SOD and tempol) and NAC, moderate for Fe-TTPS, -epicatechin, ebselen and NBT, but poor for ascorbic acid, α-tocopherol and trolox. These data may help to explain the widespread effectiveness of catalytic agents such as tempol in reducing markers of O2−, and the associated cardiovascular and renal damage and hypertension, in a wide range of animal models (Wilcox, 2005), whereas studies using supplement of vitamin E or C have generally been disappointing (Diaz et al., 1997; Frei, 1999; Lonn et al., 2001; Brasen et al., 2002; Waters et al., 2002; Yusoff, 2002; Versari et al., 2006), perhaps because they fail to effectively prevent cellular accumulation of O2− even at high doses.
Acknowledgments
We thank Ms. Emily Wing Kam Chan for preparing and editing the manuscript. Work in the authors' laboratory is supported by grants from the National Institute of Diabetes, Digestive and Kidney Diseases (DK-36079 and DK-49870) and National Heart, Lung and Blood Institute (HL-68686-01) and by funds from the George E. Schreiner Chair of Nephrology and American Heart Association. Zaiming Luo is supported by a Nephrology Fellowship Training Grant (DK-59274).
Glossary
Abbreviations:
- Ang II
angiotensin II
- DPI
diphenyliodonium
- DMEM/F-12
Dulbecco's modified Eagle's medium: Ham's nutrient mixture F-12
- ebselen
2-phenyl-1,2-benzisoselenazol-3(2H)-one
- -epicatechin
(-)-cis-3,3′,4′,5,7-pentahydroxyflavane (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol
- Fe-TTPS
5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinate iron (III)
- GPx
glutathione peroxidase
- NAC
N-acetyl-L-cysteine
- NBT
nitroblue tetrazolium
- O2−
superoxide anion
- ONOO·
peroxynitrite
- PBS
phosphate buffered saline
- PEG-SOD
polyethylene glycol covalently linked superoxide dismutase
- PGVSMCs
preglomerular vascular smooth muscle cells
- RLU
relative light units
- ROS
reactive oxygen species
- SHRs
spontaneously hypertensive rats
- SOD
superoxide dismutase
- tempol
nitroxide 4-hydroxy-2,2,6,6,-tetramethylpiperidine-1-oxyl
- tempol-H
reduced hydroxylamine form
- TEMPON
4-oxo-2,2,6,6-tetramethylpiperidine-N-oxyl
- trolox
6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxy acid
- VSMC
vascular smooth muscle cell
Conflict of interest
None.
References
- Alvarez E, Campos-Toimil M, Justiniano-Basaran H, Lugnier C, Orallo F. Study of the mechanisms involved in the vasorelaxation induced by (-)-epigallocatechin-3-gallate in rat aorta. Br J Pharmacol. 2006;147:269–280. doi: 10.1038/sj.bjp.0706507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen BT, Jackson EK, Romero GG. Angiotensin II signaling to phospholipase D in renal microvascular smooth muscle cells in SHR. Hypertension. 2001;37:635–639. doi: 10.1161/01.hyp.37.2.635. [DOI] [PubMed] [Google Scholar]
- Antonello M, Montemurro D, Bolognesi M, Di PM, Piva A, Grego F, et al. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am J Hypertens. 2007;20:1321–1328. doi: 10.1016/j.amjhyper.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Baljinnyam E, Hasebe N, Morihira M, Sumitomo K, Matsusaka T, Fujino T, et al. Oral pretreatment with ebselen enhances heat shock protein 72 expression and reduces myocardial infarct size. Hypertens Res. 2006;29:905–913. doi: 10.1291/hypres.29.905. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Minor RL, Jr, White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- Bhunia AK, Han H, Snowden A, Chatterjee S. Redox-regulated signaling by lactosylceramide in the proliferation of human aortic smooth muscle cells. J Biol Chem. 1997;272:15642–15649. doi: 10.1074/jbc.272.25.15642. [DOI] [PubMed] [Google Scholar]
- Brasen JH, Koenig K, Bach H, Kontush A, Heinle H, Witting PK, et al. Comparison of the effects of alpha-tocopherol, ubiquinone-10 and probucol at therapeutic doses on atherosclerosis in WHHL rabbits. Atherosclerosis. 2002;163:249–259. doi: 10.1016/s0021-9150(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, et al. Effects of Ang II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase and SOD expression. Am J Physiol. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- Chabrashvili T, Tojo A, Onozato M, Kitiyakara C, Quinn MT, Fujita T, et al. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol. 2007;293:H2085–H2092. doi: 10.1152/ajpheart.00968.2006. [DOI] [PubMed] [Google Scholar]
- Cruzado MC, Risler NR, Miatello RM, Yao G, Schiffrin EL, Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: effect of angiotensin II and role of insulin-like growth factor-1 receptor transactivation. Am J Hypertens. 2005;18:81–87. doi: 10.1016/j.amjhyper.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Diaz MN, Frei B, Vita JA, Keaney JF., Jr Antioxidants and atherosclerotic heart disease. New Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Roy A, Overbeck HW. Culture of renal arteriolar smooth muscle cells. Mitogenic responses to angiotensin II. Circ Res. 1992;71:1143–1152. doi: 10.1161/01.res.71.5.1143. [DOI] [PubMed] [Google Scholar]
- Frei B. On the role of vitamin C and other antioxidants in atherogenesis and vascular dysfunction. Proc Soc Exp Biol Med. 1999;222:196–204. doi: 10.1046/j.1525-1373.1999.d01-136.x. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Wang HD, McNeill JR. Tiron exerts effects unrelated to its role as a scavenger of superoxide anion: effects on calcium binding and vascular responses. Can J Physiol Pharmacol. 2002;80:755–760. doi: 10.1139/y02-106. [DOI] [PubMed] [Google Scholar]
- Gorg B, Qvartskhava N, Voss P, Grune T, Haussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Andresen BT, Seasholtz TM, Zhu C, Romero GG. Enhanced activation of RhoA by angiotensin II in SHR preglomerular microvascular smooth muscle cells. J Cardiovasc Pharmacol. 2005;45:283–285. doi: 10.1097/01.fjc.0000155383.83927.9f. [DOI] [PubMed] [Google Scholar]
- Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, et al. Salt intake, oxidative stress and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–2782. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- Kruglov AG, Subbotina KB, Saris NE. Redox-cycling compounds can cause the permeabilization of mitochondrial membranes by mechanisms other than ROS production. Free Radic Biol Med. 2008;44:646–656. doi: 10.1016/j.freeradbiomed.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. Validation of lucigenin (Bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, et al. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE) Circulation. 2001;103:919–925. doi: 10.1161/01.cir.103.7.919. [DOI] [PubMed] [Google Scholar]
- Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled NOS in the rat aorta. J Pharmacol Exp Ther. 2005;315:1058–1064. doi: 10.1124/jpet.105.091728. [DOI] [PubMed] [Google Scholar]
- MacKenzie A, Filippini S, Martin W. Effects of superoxide dismutase mimetics on the activity of nitric oxide in rat aorta. Br J Pharmacol. 1999;127:1159–1164. doi: 10.1038/sj.bjp.0702670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A, Martin W. Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: restoration by superoxide dismutase mimetics. Br J Pharmacol. 1998;124:719–728. doi: 10.1038/sj.bjp.0701899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Fukuda K, Yamamoto H, Yamazaki K, Tomiyama T, Oh M, et al. Effect of ebselen, a scavenger of reactive oxygen species, on chondrocyte metabolism. Mod Rheumatol. 2004;14:25–30. doi: 10.1007/s10165-003-0261-6. [DOI] [PubMed] [Google Scholar]
- Munzel T, Afanas'ev IB, Kleschyov AL, Harrison DG. Detection of superoxide in vascular tissue. Arterioscler Thromb Vasc Biol. 2002;22:1761–1768. doi: 10.1161/01.atv.0000034022.11764.ec. [DOI] [PubMed] [Google Scholar]
- Nouri P, Chen Y, Wilcox CS. Tempol prevents the attenuation of endothelium-dependent relaxation response to acetylcholine caused by intravenous injection of iron dextran. J Am Soc Nephrol. 2007;18:638A. [Google Scholar]
- Patel K, Chen Y, Dennehy K, Blau J, Mendonca M, Tarpey M, et al. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R37–R43. doi: 10.1152/ajpregu.00469.2005. [DOI] [PubMed] [Google Scholar]
- Perez-De La Cruz V, Gonzalez-Cortes C, Galvan-Arzate S, Medina-Campos ON, Perez-Severiano F, Ali SF, et al. Excitotoxic brain damage involves early peroxynitrite formation in a model of Huntington's disease in rats: protective role of iron porphyrinate 5,10,15,20-tetrakis (4-sulfonatophenyl)porphyrinate iron (III) Neuroscience. 2005;135:463–474. doi: 10.1016/j.neuroscience.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Puertollano MA, de Pablo MA, varez de CG. Anti-oxidant properties of N-acetyl-L-cysteine do not improve the immune resistance of mice fed dietary lipids to Listeria monocytogenes infection. Clin Nutr. 2003;22:313–319. doi: 10.1016/s0261-5614(03)00031-1. [DOI] [PubMed] [Google Scholar]
- Quine SD, Raghu PS. Effects of (-)-epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol Rep. 2005;57:610–615. [PubMed] [Google Scholar]
- Roberts LJ, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, et al. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Takeshita K, Ueda J, Ozawa T. Two reaction sites of a spin label, TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), with hydroxyl radical. J Pharm Sci. 2003;92:275–280. doi: 10.1002/jps.10304. [DOI] [PubMed] [Google Scholar]
- Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, et al. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Versari D, Daghini E, Rodriguez-Porcel M, Sattler K, Galili O, Pilarczyk K, et al. Chronic antioxidant supplementation impairs coronary endothelial function and myocardial perfusion in normal pigs. Hypertension. 2006;47:475–481. doi: 10.1161/01.HYP.0000201445.77125.26. [DOI] [PubMed] [Google Scholar]
- Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Grimminger F, Voswinckel R, Conzen J, Seeger W. Nitro blue tetrazolium inhibits but does not mimic hypoxic vasoconstriction in isolated rabbit lungs. Am J Physiol. 1998;274:L721–L727. doi: 10.1152/ajplung.1998.274.5.L721. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Baumgärtl H, Lübbers D, Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat: role of AT1 receptors. Kidney Int. 2003;63:202–208. doi: 10.1046/j.1523-1755.2003.00729.x. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill P, Aslam S, et al. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension. 2006;48:934–941. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol. 2000;278:F769–F776. doi: 10.1152/ajprenal.2000.278.5.F769. [DOI] [PubMed] [Google Scholar]
- Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hoofnagle MH, Owens GK. Myocardin and Prx1 contribute to angiotensin II-induced expression of smooth muscle alpha-actin. Circ Res. 2004;94:1075–1082. doi: 10.1161/01.RES.0000125622.46280.95. [DOI] [PubMed] [Google Scholar]
- Yusoff K. Vitamin E in cardiovascular disease: has the die been cast? Asia Pac J Clin Nutr. 2002;11:S443–S447. doi: 10.1046/j.1440-6047.11.s.7.11.x. [DOI] [PubMed] [Google Scholar]


