Abstract
Background and purpose:
Renal function can be assessed by measuring albuminuria and glomerular filtration rate, and the latter is often estimated by creatinine clearance rate (Ccr). Transforming growth factor-β1 (TGF-β1) and vascular endothelial growth factor (VEGF) are two important factors involved in the progressive loss of renal function in diabetic nephropathy (DN), especially in terms of albuminuria. We investigated the effect of urocortin 1 on renal function of rats with DN and the mechanisms involved.
Experimental approach:
A modified rat model of DN (multiple injections of low-dose streptozotocin and complete Freund's adjuvant) and a rat mesangial cell line were used. Albuminuria and Ccr were measured or calculated. Expression and secretion of TGF-β1 and VEGF were measured by immunohistochemistry, reverse transcription–polymerase chain reaction (RT-PCR) or enzyme-linked immunosorbent assay (R&D System, Inc., Minneapolis, MA, USA). Urocortin 1 and astressin [a non-selective antagonist of corticotrophin-releasing factor (CRF) receptors] were given as daily injections for 8 weeks.
Key results:
Treatment of DN rats with urocortin 1 decreased albuminuria, renal weight and overexpression of TGF-β1 and VEGF but enhanced Ccr. Furthermore, VEGF mRNA was increased in kidneys of DN rats, and this increase was reduced by treatment with urocortin 1. The secretion of VEGF induced by TGF-β1 in mesangial cells was inhibited by urocortin 1 pretreatment. Astressin given with urocortin 1 prevented most of the effects of urocortin 1, in our models, in vivo or in vitro.
Conclusion and implications:
Our results strongly suggest that urocortin 1 improved renal function in rats with DN by inhibiting the overproduction of TGF-β1 and VEGF.
Keywords: diabetic nephropathy, urocortin 1, transforming growth factor-beta 1, vascular endothelial growth factor, extracellular matrix
Introduction
Diabetic nephropathy (DN), one of the microvascular complications of diabetes, is the primary aetiology of chronic kidney disease and kidney failure. Estimation of proteinuria and measurement of glomerular filtration rate (GFR) are often used to assess the renal function and its changes (Rahn et al., 1999). Microalbuminuria is the indicator of the incipient phase of DN in diabetes mellitus, and progressive albuminuria is a generally recognized criterion for determining the degree of DN (de Zeeuw, 2007). GFR is an important parameter to be monitored in diabetic patients for screening for DN. GFR is estimated by measurement of serum creatinine and creatinine excretion in a 24-h urine sample and then computation of creatinine clearance rate (Ccr) (Jafar et al., 2005).
The progression of DN is related to many factors, including overproduction of reactive oxygen species (ROS) and haemodynamic changes. Most of the factors participate in DN progression by affecting the expression of vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1). VEGF was originally described as a permeability-increasing activity protein (Senger et al., 1983). In the kidney, it is a major mediator of increased protein filtration (Wolf et al., 2005) and regarded as providing one of the mechanisms underlying albumin filtration (Ziyadeh et al., 2000). Recent studies have demonstrated an upregulation of VEGF and its receptors in kidneys of diabetic rats (Cooper et al., 1999). VEGF also enhances TGF-β1-induced synthesis of extracellular matrix (ECM). TGF-β1 stimulates ECM accumulation, which is recognized as one of the most important pathological characteristics of DN and leads to glomerulosclerosis and results in increasing proteinuria (Cooper, 2001). Concentrations of active TGF-β1 correlate with urinary albumin excretion and serum creatinine in patients with DN and type 2 diabetes (Hellmich et al., 2000). A strong correlation between serum TGF-β 1 level, serum creatinine and 24 h urinary protein excretion has been reported (Ibrahim and Rashed, 2007). TGF-β1 takes part in the uptake of albumin by renal proximal tubule cells and in the formation of albuminuria (Russo et al., 2007). TGF-β1 also induces increased production of VEGF (Wang et al., 2004).
Urocortin 1, a 40-amino-acid peptide related to the corticotrophin-releasing factor (CRF) family, is expressed in kidney and sustains endothelial-dependent relaxation of rat artery (Huang et al., 2002) and beneficial haemodynamic effects in experimental heart failure (Rademaker et al., 2005). We have already found that urocortin 1 ameliorated DN in obese db/db mice, which was ascribed to the inhibitory effect of urocortin 1 on TGF-β1 and CTGF overexpression in mesangial cells (MCs) induced by high glucose, leading to reduction of ECM accumulation (Li et al., 2008). Moreover, our previous data indicated that urocortin 1 down-regulated VEGF expression in vivo via the CRF2 receptor (Wang et al., 2008). Taken together, these results suggest that urocortin 1 may improve renal function by inhibiting TGF-β1 and VEGF overproduction.
In the present study, we explored the role of urocortin 1 in the progression of DN and the relevant mechanisms involving TGF-β1 and VEGF. A modified rat model of diabetes and a MC cell line were used to estimate the effect of urocortin 1 on albuminuria and Ccr and the possible mechanisms involving the VEGF/TGF-β1 pathway, mediated by CRF receptors.
Methods
Animals
All animal care and experiments were conducted in accordance with the official recommendations of the Chinese Community Guidelines. Male Wistar rats were purchased from Shanghai SLAC Laboratory Animal Co. Ltd at ∼6 weeks of age and were housed in the Nanjing University of Chinese Medicine Animal Resource Center.
Experimental protocols
Streptozotocin (STZ) (dissolved in 0.1 mol·L−1 sodium citrate buffer, pH 4.0) plus complete Freund's adjuvant (CFA) was injected i.p. (30 mg·kg−1 STZ and 0.1 mL·CFA per rat) once a week to male Wistar rats (45 animals) for 3 weeks to induce the model of DN. Seven weeks later, blood samples from the tail vein were used to determine the non-fasting blood glucose with blood glucose test strips (Roche diagnostics GmbH, D-68298, Mannheim, Germany), and the presence of diabetes was defined by the blood glucose level (between 16 and 30 mmol·L−1). The diabetic rats were divided into three groups (10 animals per group): DN group, urocortin 1-treated group and urocortin 1 + astressin group. Astressin is a non-selective CRF receptor antagonist. There was no significant difference in non-fasting blood glucose levels among these three groups. Rats in the urocortin 1 group received 6 µg·kg−1 urocortin 1, and rats in urocortin 1 + astressin group received 6 µg·kg−1+ 30 µg·kg−1 astressin intraperitoneally per day for 8 weeks. Rats in DN group received the same volume of normal saline. Age-matched male Wistar rats were used as normal controls (10 animals). Normal control and diabetic rats were housed five per cage in a room with a 12 h artificial light cycle and free access to a high-fat diet (18% fat; the standard rat diet has 8% fat) and water. Body weight and non-fasting blood glucose were measured weekly.
At the end of the experiments, rats were put in a metabolic cage to collect 24 h urine samples. Rats were anaesthetized (ketamine/xylazine; 70/10 mg·kg−1 i.p.), and blood samples were withdrawn from the retrobulbar venous plexus with a capillary. Kidneys were removed, decapsulated and weighed. One kidney (the right one) from each rat was frozen at −80°C until they were processed for RNA extraction; the other (the left one) was removed for fixation in 10% formaldehyde for 24 h before being processed for histology and immunohistochemistry.
Biochemical and radioimmunoassay(Beijing Atom High Tech. Co., Ltd., Beijing, China) analysis
Serum glucose and creatinine were measured by using the commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Plasma insulin was measured by RIA.
Urinary index analysis
Urinary creatinine was detected by a commercial kit (Nanjing Jiancheng Bioengineering Institute) and albuminuria was measured by RIA (Beijing Atom High Tech. Co. Ltd.). Ccr was calculated as follows (Sun et al., 2006):
 |
(1) |
Histological evaluation
Kidneys were processed for light microscopic examination by using periodic acid Schiff (PAS) and haematoxylin-eosin (HE) stain sections, and degree of glomerular injury (glomerulosclerosis) was evaluated by a semi-quantitative method as described previously (Bilous et al., 1989). In addition, the ratio of mesangial area to total glomerular area (‘% mesangial area’) was used to determine the severity of glomerulosclerosis (Lenz et al., 1998). Both total glomerular area (including nuclei and spaces within capillary loops) and mesangial area (excluding nuclei) were obtained, and per cent mesangial area was calculated by using Olympus microscope and Analysis Imaging System (OPTMAS, Imaging Research, St. Catherine.s, Ontario, Canada). We recorded and assessed ≥20 glomeruli from each rat.
Immunohistochemistry assay
A modification of the ABC immunoglobulin enzyme bridge technique was used for immunohisochemistry, according to the manufacturer's instructions. In brief, primary antibodies (mouse anti-VEGF and mouse anti TGF-β1 optimal concentrations were 1/300 and 1/250 respectively) were added and incubated for 24 h at 4°C. Slides were rinsed in phosphate buffered saline (PBS). Secondary antibody (optimal concentration was 1/100) was added and incubated for 30 min with gentle rocking. Substrate [3,3-diaminobenzidine (DAB)] solution was prepared before use and added to the slides then incubated in the dark for about 20 min. For each glomerulus, the ratio of positive staining area (shown in brown) to total glomerular area was calculated by using Analysis Imaging System.
RNA extraction, cDNA synthesis and semi-quantitative RT-PCR analysis of VEGF
Total RNA was isolated from tissues harvested from kidney of the same site by using Trizol reagent, according to the protocol provided by the manufacturer, and cDNA was generated from total RNA by RT in a reaction containing 5 µg total RNA, 0.5 µg Oligo (dT)12–18, 10 µmol·L−1 dNTP Mix, 4 µL 5 × First-Strand Buffer, 0.2 mmol·L−1 DL-Dithiothreitol (DTT), 40 units RNase inhibitor, 200 units of moloney murine leukemia virus (M-MLV) reverse transcriptase and diethypyrocarbonate (DEPC) water (Ultrapure, DNase and RNase free) in a total volume of 20 µL. The RT reaction was performed at 37°C for 50 min, then heated at 70°C for 15 min.
PCR was performed by using a Mastercycler gradient PCR instrument (Eppendorf, Hamburg, Germany). Gene-specific primers are shown in Table 1, synthesized by Invitrogen Corporation, Carlsbad, CA, USA.
Table 1.
Primer sequences used for the RT-PCR amplification of VEGF
| Gene | Sequence (5′→3′) | Product size (bp) |
|---|---|---|
| VEGF(GenBank:NM_031836): Forward primer | GATGAGATAGAGTATATCTTCAAGCCGT | 210 bp |
| Reverse primer | TCTATCTTTCTTTGGTCTGCATTCAC | |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): Forward primer | TCCCAGAGCTGA CGGGAAGCTCACTG | 339 bp |
| Reverse primer | TGGAGGCCATGTAGGCCATGAGGTCCA |
The reaction was performed in a total volume of 50 µL containing cDNA (2 µL), dNTP Mix (10 µmol·L−1), MgCl2 (0.1 mmol·L−1), Taq DNA polymerase (0.4 U), forward and reverse primers (0.01 µmol·L−1 respectively), 10 × PCR buffer (5 µL) and DEPC water (35.6 µL). The optimal amplified procedures of VEGF/glyceraldehyde-3-phosphate dehydrogenase primer (GAPDH) were as follows: after a predenaturing of 5 min at 95°C, 30 cycles were performed with denaturing of 30 s at 94°C, annealing of 40 s at 58.5°C/62.6°C (respectively), extension of 40 s at 72°C, a final extension step of 7 min at 72°C and hold at −4°C. Positive and negative controls were included to confirm the PCR product size and that no genomic DNA was present in the RT samples. RT-PCR products (5 µL) were resolved on 2% agarose gels prepared with 1× Tris acetate EDTA containing ethidium bromide (0.06 µg·mL−1). Product sizes were confirmed by using a 100–1500 bp DNA molecular marker (Promega, Madison, WI, USA). Semi-quantification of each PCR product was performed by densitometric scanning (Gene Tools, Syngene, Philomath, OR, USA) normalizing values to those of GAPDH.
Cell culture
Rat MCs (HBZY1 cell line) were purchased from China Center for Type Culture Collection. This cell line originated from Sprague-Dawley rats. MCs were maintained at 37°C in a humidified incubator with 5% CO2/95% air and propagated in low glucose Dulbecco's modified Eagle's medium (DMEM; GIBCO BRL, Gaithersburg, MD, USA) supplemented with 10% newborn bovine serum.
Measurement of VEGF secretion of MCs by enzyme-linked immunosorbent assay
Cells were seeded in 24-well plates (5 × 104 cells per well) and incubated in growth medium for 24 h. The medium was then changed to serum-free DMEM to render the cell quiescent. After 24 h, serum-free medium containing 2 ng·mL−1 TGF-β1 (PeproTech Inc., Rocky Hill, NJ, USA) was added to the cultures, and cells were divided into six groups: control; treatment 1, 0.1 nmol·L−1 urocortin 1; treatment 2, 1 nmol·L−1 urocortin 1; treatment 3, 10 nmol·L−1 urocortin 1; treatment 4, 100 nmol·L−1 urocortin 1; treatment 5, 1 nmol·L−1 urocortin 1 + 5 nmol·L−1 astressin. Astressin was added 0.5 h before urocortin 1 was given. Cells cultured in serum-free DMEM were used as blanks. Every group was repeatedly tested six times in different 24-well plates. Twelve hours later, the medium was harvested and its content of VEGF assayed by elisa kit according to the protocol provided by the manufacturer.
Data analysis and statistical procedures
Data are presented as means ± standard deviation (SD). anova was used for comparison of group means followed by the least significant difference post hoc test for between-group differences. Differences were considered significant if P < 0.05.
Materials
Urocortin 1 and astressin were purchased from Sigma; mouse monoclonal antibody to VEGF was purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA, USA; product code: sc-53462); mouse monoclonal antibody to TGF-β1 was purchased from Abcam Ltd. (Cambridge, UK; product code: ab27969); secondary antibodies were purchased from JINGMEI Biotech (Guangzhou, China); DAB was purchased from Gene Tech company Ltd. (Hongkong, China); Trizol, Oligo (dT)12-18, RNasin, Dntp Mix, M-MLV reverse transcriptase and Taq DNA were purchased from Promega, USA.
Results
Effects of urocortin 1 on blood glucose, insulin level and kidney weight
Blood glucose level of diabetic rats was increased obviously at the fourth week from the onset of the experiment (Figure 1A), and urocortin 1 or urocortin 1 + astressin treatment did not affect the blood glucose level of DN rats at any time during the experiment (Data in Figure 1A show blood glucose levels at the end of the experiment; the weekly data are not shown). There were no changes in blood insulin levels in any of the groups (Figure 1B). Kidney weight of DN rats was increased compared with that of normal rats, and treatment with urocortin 1 restored kidney weights in DN rats to normal values (Figure 1C). Adding astressin, a non-selective CRF receptor antagonist, to the urocortin 1 treatment of DN rats did not change the effect of urocortin 1 on kidney weight.
Figure 1.
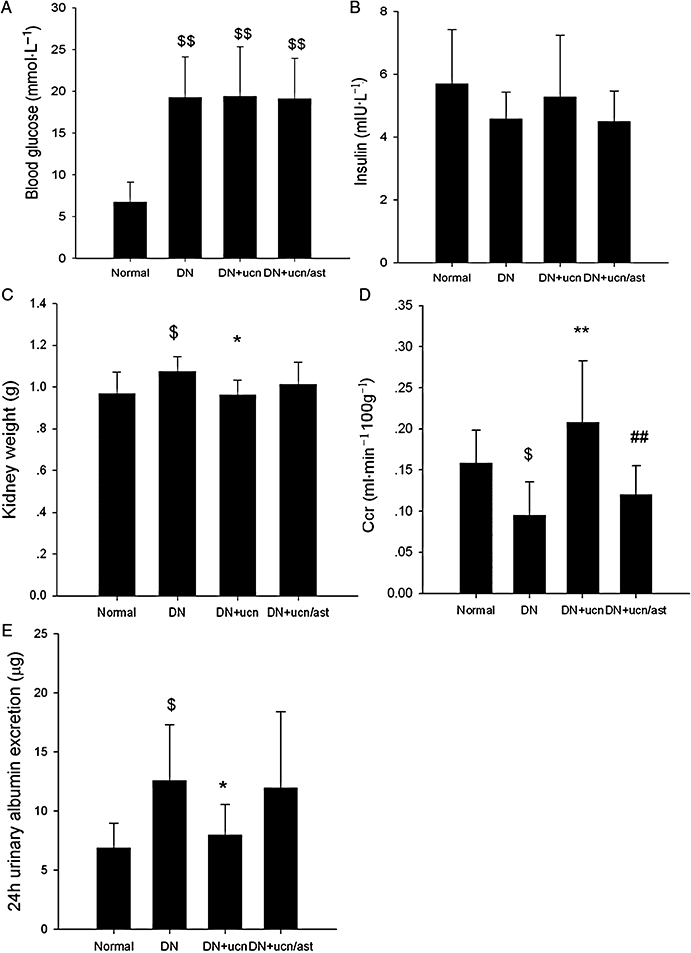
Effects of urocortin 1 on blood glucose, insulin level, kidney weight, Ccr and albuminuria of DN rats. Blood glucose (A) and insulin (B) level were not affected; however, average kidney weight (C) was decreased by urocortin 1. Ccr (D) was enhanced, and 24 h urinary albumin excretion (E) was decreased by urocortin 1 treatment. Astressin blunted the effect of urocortin 1. *P < 0.05, **P < 0.01 compared with DN; ##P < 0.01 compared with DN + ucn; $P < 0.05, $$P < 0.01 compared with normal. n= 10.Normal, non-diabetic control; DN, untreated diabetic; DN + ucn, diabetic rats treated with urocortin 1 (6 µg·kg−1); DN + ucn/ast, diabetic rats treated with urocortin 1 + astressin (6 µg·kg−1 and 30 µg·kg−1 respectively,). Ccr, creatinine clearance rate; DN, diabetic nephropathy.
Urocortin 1-induced improvement of Ccr and albuminuria
As shown in Figure 1D and E, Ccr of DN rats was clearly decreased (to about two-thirds of values in normal rats), and urocortin 1 treatment returned the Ccr in DN rats to normal levels. Combining astressin with urocortin 1 abolished the effect of urocortin 1 on Ccr in DN rats. Albumin excretion over 24 h in DN rats (approximately double that in normal rats) was decreased by urocortin 1. Adding astressin to the urocortin1 seemed to reduce the beneficial effect of urocortin 1 on albuminuria, but these data were too variable to provide significant changes.
Inhibition of glomerular ECM accumulation, TGF-β1 and VEGF overexpression by urocortin 1
As we know, DN is characterized by an expansion of glomerular mesangium, which is caused by MC proliferation, and excessive accumulation of ECM (Ahn et al., 2004). As demonstrated in Figure 2, in the normal rat, the mesangium contained the usual complement of cells and matrix without ECM accumulation (Figure 2C,G). In distinction, the glomeruli from DN rats had severe mesangial matrix expansion, and the mesangium was diffusely and markedly expanded with PAS-positive matrix material (Figure 2D,H). Urocortin 1 reduced the pathological changes significantly (Figure 2E,I), and astressin treatment reversed the effect of urocortin 1 (Figure 2F,J). As shown in Figures 3 and 4, glomeruli of DN rats after immunostaining for TGF-β1 and VEGF showed clearly increased amounts of these proteins, and such overexpression was inhibited by urocortin 1. VEGF mRNA of the kidney from DN rats was overexpressed, and urocortin 1 significantly diminished such overexpression (Figure 5). All these effects of urocortin 1 were reversed by astressin.
Figure 2.
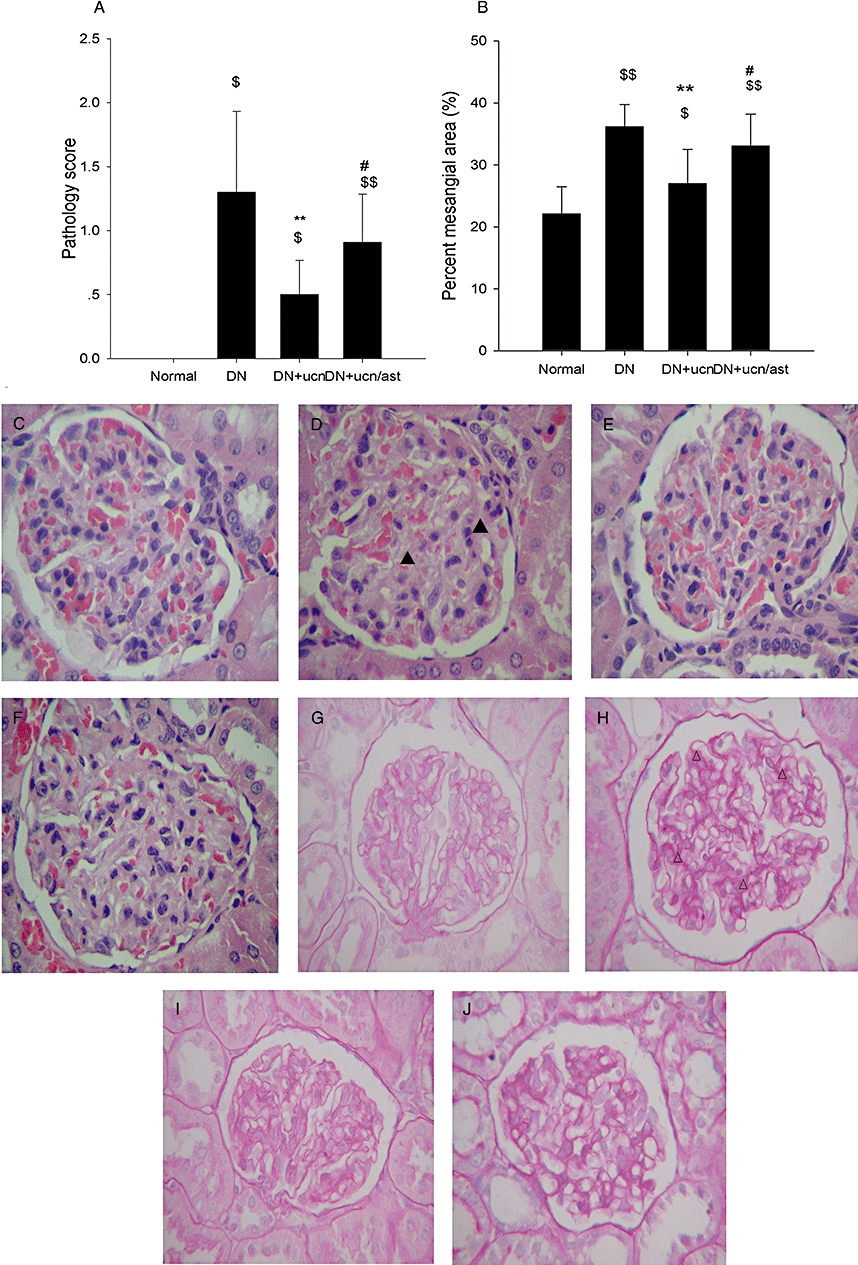
Urocortin 1-induced protective effects on pathological lesions in kidneys of DN rats. The pathology score (A) and percent mesangial area (B) of DN rats were decreased significantly by urocortin 1. Representative micrographs of HE-stained (C–F) and PAS-stained (G–J) paraffin sections showed effect of urocortin 1 on glomerular histology changes and ECM expansion and accumulation in DN rats (magnification ×400). (C, G) Glomeruli of normal rats. (D, H) Glomeruli of DN rats. (E, I) Glomeruli of DN rats treated with urocortin 1. (F, J) Glomeruli of DN rats treated with urocortin 1 + astressin. Treatment with urocortin 1 alleviated the pathology change and ECM expansion and accumulation. The effects of urocortin 1 were reversed by astressin. **P < 0.01 compared with DN; #P < 0.05 compared with DN + ucn; $P < 0.05, $$P < 0.01 compared with normal. n= 10. ▴, expanded mesangial area; ▵, accumulated ECM. DN, diabetic nephropathy; ECM, extracellular matrix; HE, haematoxylin-eosin; PAS, periodic acid Schiff.
Figure 3.
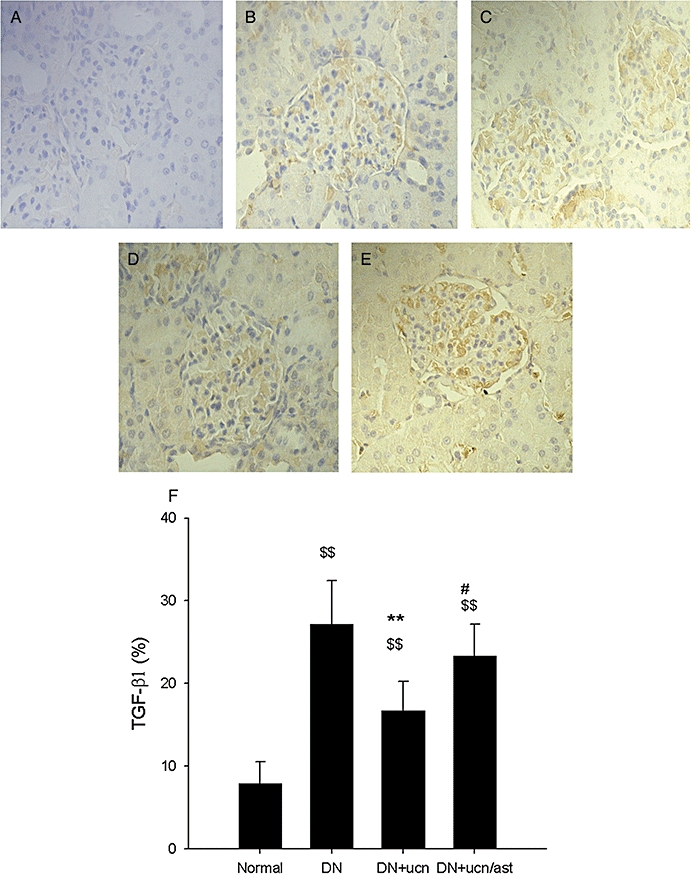
Urocortin 1-induced reduction of overexpression of TGF-β1 in kidneys of DN rats. Representative micrographs of DAB-stained paraffin sections showed urocortin 1 treatment decreased the overexpression of TGF-β1 (magnification ×400). (A). Negative. (B). Glomeruli of normal rats. (C). Glomeruli of DN rats. (D). Glomeruli of DN rats treated with urocortin 1. (E). Glomeruli of DN rats treated with urocortin 1 + astressin. The percent TGF-β1 area (F) was decreased by urocortin 1, and astressin reversed such effects. **P < 0.01 compared with DN; #P < 0.05 compared with DN + ucn; $$P < 0.01 compared with normal. n= 10. DAB, 3,3-diaminobenzidine; DN, diabetic nephropathy; TGF-β1, transforming growth factor-β1.
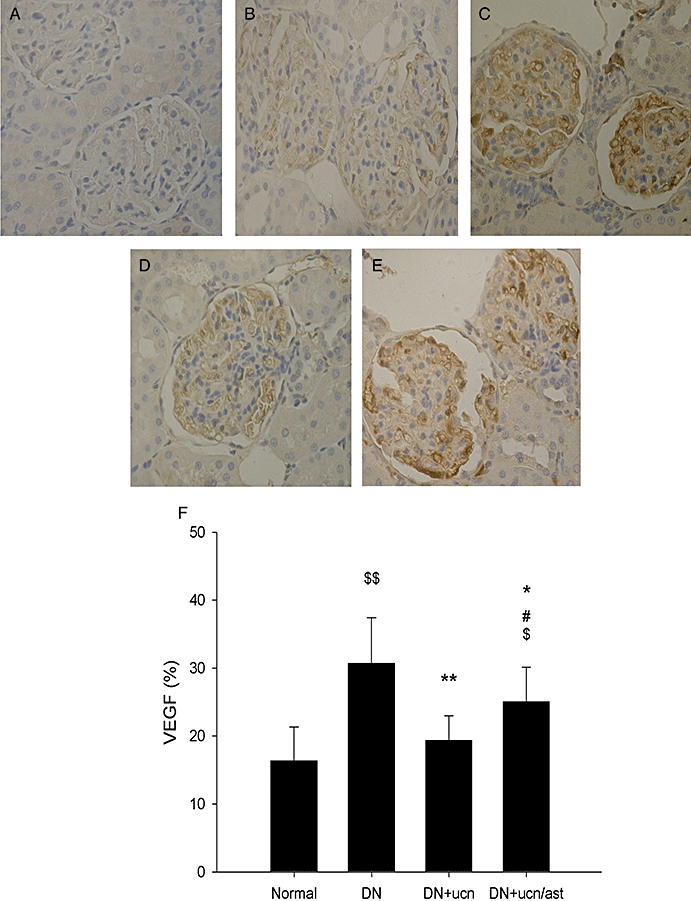
Urocortin 1-induced reduction in overexpression of VEGF in kidneys of DN rats. Representative micrographs of DAB-stained paraffin sections showed urocortin 1 treatment decreased the overexpression of VEGF (magnification ×400). (A). Negative. (B). Glomeruli of normal rats. (C). Glomeruli of DN rats. (D). Glomeruli of DN rats treated with urocortin 1. (E). Glomeruli of DN rats treated with urocortin 1 + astressin. The per cent VEGF area (F) was decreased by urocortin 1, and astressin reversed such effect. *P < 0.05, **P < 0.01 compared with DN; #P < 0.05 compared with DN + ucn; $P < 0.05, $$P < 0.01 compared with normal. n= 10. DAB, 3,3-diaminobenzidine; DN, diabetic nephropathy; VEGF, vascular endothelial growth factor.
Figure 5.
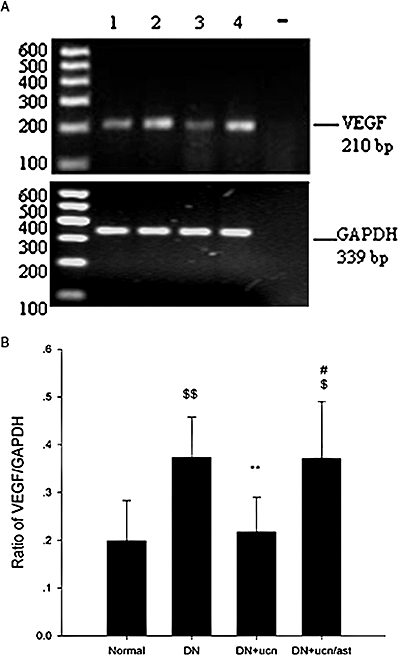
Effects of urocortin 1 on expression of VEGF mRNA in kidneys of DN rats. Representative agarose gel electrophoresis of PCR products (A). 1: kidneys of normal rats; 2: kidneys of DN rats; 3: kidneys of DN rats treated with urocortin 1; 4: kidneys of DN rats treated with urocortin 1 + astressin; −: control reaction without reverse transcriptase. Optical density analysis shown as ratio of VEGF mRNA/GAPDH mRNA (B). The increased expression of VEGF mRNA in the kidney of DN rats was decreased by urocortin 1 treatment, and astressin abolished such effects of urocortin 1. **P < 0.01 compared with DN; #P < 0.05 compared with DN + ucn; $P < 0.05,$$P < 0.01 compared with normal. n= 10. DN, diabetic nephropathy; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PCR, polymerase chain reaction; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor.
Effects of urocortin 1 on VEGF over-secretion in MC cell line induced by TGF-β1
Cultures of rat MCs serve as a model to investigate the signal transduction pathways involved in high-glucose-induced VEGF expression and TGF-β1-induced VEGF secretion in MCs (Gruden et al., 1999). Our results (Figure 6) showed that secretion of VEGF164 was enhanced by stimulation with 2 ng·mL−1 TGF-β1 (about 1.5- to 2-fold increase over controls). Urocortin 1 significantly reduced the TGF-β1-induced VEGF secretion in MCs over a range of concentrations, and this effect was blunted by astressin pretreatment.
Figure 6.
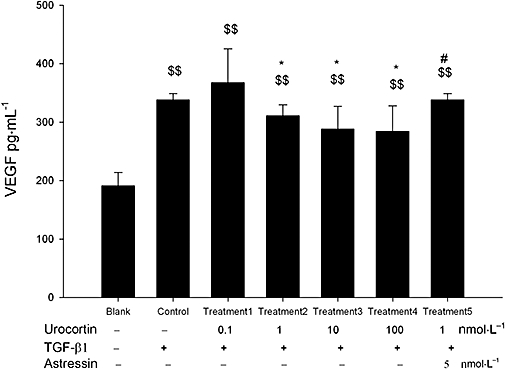
Urocortin 1 reduced the oversecretion of VEGF in MCs, induced by TGF-β1. Urocortin 1 decreased TGF-β1 induced VEGF secretion in MCs and pretreatment with astressin reversed the effect of urocortin 1. Blank, MCs were cultured in normal DMEM medium; Control, MCs cultured in DMEM containing 2 ng·mL−1 TGF-β1; Treatment 1, 2, 3, 4, MCs cultured in DMEM containing 2 ng·mL−1 TGF-β1 + 0.1 nmol·L−1, 1 nmol·L−1, 10 nmol·L−1 or 100 nmol·L−1 urocortin 1 respectively. Treatment 5, MCs cultured in DMEM containing 2 ng·mL−1 TGF-β1 + 1 nmol·L−1 urocortin 1 + 5 nmol·L−1 astressin. *P < 0.05 compared with control; $$P < 0.01 compared with blank; #P < 0.05 compared with treatment 2. n= 6. DMEM, Dulbecco's modified Eagle's medium; MC, mesangial cell; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor.
Discussion and conclusions
Our major new finding is that urocortin 1 could be beneficial in reversing effects of DN on renal function. Our previous work showed that urocortin 1 inhibited the accumulation of ECM and advanced glycation end-products, activation of the polyol pathway and ROS generation in db/db mice, an obese diabetic animal model, and all these results implied that urocortin 1 could ameliorate DN. In the current study, urocortin 1 was found to decrease albuminuria and increase Ccr, and this beneficial effect involved CRF receptors. The underlying mechanisms also involve the inhibitory effects of urocortin 1 on the overexpression and secretion of VEGF and TGF-β1, two well-known growth factors.
Here, we used a model of diabetes in rats, induced by multiple injections of low doses of STZ and CFA, to avoid the higher death rate in rats given a single high dose of STZ. Nevertheless, our model still depended on the destruction of beta cells and reduction of insulin secretary capacity. Clinically, DN is divided into five stages; stage 3 is characterized by early nephropathy with microalbuminuria, and stage 4 is overt DN characterized by persistent proteinuria and decreased GFR (Mogensen et al., 1983). With our pathophysiological and biochemical results, we are confident that our DN model would correspond to stages 3 and 4 and was thus suitable for assessing effect of urocortin 1′ on renal function and the relevant mechanisms.
Baldamus et al. (1975) reported that the glomerulus was an effective barrier for protein under control conditions. Increase in the width of glomerular and tubular basement membranes and mesangial matrix expansion, both of which indicate ECM accumulation, are hallmarks of DN (Caramori et al., 2002). Mesangial fractional volume is highly correlated with albumin excretion rate and is the strongest independent predictor of albumin excretion (Caramori et al., 2002). Mesangial expansion is strongly related to the manifestation of DN and has strong inverse correlations with capillary filtering surface area density (Mauer et al., 1984). TGF-β1 is a well-known growth factor that plays a central role in ECM accumulation. With our earlier data (Li et al., 2008), we found that urocortin 1 decreased TGF-β1 overexpression both in vivo and in vitro, via CRF receptors.
However, there is only limited evidence to support a direct role of TGF-β1 in the development of albuminuria. According to Sung et al. (2006), correlations between mesangial expansion and albuminuria are only an association and not directly causal. On the other hand, recent research has focused on the podocyte as a central target for the effects of the metabolic milieu in the development and progression of diabetic albuminuria (Ziyadeh and Wolf, 2008). Podocyte-derived VEGF appears to be a major cause of the development of proteinuria because of its property as a vascular permeability factor. Treatment with monoclonal anti-VEGF antibodies improved early renal function in experimental diabetic rats (de Vriese et al., 2001), and a VEGF receptor inhibitor ameliorated diabetic albuminuria in db/db mice (Sung et al., 2006). VEGF signalling is found to affect a number of podocyte-driven manifestations such as glomerular basement membrane thickening, slit pore density and nephrin quantity, all of which are associated with the extent of diabetic albuminuria (Sung et al., 2006). In the kidney, TGF-β1 and VEGF are factors secreted by several types of cells including MCs. MCs are important cells in maintaining renal function, as they are capable of producing ECM proteins and regulating GFR through their contractility (Haneda et al., 2003). In addition to the stimulation by TGF-β1 of VEGF synthesis in WT MCs, VEGF itself stimulates collagen and fibronectin expression in MCs and thus, in turn, enhances TGF-β1-induced ECM accumulation (Wang et al., 2004). TGF-β1 and VEGF interact with each other, and oversecretion of VEGF, induced by TGF-β1, may be a possible explanation of the role of TGF-β1 in the development of albuminuria. Inhibition of VEGF secretion in vitro and in vivo by urocortin 1 suggested that urocortin 1 may block the overproduction of VEGF induced by many mechanisms in the DN rat, such as TGF-β1, ROS and angiotensin II, via CRF receptors. However, the actual CRF receptor subtype involved is not clear, as astressin is a non-selective antagonist of these receptors.
On the other hand, diabetes-specific microvascular diseases, including DN, display abnormalities in blood flow and increased vascular permeability in the early stage. As urocortin 1 is known to dilate blood vessels, it is reasonable to believe that the amelioration of the effects of DN by urocortin may be partially attributed to the changes in microcirculation in the early stage.
In summary, urocortin 1 improved the renal function of DN rats, which was manifested by its decrease of 24 h urinary albumin excretion and increase of Ccr in DN rats. This beneficial effect was related not only to urocortin 1's inhibitory effect on overexpression of TGF-β1, VEGF and VEGF mRNA in the kidneys of DN rats but also to the inhibitory effect of TGF-β1-induced VEGF secretion in MCs. Overall, it is possible that urocortin 1 could be an important beneficial regulator of the progression of DN, through its favourable effects on TGF-β1 and VEGF overproduction.
Acknowledgments
This work was supported by Natural Scientific Fund of Jiangsu Province (No.BK2006727), Program for New Century Excellent Talents in University (NECT-06-0507), Administration of Traditional Chinese Medicine of Jiangsu Province (H05082) and Department of Education of Jiangsu Province (05KJD360158).
Glossary
Abbreviations:
- Ccr
creatinine clearance rate
- CFA
complete Freund's adjuvant
- CRF
corticotrophin-releasing factor
- DAB
3,3-diaminobenzidine
- DN
diabetic nephropathy
- ECM
extracellular matrix
- RIA
radioimmunoassay
- ROS
reactive oxygen species
- STZ
streptozotocin
- TGF-β1
transforming growth factor-β1
- VEGF
vascular endothelial growth factor
Conflict of interest
The authors state no conflict of interest.
References
- Figure 4.Ahn JD, Morishita R, Kaneda Y, Kim HJ, Kim YD, Lee HJ, et al. Transcription factor decoy for AP-1 reduces mesangial cell proliferation and extracellular matrix production in vitro and in vivo. Gene Ther. 2004;11:916–923. doi: 10.1038/sj.gt.3302236. [DOI] [PubMed] [Google Scholar]
- Baldamus CA, Galaske R, Eisenbach GM, Krause HP, Stolte H. Glomerular protein filtration in normal and nephritic rats. A micropuncture study. Contrib Nephrol. 1975;1:37–49. [PubMed] [Google Scholar]
- Bilous RW, Mauer SM, Sutherland DE, Steffes MW. Mean glomerular volume and rate of development of diabetic nephropathy. Diabetes. 1989;38:1142–1147. doi: 10.2337/diab.38.9.1142. [DOI] [PubMed] [Google Scholar]
- Caramori ML, Kim Y, Huang C, Fish AJ, Rich SS, Miller ME, et al. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes. 2002;51:506–513. doi: 10.2337/diabetes.51.2.506. [DOI] [PubMed] [Google Scholar]
- Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001;44:1957–1972. doi: 10.1007/s001250100000. [DOI] [PubMed] [Google Scholar]
- Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48:2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- Gruden G, Thomas S, Burt D, Zhou W, Chusney G, Gnudi L, et al. Interaction of angiotensin II and mechanical stretch on vascular endothelial growth factor production by human mesangial cells. J Am Soc Nephrol. 1999;10:730–737. doi: 10.1681/ASN.V104730. [DOI] [PubMed] [Google Scholar]
- Haneda M, Koya D, Isono M, Kikkawa R. Overview of glucose signaling in mesangial cells in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1374–1382. doi: 10.1097/01.asn.0000064500.89551.76. [DOI] [PubMed] [Google Scholar]
- Hellmich B, Schellner M, Schatz H, Pfeiffer A. Activation of transforming growth factor-beta1 in diabetic kidney disease. Metabolism. 2000;49:353–359. doi: 10.1016/s0026-0495(00)90264-6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Chan FL, Lau CW, Tsang SY, He GW, Chen ZY, et al. Urocortin-induced endothelium-dependent relaxation of rat coronary artery: role of nitric oxide and K+ channels. Br J Pharmacol. 2002;135:1467–1476. doi: 10.1038/sj.bjp.0704587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S, Rashed L. Estimation of transforming growth factor-beta 1 as a marker of renal injury in type II diabetes mellitus. Saudi Med J. 2007;28:519–523. [PubMed] [Google Scholar]
- Jafar TH, Schmid CH, Levey AS. Serum creatinine as maker of kidney function in south Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16:1413–1419. doi: 10.1681/ASN.2004121100. [DOI] [PubMed] [Google Scholar]
- Lenz O, Zheng F, Vilar J, Doublier S, Lupia E, Schwedler S, et al. The inheritance of glomerulosclerosis in mice is controlled by multiple quantitative trait loci. Nephrol Dial Transplant. 1998;13:3074–3078. doi: 10.1093/ndt/13.12.3074. [DOI] [PubMed] [Google Scholar]
- Li X, Hu J, Zhang R, Sun X, Zhang Q, Guan X, et al. Urocortin ameliorates diabetic nephropathy in obese db/db mice. Br J Pharmacol. 2008;154:1025–1034. doi: 10.1038/bjp.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl. 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- Rademaker MT, Charles CJ, Espiner EA, Frampton CM, Lainchbury JG, Richards AM. Four-day urocortin-I administration has sustained beneficial haemodynamic, hormonal, and renal effects in experimental heart failure. Eur Heart J. 2005;26:2055–2062. doi: 10.1093/eurheartj/ehi351. [DOI] [PubMed] [Google Scholar]
- Rahn KH, Heidenreich S, Brückner D. How to assess glomerular function and damage in humans. J Hypertens. 1999;17:309–317. doi: 10.1097/00004872-199917030-00002. [DOI] [PubMed] [Google Scholar]
- Russo LM, del Re E, Brown D, Lin HY. Evidence for a role of transforming growth factor (TGF)-beta1 in the induction of postglomerular albuminuria in diabetic nephropathy: amelioration by soluble TGF-beta type II receptor. Diabetes. 2007;56:380–388. doi: 10.2337/db06-1018. [DOI] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Sun SZ, Wang Y, Li Q, Tian YJ, Liu MH, Yu YH. Effects of benazepril on renal function and kidney expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in diabetic rats. Chin Med J (Engl) 2006;119:814–821. [PubMed] [Google Scholar]
- Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol. 2006;17:3093–3104. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu Y, Xu Y, Zhu H, Zhang R, Zhang G, et al. Urocortin's inhibition of tumor growth and angiogenesis in hepatocellular carcinoma via corticotrophin-releasing factor receptor 2. Cancer Invest. 2008;26:359–368. doi: 10.1080/07357900701788106. [DOI] [PubMed] [Google Scholar]
- Wang L, Kwak JH, Kim SI, He Y, Choi ME. Transforming growth factor-beta1 stimulates vascular endothelial growth factor 164 via mitogen-activated protein kinase kinase 3-p38alpha and p38delta mitogen-activated protein kinase-dependent pathway in murine mesangial cells. J Biol Chem. 2004;279:33213–33219. doi: 10.1074/jbc.M403758200. [DOI] [PubMed] [Google Scholar]
- Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D. Albuminuria: a target for treatment of type 2 diabetic nephropathy. Semin Nephrol. 2007;27:172–181. doi: 10.1016/j.semnephrol.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45. doi: 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]


