Abstract
Background and purpose:
Escherichia coli Nissle 1917 is a probiotic strain used in the treatment of intestinal immune diseases, including ulcerative colitis. The aim of the present study was to test if this probiotic bacterium can also show systemic immunomodulatory properties after oral administration.
Experimental approach:
The probiotic strain was administered to rats or mice for 2 weeks before its assay in two experimental models of altered immune response, the trinitrobenzenesulphonic acid (TNBS) model of rat colitis, localized in the colon, and the lipopolysaccharide (LPS) model of systemic septic shock in mice. Inflammatory status was evaluated both macroscopically and biochemically after 1 week in the TNBS model or after 24 h in the LPS shock model. In addition, splenocytes were obtained from mice and stimulated, ex vivo, with concanavalin A or LPS to activate T or B cells, respectively, and cytokine production (IL-2, IL-5 and IL-10) by T cells and IgG secretion by B cells measured.
Key results:
E. coli Nissle 1917 was anti-inflammatory in both models of altered immune response. This included a reduction in the pro-inflammatory cytokine tumour necrosis factor-α both in the intestine from colitic rats, and in plasma and lungs in mice treated with LPS. The systemic beneficial effect was associated with inhibited production of the T cell cytokines and by down-regulation of IgG release from splenocyte-derived B cells.
Conclusions and implications:
The anti-inflammatory effects of E. coli Nissle 1917 given orally were not restricted to the gastrointestinal tract.
Keywords: Escherichia coli Nissle 1917, mice LPS septic shock, cytokines, IgG, intestinal anti-inflammatory activity, TNBS rat colitis
Introduction
The non-pathogenic strain of Escherichia coli Nissle 1917 (O6:K5:H1) is the active component of the microbial drug Mutaflor (Ardeypharm GmbH, Herdecke, Germany). This is a probiotic drug, successfully used in several European countries for the treatment of various diseases of the digestive tract, including diarrhoea (Henker et al., 2008), diverticulitis (Fric and Zavoral, 2003) and inflammatory bowel disease (IBD) (Schultz, 2008). In the latter, it is particularly used for maintenance therapy in patients with ulcerative colitis in remission, showing an equivalent efficacy to the standard drug mesalazine, used in this condition (Kruis et al., 2004). Different mechanisms of action have been proposed to account for the intestinal anti-inflammatory effects exerted by this probiotic strain. Thus, E. coli Nissle 1917 inhibits adhesion and invasion by adherent invasive E. coli of intestinal epithelial cells (Boudeau et al., 2003), an effect that can be mediated by the induction, in the mucosal epithelium, of antimicrobial agents including peptides like the human β-defensin 2, which have a broad antibiotic spectrum against Gram-negative and -positive bacteria, as well as against fungi and viruses (Wehkamp et al., 2004). As a result, the probiotic bacteria limit adherence and invasion of other bacteria to the intestinal mucosa, a process with a key role in triggering the exacerbated immune response that occurs in IBD (Sartor, 2004). Furthermore, this probiotic strain may reinforce the mucosal barrier and restore it when it is disrupted, specifically through the up-regulation of expression of the mRNA for the zonula occludens proteins ZO-1 and ZO-2 in intestinal epithelial cells (Ukena et al., 2007; Zyrek et al., 2007).
In addition to its effects on the adhesion of pathogens and on mucosal barrier function, E. coli Nissle 1917 can modulate intestinal immune function with evident beneficial consequences in IBD. Of note, this microorganism possesses a specific lipopolysaccharide (LPS) that renders it immunogenic, without showing any immunotoxic properties (Grozdanov et al., 2002). Furthermore, this probiotic strain may down-regulate the expansion of newly recruited T cells into the mucosa and limit intestinal inflammation, without affecting already activated lamina propria T cells, thus preserving their role in eliminating deleterious antigens in order to maintain immunological homeostasis, which is clearly beneficial in the treatment of IBD (Sturm et al., 2005). In addition, E. coli Nissle 1917 decreased the secretion of pro-inflammatory cytokines, including interleukin (IL)-2, tumour necrosis factor (TNF)-α and interferon (IFN)-γ, and increased the secretion of anti-inflammatory cytokines, like IL-10 (Sturm et al., 2005), which can undoubtedly contribute to the reported intestinal anti-inflammatory activity of this probiotic E. coli, given the role ascribed to these cytokines in intestinal inflammation (Fiocchi, 1998).
It is evident that the research involving the beneficial effects of probiotics has mainly focused on intestinal function. Different studies have proposed that the potential beneficial effects of these bacteria are not restricted to the intestine, as different probiotic bacteria are able to ameliorate the inflammation associated with rheumatoid arthritis (Baharav et al., 2004; Sheil et al., 2004) or atopic disease (Isolauri et al., 2000; Rosenfeldt et al., 2003). However, there is little information on the potential effect of E. coli Nissle 1917 on the systemic immune response, and this may be of clinical relevance, as it has been reported that the prevalence of extra-intestinal diseases in IBD patients is around 6% (excluding arthritis), many showing an immunological basis, like primary sclerosing cholangitis, ankylosing spondylitis, iritis/uveitis, pyoderma gangrenosum and erythema nodosum (Bernstein et al., 2001). For this reason, it would be interesting to investigate if this probiotic strain, in addition to its local, intestinal anti-inflammatory activity, shows beneficial effects when the systemic immune response is altered.
Therefore, the present study describes the effects of E. coli Nissle 1917, given orally, in two experimental models of altered immune response in rodents: the trinitrobenzenesulphonic acid (TNBS) model of rat colitis, in which there is an immunological disorder localized in the large intestine, and LPS-induced shock in mice, as a model of systemic alteration of the immune response. From the results obtained, we have concluded that this probiotic E. coli ameliorated both TNBS-induced colitis in rats and LPS-induced organ damage in mice. In consequence, the anti-inflammatory effects of this probiotic treatment were not restricted to the gastrointestinal tract, thus supporting the use of this probiotic E. coli in intestinal conditions associated with systemic symptoms, related to the immune system.
Methods
Animals
All animal care and procedures in this study were carried out in accordance with the Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes of the European Union (86/609/EEC). Female Wistar rats (200–210 g) or male BALB/c mice (20–22 g) obtained from the Laboratory Animal Service of the University of Granada were housed in makrolon cages, maintained in air-conditioned animal quarters with a 12 h light–dark cycle and fed standard rodent chow (Panlab A04, Panlab, Barcelona, Spain) and water ad libitum throughout the experiment.
Evaluation of the intestinal anti-inflammatory effect of E. coli Nissle 1917 in the TNBS model of rat colitis
The rats were randomly assigned to three groups (n= 10); two of them (non-colitic and control groups) received phosphate-buffered saline (PBS) solution (1 mL) orally and the other (treated group) received the probiotic E. coli orally (109 CFU suspended in 1 mL of PBS solution) by means of an oesophageal catheter, daily for 3 weeks. Two weeks after starting the experiment, the rats were fasted overnight, and those from the control and treated groups were rendered colitic by the method originally described by Morris et al. (1989). Briefly, they were anaesthetized with halothane and given 10 mg of TNBS dissolved in 0.25 mL of 50% ethanol (v/v) by means of a Teflon cannula inserted 8 cm through the anus. Rats from the non-colitic group were administered intracolonically, 0.25 mL of PBS instead of TNBS. The body weight, water and food intake, as well as stool consistency, were recorded daily throughout the experiment. All rats were killed with an overdose of halothane 1 week after induction of colitis, and the colon was removed for the assessment of colonic damage. The colonic segment was cleaned of fat and mesentery, blotted on filter paper; each specimen was weighed and its length measured under a constant load (2 g), and the weight/length ratio determined. The colon was scored for macroscopically visible damage on a 0–10 scale by two observers unaware of the treatment, according to the criteria described by Bell et al. (1995), which takes into account the extent as well as the severity of colonic damage. The colon was longitudinally opened, and a cross-section from the distal diseased area was immediately fixed in 4% formaldehyde and embedded in paraffin for histological analysis. Subsequently, it was divided into different segments for biochemical determinations. One fragment was frozen at −80°C for assay of myeloperoxidase (MPO) activity, and the other was immediately processed for the measurement of colonic TNF-α, IL-10 and leukotriene B4 (LTB4) levels.
For the histological analysis, colonic full-thickness sections of 5 µm were stained with haematoxylin and eosin, and graded by two pathologists (A Nieto and A Concha) unaware of the experimental groups, according to the criteria previously described (Camuesco et al., 2004) (Table 1). Microphotograps were taken with a Leika DM 5000B microscope (Barcelona, Spain).
Table 1.
Scoring criteria of full-thickness distal colon sections
| Mucosal epithelium |
| Ulceration: none (0); mild surface (1); moderate (2); extensive full thickness (3) |
| Crypts |
| Mitotic activity: lower third (0); mild mid-third (1); moderate mid-third (2); upper third (3) |
| Neutrophilic infiltrate |
| Mucus depletion |
| Lamina propria |
| Plasmacytoid infiltrate |
| Neutrophilic infiltrate |
| Vascularity |
| Fibrin deposition: none (0); mucosal (1); submucosal (2); transmural (3) |
| Submucosal |
| Neutrophilic infiltrate |
| Oedema |
Scoring scale: 0, none; 1, mild; 2, moderate; 3, severe. Maximum score: 30.
Evaluation of the effects of E. coli Nissle 1917 in the LPS-induced model of septic shock in mice
The mice were randomly assigned to three groups (n= 10); two of them (healthy and control groups) received tap water and the other (treated group) received the probiotic strain suspended in drinking water at the final concentration of 108 CFU·mL−1, prepared daily. Oral gavage was not used in mice to avoid any stressful situation; however, the total daily dose of probiotic E. coli administered to mice was quite similar to that used for rats, as water intake for each mouse was between 8 and 10 mL per day. Food and water intake was recorded daily for all groups. Two weeks after starting the experiment, endotoxic shock was induced in treated and control mice with an intraperitoneal injection of LPS (20 mg·kg−1) in a final volume of 200 µL; healthy mice received sterile saline solution. Previous assays have revealed that this dose of LPS did not induce the death of any mouse in the following 24 h. Then, the mice were anaesthetized with halothane, blood samples were taken from the retro-orbital venous plexus and then killed immediately. The following tissues were quickly removed and weighed: spleen, lungs, liver and kidneys. Colon specimens, after weighing and measuring their length, were frozen at −80°C for assay of MPO activity and inducible NO synthase (iNOS) expression. Liver samples were frozen in 1 mL of 50 g·L−1 trichloroacetic acid for total glutathione content determination. One of the lungs was immediately processed for the measurement of TNF-α levels and the other was frozen at −80°C for MPO activity.
Biochemical determinations
All biochemical measurements were completed within 1 week from the time of sample collection and were performed in duplicate. MPO activity was measured as previously described (Krawisz et al., 1984); the results were expressed as MPO units g−1 wet tissue; one unit of MPO activity was defined as that degrading 1 µmol·H2O2·min−1 at 25°C. Total glutathione content was quantified in liver with the recycling assay described by Anderson (1985), and the results were expressed as nmol g−1 wet tissue. Lung or colonic samples for TNF-α, IL-10 or LTB4 determination were immediately weighed, minced on an ice-cold plate and suspended in a tube with sodium phosphate buffer 10 mmol·L−1 (pH 7.4) (1:5 w/v). The tubes were placed in a shaking water bath (37°C) for 20 min and centrifuged at 9000× g for 30 s at 4°C; the supernatants were frozen at −80°C until assay. TNF-α and IL-10 were quantified by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Abingdon, UK for rat samples, and Biosource, Nivelles, Belgium for mouse samples), and the results were expressed as pg·g−1 wet tissue. Colonic specimens from mice were also used for protein extraction to evaluate iNOS expression by Western blotting, which was performed as described elsewhere (Comalada et al., 2005). Blood samples were centrifuged (300× g for 10 min), and plasma TNF-α and IgG levels were quantified as explained elsewhere.
Spleen-derived cell culture
In order to obtain primary lymphocyte cultures, mouse spleens were immediately disaggregated in Dulbecco's modified Eagle's medium (DMEM) plus 1% penicillin/streptomycin/amphotericin after collection, centrifuged (300× g, 5 min) and erythrocytes were lysed with a lysis buffer (NH4Cl 1.7 mol·L−1, KHCO3 0.12 mol·L−1, EDTA 9 mmol·L−1) for 30 min at 4°C. Resting cells were counted using a haemocytometer and cultured to carry out stimulation assays (see below) in current culture medium (DMEM + 10% fetal bovine serum). The cells were incubated at 37°C in a humidified 5% CO2 atmosphere.
Spleen-derived lymphocytes were cultured in six-well plates (1 × 107 cells per well) in 3 mL of media and stimulated with concanavalin A (Con A) (5 µg·mL−1) or LPS (50 µg·mL−1) to activate T or B cells, correspondingly, and supernatants were collected after 48 or 72 h, respectively, and frozen until ELISA analysis. Cytokine production (IL-2, IL-5 and IL-10) by T cells and IgG secretion by B cells were measured with commercial murine ELISA kits (R&D Systems for cytokines; Bethyl, Montgomery, TX for IgG), following the manufacturers' protocols.
Statistical analysis
The results are expressed as mean ± SEM. Differences among means were tested for statistical significance using one-way analysis of variance and post hoc least significance tests. Non-parametric data (macroscopic and microscopic scores) were expressed as median (range) and analysed by the Mann–Whitney test. All statistical analyses were carried out with the Statgraphics 5.0 software package (STSC, Bethesda, MD), with statistical significance set at P < 0.05.
Materials
All chemicals, including LPS from E. coli serotype 055:B5, were obtained from Sigma Chemical (Madrid, Spain), unless otherwise stated. Escherichia coli strain Nissle 1917 (O6:K5:H1) (Ardeypharm GmbH) was prepared daily after its suspension in sterile PBS solution (109 CFU·mL−1).
Results
Preventative effects of E. coli Nissle 1917 in TNBS-induced rat colitis
The administration of the probiotic E. coli Nissle 1917 for 2 weeks before colitis induction did not result in any symptoms of diarrhoea or affect development of weight (data not shown). However, once the colitis was induced, the rats pretreated with E. coli Nissle 1917 showed an overall lower level of TNBS-induced colonic damage, compared to the TNBS control group. The anti-inflammatory effect was observed macroscopically by a significantly lower colonic damage score than that of control rats (P < 0.05), with a significant reduction of the extent of colonic necrosis and/or inflammation (Table 2). However, this anti-inflammatory effect was not associated with a significant reduction of the colonic weight/length ratio between both colitic groups, an index of colonic oedema that is increased significantly as a consequence of the inflammatory process (Table 2).
Table 2.
Effects of treatment with Escherichia coli Nissle 1917 (1 × 109 CFU·day−1 per rat) on macroscopic and microscopic damage score, changes in colon weight/length ratio and colonic myeloperoxidase (MPO) activity in trinitrobenzenesulphonic acid (TNBS) experimental colitis in rats
| Group (n =10) | Macroscopic score (0–10) | Weight/Length (mg·cm−1) | MPO (U·g−1 tissue) | Microscopic score (0–30) |
|---|---|---|---|---|
| Non-colitic | 0 | 67.9 ± 3.1 | 38 ± 6 | 0 |
| TNBS control | 7 (5–8) | 246.3 ± 22.6 | 226 ± 23 | 13 (7–21) |
| TNBS probiotic | 6 (4–7)* | 201.7 ± 21.0 | 121 ± 19* | 6 (1–11)* |
Damage score for each rat was assigned according to the criteria described by Bell et al. (1995), and data are expressed as median (range). The microscopic score for each rat was assigned according to the criteria from Table 1, and data are expressed as median (range). Colon weight and MPO activity data are expressed as mean ± SEM.
P < 0.05 versus TNBS control. All colitic groups differ significantly from non-colitic group (P < 0.05, not shown).
Histological assessment of colonic samples from the TNBS control group revealed severe transmural disruption of the normal architecture of the colon, showing epithelial ulceration and inflammation involving all the intestinal layers of the colon, giving a median score (range) value of 13 (7–21) (Figure 1; Table 2). Colonic samples were characterized by severe oedema and diffuse leukocyte infiltration, mainly composed of neutrophils in the mucosal layer and, to a lesser extent, lymphocytes and histiocytes in the submucosa. The inflammatory process was associated with crypt hyperplasia and dilation, and severe goblet cell depletion. Conversely, histological analysis of the colonic specimens from rats treated with the probiotic strain revealed a more pronounced recovery in the intestinal architecture than in TNBS controls, with a median score (range) of 6 (1–11) (P < 0.01 vs. TNBS control group) (Figure 1; Table 2). Thus, most of the samples (9 of 10) showed almost complete restoration of the epithelial cell layer, in contrast to the extensive ulceration observed in untreated animals. The transmural involvement of the lesions was reduced and goblet cell depletion was less severe and, hence, these cells appeared replenished with their mucin content, and no dilated crypts were observed. The improvement in colonic histology was accompanied by a reduction in the inflammatory infiltrate, which was slight to moderate with a patchy distribution, although neutrophils were still the predominant cell type.
Figure 1.
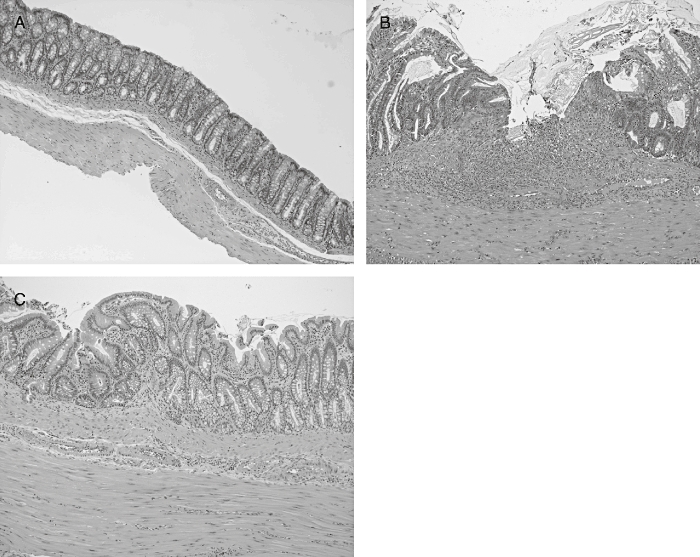
Histological sections of colonic mucosa from colitic rats 1 week after trinitrobenzenesulphonic acid (TNBS) treatment, stained with haematoxylin and eosin. (A) Non-colitic group showing the normal histology of the rat colon (original magnification ×20). (B) TNBS control group showing destruction of the mucosa, which has been replaced by inflammatory granulation tissue. There is evident edema and intense diffuse transmural inflammatory infiltrate (original magnification ×100). (C) Escherichia coli Nissle 1917-treated group showing amelioration in the inflammatory process and ‘restoration’ of the mucosal tissue with the presence of mucin-replenished goblet cells (original magnification ×100).
Biochemically, the preventative beneficial effects showed by the probiotic treatment were exhibited as a reduction of colonic MPO activity, a marker of neutrophil infiltration that was enhanced in the TNBS control group (Table 2), suggesting a lower leukocyte infiltration into the inflamed tissue, and corroborating the results obtained after histological evaluation of the colonic samples. In comparison with non-colitic animals, the colonic inflammation induced by TNBS was characterized by increased levels of colonic TNF-α and LTB4, as well as by a decrease in IL-10 levels (Table 3). The treatment of colitic rats with E. coli Nissle 1917 resulted in a significant reduction of colonic TNF-α, but no significant modification of colonic LTB4 or IL-10 levels was obtained when compared with untreated colitic rats, although a non-significant trend towards increased IL-10 was observed (P= 0.13 vs. colitic control group) (Table 3).
Table 3.
Effects of treatment with Escherichia coli Nissle 1917 (1 × 109 CFU·day−1 per rat) on cytokine [tumour necrosis factor (TNF)-α and IL-10] and leukotriene B4 (LTB4) production in trinitrobenzenesulphonic acid (TNBS) experimental colitis in rats
| Group (n =10) | TNF-α (pg·g−1 tissue) | IL-10 (pg·g−1 tissue) | LTB4(pg·g−1 tissue) |
|---|---|---|---|
| Non-colitic | 365 ± 9 | 937 ± 99 | 2.6 ± 1.8 |
| TNBS control | 1322 ± 124 | 418 ± 56 | 9.9 ± 1.8 |
| TNBS probiotic | 870 ± 93* | 516 ± 14 | 10.2 ± 0.8 |
All data are expressed as mean ± SEM.
P < 0.05 versus TNBS control. All colitic groups differ significantly from non-colitic group (P < 0.05, not shown).
Preventative effects of E. coli Nissle 1917 in the LPS-induced model of septic shock in mice
Treatment of mice with the probiotic E. coli for 2 weeks before induction of septic shock did not affect body weight gain compared with untreated groups (data not shown), and no sign of toxicity was observed, as described for rats above. After 2 weeks of probiotic consumption, the mice received an intraperitoneal injection of saline or a sub-lethal dose of 20 mg·kg−1 of LPS. The mice showed evident symptoms of endotoxic shock, including decreased motor activity, ruffled fur and ocular exudates, within 8 h, as previously described (Leite et al., 2005). At that time, mice pretreated with the probiotic strain showed a better general appearance and motor activity, although signs of illness were still evident when compared with the healthy ones. All mice survived 24 h after LPS injection, when they were killed. Macroscopic tissue modifications were observed as a consequence of the septic shock. Significant increases in colon weight/length ratio, as well as in spleen and lung weights, were observed in the LPS control group when compared with healthy mice (P < 0.05; Table 4), whereas no significant modification was observed in the weights of liver and kidneys (Table 4). The probiotic treatment resulted in a significant reduction in spleen and lung weights to values not different from those in the healthy group; however, colonic weight was not significantly affected by probiotic administration (Table 4).
Table 4.
Effects of treatment with Escherichia coli Nissle 1917 on tissue weights in lipopolysaccharide (LPS)-induced septic shock in mice
| Group | Liver (mg·g−1 mice) | Kidneys (mg·g−1 mice) | Colon weight/length (mg·cm−1) | Spleen (mg·g−1 mice) | Lungs (mg·g−1 mice) |
|---|---|---|---|---|---|
| Healthy | 52.2 ± 0.9 | 19.6 ± 0.9 | 24.1 ± 0.8 | 3.7 ± 0.1 | 14.6 ± 0.8 |
| Control LPS | 54.1 ± 4.7 | 20.7 ± 0.4 | 28.4 ± 1.8# | 4.4 ± 0.1# | 18.6 ± 0.9# |
| E. coli Nissle 1917 | 51.1 ± 2.0 | 19.9 ± 0.6 | 29.2 ± 1.1# | 3.7 ± 0.2* | 13.7 ± 1.8* |
All data are expressed as mean ± SEM (n= 10).
P < 0.05 versus control;
P < 0.05 versus healthy group.
Biochemically, pretreatment with E. coli Nissle 1917 also produced beneficial effects in this experimental model of septic shock. TNF-α levels were significantly increased 24 h after i.p. administration of LPS to mice, both in plasma and in lungs, in comparison with normal mice. The group of mice receiving E. coli Nissle 1917 for 2 weeks prior to LPS injection showed a significant reduction in TNF-α production when compared with untreated control mice (Figure 2). Sepsis promotes the activation and migration of leukocytes into various organs (Annane et al., 2005). The most common event is the infiltration of activated neutrophils into lung tissue (Welbourn and Young, 1992), which can also occur in the large intestine, as found in the present study. Thus, a higher neutrophil influx was observed both in the lungs and in the colon of control LPS-treated mice, as shown by the 10- and 5-fold increases, respectively, in MPO activities in comparison with healthy animals (P < 0.05) (Table 5). In both organs, administration of the probiotic E. coli partially reversed the increased enzyme activity observed in the LPS control mice (P < 0.05; Table 5).
Figure 2.
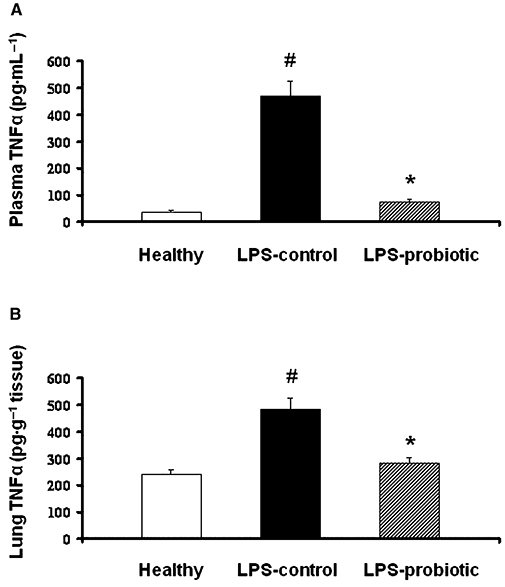
Effect of Escherichia coli Nissle 1917 on tumour necrosis factor-α production in lipopolysaccharide-induced septic shock in mice. Probiotic pretreatment inhibited production of this cytokine in plasma (A) and lungs (B). The concentrations (means ± SEM) of the cytokine were analysed by enzyme-linked immunosorbent assay (n= 10). *P < 0.05 versus control group; #P < 0.05 versus healthy group.
Table 5.
Effects treatment with Escherichia coli Nissle 1917 on lung and colonic myeloperoxidase (MPO) activity and hepatic glutathione (GSH) content in lipopolysaccharide-induced septic shock in mice
| Group (n =10) | Colonic MPO activity (U·g−1 tissue) | Lung MPO activity (U·g−1 tissue) | Hepatic GSH content (nmol·g−1 tissue) |
|---|---|---|---|
| Healthy | 8.1 ± 1.9 | 7.3 ± 0.8 | 7098 ± 455 |
| Control | 40.2 ± 7.0 | 85.3 ± 2.3 | 2266 ± 218 |
| E. coli Nissle 1917 | 24.3 ± 5.2* | 62.1 ± 5.8* | 3240 ± 287* |
All data are expressed as mean ± SEM (n= 10).
P < 0.05 versus control; all septic groups differ significantly from non-colitic group (P < 0.05, not shown).
When we assessed the overproduction of free radicals due to the systemic oxidative stress induced after LPS administration, we observed a significant decrease in hepatic glutathione levels in control LPS mice (P < 0.01 vs. healthy group; Table 5). The hepatic levels of glutathione were significantly increased in the group of mice receiving the probiotic strain (P < 0.05 vs. control LPS group; Table 5). Furthermore, previous studies have reported that an excessive production of NO, most probably derived from LPS-dependent induction of the inducible isoform of NO synthase (iNOS), plays an important role in septic shock (Cuzzocrea et al., 2006). Such induction was confirmed in the present study, as colon iNOS expression was increased in control LPS mice and was down-regulated after pretreatment of LPS mice with the probiotic E. coli (Figure 3).
Figure 3.
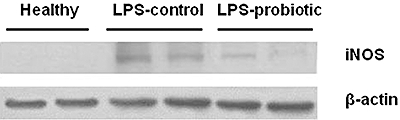
Effect of Escherichia coli Nissle 1917 on colonic inducible NO synthase (iNOS) expression in lipopolysaccharide-induced septic shock in mice. iNOS expression was analysed by Western blot using tissue homogenates as described in Methods; 150 µg of protein was loaded in each lane. β-Actin expression was used as control for loading and transfer.
The expression of different Th1 and Th2 cytokines (IL-2 and IL-5, respectively), the anti-inflammatory cytokine IL-10 in Con A-stimulated splenocytes and IgG production in LPS-stimulated splenocytes were analysed by ELISA, in order to evaluate the effects of the probiotic treatment on altered immunological response after LPS administration. The results revealed that Con A-stimulated cytokine production was altered in the splenocytes from septic mice (Figure 4). In this regard, a clearly increased expression of IL-2 and IL-5 was observed in control LPS mice in comparison with healthy ones, while a decreased expression of the anti-inflammatory cytokine IL-10 was detected. Probiotic treatment significantly reduced the expression of the pro-inflammatory cytokines and increased that of IL-10. Similarly, LPS-stimulated splenocytes from control mice with LPS-induced shock showed increased production of IgG in comparison with those from healthy mice, which was significantly reduced in the group of mice receiving the probiotic E. coli for 2 weeks, down to the levels observed in healthy mice (Figure 5).
Figure 4.
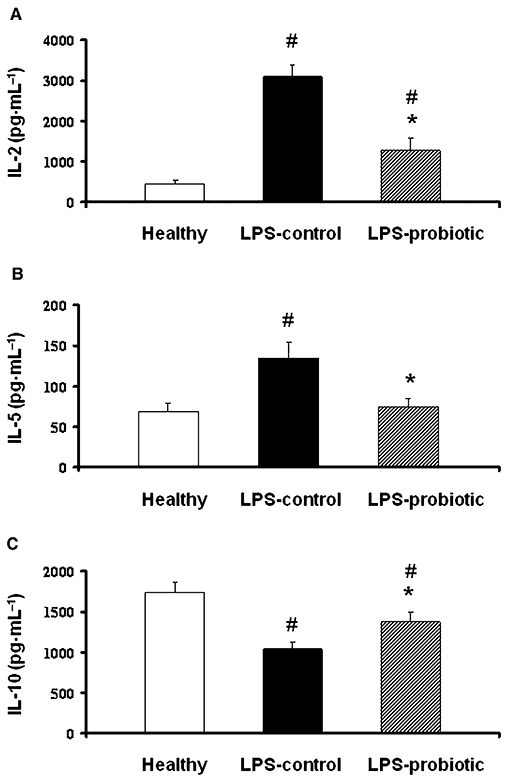
Effects of Escherichia coli Nissle 1917 on the secretion of the cytokines IL-2, IL-5 and IL-10 in Concanavalin (Con A)-activated splenocytes obtained from mice after lipopolysaccharide-induced septic shock. Splenocytes were incubated with Con A (5 µg·mL−1) for 48 h and the concentration (means ± SEM) of the cytokine in the supernatant was analysed by enzyme-linked immunosorbent assay (n= 10). *P < 0.05 versus control group; #P < 0.05 versus healthy group.
Figure 5.
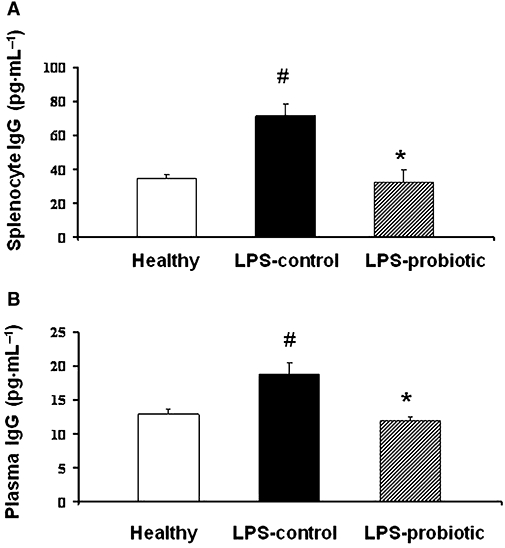
Effects of treatment with Escherichia coli Nissle 1917 on IgG secretion in lipopolysaccharide (LPS)-stimulated splenocytes (A) and plasma (B) from mice with LPS-induced septic shock. Splenocytes were incubated with LPS (50 µg·mL−1) for 72 h and the concentration (means ± SEM) of IgG in the supernatant was analysed by enzyme-linked immunosorbent assay (n= 10). *P < 0.05 versus control group; #P < 0.05 versus healthy group.
Furthermore, plasma IgG production was also increased as a result of the LPS-induced septic shock, which was completely prevented by pretreatment with E. coli Nissle 1917 (Figure 5).
Discussion
As recommended by FAO/WHO, probiotic bacteria can be defined as live microorganisms which, when administered in adequate amounts, confer a health benefit on the host. The present study supports the proposed idea that, although these microorganisms may exert their beneficial effects due to their well-known ability to modulate intestinal microbiota, these effects are not restricted to the gastrointestinal tract. Considering this, we have selected the probiotic strain E. coli Nissle 1917 which has shown efficacy in different intestinal conditions, including diarrhoea (Henker et al., 2008), diverticulitis (Fric and Zavoral, 2003) and IBD (Schultz, 2008). These intestinal anti-inflammatory effects have been confirmed in the present study as the oral administration of E. coli Nissle 1917 to rats was able to ameliorate the colonic damage induced by TNBS, shown both histologically and biochemically, and as previously reported for this probiotic strain in the dextran sodium sulphate (DSS) experimental model of intestinal inflammation in mice (Schultz et al., 2004; Grabig et al., 2006). The beneficial effect shown in these experimental models of colitis was associated with the inhibition of the production of pro-inflammatory cytokines, such as TNF-α (TNBS colitis) or IFN-γ and IL-6 (DSS colitis), which have been postulated to play a key role in IBD. Comparable conclusions have been obtained with other probiotic microorganisms, including lactobacilli like Lactobacillus fermentum and yeasts like Saccharomyces boulardii, in the same model of rat colitis (Peran et al., 2006; Lee et al., 2008).
Once we had confirmed the ability of this probiotic E. coli to down-regulate an increased immune response in the intestine, and in order to evaluate its beneficial effect in a condition with systemic altered immune responses, we assayed the same probiotic treatment in an experimental model of sepsis induced in mice by i.p. administration of LPS. Sepsis is a generalized, exacerbated and not properly regulated inflammatory response to bacterial translocation, which frequently appears as a secondary complication to a marked depression of the cell-mediated immune response, as in patients suffering from severe trauma (Ayala et al., 1994a). The exact mechanisms involved in this form of immune dysregulation are not completely understood, although the release of LPS from bacteria and its recognition by host cells are generally thought to be the initial events in the development of sepsis, thus triggering the inflammatory reaction that may subsequently result in an enhanced generation of inflammatory mediators, including cytokines, chemokines, adhesion molecules, reactive oxygen and nitrogen species, from activated immune cells like macrophages and polymorphonuclear cells (Liu and Malik, 2006), comparable to that observed in intestinal inflammation (Fiocchi, 1998). In this setting, the probiotic E. coli Nissle 1917 can be of particular interest as, in addition to its immunodulatory properties, it interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens (Altenhoefer et al., 2004), thus preventing their access to the systemic circulation.
Many studies have attributed a critical role to TNF-α in the development of LPS-mediated shock in mice (i.e. an excess of this cytokine promotes the major alterations observed during septic shock, including vasodilatation, impaired coagulation and fibrinolysis) (Annane et al., 2005). Simultaneously, anti-inflammatory pathways are also activated, leading to the release of anti-inflammatory cytokines, including IL-10, that serve as counter-regulatory mechanisms to dampen the inflammatory response. However, there is a clear predominance of the inflammatory response, leading to the development of septic shock (Liu and Malik, 2006).
The results obtained in this study clearly reveal that mice treated with the probiotic E. coli Nissle 1917 noticeably improved the altered immune response and decreased the production of inflammatory mediators associated with endotoxic shock induced by LPS. Thus, the probiotic strain was able to downregulate the increased levels of TNF-α both in plasma and lungs. This effect was dose dependent, which was shown after performing additional experiments with different doses of the probiotic E. coli (106–109 CFU per mice) (data not shown), in accordance with the results of Hockertz (1997). The ability of this probiotic bacterium to reduce TNF-α production in inflammatory conditions was demonstrated in the present study when assayed in the TNBS model of rat colitis or when it was evaluated in the DSS-induced colitis in mice (Grabig et al., 2006).
As observed in the TNBS colitis model, which is considered to display a Th1 cytokine profile, or in the DSS colitis model, with a Th1 as well as Th2 cytokine profile (Strober et al., 2002), the beneficial effects showed by this strain of E. coli can be derived from an improvement in the altered immune response induced by LPS. The onset of sepsis is characterized by hyperactivation of the inflammatory cascade, in which T-cell activation and proliferation play a key role. Activated T cells are programmed to secrete either cytokines with inflammatory properties, including TNF-α, IFN-γ, IL-2 (from type 1 helper T-cell), IL-4 and IL-5 (from type 2 helper T-cell) or cytokines with anti-inflammatory properties, like IL-10 (Hotchkiss and Karl, 2003). Consequently, the altered immune response induced in mice after i.p. injection of LPS was shown by an increased production of IL-2 in Con A-stimulated splenocytes, similarly to that previously described in this experimental model of septic shock (Kim et al., 2003). The probiotic treatment reduced the production of this cytokine, suggesting that splenic T cells are part of the mechanism by which it exerts the beneficial effect, in accordance with previous observations reported for different probiotic treatments in the experimental TNBS model of rat colitis (Damaskos and Kolios, 2008), which represents an experimental model of T cell-mediated intestinal inflammation (Strober et al., 2002).
The expression of the Th2 cytokines has been also reported to be up-regulated in mice with polymicrobial sepsis (Ayala et al., 1994b). In this regard, in the present study, IL-5 expression was increased in Con A-stimulated splenocytes, whereas probiotic treatment down-regulated its expression. The concomitant production of anti-inflammatory cytokines such as IL-10 is an attempt to counterbalance the actions of pro-inflammatory cytokines, after LPS administration (Haveman et al., 1999). In fact, experimental studies have revealed that the primary inducers of IL-10 synthesis are pro-inflammatory cytokines, such as TNF-α and IL-1β, and once released it can suppress the production of TNF-α and IL-6 (Oberholzer et al., 2002). When Con A-stimulated production of IL-10 was evaluated in mouse splenocytes, a decreased production was observed in cells from control LPS mice, compared to the healthy group. This depicts the failure of this anti-inflammatory cytokine to control systemic inflammatory situation following the up-regulated release of pro-inflammatory cytokines, including TNF-α. Probiotic treatment of LPS mice resulted in a significantly increased production of IL-10 which contributed to the down-regulation of pro-inflammatory cytokines, including TNF-α. Previous ex vivo experiments have revealed that E. coli Nissle 1917-conditioned medium exerts differential effects on T cells depending on their origin (i.e. circulating or tissue-bound) (Sturm et al., 2005), which may explain the beneficial activity of the probiotic strain against both intestinal inflammation and septic shock. These observations are in accordance with the results obtained in the present study in Con A-stimulated splenocytes from mice with LPS-induced septic shock treated with this probiotic E. coli.
In addition to T-cell activation, B cells, through the production of immunoglobulins (Igs), have been also involved in septic shock (Arad et al., 2004). Thus, the LPS- induced septic shock in mice is associated with a significant increase of IgG antibodies, reflecting the presence of a second host defence system that attempts to eliminate harmful antigens. The pretreatment with E. coli Nissle 1917 resulted in a decreased production of IgG, both in splenocytes and in plasma, thus confirming the immunomodulatory effects observed with this probiotic strain.
The consequences of the immunomodulatory actions of E. coli Nissle 1917 in septic mice and in colitic rats are the amelioration of the tissue damage induced by LPS or TNBS respectively. Thus, TNF-α overproduction promotes polymorphonuclear leukocyte sequestration and activation that, in turn, damages the different organs, including lungs (Ito et al., 2006) and colon (Naito et al., 2007). This was confirmed in our study as MPO activity was increased in the lungs and colons from mice with LPS-induced septic shock and in the colon of colitic rats when compared to healthy animals, as described previously (Sener et al., 2005; Bailón et al., 2007). However, in those groups treated with the probiotic strain, the reduction of TNF-α content in the different organs was associated with a lower MPO activity in comparison with the untreated, control LPS group. This suggests that the probiotic treatment was able to reduce the neutrophil infiltration that takes place in the lungs or in colonic specimens, thus preventing, at least partially, the deleterious effects in the infiltrated organs.
In conclusion, we have demonstrated that the immunomodulatory properties of E. coli Nissle 1917 protect against TNBS-induced colitis in rats and LPS-induced organ damage in mice. Therefore, the anti-inflammatory effects of this probiotic E. coli were not restricted to the gastrointestinal tract.
Acknowledgments
The authors want to thank Shanita Kara for the English correction of the manuscript. This study was supported by the Spanish Ministry of Science and Technology (SAF2005-03199 and SAF2008-02616), with funds from the European Union, by Junta de Andalucia (CTS 164), and by Casen Fleet S.L.U. M Comalada is a recipient of the Ramon y Cajal Program from the Spanish Ministry of Science and Innovation; E Bailón and D Camuesco are recipients of support from the Spanish Ministry of Science and Innovation. CIBER-EHD is funded by the Instituto de Salud Carlos III.
Glossary
Abbreviations:
- CFU
colony-forming unit
- iNOS
inducible NO synthase
- Ig
immunoglobulin
- LTB4
leukotriene B4
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- TNBS
trinitrobenzenesulphonic acid
Conflicts of interest
None.
References
- Altenhoefer A, Oswald S, Sonnenborn U, Enders C, Schulze J, Hacker J, et al. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol Med Microbiol. 2004;40:223–229. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- Arad G, Hillman D, Levy R, Kaempfer R. Broad-spectrum immunity against superantigens is elicited in mice protected from lethal shock by a superantigen antagonist peptide. Immunol Lett. 2004;91:141–145. doi: 10.1016/j.imlet.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ayala A, Lehman DL, Herdon CD, Chaudry IH. Mechanism of enhanced susceptibility to sepsis following hemorrhage. Interleukin-10 suppression of T-cell response is mediated by eicosanoid-induced interleukin-4 release. Arch Surg. 1994a;129:1172–1178. doi: 10.1001/archsurg.1994.01420350070009. [DOI] [PubMed] [Google Scholar]
- Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-gamma release while increasing IL-4/IL-10 production. J Surg Res. 1994b;56:579–585. doi: 10.1006/jsre.1994.1092. [DOI] [PubMed] [Google Scholar]
- Baharav E, Mor F, Halpern M, Weinberger A. Lactobacillus GG bacteria ameliorate arthritis in Lewis rats. J Nutr. 2004;134:1964–1969. doi: 10.1093/jn/134.8.1964. [DOI] [PubMed] [Google Scholar]
- Bailón E, Camuesco D, Nieto A, Concha A, Fernández de Arriba A, Román J, et al. The intestinal anti-inflammatory effects of the novel agent UR-1505 in the TNBS model of rat colitis are mediated by T-lymphocyte inhibition. Biochem Pharmacol. 2007;74:1496–1506. doi: 10.1016/j.bcp.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol. 1995;268:G622–G630. doi: 10.1152/ajpgi.1995.268.4.G622. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122. doi: 10.1111/j.1572-0241.2001.03756.x. [DOI] [PubMed] [Google Scholar]
- Boudeau J, Glasser AL, Julien S, Colombel JF, Darfeuille-Michaud A. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn's disease. Aliment Pharmacol Ther. 2003;18:45–56. doi: 10.1046/j.1365-2036.2003.01638.x. [DOI] [PubMed] [Google Scholar]
- Camuesco D, Comalada M, Rodriguez-Cabezas ME, Nieto A, Lorente MD, Concha A, et al. The intestinal anti- inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol. 2004;143:908–918. doi: 10.1038/sj.bjp.0705941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J, et al. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35:584–592. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Di Paola R, Esposito E, Macarthur H, Matuschak GM, et al. A role for nitric oxide-mediated peroxynitrite formation in a model of endotoxin-induced shock. J Pharmacol Exp Ther. 2006;319:73–81. doi: 10.1124/jpet.106.108100. [DOI] [PubMed] [Google Scholar]
- Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: microflora ‘on the scope’. Br J Clin Pharmacol. 2008;65:453–467. doi: 10.1111/j.1365-2125.2008.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Fric P, Zavoral M. The effect of non-pathogenic Escherichia coli in symptomatic uncomplicated diverticular disease of the colon. Eur J Gastroenterol Hepatol. 2003;15:313–315. doi: 10.1097/01.meg.0000049998.68425.e2. [DOI] [PubMed] [Google Scholar]
- Grabig A, Paclik D, Guzy C, Dankof A, Baumgart DC, Erckenbrecht J, et al. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect Immun. 2006;74:4075–4082. doi: 10.1128/IAI.01449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov L, Zahringer U, Blum-Oehler G, Brade L, Henne A, Knirel YA, et al. A single-nucleotide exchange in the wzy gene is responsible for the semi-rough O6 LPS phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J Bacteriol. 2002;184:5912–5925. doi: 10.1128/JB.184.21.5912-5925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haveman JW, Muller Kobold AC, Tervaert JW, van den Berg AP, Tulleken JE, Kallenberg CG, et al. The central role of monocytes in the pathogenesis of sepsis: consequences for immunomonitoring and treatment. Neth J Med. 1999;55:132–141. doi: 10.1016/s0300-2977(98)00156-9. [DOI] [PubMed] [Google Scholar]
- Henker J, Laass MW, Blokhin BM, Maydannik VG, Bolbot YK, Elze M, et al. Probiotic Escherichia coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. Pediatr Infect Dis J. 2008;27:494–499. doi: 10.1097/INF.0b013e318169034c. [DOI] [PubMed] [Google Scholar]
- Hockertz S. Augmentation of host defence against bacterial and fungal infections of mice pretreated with the non-pathogenic Escherichia coli strain Nissle 1917. Arzneimittelforschung. 1997;47:793–796. [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Isolauri E, Arvola T, Sütas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604–1610. doi: 10.1046/j.1365-2222.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- Ito H, Koide N, Hassan F, Islam S, Tumurkhuu G, Mori I, et al. Lethal endotoxic shock using alpha-galactosylceramide sensitization as a new experimental model of septic shock. Lab Invest. 2006;86:254–261. doi: 10.1038/labinvest.3700388. [DOI] [PubMed] [Google Scholar]
- Kim GY, Roh SI, Park SK, Ahn SC, Oh YH, Lee JD, et al. Alleviation of experimental septic shock in mice by acidic polysaccharide isolated from the medicinal mushroom Phellinus linteus. Biol Pharm Bull. 2003;26:1418–1423. doi: 10.1248/bpb.26.1418. [DOI] [PubMed] [Google Scholar]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kim YW, Chi SG, Joo YS, Kim HJ. The effect of Saccharomyces boulardii on human colon cells and inflammation in rats with trinitrobenzene sulfonic acid-induced colitis. Dig Dis Sci. 2008;54:255–263. doi: 10.1007/s10620-008-0357-0. [DOI] [PubMed] [Google Scholar]
- Leite MS, Pacheco P, Gomes RN, Guedes AT, Castro-Faria-Neto HC, Bozza PT, et al. Mechanisms of increased survival after lipopolysaccharide-induced endotoxic shock in mice consuming olive oil-enriched diet. Shock. 2005;23:173–178. doi: 10.1097/01.shk.0000148072.12094.77. [DOI] [PubMed] [Google Scholar]
- Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge W, Depew W, Szewczuk MR, Wallace JL. Hapten induced model of chronic inflammation and ulceration in rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Naito Y, Takagi T, Yoshikawa T. Molecular fingerprints of neutrophil-dependent oxidative stress in inflammatory bowel disease. J Gastroenterol. 2007;42:787–798. doi: 10.1007/s00535-007-2096-y. [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30:S58–S63. [PubMed] [Google Scholar]
- Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Adrio JL, et al. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int J Colorectal Dis. 2006;21:737–746. doi: 10.1007/s00384-005-0773-y. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003;111:389–395. doi: 10.1067/mai.2003.389. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- Schultz M, Strauch UG, Linde HJ, Watzl S, Obermeier F, Göttl C, et al. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol. 2004;11:372–378. doi: 10.1128/CDLI.11.2.372-378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener G, Toklu H, Kapucu C, Ercan F, Erkanli G, Kaçmaz A, et al. Melatonin protects against oxidative organ injury in a rat model of sepsis. Surg Today. 2005;35:52–59. doi: 10.1007/s00595-004-2879-1. [DOI] [PubMed] [Google Scholar]
- Sheil B, McCarthy J, O'Mahony L, Bennett MW, Ryan P, Fitzgibbon JJ, et al. Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut. 2004;53:694–700. doi: 10.1136/gut.2003.027789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Sturm A, Rilling K, Baumgart DC, Gargas K, Abou-Ghazalé T, Raupach B, et al. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling. Infect Immun. 2005;73:1452–1465. doi: 10.1128/IAI.73.3.1452-1465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE. 2007;2(12):e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Harder J, Wehkamp K, Wehkamp-Von Meissner B, Schlee M, Enders C, et al. NF-κB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbourn CR, Young Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br J Surg. 1992;79:998–1003. doi: 10.1002/bjs.1800791006. [DOI] [PubMed] [Google Scholar]
- Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]


