Abstract
Background and purpose:
The effect of age on the distribution of morphine and morphine-3-glucuronide (M3G) across the blood–brain barrier (BBB) was studied in a sheep model utilizing intracerebral microdialysis. The effect of neonatal asphyxia on brain drug distribution was also studied.
Experimental approach:
Microdialysis probes were inserted into the cortex, striatum and blood of 11 lambs (127 gestation days) and six ewes. Morphine, 1 mg·kg−1, was intravenously administered as a 10 min constant infusion. Microdialysis and blood samples were collected for up to 360 min and analysed using liquid chromatography-tandem mass spectrometry. The half-life, clearance, volume of distribution, unbound drug brain : blood distribution ratio (Kp,uu) and unbound drug volume of distribution in brain (Vu,brain) were estimated.
Key results:
Morphine Kp,uu was 1.19 and 1.89 for the sheep and premature lambs, respectively, indicating that active influx into the brain decreases with age. Induced asphyxia did not affect transport of morphine or M3G across the BBB. Morphine Vu,brain measurements were higher in sheep than in premature lambs. The M3G Kp,uu values were 0.27 and 0.17 in sheep and premature lambs, indicating a net efflux from the brain in both groups.
Conclusions and implications:
The morphine Kp,uu was above unity, indicating active transport into the brain; influx was significantly higher in premature lambs than in adult sheep. These results in sheep differ from those in humans, rats, mice and pigs where a net efflux of morphine from the brain is observed.
Keywords: microdialysis, morphine, active efflux, active influx, blood–brain barrier
Introduction
In neonatal intensive care, morphine is used for analgesia and sedation. Dosage recommendations at our clinical institutions for administration of morphine differ according to age; children, and newborns in particular, are known to need lower dosages than adults, suggesting that the pharmacokinetics/pharmacodynamics of morphine differ with the age of the patient.
Morphine has affinity for µ-opiod receptors and must enter the CNS to exert the greater part of its analgesic and sedative effect. To enter the CNS, the drug must pass the blood–brain barrier (BBB), which consists of endothelial cells connected by tight junctions. These tight junctions restrict diffusion across the BBB of water-soluble substances. In addition, active transporters on the luminal and abluminal sides of the BBB are able to transport molecules in both directions against the concentration gradients. For example, both P-glycoprotein (Pgp) and probenecid-sensitive transporters can actively transport morphine (Xie et al., 1999; Tunblad et al., 2003). Morphine is actively transported out of the brains of adult humans (Ederoth et al., 2004), rats (Bengtsson et al., 2008), mice (Xie et al., 1999) and pigs (Tunblad et al., 2004). However, although the net effect is that of efflux, there are indications that some active transport into the brains of mice (Xie et al., 1999) and rats also occurs (Groenendaal et al., 2007).
Some age-related differences in the pharmacokinetics of morphine have been demonstrated. For example, the serum clearance (CL) of morphine in newborn and infant humans is lower than that in adults (Scott et al., 1999; Saarenmaa et al., 2000). In rats, the extent of BBB morphine transport, measured as the brain-to-plasma ratio (Kp), in newborn rats is threefold higher during the first 10 days postpartum than in 30-day-old rats (Auguy-Valette et al., 1978). It thus appears that BBB permeability (i.e. the net sum of diffusion and active transport) decreases with increasing age.
Only unbound drug is able to cross the BBB to interact with the receptor. Microdialysis has allowed measurement of the concentrations of unbound morphine on both sides of the BBB. The ratio of unbound drug in brain interstitial fluid to that in blood, expressed as Kp,uu, can be calculated by comparing the concentrations over time of unbound morphine in the interstitial fluid (ISF) of the brain with the corresponding concentrations of unbound morphine in the blood (Gupta et al., 2006). A Kp,uu value of 0.64 has been reported in the adult human brain after trauma (Ederoth et al., 2004), while 0.49, 0.3 and 0.47 have been reported for rats (Bengtsson et al., 2008), mice (Xie et al., 1999) and pigs (Tunblad et al., 2004) respectively. As mentioned earlier, this indicates that morphine is actively transported from the brain to the blood across the BBB in adults of these species.
Reduction in the permeability of the BBB with age, that is, continuing development of the tight junctions and/or active transporters after birth, could be contributing to the lower dosages of morphine required for newborns as compared with adults. In addition, the neonatal asphyxia experienced by some newborns in neonatal intensive care units can induce hypoxic ischemic encephalopathy. The effect of this clinically important condition on the pharmacokinetics of morphine across the BBB has not been well studied.
The aim of this study was therefore to characterize the effect of age on the distribution of morphine and its metabolites morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) across the BBB. A secondary aim was to investigate whether experimentally induced asphyxia could influence the distribution of morphine in the brain. Microdialysis was used to characterize the pharmacokinetics of morphine transport across the BBB in premature lambs and adult sheep.
Methods
Animals
Animal care and experimental protocols were approved by the Ethical Committee for Laboratory Animal Experiments at the Medical Faculty of Lund University (M 223-02 and M 136-06). Eleven lambs (mixed gender) aged 127 gestation days (term 145 days) and six female adult (5-year-old) sheep were included in the study. The animals were of mixed breed and non-fasting. The average weights (SD) of the lambs and adults were 3.1 (0.7) kg and 77.5 (12.1) kg respectively.
Surgery
The lambs were prepared as previously described (Markus et al., 2007). Briefly, the pregnant ewes were intubated after induction of anaesthesia with ketamine and thiopenthal i.v. Cesarean section was performed during isoflurane anaesthesia supplemented with remifentanil infusion and the lambs were prepared in utero. Catheters were placed in the axillary artery and in the jugular vein for baseline blood sampling. Via separate drill holes in the skull, two CMA/70 microdialysis probes with a 10 mm microdialysis membrane and 20 kDa cut-off (CMA Microdialysis, Stockholm, Sweden) were inserted into the superficial brain tissue (as this was mainly into cortex tissue, this probe is hereafter referred to as the cortex probe) and deep into the striatum. An endotracheal tube was inserted by tracheotomy.
In the group used to investigate the effects of asphyxia, the following procedure was undertaken. The umbilical cord of the in utero foetus was ligated. An additional catheter was then positioned in the foetal aorta via the umbilical artery and used for continuous monitoring of foetal mean arterial blood pressure. The foetuses were delivered 8 min after cord ligation, and were then weighed, sedated and connected to a pressure regulated ventilator (Servo 900 C, Siemens-Elema, Solna, Sweden). The inspiratory pressure was adjusted to achieve a PaCO2 of 4.5–6 kPa and the oxygen gas mixture was adjusted to achieve normoxaemia, targeting a PaO2 of 7–9 kPa and a percutaneous oxygen saturation between 90% and 95%. If respiratory problems developed, the lambs were treated with intratracheally administered surfactant (100 mg·kg−1) and/or inhaled nitric oxide (NO) distributed via the ventilator. Lambs in the control group were prepared in the same way but without induced asphyxia.
After delivery of the lambs, anaesthesia and analgesia were obtained with an initial bolus dose of 10 µg of fentanyl i.v. followed by a continuous i.v. infusion of fentanyl at 10 µg·kg−1·h−1. The lambs were treated in an open incubator and the body temperature was kept at 38.5°C. An isotonic crystalloid solution (Ringer-Acetate) and a glucose solution were infused throughout the experiment; flow rates were adjusted according to arterial blood pressure and blood glucose levels respectively.
The adult, non-pregnant sheep were anaesthetized with ketamine and thiopental. Endotracheal intubation was performed and the animals were ventilated using a Servo 900C with the same respiratory goals as for the lambs. Anaesthesia was maintained by intravenous infusion of fentanyl (initially 20 µg·kg−1·h−1) and thiopental (initially 10 mg·kg−1·h−1) with dose adjustment when necessary. The same isotonic crystalloid and glucose solutions as those used for the lambs were infused throughout the study with flow rates adjusted according to arterial blood pressure and blood glucose levels respectively. Two CMA/70 microdialysis probes with a 10 mm membrane and 20 kDa cut-off were inserted into the left and right frontal superficial brain tissues (mainly cortex) via separate drill holes and one CMA/70 microdialysis probe was inserted into the jugular vein. Catheters were placed in an artery in the ear or the front leg for blood sampling and measurements of blood pressure and heart rate. The arterial blood pressure, heart rate, and blood gas and glucose levels were analysed as for the lambs.
Experimental set-up
The microdialysis probes were perfused at 1 µL·min−1 with Perfusion fluid CNS using a CMA/100 perfusion pump (CMA Microdialysis, Stockholm, Sweden). The perfusion fluid contained deuterated (D3-) morphine, D3-M3G and D3-M6G at a concentration of 50 ng·mL−1 for continuous estimation of the relative recovery of morphine, M3G and M6G in the microdialysis probes (Bengtsson et al., 2008). The animals were given 1 mg·kg−1 morphine as a 10 min constant intravenous infusion using a Teufusion Syringe pump (Terumo STC-521, Terumo Inc., Tokyo, Japan) at least 90 min after probe insertion. Dialysate samples were collected using a micro fraction collector CMA/140 (CMA Microdialysis, Stockholm, Sweden) every 5 min for 30 min and then every 10 min throughout the study. The dialysate samples were frozen and kept at −20°C until analysis. Arterial blood samples of 0.5 mL (premature lamb) and 4 mL (adult sheep) were collected in 5 mL of Becton-Dickinson Na-Heparin 143 U.S.P at 0, 2.5, 7.5, 10, 12.5, 17.5, 22.5, 35, 55, 85, 115, 175 and 235 min. Blood samples were also collected from the adult sheep at 295 and 355 min. The plasma was separated by centrifugation for 10 min (630×g) and frozen (−20°C) until analysis of drug concentrations. The lambs were studied over 4 h and the adult sheep over 6 h. After the study, the animals were killed by i.v. thiopental in ethanol and the brain was immediately removed, frozen on dry ice (−70°C) and stored at −20°C pending analysis of morphine and metabolites in the brain tissue. Removal of the brain took less than 15 min.
Chemical analysis
Plasma and microdialysis samples were analysed by liquid chromatography followed by detection with a tandem mass spectrometer (LC-MS/MS) using electro-spray ionization (Bengtsson et al., 2005). Briefly, 5 µL of the microdialysate was directly injected onto the system. For the plasma samples, 100 µL were precipitated with 200 µL acetonitrile (ACN) containing deuterated analogues. The sample was vortexed and centrifuged. Thereafter, 50 µL of the supernatant was evaporated under N2 at 45°C. The residue was dissolved in 200 µL 0.05% trifluoro acetic acid (TFA) for the premature lambs and 0.02% TFA for the adult sheep and transferred to a polypropylene autosampler vial. The plasma sample injection volume was 10 µL. The samples were kept at 10°C in the autosampler. The chromatography system consisted of a pump (Shimadzu LC-10AD, Shimadzu Kyoto, Japan), an injector (Triathlon, Spark Holland, The Netherlands) and a detector – a triple quadropole mass spectrometer (Quattro Ultima, Micromass, UK). A column switch system was used to remove salts from the samples. A HyPurity C 18 × 3 mm column (Chrom Tech, Hägersten, Sweden) was used for purification and a ZIC HILIC 50 × 4.6 mm column (SeQuant AB, Umeå, Sweden) was used for the analytical separation. The mobile phase consisted of 70% ACN in 5 mmol·L−1 ammonium acetate. For the purification, 0.05% TFA was used for the premature lambs, while 0.02% was used for analysis of the adult sheep samples. The flow rate was 500 µL·min−1, decreased to 260 µL·min−1 before the sample entered the mass spectrometer. The detector was set in positive ion mode. The parameters included cone gas (N2) at 280 L·h−1 and desolvation gas (N2) at 1180 L·h−1. The source temperature was set at 130°C and the desolvation temperature was set at 400°C. The capillary voltage was 3.00 kV and the cone voltage was 75 V. The transitions were 286.0→152.0 m/z for morphine, 289.0→152.0 m/z for D3-morphine, 462.1→286.0 m/z for M3G and 465.1→289.0 m/z for D3-M3G. The brain samples were homogenized with fivefold volume (w/v) perchloric acid (0.1 mol·L−1) and then centrifuged at 1500×g for 10 min. A slightly modified solid phase extraction method by Joel et al. (Joel et al., 1988) was used to pretreat 100 µL of the supernatant and 50 µL internal standards at a concentration of 25 ng·mL−1. Methanol (3 mL) was used for elution and the eluate was evaporated at 45°C under nitrogen. The residue was redissolved in 200 µL 0.02% TFA and 10 µL were injected onto the system as described above.
Microdialysis probe calibration
D3-morphine, D3-M3G and D3-M6G were added to the perfusion fluid for continuous estimation of the relative in vivo recovery by retrodialysis using a deuterated calibrator (Bengtsson et al., 2008). The recovery from each probe and each microdialysate sample was calculated by
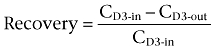 |
(1) |
where CD3-in is the concentration of the deuterated calibrator in the perfusion fluid and CD3-out is the concentration of the deuterated calibrator in the collected dialysate.
The true tissue concentrations of unbound morphine and M3G in blood and brain ISF were calculated by
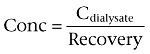 |
(2) |
where Cdialysate is the concentration of morphine or M3G in the microvials and the recovery was calculated using Eq 1. To correctly estimate the concentrations of unbound morphine and M3G in the blood of adult sheep, a moving average (n= 3) of the recovery values was used for each time point, as recovery was found to decrease over time (Bouw and Hammarlund-Udenaes, 1998).
Data analysis
Non-compartmental analysis was used in the calculation of the terminal elimination rate constant (λ2), terminal half-life (t1/2), plasma clearance (CL), volume of distribution (Vβ), Kp,uu and volume of distribution of unbound drug in the brain in mL·g−1 brain (Vu,brain). The elimination rate constant was calculated from the terminal slope (min−1) of morphine and M3G concentration versus time data. Morphine λ2 was estimated from 85 min for plasma and from 75 min for microdialysis data, respectively, for premature lambs. For adult sheep, morphine λ2 was estimated from 115 min for both plasma and microdialysis data. The elimination rate constant for M3G was calculated in adult sheep from 235 min for both plasma and microdialysis data. It was not possible to estimate the half-life of M3G in premature lambs because the blood, plasma and ISF concentrations did not decrease in the studied time frame.
The t1/2 of morphine was calculated as ln(2)/λ2. The CL was calculated as the total dose of morphine divided by the area under the concentration-time curve (AUC), and the AUC was calculated according to the trapezoidal method extrapolated to infinity. The residual area under the morphine concentration-time curve was estimated as Clast/λ2, where Clast is the last observed plasma morphine concentration. Vβ was calculated as CL ×t1/2/ln(2).
The distribution of unbound morphine across the BBB, expressed as Kp,uu, was calculated as
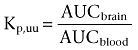 |
(3) |
As the concentration of M3G in the premature lamb brain cortex increased throughout the study, the Kp,uu for this compound was calculated using the concentration of unbound M3G in the cortex and blood at the last sampling time.
The volume of distribution of unbound drug in the brain measures how the substance is distributed within the brain compartment. If a substance is distributed only into the ISF and not into brain parenchymal cells, the Vu,brain is equal to the ISF volume, which is 0.15 mL·g−1 brain for the rat (Goodman et al., 1973) and 0.17–0.20 for rabbits, cats, dogs and monkeys (Levin et al., 1970). The ISF volume does not appear to have been reported for sheep. If a substance is distributed into the brain cells or binds to cellular components, the Vu,brain will be larger than the ISF volume. The Vu,brain values for morphine and M3G were calculated as
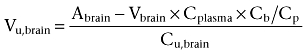 |
(4) |
where Abrain is the total amount of morphine or M3G per gram of cortex (ng·g−1 brain), with the assumption that the cortex was representative of the brain as a whole. Vbrain is the vascular volume (mL·g−1 brain) of the brain tissue, Cplasma is the plasma drug concentration (ng·mL−1), Cb/Cp is the partitioning constant between blood and plasma and Cu,brain is the concentration of unbound drug (ng·mL−1), obtained from the microdialysis experiment. Vbrain has been estimated as 25 µL·g−1 brain tissue in premature lambs at 92 gestation days (Barfield et al., 1999). No value for Vbrain was found in the literature for adult sheep, and the value for premature lambs was consequently used for both groups. The values for Cb/Cp are 1.32 and 0.87 for morphine and M3G, respectively, at a haematocrit of 34% (Milne et al., 1997).
Statistical methods
All values are presented as mean values (SD). Student's t-test was used to compare recovery, CL, Vβ, t1/2, Kp,uu and Vu,brain between adult sheep and control premature lambs. Student's t-test was also used to compare recovery, CL, Vβ, t1/2 and Kp,uu between the two premature groups. A P value <0.05 was considered statistically significant.
Materials
Morphine hydrochloride trihydrate (10 mg·mL−1) and pentobarbital sodium (thiopental) in ethanol (60 mg·mL−1) were obtained from Apoteket AB, Stockholm, Sweden. Ammonium acetate (AmAc), ACN, perchloric acid, ammonium sulphate, phosphoric acid and TFA were purchased from Merck, Darmstadt, Germany. Methanol was purchased from J.T. Baker, Deventer, The Netherlands. Deuterated (D3) morphine hydrochloride, morphine-3-glucuronide (D3-M3G) and morphine-6-glucuronide (D3-M6G) were purchased from Lipomed, Arleshem, Switzerland. Perfusion Fluid CNS, purchased from CMA Microdialysis, Stockholm, Sweden, consisted of 147 mmol·L−1 NaCl, 2.7 mmol·L−1 KCl, 1.2 mmol·L−1 CaCl2 and 0.85 mmol·L−1 MgCl2. Ketamine (50 mg·mL−1) was obtained from Pfizer AB, New York, USA; thiopental (50 mg·mL−1) was purchased from Hospira Enterprises B.V., Hoofddorp, The Netherlands; isoflurane was obtained from Abbot Laboratories, Abbot Park, Illinois, USA; and remifentanil was obtained from GlaxoSmithKline, Philadelphia, USA. Fentanyl (50 µg·mL−1) was obtained from B. Braun Melsungen AG, Melsungen, Germany. Ringer Acetate was purchased from Fresenius Kabi AB, Úppsala, Sweden. Glucose (100 mg·mL−1) and NaCl (0.9%) were obtained from Baxter Medical AB, Kista, Sweden. Sep-Pak® C18 (360 mg cartridges) were supplied by Waters, Milford, MA, USA. Surfactant (Curosurf® 80 mg·mL−1) was purchased from Nycomed Pharma, Roskilde, Denmark.
Results
The physiological variables in lambs and adult sheep are presented in Table 1.The position of the probe (in the striatum or the cortex in lambs, or in the right or left sides of the cortex in adult sheep) had no significant effect on brain morphine or M3G pharmacokinetic results. Data from the right brain cortex probes are therefore presented throughout the paper. The relative recovery of drug using the microdialysis probes are presented in Table 2. The recovery of morphine and M3G in the blood probe in adult sheep decreased over time (Figure 1A,B), while recovery from other areas remained stable throughout the study (Table 2). The moving average for the recovery is presented in Figure 1A,B. It is observed that the change in recovery was probe specific and the same pattern was detected for morphine and M3G. No M6G was detected in brain ISF samples, with a lower limit of quantification of 0.55 ng·mL−1.
Table 1.
Physiological variables for adult sheep and premature lambs
|
Adult sheep |
Premature lambs |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
(n =6) |
Control (n =6) |
Asphyxial (n =5) |
|||||||
| 0 h | 2 h | 4 h | 0 h | 2 h | 4 h | 0 h | 2 h | 4 h | |
| pH | 7.51 (0.06) | 7.51 (0.05) | 7.51 (0.04) | 7.31 (0.08) | 7.23 (0.05) | 7.26 (0.10) | 7.25 (0.12) | 7.27 (0.08) | 7.28 (0.12) |
| PaCO2 (kPa) | 5.5 (0.9) | 5.7 (0.7) | 5.6 (0.7) | 6.1 (1.2) | 7.3 (0.9) | 7.0 (1.6) | 7.2 (2.1) | 6.2 (1.4) | 5.7 (2.0) |
| PaO2 (kPa) | 14.0 (6.2) | 18.8 (12.6) | 19.5 (13.1) | 5.8 (1.8) | 4.5 (2.0) | 11.2 (11.4) | 5.3 (1.6) | 9.0 (8.1) | 15.1 (10.5) |
| BE (mmol·L−1) | – | – | – | −4.2 (3.2) | −5.3 (3.0) | −4.4 (3.0) | −4.7 (4.8) | −6.3 (2.1) | −7.4 (2.1) |
| Hb (g·L−1) | 77 (15) | 80 (14) | 77 (16) | 143 (20) | 133 (11) | 127 (16) | 145 (19) | 134 (23) | 122 (25) |
| SaO2 (%) | 97 (3) | 99 (1) | 99 (2) | 94 (11) | 80 (22) | 97 (6) | 92 (7) | 93 (6) | 99 (1) |
| B-Glu (mmol·L−1) | 7.7 (4.6) | 9.7 (3.8) | 7.6 (1.9) | 5.1 (1.9) | 6.7 (5.9) | 3.8 (1.8) | 4.0 (3.1) | 2.5 (3.0) | 3.8 (1.9) |
| Lactate (mmol·L−1) | – | – | – | 5.6 (1.8) | 7.0 (0.4) | 7.26 (0.10) | 6.1 (2.6) | 6.8 (3.1) | 7.1 (3.4) |
–, not estimated; B-Glu, blood glucose; BE, base excess; Hb, haemoglobin; PaCO2, arterial carbon dioxide pressure; PaO2, arterial oxygen pressure; SaO2, arterial oxygen saturation.
Table 2.
Relative recovery of morphine and M3G from microdialysis probes in sheep and lambs
|
Adult sheep |
Premature lambs |
||
|---|---|---|---|
| (n =6) | Control (n =6) | Asphyxial (n =5) | |
| Morphine | |||
| Blood | 0.71 (0.10)–0.45 (0.09)a | 0.80 (0.05) | 0.84 (0.05) |
| Brain | 0.13 (0.05) | 0.32 (0.10) | 0.36 (0.16) |
| M3G | |||
| Blood | 0.56 (0.08)–0.32 (0.07)a | 0.63 (0.06) | 0.68 (0.06) |
| Brain | 0.052 (0.02) | 0.20 (0.19) | 0.21 (0.16) |
Decreased throughout the study.
Recovery was calculated as described in Methods. Values in the table are means (SD) from the number of animals shown (n).
M3G, morphine-3-glucuronide.
Figure 1.
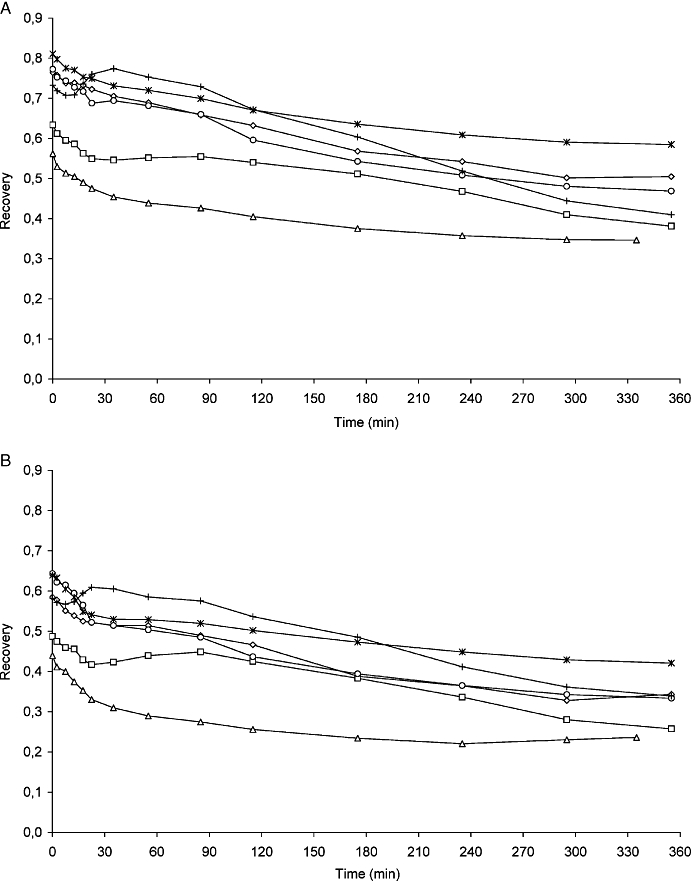
Recovery of morphine and M3G in adult sheep. In A, moving average (n= 3 samples) of the recovery for morphine blood probe in the adult sheep. In B, moving average (n= 3 samples) of the recovery for M3G blood probe in the adult sheep. Results from individual sheep are indicated by the different symbols. M3G, morphine-3-glucuronide.
Individual plasma concentrations of morphine and M3G are presented in Figure 2A for the control premature lambs and in Figure 2B for the adult sheep. Mean plasma concentrations for the adult sheep and the control and asphyxial lamb groups are presented together in Figure 2C for comparison. Morphine CL from plasma was significantly higher in adult sheep than in premature lambs, as was the Vβ (Table 3). These combined differences lead to a somewhat longer plasma half-life for morphine in adult sheep than in premature lambs, although the difference did not reach statistical significance. In premature lambs, plasma concentrations of M3G reached a plateau and did not change thereafter throughout the study (Figure 2A,C). M3G was slowly eliminated from adult sheep (Figure 2B,C).
Figure 2.
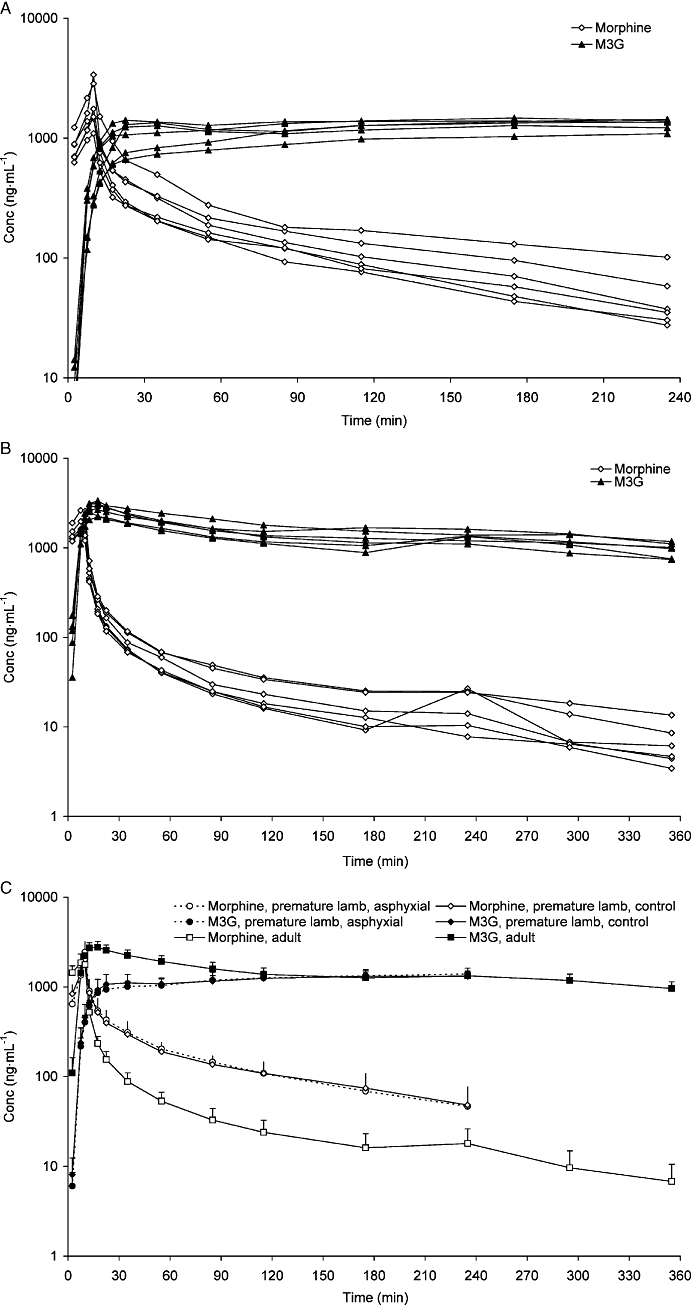
Plasma concentrations (ng·mL−1) of morphine and M3G after 1 mg·kg−1 morphine given as a 10 min constant infusion. In A, results from control premature lambs and in B, results from adult sheep. In C, values from adults, premature lambs and premature asphyxial lambs are shown. M3G, morphine-3-glucuronide.
Table 3.
Pharmacokinetic parameters presented as mean values (SD) for morphine and M3G in adult sheep, and in control and asphyxial premature lambs
|
Adult sheep |
Premature lambs |
||||
|---|---|---|---|---|---|
| Control (n =6) | Control (n =6) | Asphyxial (n =5) | P value |
||
| Control groupsa | Lamb groupsb | ||||
| Morphine | |||||
| CL (mL·min−1·kg−1) | 34.3 (8.5)c | 20.3 (6.6) | 18.4 (2.5) | 0.013 | 0.549 |
| Vβ (L·kg−1) | 6.38 (0.90)c | 2.74 (0.52) | 2.45 (0.45) | 0.00001 | 0.354 |
| Kpuu | 1.19 (0.20) | 1.89 (0.51) | 1.80 (0.68) | 0.018 | 0.811 |
| Vubrain (mL·g−1) | 3.15 (0.34)c | 1.76 (0.47) | – | 0.002 | |
| t1/2 blood probe (min) | 119 (23) | 78 (23) | 83 (17) | 0.011 | 0.700 |
| t1/2 cortex probe (min) | 320 (87)d | 157 (47)d | 140 (27)d | 0.002 | 0.486 |
| Residual area blood probe (%) | 5.5 (2.0) | 9.5 (6.2) | 10.6 (4.8) | ||
| Residual area cortex probe (%) | 44.3 (7.6) | 35.4 (9.3) | 32.2 (6.6) | ||
| M3G | |||||
| Kpuu | 0.27 (0.16) | 0.17 (0.15) | 0.24 (0.21) | 0.251 | 0.527 |
T-test comparing adult sheep with the premature control groups.
T-test comparing the premature lamb groups.
Based on five individuals.
T-test comparing t1/2 in cortex and blood (P values were 0.0008, 0.0008 and 0.0118 for adults, the premature control group and the premature asphyxial group respectively).
–, not estimated; M3G, morphine-3-glucuronide.
Morphine brain distribution
Individual unbound morphine concentrations in the blood and cortex ISF of control premature lambs and adult sheep are presented in Figure 3A,B respectively. The brain-to-blood ratio for unbound morphine (Kp,uu) was 1.19 in adult sheep and 1.89 in control premature lambs (Table 3), indicating a net active uptake of morphine into the brain. Kp,uu was significantly higher in lambs than in sheep. The half-life of morphine in both brain cortex ISF and blood was longer in sheep than in premature lambs (Table 3). This was mainly the result of higher intra-brain binding of morphine in adult sheep, as demonstrated by the Vu,brain value of 3.15 mL·g−1 brain in sheep versus 1.76 mL·g−1 brain in premature lambs, and the greater Vβ in sheep (Table 3). The half-life of morphine was significantly longer in the brain cortex ISF than in blood for both groups.
Figure 3.
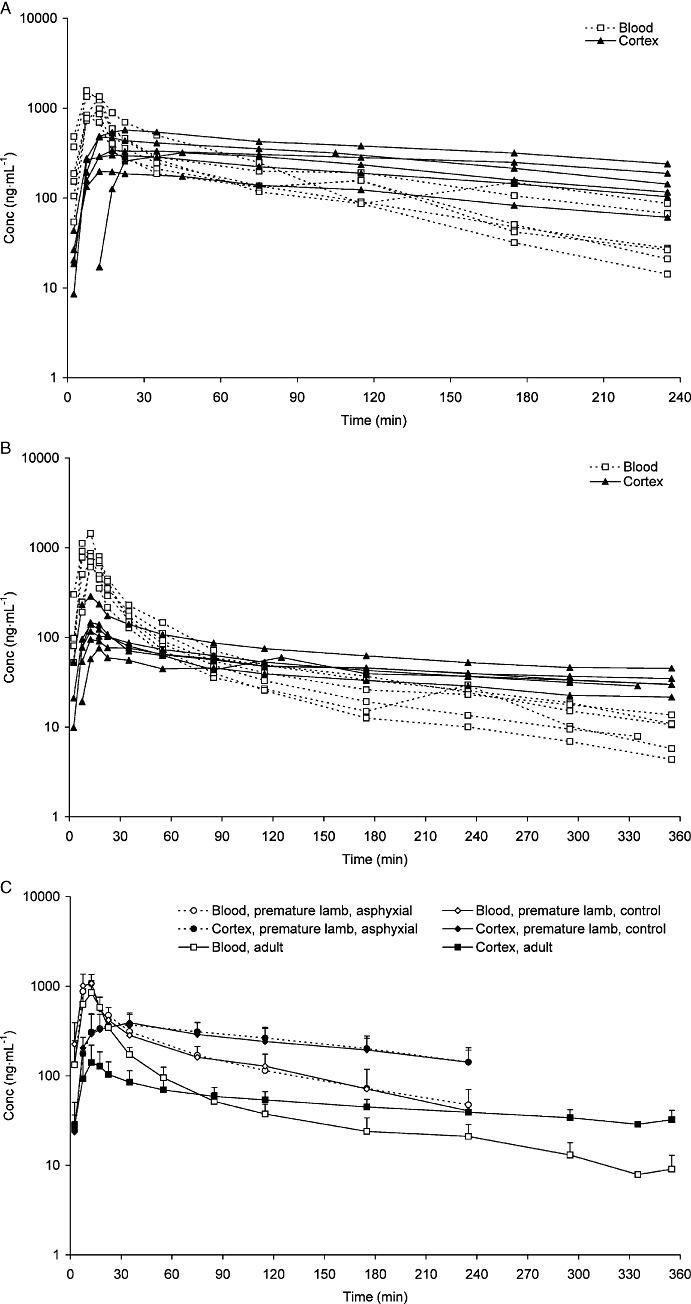
Unbound morphine concentrations (ng·mL−1) in blood and cortex ISF after 1 mg·kg−1 morphine given as a 10 min constant infusion. In A, results from premature control lambs and in B, results from adult sheep. In C, values from adults, premature control lambs and premature asphyxial lambs are shown. ISF, interstitial fluid.
Mean (SD) unbound morphine concentrations in blood and brain cortex ISF for all three groups are presented in Figure 3C for comparison. The Kp,uu value for the asphyxial group was 1.80, similar to that of the control group (Table 3). In addition, no pharmacokinetic differences were found between the control and asphyxial premature groups for either morphine or M3G.
M3G brain distribution
In contrast to the results for morphine, the unbound M3G concentrations in brain cortex were lower than the corresponding concentrations in the blood at all times for all groups (Figure 4A–C). Individual data are presented for control premature lambs and adult sheep in Figure 4A,B, while mean values (SD) for the groups are presented in Figure 4C. As the unbound M3G concentrations in the premature lamb brain cortex increased somewhat throughout the whole study, it was not possible to estimate a stable relationship between brain ISF and blood concentrations for M3G in premature lambs, so the last sampling time (235 min for lambs and 355 min for adult sheep) was used for estimation of Kp,uu for this group. Given these restrictions, the Kp,uu of M3G was 0.27 and 0.17 for adult sheep and premature lambs respectively (Table 3). The Kp,uu of M3G for the asphyxial lamb group was 0.24, which was similar to that for the control lamb group (Table 3). There were thus no significant differences between the groups in M3G partitioning across the BBB. The Vu,brain of M3G was 0.16 mL·g−1 brain for premature lambs, close to the volume of brain ISF. It was not possible to estimate the corresponding value in adult sheep.
Figure 4.
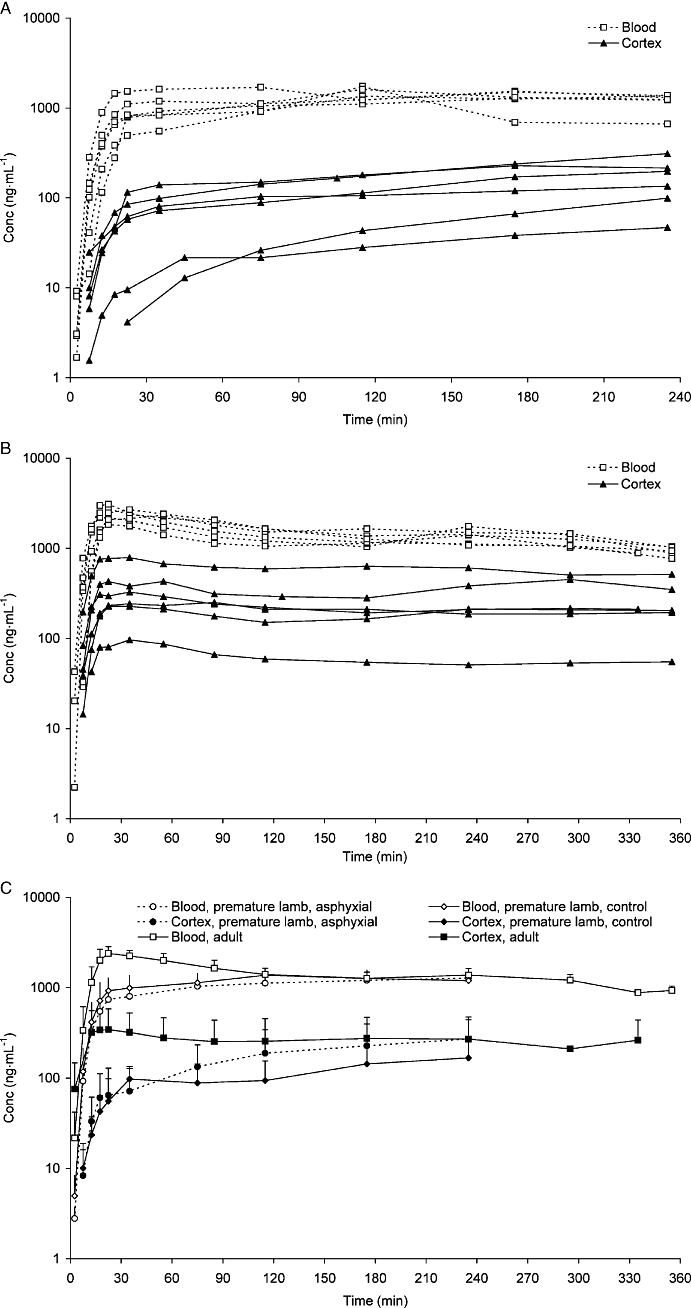
Unbound M3G concentrations (ng·mL−1) in the blood and cortex ISF after 1 mg·kg−1 morphine given as a 10 min constant infusion. In A, results from premature control lambs and in B, results from adult sheep. In C, values from adults, premature control lambs and premature asphyxial lambs are shown. ISF, interstitial fluid; M3G, morphine-3-glucuronide.
Discussion and conclusions
Our results indicated three main findings: first, exposure to unbound morphine in the brain, expressed as Kp,uu, was significantly higher in premature lambs than in adult sheep. Then, the ratio of unbound morphine concentrations in brain ISF and blood was greater than unity in both lambs and sheep, indicating active influx of morphine across the BBB. Finally, BBB transport of morphine in sheep, independent of age, differed from that in other species, including humans (Ederoth et al., 2004), rats (Bengtsson et al., 2008), mice (Xie et al., 1999) and pigs (Tunblad et al., 2004), in historical reports.
Premature versus adult
The ratio of unbound morphine in brain to that in blood was higher in premature lambs than in adult sheep. This means that a given cerebral concentration of unbound morphine would be achieved at lower plasma concentrations in premature lambs than in adult sheep. In addition, the lower plasma clearance rate in premature lambs would keep plasma morphine concentrations higher, further increasing the cerebral concentration of a given dose, with the associated potentially increased risk of side effects and/or toxicity. It is possible, but not proven because of the clear species differences between sheep and humans in morphine BBB transport, that the age dependency of morphine BBB pharmacokinetics in sheep is also applicable to humans. This is backed by the lower plasma clearance of morphine in newborn humans than in adults (Scott et al., 1999; Saarenmaa et al., 2000), and the lower doses of morphine given to newborn humans (recommended dose 25 µg·kg−1 at Lund University Hospital) than those given to adults (up to 10 mg is recommended, i.e. 143 µg·kg−1 for a 70 kg person).
The decreasing Kp,uu with increasing age seen in our study could be interpreted as decreased active influx, increased counteracting active efflux, a change in the tight junction function or a combination of these. The design of the study precluded further discrimination between these possible explanations.
It initially seemed reasonable to assume that brain ischemia, as in asphyxia neonatorum, would affect drug perfusion and BBB function. In this study, however, the induced asphyxia did not affect BBB transport of morphine or M3G in the acute situation, as Kp,uu and all other pharmacokinetic parameters were similar in the two premature lamb groups.
These results stress the importance of measuring the pharmacologically active concentrations of unbound drug close to the site of action. Calculating Kp (Cbrain/Cplasma), as often occurs, or Kp,u (Cbrain/Cunbound plasma) from the results in this study would indicate that uptake of morphine into the adult brain is higher than that into the premature brain, which is the opposite result to that indicated by the unbound drug data. The higher Kp and Kp,u values are the result of the higher affinity of morphine for brain tissue (greater Vu,brain) in adulthood, probably because of non-specific binding, while the pharmacologically active fraction reaching the brain is actually lower in this group than in younger animals, as reflected in the lowe r Kp,uu for adult sheep. Auguy-Valette et al. (1978) described an almost threefold decrease in total brain-to-blood ratios (Kp) of morphine from postnatal day 10 to day 30 in rats. However, further details specifying unbound or total drug data are required before these results can be interpreted in light of the discussion above.
Higher than unity Kp,uu
The net active influx into the brain of morphine in premature lambs and adult sheep (Kpuu above unity) was less pronounced in the adult sheep. There are a few historical reports suggesting that morphine is a substrate for active uptake transport into the brain. Groenendaal et al. (2007) came to the conclusion that morphine is transported into the rat brain by both passive and active low-capacity processes that are saturated at low concentrations. Xie et al. (1999) found different Kp,uu values for morphine in mice with changing blood concentrations, which could indicate the presence of a saturable influx process. However, the net flux of morphine in these studies was efflux, due to transport by Pgp and other transporters (Xie et al., 1999; Tunblad et al., 2003; Groenendaal et al., 2007). Other substances actively taken up by the brain include oxycodone (Bostrom et al., 2006), apomorphine (Sam et al., 1997), gacyclidine (Hoizey et al., 2000), diphenhydramine (Au-Yeung et al., 2007) and possibly morphine-6-glucuronide (Bourasset et al., 2003).
Species differences
In contrast to all other species studied, the BBB morphine transport results for sheep indicated a net influx into the brain, as compared with the net morphine efflux in the other species. Kp,uu values reported for rats (Bengtsson et al., 2008), mice (Xie et al., 1999), pigs (Tunblad et al., 2004) and humans with brain trauma (Ederoth et al., 2004) are 0.49, 0.3, 0.47 and 0.64 respectively. This marked difference in morphine brain transport in sheep compared with other species raises the question of whether sheep lack the efflux transporter responsible for efflux of morphine in mice, rats, pigs and humans. It remains to be shown if the same species differences are present for other drugs.
Methods and design
The use of a deuterated calibrator allowed continuous measurement of the relative recovery of morphine and, thus, calculation of the correct concentration of unbound drug for each microdialysis sample. The drug recovery values from the brain probes in both premature lambs and adult sheep and from the blood probe in premature lambs were stable over time (data not shown). The decreasing recovery of both morphine and M3G from the blood probe in adult sheep was unexpected. This may have been the result of clotting around the probe, but this has not been observed in earlier studies (Bostrom et al., 2006; Bengtsson et al., 2008). Because of this discrepancy, the average of three samples was used to calculate recovery for the data from the adult blood probes (Bouw and Hammarlund-Udenaes, 1998).
Circulation, respiration and metabolism were unstable in all the premature lambs. The respiratory goals defined in the Materials and Methods section were not met. pH, oxygenation and blood glucose were lower and pCO2 was higher in lambs than in adult sheep. Several lambs required treatment with intratracheal surfactant and/or inhaled NO to achieve acceptable lung function. In contrast, a mild alkalosis was found in the adult sheep despite stable physiological parameters during the whole study. Although it is acknowledged that the instability of the lambs and the alkalosis in the sheep might have affected the pharmacokinetic results, morphine is a weak base, and a lower blood pH in the lambs would theoretically have resulted in a lower Kp,uu and correspondingly, a higher pH in the sheep would yield a higher Kp,uu (Bouw et al., 2000). However, this model studies lambs during and immediately after acute delivery and somewhat unstable conditions are therefore anticipated. Given this, the model itself, in our opinion, produces robust results (Ley et al., 2004; Markus et al., 2007). Finally, comparably unstable conditions are also encountered in the neonatal intensive care unit.
The lambs were adequately sedated with continuous administration of fentanyl only, while the adult sheep required an additional thiopental infusion for adequate sedation. Adult pigs sedated with fentanyl/thoipental (Tunblad et al., 2004) and humans sedated with fentanyl/midazolam (Ederoth et al., 2004) infusions have demonstrated similar morphine pharmacokinetics to those in this study (Kp,uu values of 0.64 and 0.47 vs. 1.19 in adult sheep). Thus, it is unlikely that the intravenous thiopental infusion per se exerted a major effect on morphine BBB pharmacokinetics. Also, fentanyl had no effect on morphine steady-state concentrations when given to rats (J. Bengtsson, unpubl. data).
In conclusion, there was an age-dependent net influx of morphine into the brain in sheep. This influx decreased with age but was still a net influx in the adult sheep. These results are in contrast to findings in other species (mice, rats, pigs and humans) where a net efflux was observed. This indicates that sheep may lack an efflux transporter present in the other species, which is in itself an interesting finding. Acute disease states like asphyxia and seizures did not influence morphine BBB transport in premature lambs.
Acknowledgments
The study was supported by grants from the Swedish Medical Research Council (Grants 0037 and 5980) and Lund University Hospital Funds. Technical support from Ingela Mattisson-Sandström, Ulla Ganestam and Britt Jansson during the study was much appreciated.
Glossary
Abbreviations:
- AUC
area under the concentration-time curve
- BBB
blood–brain barrier
- ISF
interstitial fluid
- Kp,uu
ratio of unbound drug in brain interstitial fluid to that in blood
- Vu,brain
volume of distribution of unbound drug in the brain in mL·g−1 brain
Conflict of interest
The authors state no conflict of interest.
References
- Au-Yeung SC, Riggs KW, Gruber N, Rurak DW. The use of microdialysis for the study of drug kinetics: central nervous system pharmacokinetics of diphenhydramine in fetal, newborn, and adult sheep. Drug Metab Dispos. 2007;35:1285–1291. doi: 10.1124/dmd.106.013995. [DOI] [PubMed] [Google Scholar]
- Auguy-Valette A, Cros J, Gouarderes C, Gout R, Pontonnier G. Morphine analgesia and cerebral opiate receptors: a developmental study. Br J Pharmacol. 1978;63:303–308. doi: 10.1111/j.1476-5381.1978.tb09761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield CP, Yu VY, Noma O, Kukita J, Cussen LJ, Oates A, et al. Cerebral blood volume measured using near-infrared spectroscopy and radiolabels in the immature lamb brain. Pediatr Res. 1999;46:50–56. doi: 10.1203/00006450-199907000-00009. [DOI] [PubMed] [Google Scholar]
- Bengtsson J, Jansson B, Hammarlund-Udenaes M. On-line desalting and determination of morphine, morphine-3-glucuronide and morphine-6-glucuronide in microdialysis and plasma samples using column switching and liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2116–2122. doi: 10.1002/rcm.2035. [DOI] [PubMed] [Google Scholar]
- Bengtsson J, Bostrom E, Hammarlund-Udenaes M. The use of a deuterated calibrator for in vivo recovery estimations in microdialysis studies. J Pharm Sci. 2008;97:3433–3441. doi: 10.1002/jps.21217. [DOI] [PubMed] [Google Scholar]
- Bostrom E, Simonsson US, Hammarlund-Udenaes M. In vivo blood-brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug Metab Dispos. 2006;34:1624–1631. doi: 10.1124/dmd.106.009746. [DOI] [PubMed] [Google Scholar]
- Bourasset F, Cisternino S, Temsamani J, Scherrmann JM. Evidence for an active transport of morphine-6-beta-d-glucuronide but not P-glycoprotein-mediated at the blood-brain barrier. J Neurochem. 2003;86:1564–1567. doi: 10.1046/j.1471-4159.2003.01990.x. [DOI] [PubMed] [Google Scholar]
- Bouw MR, Hammarlund-Udenaes M. Methodological aspects of the use of a calibrator in in vivo microdialysis-further development of the retrodialysis method. Pharm Res. 1998;15:1673–1679. doi: 10.1023/a:1011992125204. [DOI] [PubMed] [Google Scholar]
- Bouw MR, Gardmark M, Hammarlund-Udenaes M. Pharmacokinetic-pharmacodynamic modelling of morphine transport across the blood-brain barrier as a cause of the antinociceptive effect delay in rats – a microdialysis study. Pharm Res. 2000;17:1220–1227. doi: 10.1023/a:1026414713509. [DOI] [PubMed] [Google Scholar]
- Ederoth P, Tunblad K, Bouw R, Lundberg CJ, Ungerstedt U, Nordstrom CH, et al. Blood-brain barrier transport of morphine in patients with severe brain trauma. Br J Clin Pharmacol. 2004;57:427–435. doi: 10.1046/j.1365-2125.2003.02032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman FR, Weiss GB, Alderdice MT. On the measurement of extracellular space in slices prepared from different rat brain areas. Neuropharmacology. 1973;12:867–873. doi: 10.1016/0028-3908(73)90039-7. [DOI] [PubMed] [Google Scholar]
- Groenendaal D, Freijer J, de Mik D, Bouw MR, Danhof M, de Lange EC. Population pharmacokinetic modelling of non-linear brain distribution of morphine: influence of active saturable influx and P-glycoprotein mediated efflux. Br J Pharmacol. 2007;151:701–712. doi: 10.1038/sj.bjp.0707257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Chatelain P, Massingham R, Jonsson EN, Hammarlund-Udenaes M. Brain distribution of cetirizine enantiomers: comparison of three different tissue-to-plasma partition coefficients: K(p), K(p,u), and K(p,uu) Drug Metab Dispos. 2006;34:318–323. doi: 10.1124/dmd.105.007211. [DOI] [PubMed] [Google Scholar]
- Hoizey G, Kaltenbach ML, Dukic S, Lamiable D, Lallemand A, D'Arbigny P, et al. Distribution of gacyclidine enantiomers in spinal cord extracellular fluid. Pharm Res. 2000;17:148–153. doi: 10.1023/a:1007557028313. [DOI] [PubMed] [Google Scholar]
- Joel SP, Osborne RJ, Slevin ML. An improved method for the simultaneous determination of morphine and its principal glucuronide metabolites. J Chromatogr. 1988;430:394–399. doi: 10.1016/s0378-4347(00)83176-x. [DOI] [PubMed] [Google Scholar]
- Levin VA, Fenstermacher JD, Patlak CS. Sucrose and inulin space measurements of cerebral cortex in four mammalian species. Am J Physiol. 1970;219:1528–1533. doi: 10.1152/ajplegacy.1970.219.5.1528. [DOI] [PubMed] [Google Scholar]
- Ley D, Oskarsson G, Bellander M, Hernandez-Andrade E, Lingman G, Marsal K, et al. Different responses of myocardial and cerebral blood flow to cord occlusion in exteriorized fetal sheep. Pediatr Res. 2004;55:568–575. doi: 10.1203/01.PDR.0000113785.66455.E7. [DOI] [PubMed] [Google Scholar]
- Markus T, Hansson S, Amer-Wahlin I, Hellstrom-Westas L, Saugstad OD, Ley D. Cerebral inflammatory response after fetal asphyxia and hyperoxic resuscitation in newborn sheep. Pediatr Res. 2007;62:71–77. doi: 10.1203/PDR.0b013e31811ead6e. [DOI] [PubMed] [Google Scholar]
- Milne RW, McLean CF, Mather LE, Nation RL, Runciman WB, Rutten AJ, et al. Influence of renal failure on the disposition of morphine, morphine-3-glucuronide and morphine-6-glucuronide in sheep during intravenous infusion with morphine. J Pharmacol Exp Ther. 1997;282:779–786. [PubMed] [Google Scholar]
- Saarenmaa E, Neuvonen PJ, Rosenberg P, Fellman V. Morphine clearance and effects in newborn infants in relation to gestational age. Clin Pharmacol Ther. 2000;68:160–166. doi: 10.1067/mcp.2000.108947. [DOI] [PubMed] [Google Scholar]
- Sam E, Sarre S, Michotte Y, Verbeke N. Distribution of apomorphine enantiomers in plasma, brain tissue and striatal extracellular fluid. Eur J Pharmacol. 1997;329:9–15. doi: 10.1016/s0014-2999(97)10082-6. [DOI] [PubMed] [Google Scholar]
- Scott CS, Riggs KW, Ling EW, Fitzgerald CE, Hill ML, Grunau RV, et al. Morphine pharmacokinetics and pain assessment in premature newborns. J Pediatr. 1999;135:423–429. doi: 10.1016/s0022-3476(99)70163-0. [DOI] [PubMed] [Google Scholar]
- Tunblad K, Jonsson EN, Hammarlund-Udenaes M. Morphine blood-brain barrier transport is influenced by probenecid co-administration. Pharm Res. 2003;20:618–623. doi: 10.1023/a:1023250900462. [DOI] [PubMed] [Google Scholar]
- Tunblad K, Ederoth P, Gardenfors A, Hammarlund-Udenaes M, Nordstrom CH. Altered brain exposure of morphine in experimental meningitis studied with microdialysis. Acta Anaesthesiol Scand. 2004;48:294–301. doi: 10.1111/j.0001-5172.2003.0311.x. [DOI] [PubMed] [Google Scholar]
- Xie R, Hammarlund-Udenaes M, de Boer AG, de Lange EC. The role of P-glycoprotein in blood-brain barrier transport of morphine: transcortical microdialysis studies in mdr1a (−/−) and mdr1a (+/+) mice. Br J Pharmacol. 1999;128:563–568. doi: 10.1038/sj.bjp.0702804. [DOI] [PMC free article] [PubMed] [Google Scholar]


