Abstract
Background
Medullary thyroid carcinoma (MTC) is a neuroendocrine (NE) malignancy that frequently metastasizes and has limited treatments. This study was aimed at assessing the antitumor effects of suberoyl bishydroxamic acid (SBHA) in an in vivo model of MTC.
Methods
Nude mice were injected with human MTC cells (TT), and the groups were treated with SBHA (200 mg/kg) or vehicle (DMSO) in saline injection every other day for 12 days. Tumors were measured every 4 days and harvested at 12 days for Western analysis.
Results
Treatment with SBHA resulted in an average 55% inhibition of tumor growth in the treatment group (P < 0.05). Analysis of SBHA-treated MTC tumors revealed a marked increase in the active form of Notch1 (NICD) with a concomitant decrease in achaete-scute complex-like 1 (ASCL1), a downstream target of Notch1 signaling, as well as the NE tumor marker chromogranin A (CgA). Importantly, SBHA treatment resulted in an increase in protein levels of p21CIP1/WAF1, p27KIP1, cleaved caspase-9, cleaved caspase-3 and cleaved poly ADP-ribose polymerase (PARP) and concomitant with a decrease in cyclin D1 and cyclin B1, indicating that the growth inhibition was due to both cell cycle arrest and apoptosis. Moreover, SBHA downregulated cell survival proteins Bcl-2 and Bcl-XL, but upregulated apoptotic proteins Bax, Bad, and Bmf.
Conclusions
These results demonstrate that SBHA inhibits MTC growth in vivo. SBHA is a promising candidate for further preclinical and clinical studies in MTC.
Keywords: Histone Deacetylase Inhibitor, Suberoyl Bis-Hydroxamic Acid (SBHA), Medullary Thyroid Carcinoma, Local Delivery, Notch1, Apoptosis
INTRODUCTION
Medullary thyroid carcinoma (MTC) is a neuroendocrine (NE) tumor, characterized by its production of NE tumor markers such as calcitonin and chromogranin A (CgA).1 Although surgery is currently the only effective treatment for MTC, approximately 50–80% of patients already have metastatic disease at the time of initial diagnosis, and thus complete surgical resection is often not possible.2–4 This emphasizes the need for the development of new treatment strategies.
It has been well established that the occurrence of many cancers are accompanied by a genome-wide histone hypoacetylation.5 In recent years, a great deal of research interest has been focused on efforts aimed at the restoration of the acetylation/deacetylation balance by using histone deacetylase (HDAC) inhibitors, with the hope that a new strategy of cancer treatment can be developed based on this mechanism.5 Suberoyl bis-hydroxamic acid (SBHA) is a small molecule HDAC inhibitor. Some studies reveal that SBHA exerts antitumor effects by inhibiting acetylation of histone. This results in up-regulation of tumor suppressor, proapoptotic, and growth-inhibitory genes in human malignant mesothelioma cells, melanoma cells and gastrointestinal and pulmonary carcinoid cancer cells.6–8 These studies highlight the observation that the inhibiting activity of SBHA on cancer cell growth spans many tissue types, suggesting it could be a useful agent for the treatment of a wide variety of malignancies. Therefore, we sought to investigate whether an in vitro application of SBHA against human MTC cells would lead to growth inhibition. Our findings revealed that SBHA had profound antigrowth activity against MTC cells via caspase-dependent apoptosis.9 In addition, our in vitro data demonstrated that SBHA activates the Notch1 signaling pathway, leading to the reduction of NE tumor markers.9 These results prompted us to look more closely at the effects of SBHA on MTC cells in vivo. In this study, we utilized a xenograft model of human MTC to examine whether SBHA activates the Notch1 signaling pathway and inhibits MTC growth in vivo.
MATERIALS AND METHODS
Cell Culture
Human MTC cells (TT) were obtained from American Type Culture Collection (ATCC, Manassas, VA). The TT cell line derived from MEN 2-associated MTC with a RET germline mutation in codon 634, for alternative mechanisms of tumorigenesis.10 TT cells maintained in RPMI 1640 (Life Technologies, Rockville, MD) supplemented with 18% fetal bovine serum (Sigma-Aldrich, St Louis, MO), 100 IU/mL penicillin and 100 µg/mL streptomycin (Life Technologies) in a humidified atmosphere of 5% CO2 in air at 37 °C.
Xenograft Model and SBHA Administration
Both animal care and treatment were performed in compliance with our animal experiment protocol approved by the University of Wisconsin–Madison Animal Care and Use Committee. Male nude athymic, nu/nu mice (Charles Rivers, Wilmington, Maryland, USA) received subcutaneous (s.c.) injections of TT cells (106) into the right flank. Mice with palpable tumors were randomly divided into two groups for the study. SBHA (Biomol, Plymouth Meeting, PA) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at a stock concentration of 500 mg/mL and stored at −20 °C. Fresh dilutions in PBS were made for each injection. The treatment group of mice received 200 mg/kg SBHA every two days by intraperitoneal (i.p.) injection for 12 days. A control group was injected with vehicle (DMSO) diluted in PBS. The mice were weighed three times during the experimental period to assess toxicity of the treatments, and the tumors were measured every four days using calipers. Tumor volume was calculated from the two-dimensional caliper measurements using the following formula: tumor volume = length × (width)2 × π/6. On the final day of the study, all mice were sacrificed by carbon dioxide inhalation. The s.c. tumor was removed and snap frozen in liquid nitrogen.
Western Blot Analysis
Total tissue proteins were isolated as described,11 and the protein concentrations were determined using a bicinchoninic assay kit (Pierce, Rockford, IL). Tissue extracts (30–50 µg) were denatured by boiling for 5 minutes and separated by 8%, 10% or 12% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes (Schleicher and Schuell, Keene, NH) by electroblotting. Membranes were blocked in milk (5% nonfat dry milk and 0.1% Tween 20 in phosphate-buffered saline), and exposed to primary and secondary antibodies as described.9 The following primary antibody dilutions were used: Notch1 (1:1000, Santa Cruz Biotechnology); Achaete Scute Complex Like-1 (ASCL1) (1:1000; BD Pharmingen, San Diego, CA); Glyceraldehyde 3 phosphate dehydrogenase (G3PDH) (1:10,000, Trevigen, Gaithersburg, MD); Acetyl-histone H4 (Lys12) (AH4), p21CIP1/WAF1, p27KIP1, poly (ADP-ribose) polymerase (PARP), cleaved caspase-3, cleaved caspase-9, Bcl-2, Bcl-XL, Bad, Bmf, Bax, cyclin B1, cyclin D1 (1:1000, Cell Signaling Technology, Beverly, MA) and chromogranin A (CgA) (1:1000, Zymed Laboratories, San Diego, CA). Primary antibody incubations were kept overnight at 4°C and membranes were washed 3 times for 5 minutes or 3 times for 10 minutes in wash buffer (0.1% Tween 20 in phosphate-buffered saline). The membranes were incubated with a 1:2000 dilution of horseradish peroxidase-conjugated anti-mouse secondary antibody (for ASCL1) (Cell Signaling Technology) or anti-rabbit antibody (for Notch1, AH4, p21CIP1/WAF1, p27KIP1, PARP, cleaved caspase-3, cleaved caspase-9, Bcl-2, Bcl-XL, Bad, Bmf, Bax, cyclin B1, cyclin D1, CgA, and G3PDH). Membranes were developed by Immun-Star (Bio-Rad) for CgA, PARP, Bcl-2 and G3PDH or by Super West Femto chemiluminescence substrate (Pierce) for AH4, Notch1, ASCL1, p21CIP1/WAF1, p27KIP1, cleaved caspase-3, cleaved caspase-9, Bcl-XL, Bad, Bmf, Bax, cyclin B1 and cyclin D1 according to the manufacturers’ directions.
Statistical Analysis
Analysis of variance (ANOVA) with Bonferroni post hoc testing was performed using a statistical analysis software package (SPSS version 10.0, SPSS, Chicago, IL). A P-value of < 0.05 was considered significant.
RESULTS
SBHA Suppresses Growth of MTC Xenografts in Nude Mice
We have demonstrated that SBHA inhibited the growth of MTC cells in vitro.9 Thus, we sought to investigate if SBHA could inhibit MTC growth in vivo. Nude mice with MTC tumors received SBHA (200 mg/kg/2d, i.p.) or DMSO alone for 12 days. Animal weight remained stable over the course of the experiments and no adverse effects were observed. All animals survived until the respective study end-points. As shown in figure 1, tumor growth progressively slowed with increasing injections of drug. SBHA-treated animals averaged a 55% inhibition of tumor growth compared to those of DMSO-treated controls (P < 0.05), which demonstrated that SBHA inhibits MTC growth in vivo.
Figure 1.

SBHA inhibits growth of human medullary thyroid carcinoma cells in vivo. Nude mice were injected with TT cells (106). Tumor bearing animals were treated with SBHA (200 mg/kg) or DMSO by intraperitoneal (i.p.) injections every other day for 12 days. Growth of tumors during course of experiment was measured by increases in calculated tumor volumes. P < 0.05 between control tumors and SAHA-treated tumors by Bonferroni post hoc test.
SBHA Effects of Notch1 Signaling on NE Marker in vivo
The tumors were examined for levels of AH4 to test the acetylation property of SBHA. Western blot analysis showed that AH4 significantly increased (Fig.2), verifying the activity of SBHA as an HDAC inhibitor in vivo. To provide more evidence for the in vivo efficacy of SBHA, we examined the Notch1 signaling pathway activation and levels of CgA in tumor tissues. Western blot analysis showed that treatment with SBHA resulted in a significant induction of the active cleaved Notch1 intracellular domain (NICD) (Fig.3). Moreover, we found that there was a concomitant decrease in the levels of ASCL1, which is a known downstream target of the Notch1 signaling pathway, indicating that SBHA activates the Notch1 signaling pathway in vivo. MTC secretes various bioactive hormones such as CgA. We previously reported that SBHA decreased levels of CgA by inducing Notch1 signaling pathway in MTC cells.9 In this study, we found a significant reduction in CgA in SBHA-treated mice (Fig.3).
Figure 2.
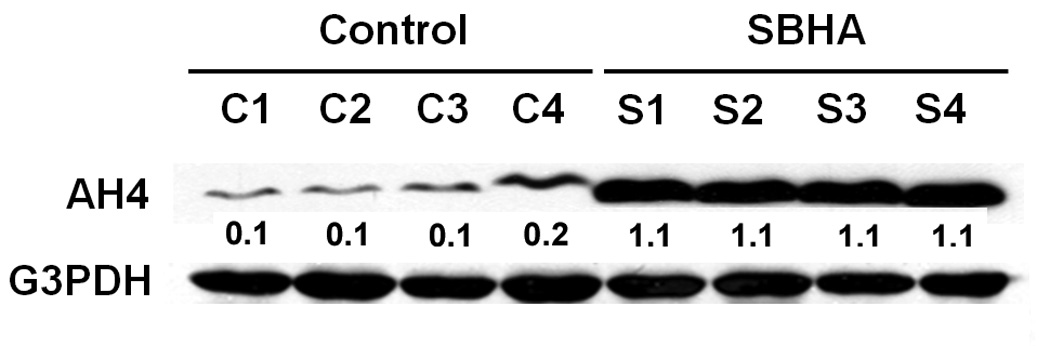
SBHA induces accumulation of acetyl-histone H4 (Lys12) (AH4). Total tumor tissue extracts were isolated from SBHA-treated or DMSO-treated mice and analyzed for the levels of AH4. Western blot analysis showed that AH4 is significantly increased in SBHA-treated samples compared to those in the control group. G3PDH was used to confirm equal protein loading. The quantification of the bands was generated by comparison with G3PDH.
Figure 3.
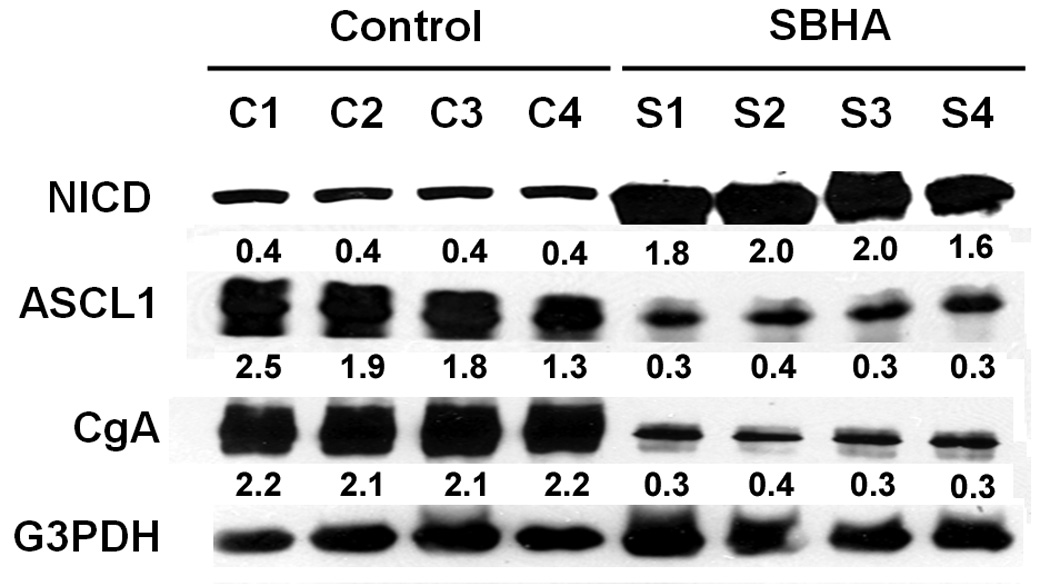
SBHA activates the Notch1 signaling pathway and decreases levels of chromogranin A (CgA). Total tumor tissue extracts were isolated from SBHA-treated or DMSO-treated mice and analyzed by Western blotting for expression of Notch1 intracellular domain (NICD), achaete-scute complex-like 1 (ASCL1) and CgA. SBHA treatment induced NICD protein, which led to a concomitant decrease in ASCL1, a downstream target of Notch1 signaling, as well as NE tumor marker CgA. G3PDH was used to confirm equal protein loading. The quantification of the bands was generated by comparison with G3PDH.
SBHA Induces Cell Cycle Arrest in MTC Xenografts
After establishing that SBHA inhibits MTC growth in vivo, we were interested in determining the mechanism of action for this effect. We have reported that SBHA induces cell cycle arrest in a variety of carcinoid cancer cell lines by modulating expression of the cyclin-dependent kinase (CDK) inhibitor p21CIP1/WAF1 and other regulatory proteins.6 In the present study, we performed Western blot analysis using MTC xenograft tissue lysates after treatment with SBHA to measure the effects of the drug on cell cycle regulators. Treatment of animals with SBHA resulted in an increase in protein levels of p21CIP1/WAF1 and p27KIP1 (Fig.4). To confirm the induction of cell cycle arrest with SBHA treatment, we further examined the levels of cell cycle promoters cyclin B1 and cyclin D1. As expected, SBHA treatment led to a decrease in the levels of these proteins (Fig.4), confirming that cell cycle arrest is one of the mechanisms of SBHA induced MTC growth suppression in vivo.
Figure 4.
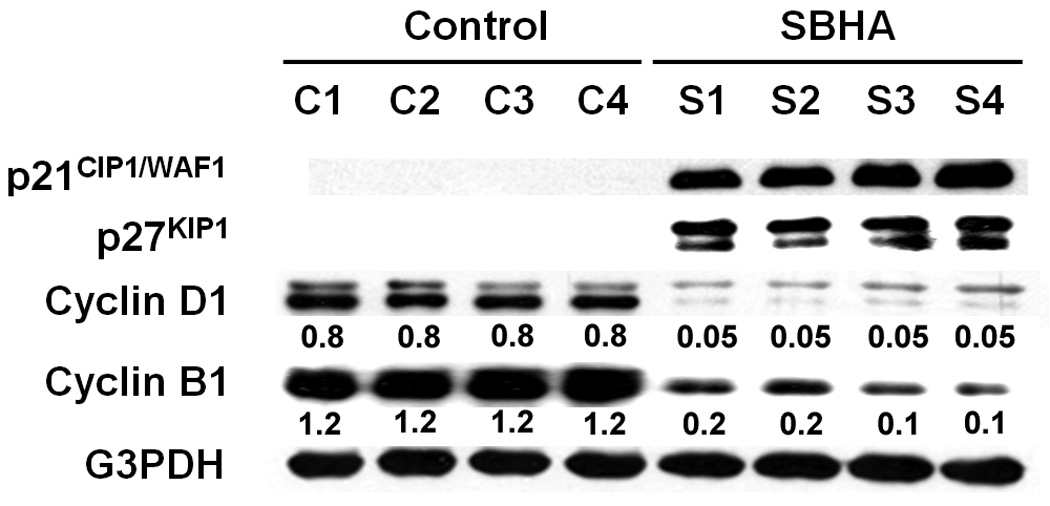
SBHA induces cell cycle arrest in MTC xenografts. Tumor tissue lysates from SBHA- or DMSO-treated mice were analyzed by Western blotting for levels of cell cycle regulators. Treatment of animals with SBHA resulted in significant increases in protein levels of p21CIP1/WAF1and p27KIP1 and significant decreases in levels of cyclin B1 and cyclin D1, indicating cell cycle arrest. G3PDH was used to confirm equal protein loading. The quantification of the bands was generated by comparison with G3PDH.
SBHA Regulates the Expression of Apoptotic Mediators and Induces Caspase-Dependent Apoptosis in vivo
It is known that tumor growth suppression is usually due to cell cycle arrest and/or apoptosis. In vitro experiments revealed that SBHA was capable of inducing apoptosis,9 therefore we wanted to determine whether or not SBHA induces apoptosis in vivo. We carried out Western blot analysis for apoptotic mediators after SBHA treatment. PARP is a well-known marker of apoptosis, and caspase-3 is the final executioner of both the extrinsic (caspase-8) and intrinsic (caspase-9) apoptotic pathways.12 As shown in figure 5A, apoptotic mediators, including cleavage of PARP, caspase-3 and caspase-9, were detected by Western blot in SBHA-treated animals. We further evaluated expression of the antiapoptotic and the proapototic proteins in MTC xenograft tumors. Western blot revealed that SBHA downregulated cell survival proteins Bcl-2 and Bcl-XL, but upregulated apoptotic proteins Bax, Bad, and Bmf (Fig.5B), confirming that SBHA was capable of inducing apoptosis in vivo. Taken together, Western blot analysis for cell cycle regulatory proteins and apoptotic mediators indicated that SBHA-induced MTC growth inhibition in vivo is due to a combination of cell cycle arrest and apoptosis.
Figure 5.
SBHA regulates the expression of apoptotic mediators and induces caspase-dependent apoptosis in a mouse model of MTC. Western blot analysis was performed for the levels of apoptotic mediators utilizing tumor tissue lysates. (A) SBHA treatment resulted in increased caspase-9, caspase-3 and PARP cleavage, indicating activation of the apoptotic pathway. (B) After SBHA treatment, the antiaopototic proteins Bcl-2 and Bcl-XL were markedly down-regulated. In contrast, the expression levels of proapoptotic proteins Bad, Bmf and Bax were up-regulated. G3PDH was used to confirm equal protein loading.
DISCUSSION
Although growth-inhibiting activity of SBHA has been described in a variety of cancer cell lines,6–9 there is a scarcity of available data on its ability to inhibit tumor growth in vivo or the mechanisms involved. In this study, we evaluated the effects of SBHA in a nude mouse model of MTC. Our data demonstrated that treatment with SBHA resulted in an average 55% inhibition of tumor growth in the treatment group (P < 0.05), suggesting that SBHA not only reduced MTC cell viability in vitro, but also had a profound effect on growth of MTC tumors in vivo. During the treatment, these mice had no signs or symptoms of toxicity from the drug. Additionally, we found that the levels of acetylated histones were higher in the tumors developed in nude mice treated with SBHA as compared to control tumor.
Epigenetic modulations of cancer-associated genes have been accepted as key mediators of tumorigenesis, and in particular, these modulations can be controlled by histone acetylation.13 Therefore, it can be expected that antitumor effects of SBHA in the MTC model involve several genes or signaling transduction pathways. We have previously demonstrated that the Notch1 signaling pathway plays a role in tumor suppression in NE tumors.14–19 We found that the global changes of MTC cells from SBHA treatment, especially regarding reduction of the NE tumor markers and growth inhibition, were due to SBHA-inducing Notch1 signaling activation, cell cycle inhibitors or apoptotic mediators.9 Consistent with the in vitro data, the results from the nude mouse model experiment further supported that SBHA activated the Notch1 signaling pathway as measured by the increase in NICD protein. Notch1 signaling activation by SBHA in vivo also led to a reduction in NE tumor markers such as ASCL1 and CgA.
In a recent publication we found that overexpression of NICD, utlizing a doxycycline-inducible NICD construct, inhibits MTC cell proliferation and this action was mediated by cell cycle arrest associated with up-regulation of the cyclin-dependent kinase (CDK) inhibitor p21CIP1/WAF1.16 More recently, we reported that apoptosis is also involved in NICD-mediated growth inhibition in SBHA-treated MTC cells.9 In the present study, we showed that SBHA increased the expression of p21CIP1/WAF1and p27KIP1 and significantly decreased the CDK inhibitors cyclin B1 and cyclin D1 in SBHA-treated mice. We also observed that levels of cleavage of caspase-3 and caspase-9, important effectors of apoptotic cell death, increased in mice treated with SBHA. Proper functioning of cleavage of caspase-3 and caspase-9 were subsequently demonstrated by the detection of one of their cleaved substrates, PARP. Moreover, we found that SBHA downregulated the antiapoptotic protein Bcl-2 and Bcl-XL, but upregulated apoptotic proteins Bax, Bad, and Bmf. Each of these actions was consistent with induction of apoptosis by SBHA in vivo. Taken together, our results indicated that the antitumor effect of SBHA in MTC animal models is due to both cell cycle arrest and apoptosis.
Recently, a phase II trial has been initiated to assess the efficacy and safety of oral suberoylanilide hydroxamic acid (SAHA), a similar drug with SBHA, in patients with recurrent and/or metastatic head and neck cancer. The results of this clinical trial revealed that SAHA at 400 mg qd appears to be well tolerated and has modest single agent activity in those patients.20 Up to now, almost 40 SAHA clinical trials are being performed for breast cancer, renal cell carcinoma, colon cancer, and other hematologic and solid tumors.21 In contrast, there has no early clinical data on SBHA in human cancer, although SBHA has in vivo activity with little or no toxicity in the experiment model. Therefore, further clinical trials are needed to evaluate the antitumor activity of SBHA in patients with MTC.
In conclusion, our results demonstrate that the HDAC inhibitor SBHA has impressive antitumor activity in vivo in MTC animal models and a lack of toxicity at doses that effectively inhibit tumor growth. The mechanisms of these effects are due to Notch1 signaling activation, apoptosis induction and cell cycle arrest. These findings strongly support that SBHA is a promising candidate for further preclinical and clinical studies in MTC.
ACKNOWLEDGEMENTS
Financial Support: American Cancer Society Research Scholars Grant 05-08301TBE; National Institutes of Health RO1 CA109053; NIH-R21CA117117; American College of Surgeons George H.A. Clowes Jr. Memorial Research Career Development Award; Vilas Foundation Research Grant; Carcinoid Cancer Foundation Research Award; Doctors Cancer Foundation Award and the Society of Surgical Oncology Clinical Investigator Award.
The authors thank Mr. Eric Wendt for editing this manuscript.
Footnotes
SYNOPSIS
We previously reported that SBHA induces apoptosis in MTC cells in vitro. In this study, we demonstrated that SBHA inhibits MTC growth in vivo by inducing tumor cell apoptosis and cell cycle arrest. Therefore, SBHA is a promising candidate for further preclinical and clinical studies in MTC.
REFERENCES
- 1.Grozinsky-Glasberg S, Benbassat CA, Tsvetov G, et al. Medullary thyroid cancer: a retrospective analysis of a cohort treated at a single tertiary care center between 1970 and 2005. Thyroid. 2007;17:549–556. doi: 10.1089/thy.2006.0229. [DOI] [PubMed] [Google Scholar]
- 2.Greenblatt DY, Elson D, Mack E, et al. Initial lymph node dissection increases cure rates in patients with medullary thyroid cancer. Asian J Surg. 2007;30:108–112. doi: 10.1016/S1015-9584(09)60141-X. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Roberts JR, Ball DW, et al. Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg. 1998;227:887–895. doi: 10.1097/00000658-199806000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sippel RS, Chen H. Current management of medullary thyroid cancer. The Oncologist. doi: 10.1634/theoncologist.2007-0239. (in press) [DOI] [PubMed] [Google Scholar]
- 5.Mahlknecht U, Hoelzer D. Histone acetylation modifiers in the pathogenesis of malignant disease. Mol Med. 2000;6:623–644. [PMC free article] [PubMed] [Google Scholar]
- 6.Greenblatt DY, Cayo M, Ning L, et al. Suberoyl bishydroxamic acid inhibits cellular proliferation by inducing cell cycle arrest in carcinoid cancer cells. J Gastrointest Surg. 2007;11:1515–1520. doi: 10.1007/s11605-007-0249-1. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie S, Borrow J, Zhang XD, et al. Bim plays a crucial role in synergistic induction of apoptosis by the histone deacetylase inhibitor SBHA and TRAIL in melanoma cells. Apoptosis. 2006;11:2251–2265. doi: 10.1007/s10495-006-0283-6. [DOI] [PubMed] [Google Scholar]
- 8.Neuzil J, Swettenham E, Gellert N. Sensitization of mesothelioma to TRAIL apoptosis by inhibition of histone deacetylase: role of Bcl-XL downregulation. Biochem Biophys Res Commun. 2004;314:186–191. doi: 10.1016/j.bbrc.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 9.Ning L, Greenblatt DY, Kunnimalaiyaan M, et al. Suberoyl bishydroxamic acid activates Notch1 signaling and induces apoptosis in medullary thyroid carcinoma cells. The Oncologist. 2008;13:98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- 10.Leong SS, Hororszewicz JS, Shimaoka K, et al. A new cell line for study of human medullary thyroid carcinoma. In: Andreoli M, Monaco F, Robbins J, editors. Advances in thyroid neoplasia. Rome: Field Educational Italia; 1981. pp. 95–108. [Google Scholar]
- 11.Sippel RS, Carpenter JE, Kunnimalaiyaan M, et al. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:245–254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol Int. 2005;29:489–496. doi: 10.1016/j.cellbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Miremadi A, Oestergaard MZ, Pharoah PD, et al. Cancer genetics of epigenetic genes. Hum Mol Genet. 2007;16:28–49. doi: 10.1093/hmg/ddm021. [DOI] [PubMed] [Google Scholar]
- 14.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. The Oncologist. 2007;12:535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 15.Greenblatt DY, Vaccaro A, Jaskula-Sztul R, et al. Valproic acid activates Notch1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. The Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 16.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, et al. Overexpression of the Notch1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–39830. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 17.Kunnimalaiyaan M, Yan S, Wong F, Zhang YW, Chen H. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138:1137–1142. doi: 10.1016/j.surg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:636–642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 19.Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by Notch signaling. J Clin Endocrinol Metab. 2005;90:4350–4356. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 20.Blumenschein G, Lu C, Kies M, et al. Phase II clinical trial of suberoylanilide hydroxamic acid (SAHA) in patients (pts) with recurrent and/or metastatic head and neck cancer (SCCHN) Journal of Clinical Oncology, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004;22:5578. [Google Scholar]
- 21.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]


