Abstract
Traumatic brain injury (TBI) is a major cause of death and disability worldwide; however, no effective treatment has been clinically identified. Our recent studies show that the combination of collagen scaffolds with human bone morrow stromal cells (hMSCs) for treatment of TBI improves functional outcome and reduces the lesion volume when this combination was applied at day 4 after TBI in rats. The mechanisms underlying these benefits remain unclear. Whether further delayed treatment with this combination will provide benefits has not been investigated. In the present study, we investigated whether the delayed (7 days post injury) transplantation would have beneficial effects on functional and histological outcome and sought to elucidate underlying mechanisms of therapeutic action. Collagen scaffolds seeded with 3 × 106 hMSCs, scaffolds alone, 3 × 106 hMSCs alone, or saline were transplanted into the lesion cavity of the injured cortex 7 days after TBI. Sensorimotor function and spatial learning were measured. Corticocortical labeling with 1, 1″-dioleyl-3, 3, 3″, 3″-tetramethylindocarbocyanine methanesulfonate (DiI) was performed at day 36 after TBI. The rats were sacrificed 43 days after TBI, and the brain tissue was processed for DiI-labeling fiber and immunohistochemical analyses. The present data show that delayed transplantation of hMSCs or scaffolds seeded with hMSCs improved spatial learning and sensorimotor function, enhanced angiogenesis in the injured cortex and the ipsilateral hippocampus and increased DiI-labeled neural fiber length in the injured cortex. hMSC-seeded scaffolds may be a new and effective way to improve neurological function after TBI.
Keywords: Angiogenesis, neural fiber length, human bone marrow stromal cell, scaffolds, sensorimotor, spatial learning, traumatic brain injury
1. Introduction
The most prevalent and debilitating features in survivors of traumatic brain injury (TBI) are cognitive deficits and motor dysfunctions. To date, there is no effective treatment to promote functional recovery except for routine medical intervention and care (1998; Narayan et al., 2002; Royo et al., 2003). Thus, the development of improved treatment modalities would be of enormous clinical and economic benefit. Cellular therapies using neural stem/progenitor cells are promising approaches for the treatment of TBI. However, the clinical use of embryonic stem cells or fetal tissues is limited by ethical considerations and other scientific problems. Thus, human bone marrow stromal cells (hMSCs) could represent an alternative source of stem cells for cell therapies. hMSCs are mesoderm-derived cells, primarily resident in adult bone marrow.
Using three different routes (i.e., intraarterial, intravenous and intracranial) to administer hMSCs into injured rat brain (Lu et al., 2001; Mahmood et al., 2001a; Mahmood et al., 2001b), we have demonstrated that hMSC transplantation after TBI reduces neurological functional deficits although the lesion volume of cortex in the controlled cortical impact model employed is not significantly reduced. Engineered scaffolds have been used for delivery of neural stem cells after ischemia (Park et al., 2002) and for treating spinal cord injury (Bakshi et al., 2004). In a recent effort to optimize hMSC therapy for TBI, we used hMSC-impregnated collagen scaffolds as hMSC delivery vehicles and demonstrated that the scaffolds provide an effective platform for therapeutic delivery of hMSCs to injured tissue 4 days after TBI and improve cellular survival in the hippocampus and overall function for the intended repair (Lu et al., 2007).
The mechanisms underlying these benefits remain unclear. Whether further delayed treatment with this combination for TBI provides benefits has not been investigated. In the present study, we investigated the effects of the delayed treatment (7 days after injury) of hMSCs alone or hMSC-impregnated collagen scaffolds on functional and histological outcome in rats subjected to TBI induced by a controlled cortical impact (CCI) injury. We tested the hypothesis that scaffolds impregnated with hMSCs amplify angiogenesis and neural fiber length after TBI.
2. Results
2.1 Scaffold/hMSC treatment improves sensorimotor functional recovery after TBI
The higher the modified neurological severity score (mNSS), the worse the sensorimotor function (Lu et al., 2007a). Figure 1 shows that there is no significant difference in the mNSS scores between TBI + scaffold and TBI + saline groups (P>0.05). However, significantly improved scores were measured at days 14, 21, 28, and 35 after TBI in the TBI + hMSC and TBI + scaffold/hMSC groups, compared with the TBI + saline and TBI + scaffold groups (P<0.05). At days 21, 28, and 35, significantly lower (i.e., improved) scores were measured in the TBI + scaffold/hMSC group versus TBI + hMSC group (P<0.05). These data demonstrate that the delayed treatment (7 days post injury) for TBI with hMSCs and hMSC-populated scaffolds promotes recovery of sensorimotor functional deficits, and scaffold/hMSC treatment shows better outcome than hMSCs alone.
Figure 1.
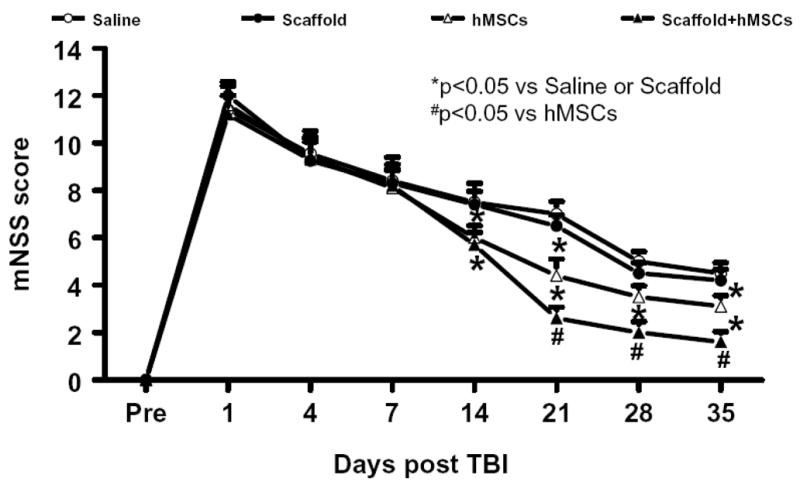
The plot shows the functional improvement detected on the modified neurological severity scores (mNSS). * P < 0.05, vs the saline or scaffold groups; # P < 0.05, vs the hMSC group. Data represent mean ± SD. N (rats/group) = 6.
2.2 Scaffold/hMSC treatment improves spatial learning
To test whether delayed scaffold/hMSC treatment promotes spatial learning in the relatively late phase (approximately 1 month) after TBI, rats were tested on the Morris water maze (MWM) during the last five days (from day 31 to day 35 after TBI) before dye injection. The mean percentages of time spent in the correct quadrant were significantly higher in the TBI + hMSC and TBI + scaffold/hMSC groups than in the TBI + saline and TBI + scaffold group at days 33, 34, and 35 after TBI (P<0.05, Figure 2). However, treatment with scaffold/hMSCs significantly improved spatial learning at 34 and 35 days after TBI as compared to TBI + hMSC group (P <0.05). These data demonstrate that both hMSCs and scaffold/hMSCs promote restoration of spatial learning in the relatively late phase after TBI. However, scaffold/hMSCs appear to be more effective in improving spatial learning outcome than hMSCs alone.
Figure 2.
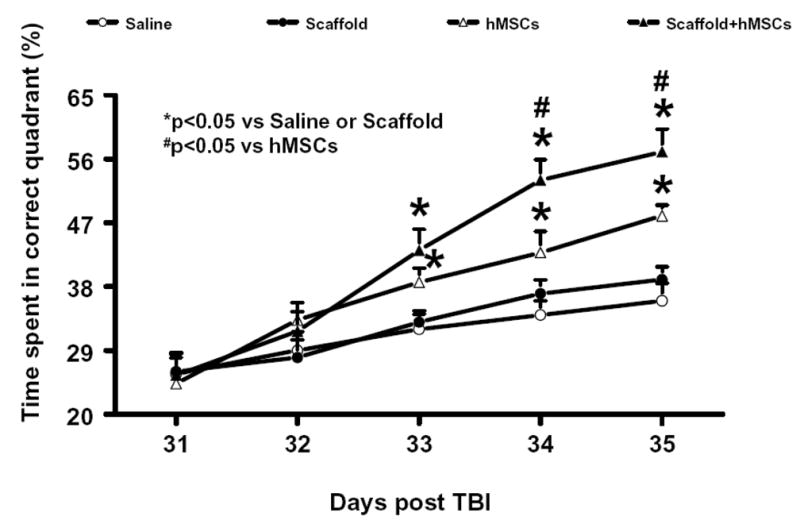
The plot shows the spatial learning deficits of rats from days 31 to 35 after TBI, and after TBI with different treatments. *P< 0.05, vs the saline or scaffold groups; # P <0.05, vs the hMSC group. Data represent mean ± SD. N (rats/group) = 6.
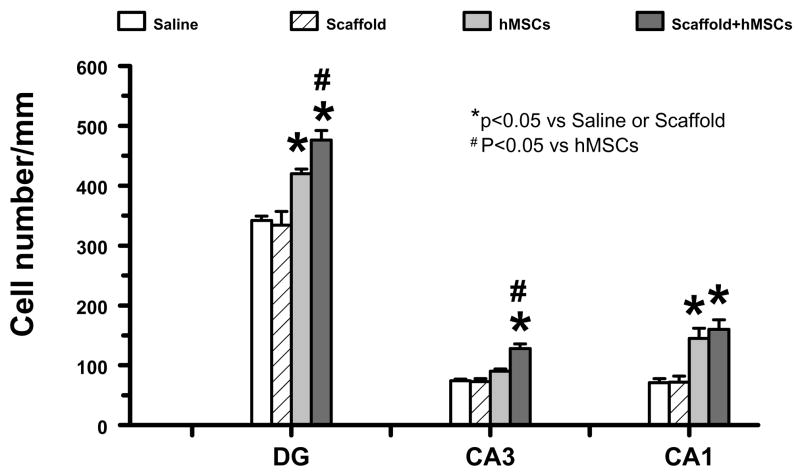
2.3 Scaffold/hMSC treatment increases neuronal cell density in the hippocampus after TBI
Although treatment with scaffold/hMSCs shows a tendency to reduce the lesion volume, this tendency did not reach a significant difference among all the groups (data not shown). However, scaffold/hMSC treatment significantly reduced neuronal cell loss in the dentate gyrus (DG), CA1, and CA3 of the ipsilateral hippocampus as compared to hMSCs alone (Figure 3). hMSCs alone also reduced the cell loss in the DG and CA1 but not in the CA3 region. Scaffold alone did not show any effect on the cell loss in these regions. The percentage of time rats spent in the correct quadrant was significantly and highly correlated to the cell number in the DG (r = 0.9814, p = 0.01858), the CA3 region (r = 0.9594, p = 0.04055), and CA1 region (r = 0.9794, p = 0.02059).
Figure 3.
The bar graph shows changes of cell numbers in the hippocampus among different groups. * P<0.05, vs the saline group; # P<0.05 vs the hMSC group. Data represent mean ± SD. N (rats/group) = 6.
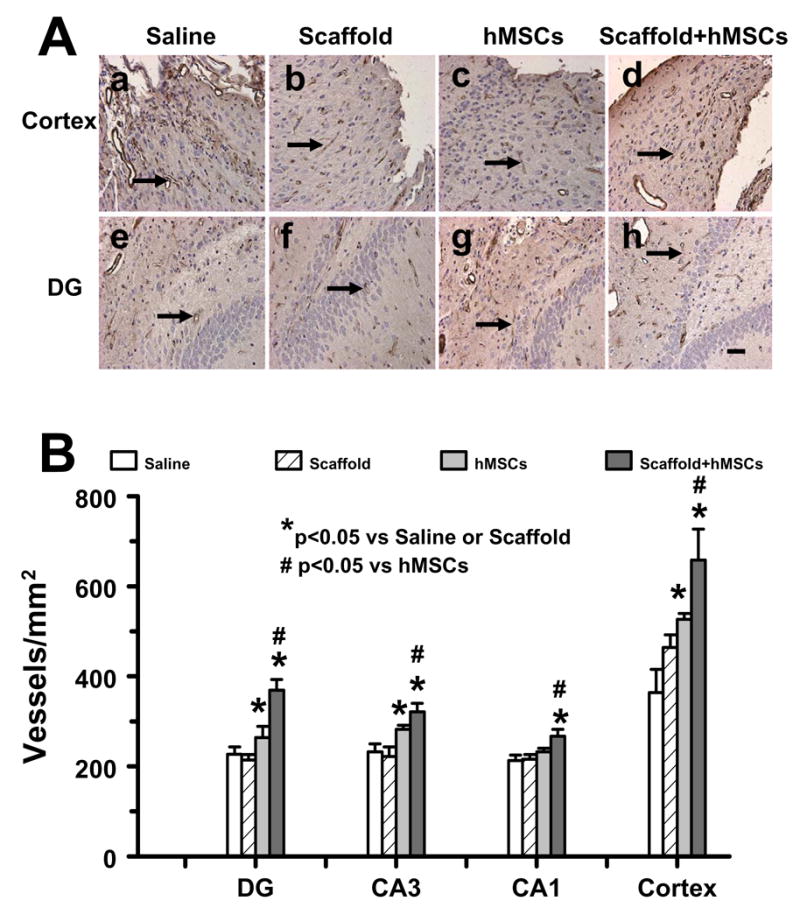
2.4 Scaffold/hMSC treatment enhances angiogenesis in the injured cortex and ipsilateral hippocampus
von Willebrandfactor (vWF)-staining has been used to identify vascular structure in the brain after TBI (Xiong et al., 2007). Figure 4 shows that there is no significant difference in the angiogenesis in the injured cortex and ipsilateral hippocampus between TBI + scaffold and TBI + saline groups (P>0.05). Treatment with hMSCs and scaffold/hMSCs significantly increased angiogenesis in the injured cortex and DG and CA3 region as compared to TBI + saline and TBI + scaffold group (P<0.05). However, treatment with scaffold/hMSCs significantly enhanced angiogenesis in these regions as compared to TBI + hMSC group (P<0.05). Interestingly, only scaffold/hMSCs significantly increased angiogenesis in the CA1 region (Figure 4B).
Fig. 4.
vWF staining for vascular structure in the ipsilateral DG and cortex among different groups at 43 days after TBI. The vasculature with vWF staining is counted (brown stained, A). Scale bar = 25μm (a–h). The density of vWF-stained vasculature is shown in (B). DG: dentate gyrus. * P < 0.05, vs the saline or scaffold groups; # P < 0.05, vs the hMSC group. Data represent mean ± SD. N (rats/group) = 6.
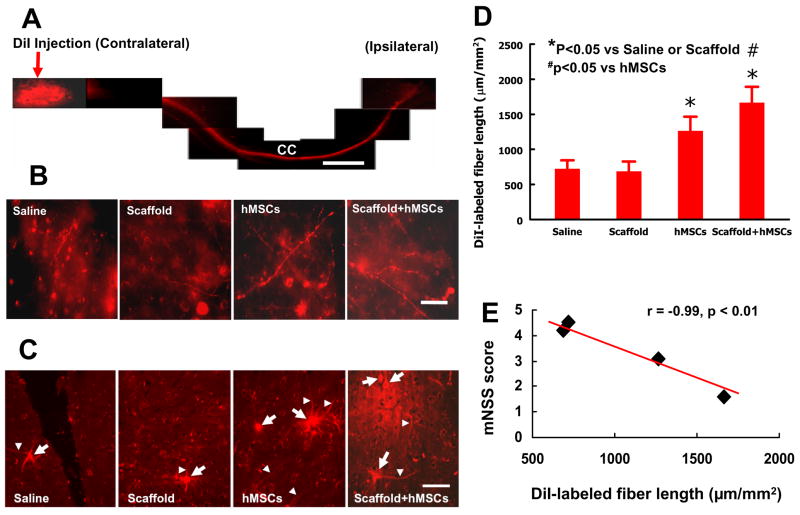
2.5 Scaffold/hMSC treatment increases DiI-labeled neural fiber length in the injured cortex
To investigate whether delayed transplantation of hMSCs or scaffold/hMSCs would affect neuronal projections extending across the corpus callosum (CC), DiI was stereotactically injected into the contralateral cortex 5 weeks after TBI. The DiI-labeled neural fibers were examined 1 week after DiI injection. Our data show that DiI was transported from the injected site in the contralateral hemisphere to the injured hemisphere through the CC (Figure 5A). Treatment with scaffold/hMSCs or hMSCs significantly increased the DiI-labeled neural fiber length in the injured cortex when compared to the scaffold alone or saline alone groups (Figure 5B and D, P<0.05). However, scaffold/hMSCs significantly increased DiI-labeled fiber length as compared to hMSCs alone (P <0.05). Figure 5C shows DiI-labeled neurons observed in the injured cortex. The number (per mm2) of DiI-labeled neurons in the injured cortex was 16 ± 5 for the TBI + saline group, 25 ± 7 for the TBI + scaffold group, 39 ± 8 for the TBI + hMSC group and 58 ± 7 for the TBI + scaffold/hMSC group. There is no significant difference between the TBI + saline and TBI + scaffold groups. The number of DiI-labeled neurons is significantly greater in the TBI + hMSC and TBI + scaffold/hMSC groups than that in TBI + saline and TBI + scaffold groups (P<0.05). However, scaffold/hMSCs significantly increased DiI-labeled neurons as compared to hMSCs alone (P<0.05). The increased number of DiI-labeled neurons suggests that treatment with hMSCs or scaffold/hMSCs promotes neural connectivity between contralateral uninjured and ipsilateral injured hemispheres. These neurons were retrogradely labeled by DiI because the axons of these neurons extend to the contralateral hemisphere where the dye was injected and transported from their axons back to the cell bodies in the ipsilateral hemisphere via the CC (Figure 5A). As presented above, functional outcome (mNSS) was significantly better (lower mNSS) in scaffold/hMSC and hMSC groups compared with scaffold alone or saline groups, with scaffold/hMSC group performing better than hMSC alone group (Figure 1). Also the mNSS scores at day 35 post injury were significantly correlated to the DiI-labeled neural fiber length (Figure 5E, r = − 0.99, p< 0.01).
Fig 5.
Neural fiber tracing with DiI cortical injection. DiI was stereotactically injected into the contralateral cortex (A). The DiI-labeled neural fibers (B) and DiI-labeled cells (C) were detected in the lesion boundary zone. Arrows indicate DiI-labeled fibers while arrowheads show cell bodies (C). The bar graph (D) shows DiI-labeled neural fiber length among different groups. The line graph (E) shows a significant correlation between the DiI-labeled fiber length and functional outcome (mNSS score at day 35). The scale bar = 1 mm (in A) and 25 μm (in B and C). CC: corpus callosum. * P < 0.05, vs the saline or scaffold groups; # P < 0.05, vs the hMSC group. Data represent mean ± SD. N (rats/group) = 6.
3. Discussion
Our present data demonstrated that delayed (7 days post injury) transplantation of scaffold/hMSCs improved spatial learning and sensorimotor function, reduced cell loss in the hippocampus, enhanced angiogenesis as well as increased neural fiber length after TBI compared to saline-, scaffold- and hMSC-treated groups.
Our previous studies have shown that transplantation (4 days after injury) of scaffolds populated with hMSCs reduces deficits of spatial learning and sensorimotor function as well as lesion volume after TBI (Lu et al., 2007a). In the present study, further delayed (7 days post injury) treatment with scaffold/hMSC did not show any significant effect on the lesion volume. Our previous study showed that the lesion volume remained relatively stable at days 7 and 35 after TBI induced by CCI (Xiong et al., 2008). The late treatment may not prevent the tissue loss. However, this delayed treatment with hMSCs or scaffold/hMSCs still exhibits significant improvement in functional outcome. Scaffold/hMSCs are more effective than hMSCs alone in improving functional outcome. MSCs significantly improved functional outcomes in animals when given 1 day after TBI (Mahmood et al., 2001b; Mahmood et al., 2005) or stroke (Li et al., 2002).
Administration of hMSCs at day 7 (Li et al., 2005) and 30 (Shen et al., 2007) after stroke and day 7 after spinal cord injury (Urdzikova et al., 2006) significantly improved long-term functional outcome in rats. The present study for the first time demonstrates that delayed (7 days post injury) transplantation of hMSCs and scaffold/hMSCs provided beneficial therapeutic effects after TBI in rats. hMSC-enhanced angiogenesis and increased DiI-labeled neural fiber length may contribute to improved sensorimotor functional recovery (lower mNSS score) while reduced cell loss and increased angiogenesis in the hippocampus could promote the spatial learning function evaluated using the modified Morris water maze test after TBI.
Reconstruction and regeneration of the central nervous system (CNS) following injury is a difficult task. However, treatment with transplanted cells such as neural progenitor cells (NPCs) or hMSCs is a promising approach that has resulted in various levels of functional recovery in animals that had experienced an experimental injury of the brain or spinal cord (Mahmood et al., 2005; Potter et al., 2008; Shen et al., 2007). Unfortunately, CNS injury often leads to significant tissue loss, limiting the survival and integration of transplanted NPCs or MSCs. Therefore, researchers have developed biomaterials that have been used to culture, transplant, and influence the differentiation and integration of transplanted NPCs or MSCs. Biomaterial scaffolds with a three-dimensional lattice can be engineered to support cells in vitro as well as serve as a temporary extracellular matrix after transplantation (Potter et al., 2008). In our present study, transplantation of scaffolds alone has no significant effect on functional outcome when compared with saline injection. Although hMSC treatment significantly improved functional outcomes compared to the saline group, the effect was reduced compared to that of the scaffolds populated with hMSCs, demonstrating that transplantation of scaffolds populated with hMSCs is an effective cell delivery vehicle for restoration and repair of neural injury after TBI (Lu et al., 2007a). The scaffolds, by temporally holding the hMSCs in space and maintaining proximity to the injured tissue, likely foster such constitutive reciprocal interactions between hMSCs and the injured brain tissue (Park et al., 2002).
The beneficial effect of MSCs after TBI is not likely attributed to cell replacement because only a fraction of transplanted MSCs survive and express parenchymal cell phenotypes after TBI (Lu et al., 2001) and stroke (Chen et al., 2001; Shen et al., 2007). The improvement in functional outcome observed after MSC treatment of TBI involves more than one mechanism. The surrounding injured brain tissue generates cytokines that stimulate and activate hMSCs to secrete growth factors, and also attract hMSCs to migrate into the lesion boundary zone (Lu et al., 2003). MSCs produce and induce within parenchymal cells many cytokines and trophic factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF) (Lu et al., 2003), morphogenetic proteins BMP2 and BMP4 or connexin 43 expression in astrocytes (Zhang et al., 2006). These factors, secreted by hMSCs as well as induced within parenchymal cells, influence several neural restorative functions such as synaptogenesis (Chopp and Li, 2002), angiogenesis (Chopp and Li, 2002; Qu et al., 2008), and neurogenesis (Chen and Chopp, 2006). Via these mechanisms, hMSCs inhibit the progressive chronic processes of cell death, or alternatively, repair the injured cerebral tissue. In concert with these restorative effects, MSCs significantly decrease glial scar formation and promote glial–axonal remodeling (Li et al., 2005). Thus, MSCs act in a pleiotropic way to stimulate injured brain remodeling after transplantation. In animal models, transplantation of MSCs into the spinal cord following injury enhances axonal regeneration and promotes functional recovery; MSC transplantation may promote axonal regeneration both by stimulating nerve growth via secreted factors and also by reducing the nerve-inhibitory effects of the extracellular molecules present (Wright et al., 2007). Our present study demonstrates that delayed treatment with hMSCs or scaffold/hMSCs after TBI increased DiI-labeled neural fiber length, indicating that hMSCs promotes neuronal connectivity by axonal projections or neurite outgrowth and elongation in the injured cortex. This finding was in agreement with a previous study with neural stem cells/scaffold to treat brain injury induced by hypoxia-ischemia (Park et al 2002). Increased neural connectivity may be one of the mechanisms for improvement in behavior after hMSC or scaffold/hMSC treatment for TBI. Our recent study demonstrated that transplantation of rat MSCs enhanced axonal sprouting and rewiring into the denervated spinal cord, which may facilitate functional recovery after focal cerebral ischemia (Liu et al., 2007). Further investigations are warranted to investigate whether hMSC or scaffold/hMSC-induced functional recovery after TBI occurs through mechanisms similar to those observed after stroke.
In conclusion, the present study shows that delayed transplantation of hMSCs or hMSC-seeded scaffolds improved spatial learning and sensorimotor functions, reduced ipsilateral hippocampal cell loss, enhanced ipsilateral cortical and hippocampal angiogenesis and increased DiI-labeled neural fiber length in the injured cortex after TBI in rats. hMSC-seeded scaffolds had better outcomes and may provide a new restorative therapy for TBI.
4. Experimental procedures
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Health System.
4.1 TBI Model
A controlled cortical impact (CCI) model of TBI in the rat was utilized for the present study (Dixon et al., 1991; Mahmood et al., 2004). Young adult male Wistar rats (300–400 g) were anesthetized intraperitoneally with 350 mg/kg/body weight chloral hydrate. Rectal temperature was kept at 37°C with a feedback-regulated water-heating pad. A controlled cortical impact device was used to induce the injury. Rats were placed in a stereotactic frame. Two 10-mm diameter craniotomies were performed adjacent to the central suture, midway between lambda and bregma. The second craniotomy allowed for movement of cortical tissue laterally. The dura was kept intact over the cortex. Injury was delivered by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 6-mm diameter tip at a rate of 4 m/s and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer.
4.2 Experimental Groups
Twenty-four male Wistar rats were randomly divided into four groups with 6 rats per group (i.e., TBI + saline, TBI + scaffold, TBI + hMSCs and TBI + scaffold/hMSCs). All treatments were performed 7 days after TBI. Rats in the TBI + scaffold group were subjected to TBI, and 7 days later, a scaffold was directly transplanted into the lesion cavity. Rats in the TBI + scaffold/hMSC group received transplantation of a scaffold seeded with 3 × 10 6 hMSCs. Rats in the TBI + hMSC group received 3 × 106 hMSCs injected directly into the lesion cavity 7 days after TBI. Rats in the TBI + saline group were subjected to TBI and injected with saline alone. The mNSS score was measured before and after TBI at different time points (1, 4, and 7 days, 2, 3, 4, and 5 weeks post injury). Spatial learning was evaluated in all rats by the modified Morris Water Maze (MWM) test during the last five days (31–35 days after TBI) before DiI injection.
4.3 Scaffold transplantation
Under aseptic conditions and general anesthesia with ketamine(40 mg/kg) and xylazine (8 mg/kg), a 1-cm incision was madealong the middle line of the scalp. The lesion cavity induced by TBI was exposed in the left hemisphere. Scaffolds were seeded with 3 × 106 hMSCs and transplanted into the lesion cavity of rats 7 days after TBI without removal of additional brain tissue, and subsequently covered by surgical foam (polyurethane foam), and the incision was closed with 4-0 absorbablegut surgical suture (Lu et al., 2007a).
4.4 Preparation of hMSCs
hMSCs were obtained from Cambrex Bioscience, Walkersville, Inc. (Walkersville, MD) and resuspended in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 0.1 mM nonessential amino acids, 100 U/ml penicillin and streptomycin, and 1 ng/ml basic fibroblast growth factor (bFGF), plated at 10 μl aspirate/cm2 in tissue culture flasks, and maintained at 37 °C in an atmosphere of 95% air and 5% carbon dioxide. All tissue culture components were obtained from Life Technologies (Rockville, MD). hMSCs were selected based on their ability to adhere to the tissue culture plastic after approximately 10 days in culture; non-adherent hematopoietic cells were removed during medium replacement. Medium was changed twice per week thereafter. hMSCs were subsequently detached using 0.25% trypsin/1 mM EDTA, replated at 5 × 103 cells/cm2, and cultured to 90% confluency to passage-one cells. hMSCs were subsequently frozen in 10% fetal calf serum (FCS) and 8% dimethyl sulfoxide (DMSO) in DMEM. They were then thawed and ex vivo expanded to passage-three cells as described above and subsequently utilized for scaffold seeding (Lu et al., 2007a).
4.5 Preparation of hMSC-seeded scaffolds
Ultrafoam scaffolds (collagen type I) were obtained from commercial sources (Davol, RI, USA). Scaffolds were pre-wet overnight at 4°C in culture medium consisting of DMEM supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, and 1 ng/ml of bFGF (Life Technologies, Rockville, MD). The scaffolds were then aseptically transferred using tweezers (1 scaffold per tube) to a 50-ml sterile centrifuge tube (Falcon) and allowed to sit at the bottom of the tube. Following trypsinization of the hMSCs from ex vivo expansion conditions, they were resuspended thoroughly and transferred (3 × 106 hMSCs per scaffold) into 200 μl of culture medium. 100 μl of culture medium was then applied two times successively to opposite sides of the body of the cylindrical scaffold. The scaffold and cell solution were incubated for 30 minutes in a humidified incubator to facilitate primary cell seeding. The scaffolds were agitated gently within the solution manually twice every 15 min during this time. Following primary seeding, the centrifuge tubes were filled with an additional 3 ml of culture medium and placed within a humidified incubator and incubated overnight until scaffold implantation (Lu et al., 2007a). Prior to implantation, the scaffolds were collected from the medium.
4.6 Evaluation of neurological outcome
All functional tests were performed by investigators blinded to the treatment status. Neurological functional measurement was performed using an mNSS test(Chen et al., 2001). The test was carried out on all rats preinjury and on days 1, 4, 7, 14, 21, 28, and 35 after TBI. The mNSS is a composite of the motor (muscle status, abnormal movement), sensory (visual, tactile and proprioceptive) and reflex tests and has been employed in previous studies (Lu et al., 2007a). In this TBI model, injury in the left hemispheric cortex of rats causes sensory and motor functional deficiency with elevated scores on motor, sensory, and Beam Balance Tests in the early phase after injury (day 1 after injury). Absent reflexes and abnormal movements can be measured on rats with severe injury. Slow recovery in asymmetry deficiency as reflected by Beam Balance Test results has been reported in unilateral brain injuries including TBI (Lu et al., 2007a) and ischemia (Chen et al., 2001). This test is suitable for evaluating long-term neurological function after unilateral brain injury.
4.7 Spatial learning function
To detect spatial learning deficits, a recent version of the Morris water maze (MWM) test was used (Choi et al., 2006; Lu et al., 2005; Xiong et al., 2007). All animals were tested during the last five days (i.e., from 31–35 days after TBI) before DiI injection. Data collection was automated by the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA.). For descriptive data collection, the pool was subdivided into four equal quadrants formed by imaging lines. At the start of a trial, the rat was placed randomly at one of four fixed starting points, randomly facing either toward the wall or the center (designated North, South, East and West) and allowed to swim for 90 seconds or until they could find the platform. If the animal found the platform, it was allowed to remain on it for 10 seconds. Throughout the test period the platform was located in the NE quadrant 2 cm below water in a randomly changing position, including locations against the wall, toward the middle of the pool or off-center, but always within the target quadrant. If the animal failed to find the platform within 90 seconds, it was placed on the platform for 10 seconds. The trial was terminated and a maximum score of 90 seconds was assigned. If the animal reached the platform within 90 seconds, the percentage of time traveled within the NE (correct) quadrant was calculated relative to the total amount of time spent swimming before reaching the platform and employed for statistical analysis. The advantage of this version of the water maze is that each trial or set of trials takes on the key characteristics of a probe trial because the platform is not in a fixed location within the target quadrant (Choi et al., 2006).
4.8 Dye injection
For corticocortical tracing, the dye injection was performed 7 days before sacrifice (i.e., Day 36 after TBI). Under deep anesthesia with an intraperitoneal injection of ketamine (44 mg/kg) and xylazine (13 mg/kg), rats underwent midline incision and re-exposure of the right hemisphere dura mate between bregma and lambda. 2.5% DMSO solution of 1,1″-dioleyl-3,3,3″,3″-tetramethylindocarbocyanine methanesulfonate (Delta 9-DiI; AnaSpec, San Jose, CA, USA) was injected through a finely drawn glass capillary with an electric injection system into four points in the left motorsensory cortex (Paxinos and Watson, 1986) (100 nl per injection; stereotaxic coordinates: 2, 3, 4, and 5 mm posterior to the bregma, 3 and 4 mm lateral to the midline, 1.5 mm depth from the surface of the cortex). The micropipette remained in place for 4 min after completion of the injection. If cortical vessels were encountered at the intended incision site, the site was moved immediately rostral or caudal to avoid the cortical vessel (Liu et al., 2007).
4.9 Tissue preparation and measurement
Animals were sacrificed under deep ketamine anesthesia at 7 days after DiI injection (i.e., 43 days after TBI). Rats were perfused transcardially with saline, followed by 4% paraformaldehyde. The brains were immersed in 4% paraformaldehyde overnight. The brain was cut into seven equally spaced (2 mm) coronal blocks using a rat brain matrix (Activational System, Warren, MI, USA). The brain blocks were processed for vibratome sections (100 μm). The DiI labeling in the red fibers on brain sections was analyzed with a Bio-Rad MRC 1024 (argon and krypton) laser-scanning confocal imaging system mounted onto a Zeiss microscope (Bio-Rad, Cambridge, MA, USA) (Liu et al., 2007). Microscopic data were acquired with a 10× objective or a 40× oil immersion objective lens. The length of DiI-labeled fibers were analyzed with NIH image software (Image J) based on the average of three histology slides from 10 continuous sections for each animal (Liu et al., 2007). After vibratome sectioning, the rest of the brain blocks were embedded in paraffin. A series of adjacent 6-μm-thick sections were cut from each block in the coronal plane and stained with hematoxylin and eosin. To measure the lesion volume, the sections were traced using a Global Laboratory image-analysis system (Data Translation, Marlboro, MA, USA). The indirect lesion area was calculated as the intact area of the ipsilateral hemisphere subtracted from the area of the contralateral hemisphere (Swanson et al., 1990). Lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere (Lu et al., 2007a).
4.10 Immunohistochemical staining for angiogenesis
To identify vascular structure, coronal sections were immunohistochemically stained with von Willebrandfactor (vWF) antibody (Xiong et al., 2007). Brain sections were deparaffinized and then incubated with 0.4% Pepsin solution at 37 °C for 1 h. After washing, the sections were blocked with 1% BSA at room temperature for 1 h, and then incubated with rabbit anti-human vWF (1:200; DakoCytomation, Carpinteria, CA) at 4 °C overnight. After washing, sections were incubated with biotinylated anti-rabbit antibody (1:200; Vector Laboratories, Inc., Burlingame, CA) and then with an avidin-biotin-peroxidase system, visualized with diaminobenzidine (brown) and counterstained with hematoxylin. vWF-stained vascular structures in the ipsilateral hippocampus and injured cortex were examined at 20× magnification and counted (Xiong et al., 2007).
4.11 Estimates of cell number and angiogenesis
Cell and vessel counts were performed by observers blinded to the individual status of the animals. Measurements of cells and vessels were performed on paraffin-embedded sections (6-μm thickness). We counted cells using the principles of stereology (Zhang et al., 2001). The cell and vessel counting results after stroke and TBI are consistent in our previous studies using this method (Chen et al., 2004; Lu et al., 2007b; Zhang et al., 2001; 2002; Xiong et al., 2008). This method permits a meaningful comparison of differences between groups. To test the effect of the hMSC-seeded scaffolds on the hippocampus, the density (cells/mm) of granular cells in the DG and pyramidal cells in the CA1 and CA3 region was calculated on the H&E stained slides (Lu et al., 2007b; Xiong et al., 2008). Although H&E staining is not a neuron-specific stain, the morphological characteristics of neuronal cells in the DG, CA3 and CA1 regions allow us to count them.
4.12 Statistics
All data are presented as the means with standard deviations (SD). For lesion volume, cell counting, DiI-labeled neural fiber length and vWF-stained vascular density, a one-way analysis of variance (ANOVA) followed by post hoc Student-Newman-Keuls (SNK) tests was used to compare the difference between different groups. Data were analyzed by ANOVA for repeated measurements of functional tests (MWM test and mNSS scores). Statistical significance was set at P<0.05.
Acknowledgments
This work was supported by NIH grants RO1 NS042259, RO1 NS 062002, PO1 NS42345 and P41 EB002520.
References
- Rehabilitation of persons with traumatic brain injury. NIH Consensus Statement. 1998;16:1–41. [PubMed] [Google Scholar]
- Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J Neurosurg Spine. 2004;1:322–9. doi: 10.3171/spi.2004.1.3.0322. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–8. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–8. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3:466–73. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Woodlee MT, Hong JJ, Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods. 2006;156:182–93. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–62. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–17. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Qu R, Shen L, Gao Q, Zhang X, Lu M, Savant-Bhonsale S, Borneman J, Chopp M. Axonal sprouting into the denervated spinal cord and synaptic and postsynaptic protein expression in the spinal cord after transplantation of bone marrow stromal cell in stroke rats. Brain Res. 2007;1149:172–80. doi: 10.1016/j.brainres.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18:813–9. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Chopp M. Biologic transplantation and neurotrophin-induced neuroplasticity after traumatic brain injury. J Head Trauma Rehabil. 2003;18:357–76. doi: 10.1097/00001199-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–7. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007a;61:596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. discussion 602–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, Mahmood A, Chen J, Li Y, Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007b;24:1132–46. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001a;49:1196–203. discussion 1203–4. [PubMed] [Google Scholar]
- Mahmood A, Lu D, Yi L, Chen JL, Chopp M. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J Neurosurg. 2001b;94:589–95. doi: 10.3171/jns.2001.94.4.0589. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–93. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57:1026–31. doi: 10.1227/01.neu.0000181369.76323.50. discussion 1026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–57. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–7. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotactic Coordinates. 2. San Diego: Academic Press; 1986. [Google Scholar]
- Potter W, Kalil RE, Kao WJ. Biomimetic material systems for neural progenitor cell-based therapy. Front Biosci. 2008;13:806–21. doi: 10.2741/2721. [DOI] [PubMed] [Google Scholar]
- Qu C, Mahmood A, Lu D, Goussev A, Xiong Y, Chopp M. Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. 2008;1208:234–9. doi: 10.1016/j.brainres.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo NC, Shimizu S, Schouten JW, Stover JF, McIntosh TK. Pharmacology of traumatic brain injury. Curr Opin Pharmacol. 2003;3:27–32. doi: 10.1016/s1471-4892(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23:1379–91. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- Wright KT, El Masri W, Osman A, Roberts S, Chamberlain G, Ashton BA, Johnson WE. Bone marrow stromal cells stimulate neurite outgrowth over neural proteoglycans (CSPG), myelin associated glycoprotein and Nogo-A. Biochem Biophys Res Commun. 2007;354:559–66. doi: 10.1016/j.bbrc.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Lu D, Qu C, Goussev A, Schallert T, Chopp M. Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res. 2007;1185:301–12. doi: 10.1016/j.brainres.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, Chopp M. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–21. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, Chopp M. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience. 2006;141:687–95. doi: 10.1016/j.neuroscience.2006.04.054. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Zhang L, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–80. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]