Abstract
Objective
To determine the frequency and possible cognitive effect of histological Alzheimer’s disease (AD) in autopsied older nondemented individuals.
Design
Senile plaques (SPs) and neurofibrillary tangles (NFTs) were assessed quantitatively in 97 cases from 7 Alzheimer’s Disease Centers (ADCs). Neuropathological diagnoses of AD (npAD) were also made with four sets of criteria. Adjusted linear mixed models tested differences between participants with and without npAD on the quantitative neuropathology measures and psychometric test scores prior to death. Spearman rank-order correlations between AD lesions and psychometric scores at last assessment were calculated for cases with pathology in particular regions.
Setting
Washington University Alzheimer’s Disease Research Center.
Participants
Ninety-seven nondemented participants who were age 60 years or older at death (mean = 84 years).
Results
About 40% of nondemented individuals met at least some level of criteria for npAD; when strict criteria were used, about 20% of cases had npAD. Substantial overlap of Braak neurofibrillary stages occurred between npAD and no-npAD cases. Although there was no measurable cognitive impairment prior to death for either the no-npAD or npAD groups, cognitive function in nondemented aging appears to be degraded by the presence of NFTs and SPs.
Conclusions
Neuropathological processes related to AD in persons without dementia appear to be associated with subtle cognitive dysfunction and may represent a preclinical stage of the illness. By age 80–85 years, many nondemented older adults have substantial AD pathology.
Keywords: preclinical Alzheimer’s disease, nondemented aging, neuropathological Alzheimer’s disease
Introduction
Senile plaques (SPs) and neurofibrillary tangles (NFTs), the neuropathological hallmarks of Alzheimer’s disease (AD),(Khachaturian, 1985) are not limited to individuals with dementia of the Alzheimer type (DAT) but also may be present in the brains of cognitively normal older adults.(Tomlinson et al., 1968; Crystal et al., 1988; Katzman et al., 1988; Price et al., 1991; Dickson et al., 1992; Troncoso et al., 1996; Troncoso et al., 1998; Hulette et al., 1998; Schmitt et al., 2000; Knopman et al., 2003) Neuropathological criteria for AD rely on densities of SPs and NFTs to discriminate AD from aging, as the distinction between the two conditions has been thought to be quantitative rather than qualitative.(Tomlinson et al., 1970; Arriagada et al., 1992) Alternatively, the presence of SPs and NFTs in nondemented older adults may represent AD at a stage prior to its clinical expression,(Morris et al., 1996; Price & Morris, 1999; Troncoso et al., 1998; Hulette et al., 1998; Schmitt et al., 2000) in which the neuropathological lesions of AD accumulate over many years before sufficient synaptic and neuronal damage occurs to produce the symptoms of AD. This notion of a preclinical (presymptomatic) stage of AD is consistent with similar latent stages in other neurodegenerative disorders.(Dickson et al., 2008) Although it is possible to explain the presence of SPs and NFTs without apparent dementia in other ways(Berlau et al., 2007), a continuous neuropathologic process for AD, regardless of clinical status, is supported by observations that the mechanisms responsible for SPs and NFTs in nondemented older adults appear identical to those found in AD(Haroutunian et al., 1998; Haroutunian et al., 1999) and the distribution of SPs and NFTs in nondemented older adults corresponds with the hierarchical topographical progression associated with symptomatic AD.(Arriagada et al., 1992; Price & Morris, 1999; Price et al., 1991)
Previous studies of neuropathological AD in nondemented aging have been based on cases collected at a single site(Price & Morris, 1999; Galvin et al., 2005; Bennett et al., 2006), and comparison of results has been difficult because neuropathological methods and criteria differed in these studies. Such methodological differences, combined with relatively small sample sizes in all but one study,(Bennett et al., 2006) may contribute to conflicting results on the relationships between SPs or NFTs and cognition in nondemented aging. Some studies have reported a negative effect for episodic memory only,(Hulette et al., 1998; Schmitt et al., 2000; Bennett et al., 2006) without effects in other cognitive domains, while other studies found no relationship between AD pathology and any cognitive measure.(Knopman et al., 2003; Driscoll et al., 2006) We therefore sought to provide a uniform assessment of neuropathological markers of AD in a large sample of nondemented older adults whose cognitive status had been evaluated antemortem at several Alzheimer’s Disease Centers (ADCs) and to evaluate relationships between these markers and cognitive function.
Material and Methods
Participants
This study capitalized on the National Alzheimer Coordinating Center-supported Neuropsychological Database Initiative (NDI; M Grundman, PI) that merged longitudinal clinical and cognitive data from more than 4,000 cognitively normal older ADC participants. Individuals in the NDI who came to autopsy at 7 participating ADCs, with a Clinical Dementia Rating (CDR)(Morris, 1993) of 0 (indicating the absence of dementia and of mild cognitive impairment) within 2 years of death, and were at least 60 years of age at death were eligible for this study. Ninety-seven cases met these criteria; 20 from the University of Kentucky, 18 from Washington University, 16 each from Mayo Clinic and Duke University, 14 from the University of California, San Diego, 9 from Oregon Health Sciences University, and 4 from the University of Rochester. The mean interval from last ADC assessment to death in the 97 cases was 0.7 +/− 0.5 years. Clinicians at each ADC verified the nondemented status for each case (the neuropathological diagnoses generated at the ADC performing the autopsy were not included in the eligibility criteria).
In addition, 11 cognitively impaired cases with CDR greater than 0 were submitted from 6 of the 7 ADCs. These 11 cases were assessed together with the nondemented cases, and all cases were coded so that the central laboratory was blind to the cognitive status of each case. Data from cases with CDR > 0 are not reported here because they did not provide an adequately sized sample.
Clinical and Psychometric Measures
Clinical variables available for this study included age at death, gender, educational attainment, CDR, and Mini Mental Status Examination (MMSE(Folstein et al., 1975)) scores at last ADC assessment. Apolipoprotein E genotypes were not available. Cognitive data included in the NDI database were available for Logical Memory–Delayed from the Wechsler Memory Scale-Revised;(Wechsler, 1987) Category Fluency (animals);(Goodglass & Kaplan, 1983) Boston Naming;(Goodglass & Kaplan, 1983) Trailmaking A and B;(Armitage, 1946) and Digit Symbol from the Wechsler Adult Intelligence Scale.(Wechsler, 1997) These tests measure episodic memory, semantic knowledge, attention, speeded psychomotor performance, and executive function. Not all individuals completed all measures.
Neuropathological Procedures
Each ADC provided a series of 10 unstained paraffin sections (10μm) of formalin-fixed tissue from each of five cerebral regions for each case: midfrontal cortex, superior/middle temporal gyrus, occipital cortex (Brodmann area 17, 18, or 19), medial temporal lobe (one block through the entorhinal cortex and rostral hippocampus, and a second, optional block through the amygdala), and midbrain. These regions were based on those proposed for the standard neuropathological assessment of AD.(Mirra et al., 1991) The Neuropathology Core (JLP, DWM) of Washington University’s Alzheimer’s Disease Research Center served as the “central laboratory” to provide a standard and uniform neuropathological assessment of each case and avoid the variability introduced by different methods, criteria, and interpretations used at individual ADCs.(Mirra et al., 1994; Alafuzoff et al., 2006) The central laboratory’s staining methods included hematoxylin and eosin, modified Bielschowsky, Gallyas, and Nissl stains, as well as immunohistochemical procedures with anti-amyloid-beta (Aβ; monoclonal antibody “10D5”, gift of Athena Neurosciences and Elan) and anti-paired helical filaments (PHF; monoclonal antibody “PHF-1”, gift of Drs Sharon Greenberg and Peter Davies, Albert Einstein Medical School, Bronx, NY). The anti-Aβ and anti-PHF stains were done both separately on different sections (Vectastain ABC peroxidase kit, DAB substrate) and as a double stain on the same sections, using different secondary antibody kits and chromogens so that Aβ was stained red (Vectastain ABC alkaline phosphatase kit, Vector red substrate) and PHF stained black (Vectastain ABC peroxidase kit, DAB-Ni substrate).
The central laboratory generated independent neuropathological diagnoses for each of the 97 nondemented cases. The diagnoses were done by joint examination of each case by DWM and JLP, using a double-headed microscope to arrive at a consensus neuropathological diagnosis. [Note: the neuropathological diagnoses generated by the central laboratory did not always coincide with the diagnoses generated at the individual sites]. Both observers were “blind” to the clinical and CDR status of the cases and to the neuropathological findings determined by the ADCs contributing the cases.
Each case was assessed in accordance with 4 published criteria for the neuropathological diagnosis of AD. Consensus criteria reported by Khachaturian(Khachaturian, 1985) are based on the highest density of total SPs (diffuse and neuritic) in any cortical field, adjusted for age (the greater the age of the individual, the greater the density required for diagnosis). The Washington University criteria(Berg et al., 1998; McKeel et al., 2004) modify the Khachaturian criteria in three ways: 1) rather than relying on the highest SP density in a single field, average SP density across 10 adjacent neocortical fields (1 mm2) is used; 2) SP densities are not adjusted for age; and 3) at least one neocortical NFT is required. Both Khachaturian and Washington University criteria yield dichotomous outcomes (a diagnosis of either npAD or no-npAD) and are the only criteria that consider diffuse SPs. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)(Mirra et al., 1991) criteria use a semiquantitative assessment of neuritic SP density and are age-adjusted to stipulate three levels of certainty that dementia is explained by the neuropathological diagnosis: Possible, Probable, and Definite AD. Finally, criteria developed at a conference sponsored by the National Institute of Aging and the Reagan Institute of the Alzheimer’s Association (NIA-Reagan)(National Institute on Aging & Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease, 1997) combine CERAD criteria for neuritic SPs and the neurofibrillary staging system proposed by Braak and Braak(Braak & Braak, 1991) to characterize individuals in terms of three probabilistic diagnoses (Low, Intermediate, and High) that dementia during life was due to AD. The NIA-Reagan criteria were not intended to characterize nondemented individuals or those with incipient dementia.(National Institute on Aging & Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease, 1997) In this report, the Washington University criteria were considered “primary” because they were based on discriminative analyses of neuropathological data.(McKeel et al., 2004) In practice and research, however, the CERAD and NIA-Reagan criteria are much more commonly used.(Schmitt et al., 2000; Knopman et al., 2003; Bennett et al., 2006)
Braak neurofibrillary staging was determined for all cases. Briefly, Braak Stage 0 corresponds to absence of NFTs, Stages I–II correspond to NFTs limited to entorhinal-perirhinal cortex, Stages III–IV correspond to NFTs additionally in hippocampus and inferior temporal cortex and Stages V–VI correspond to NFTs distributed in wider neocortical areas.
Quantitative Assessment
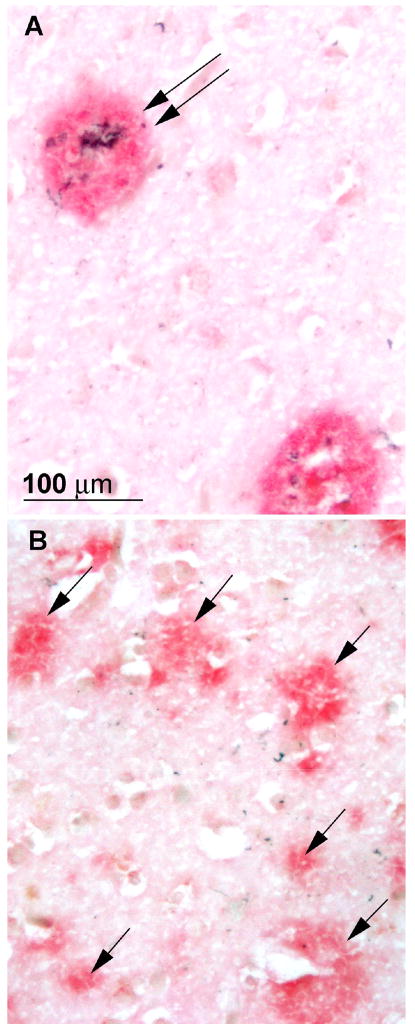
The central laboratory assessed SPs and NFTs quantitatively with the use of computerized sampling and the Computer Assisted Stereological Toolbox (CAST) system (Olympus America, Center Valley, PA 18034-0610). Analysis was based on Bielschowsky-stained sections because they were more consistent, given the variable state of fixation of the material from different sources, although immunohistochemical sections (singly stained for Aβ or PHF, or double stained for both Aβ and PHF) were routinely examined and compared with the silver stained sections. Diffuse SPs had a distinct concentration of Aβ staining without neuritic pathology; neuritic plaques had dystrophic neurites (with PHF) within an Aβ plaque and/or had a dense amyloid core (Figure 1). Variables yielded by the quantitative analysis included NFT density, diffuse SP density, neuritic SP density, and percent area occupied by SPs (plaque burden) in limbic structures (entorhinal and perirhinal cortex, field CA1 of the hippocampus, and the amygdala) and neocortical areas (middle frontal gyrus, superior temporal gyrus, and occipital cortex). Because the number of sections through each region was limited, total numbers of lesions were not obtained; only the density was measured for each marker (other than plaque burden). Although density measures may be affected by tissue shrinkage, the uniform treatment of the tissue (formalin fixation and paraffin embedding) and uniform staining in the central laboratory reduced variability. Plaque burden was defined as the percent area occupied by plaques in relation to the total area sampled and is unaffected by tissue shrinkage. Plaque burden and NFT density were least dependent on fixation differences between different cases.
Figure 1.
Photomicrographs of neuritic (A, double arrow) and diffuse plaques (B, single arrows), from adjacent areas of the temporal cortex of a case that was rated npAD with the Khachaturian and Washington University criteria, probable AD with CERAD criteria, and intermediate probability of AD with NIA-Reagan criteria. Stained with a double immunohistochemical procedure in which Aβ is stained red and paired helical filaments are stained black.
Statistical Analyses
Analyses were performed using SAS statistical software (SAS Institute, Cary, NC). General linear mixed models (PROC MIXED) were used to test differences between two groups of cases, one meeting neuropathological criteria for AD (npAD) and the other without neuropathological AD (no-npAD) on age at death, time between last assessment and death, and years of education while adjusting for an ADC effect by including that effect as a random variable. General linear mixed models were also used to test differences between the npAD and no-npAD groups after adjustment for age, sex, interval between last assessment and death, and education on the quantitative neuropathology variables, and the psychometric test scores at last assessment.
The ability of diffuse SPs in the neocortical and limbic areas to predict a neuropathological diagnosis of AD as determined by the CERAD and NIA-Reagan criteria (which do not incorporate diffuse SPs) was examined by calculating the area under the Receiver Operating Characteristic (ROC) curve, and computing sensitivity values (holding specificity fixed at 80%) for each lesion type. Subsequent comparisons of areas under the ROC curves were done using Fisher’s z-transformation in order to examine the predictive power of all lesions.
To assess whether neuropathological lesions are associated with cognitive performance, Spearman rank-order correlations between average lesion densities and psychometric scores at last assessment were calculated for cases with lesions in the specified brain regions. In the general linear mixed models, the ROC analyses, and calculation of sensitivity values, the quantitative neuropathology variables were first transformed by addition of 0.5 to each value (to account for cases with no pathology) and then conversion to the natural log.
Results
The 97 autopsied nondemented individuals had an average age ± SD at death of 84.3±8.6 years. There were 55 women, the mean years of education was 15.4±2.9, and the mean MMSE score at last assessment was 28.1±2.1.
Neuropathological Assessment
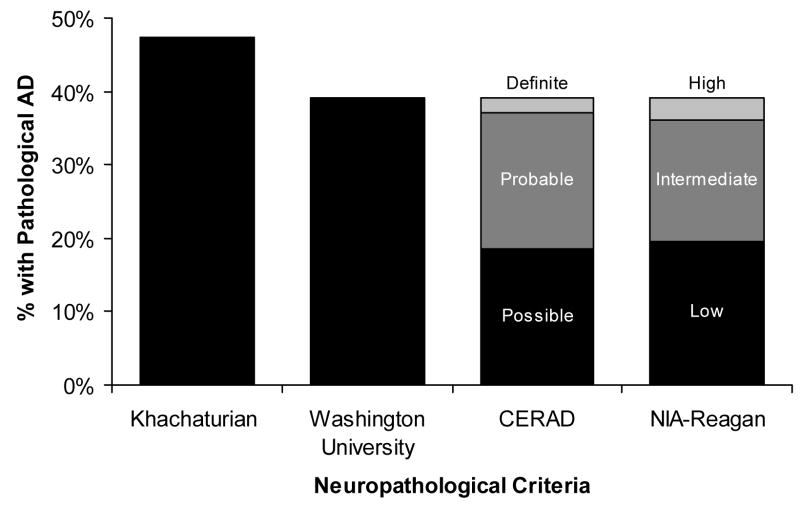
A substantial fraction of cases had lesions consistent with a neuropathological diagnosis of AD regardless of the criteria used. Figure 2 shows the percentage of cases meeting the dichotomous outcomes for npAD with the Khachaturian and Washington University criteria and the percentage of cases in each of the 3 levels denoting AD lesions with the CERAD and NIA-Reagan criteria. The least strict of the 4 sets of criteria, those proposed by Khachaturian, resulted in a neuropathological diagnosis of AD in 46 of the 97 cases (47%). The Washington University criteria yielded a neuropathological diagnosis of AD in 38 cases (39%), identical to the number of cases meeting any of the 3 levels of npAD using CERAD criteria (19% Possible, 19% Probable, and 2% Definite AD; 39% with all levels combined,) and any of the 3 levels of the NIA-Reagan criteria (20% Low, 16% Intermediate, and 3% High; 39% combined). [Note: Many investigators reserve the diagnosis of npAD only for cases meeting “Probable” and “Definite” AD with CERAD criteria or “Intermediate” and “High” probability with NIA-Reagan criteria; using these definitions, 21% were diagnosed with npAD by the CERAD Probable/Definite criteria, and 19% by the NIA-Reagan Intermediate/High criteria.] The WU, CERAD and NIA-Reagan criteria identified the same npAD cases, except for 2 cases diagnosed as npAD with the WU criteria that were not recognized by CERAD or NIA-Reagan, and 2 cases recognized by CERAD and NIA-Reagan that were not diagnosed as npAD by WU criteria.
Figure 2.
Classification of cases by four neuropathological criteria (Khachaturian, Washington University, CERAD, NIA-Reagan), N=97.
Braak neurofibrillary stages in the 97 cases ranged from 0 (no NFTs observed) to V (widespread neocortical tangles), with a median value of Stage II (Stage 0, n=4; Stage I, n=31; Stage II, n=36; Stage III, n=15; Stage IV, n=8; Stage V, n=3). No cases met criteria for stage VI (very pronounced neurofibrillary changes throughout all parts of cortex and in many subcortical structures). The 4 stage 0 cases were relatively young, aged 66, 69, 72, and 72 years at death. Older age was significantly correlated with higher Braak stages (Spearman r=.372, p<.001), consistent with observations that NFTs increase with age, even in the absence of SPs.(Arriagada et al., 1992; Price & Morris, 1999)
Comparison of npAD and no-npAD cases
There were no differences in mean age at death, interval between last clinical assessment and death, education, or psychometric performance at last assessment before death between cases with npAD versus those without npAD using any of the 4 criteria (even when restricted to Probable and Definite CERAD and Intermediate and High NIA-Reagan criteria) (Table 1).
Table 1.
Sample characteristicsa for nondemented individuals with and without npAD (Washington University criteria).
| No-npAD (N=59) | npAD (N=38) | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean | SE | N | Mean | SE | p-value | |
| Age at death (y) | 59 | 83.7 | 1.4 | 38 | 85.1 | 1.6 | .43 |
| Interval (y) from last assessment to death | 59 | 0.7 | 0.1 | 38 | 0.7 | 0.1 | .41 |
| Education (y) | 59 | 15.1 | 0.6 | 37 | 15.7 | 0.6 | .27 |
| MMSEb | 44 | 28.2 | 0.3 | 30 | 28.1 | 0.4 | .85 |
| Logical Memory Delayedb | 18 | 22.1 | 5.0 | 11 | 22.5 | 5.2 | .88 |
| Boston Namingb | 31 | 54.0 | 0.9 | 23 | 54.1 | 1.1 | .95 |
| Trailmaking Ab | 37 | 46.2 | 8.9 | 25 | 50.6 | 9.2 | .55 |
| Trailmaking Bb | 29 | 102.0 | 15.3 | 18 | 115.0 | 16.1 | .29 |
| Category Fluencyb | 36 | 16.7 | 0.9 | 23 | 15.5 | 1.2 | .44 |
| Digit Symbolb | 16 | 33.5 | 3.1 | 13 | 35.3 | 3.2 | .70 |
Abbreviations: SE = Standard Error, MMSE = Mini-Mental State Test
Least-square mean scores for psychometric tests at last assessment before autopsy after adjustment for effect of Alzheimer Disease Center. Better cognitive functioning is indicated by higher scores for the MMSE, Logical Memory Delayed, Category Fluency, Digit Symbol, and Boston Naming test; and by lower scores on Trailmaking A and Trailmaking B tests.
Also adjusted for age, interval between last assessment and death, and education.
Association with Braak Neurofibrillary Stage
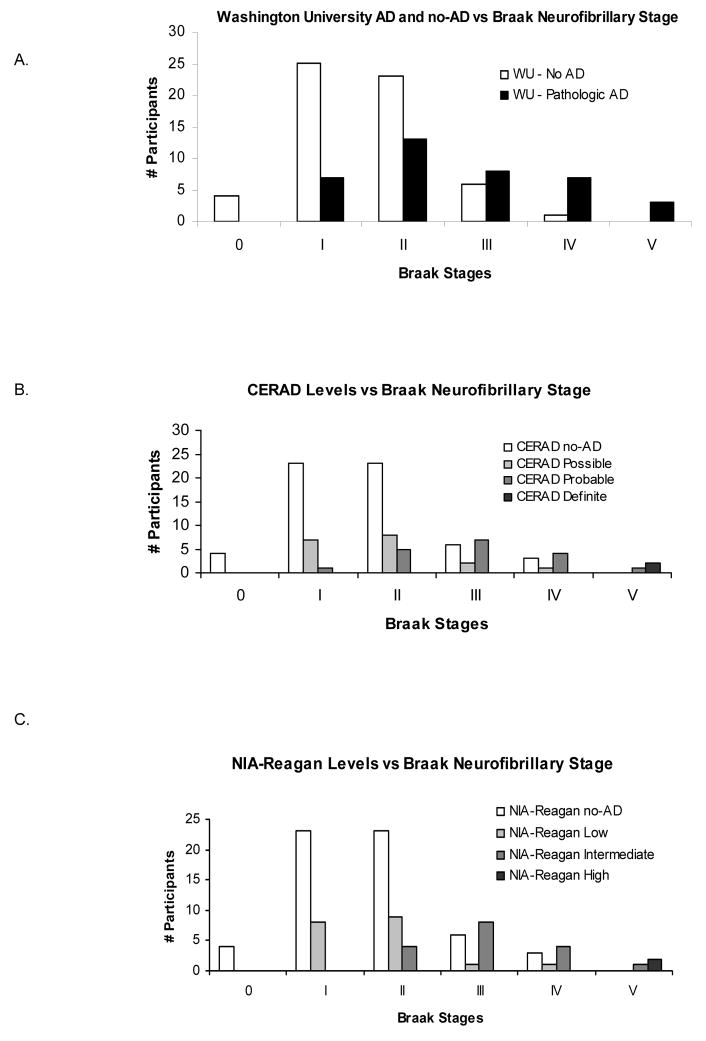
Although a greater likelihood of npAD was associated with higher Braak stage scores for all 4 criteria (chi-square, p<0.003), there was substantial overlap of the Braak stages between the npAD and no-npAD cases (Figure 3). There was virtually no difference between the Braak stages of cases at comparable levels of the CERAD and NIA-Reagan criteria (Figure 3B,C).
Figure 3.
A. Number of npAD and no-npAD participants (defined by the Washington University criteria) at each Braak neurofibrillary stage. B. & C. Number of cases rated at each level of the CERAD (B), and NIA-Reagan (C) criteria, for each Braak neurofibrillary stage. No cases were identified at Braak neurofibrillary stage VI.
Quantitative Neuropathological Assessment
ROC Analysis
To avoid the circularity inherent in using measures as outcomes when those same measures were used in classification, this analysis was limited to the CERAD and NIA-Reagan criteria, which do not incorporate diffuse SPs. ROC analyses indicated that the density of neocortical diffuse SPs best discriminated between npAD and no-npAD (defined by combination of all three diagnostic levels of either criteria), followed by the density of diffuse SPs in limbic areas. For completeness, the area under the curve and sensitivity for the other assessed neuropathological lesions (neuritic SPs, NFTs) also are shown in Table 3 although these data are confounded by the circularity noted above. The density of NFTs in limbic areas was the least discriminative (Table 2). Spearman correlations between the quantitative neuropathological measures and all outcomes of CERAD criteria (no AD or Possible, Probable, or Definite) and all outcomes of NIA-Reagan criteria (no AD or Low, Intermediate, or High) yielded almost the identical rank order for association with diagnostic level (Table 2).
Table 3.
Spearman correlations between psychometric test scores at last assessment and plaque burden, counts of diffuse plaques, and NFT density for participants with those lesions.
| MMSE | Logical Memory Delayed |
Trailmaking A | Trailmaking B | Boston Naming | Category Fluency |
Digit Symbol |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | r | N | r | N | r | N | r | N | r | N | r | N | r | ||||||
| Plaque Burden | |||||||||||||||||||
| Limbic | 55 | .06 | 24 | −.48 | * | 42 | .34 | * | 31 | .21 | 38 | .07 | 42 | −.12 | 20 | −.09 | |||
| Neocortex | 53 | −.02 | 23 | −.53 | * | 41 | .50 | *** | 31 | .25 | 36 | .01 | 39 | −.21 | 19 | .10 | |||
| Diffuse SPs | |||||||||||||||||||
| Limbic | 44 | −.29 | 18 | −.58 | * | 36 | .15 | 26 | .41 | * | 30 | .02 | 32 | −.23 | 16 | −.30 | |||
| Neocortex | 46 | .02 | 18 | −.54 | * | 38 | .38 | * | 28 | .14 | 31 | −.11 | 35 | −.25 | 17 | .12 | |||
| NFTs | |||||||||||||||||||
| Limbic | 68 | −.36 | ** | 27 | −.01 | 56 | −.19 | 43 | .00 | 49 | −.14 | 53 | −.03 | 28 | .33 | ||||
| Neocortex | 17 | −.01 | 3 | NA | 20 | −.27 | 12 | .44 | 17 | .05 | 15 | −.56 | * | 12 | .24 | ||||
p<.05;
p<.01;
p<.001
Abbreviations: SE = Standard Error, MMSE = Mini-Mental State Test, SP = senile plaques, NFT = neurofibrillary tangles
Better cognitive functioning is indicated by higher scores for the MMSE, Logical Memory Delayed, Category Fluency, Digit Symbol, and Boston
Naming test and by lower scores on Trailmaking A and Trailmaking B tests.
Table 2.
Results of ROC analyses testing the ability of the neuropathological lesions to predict no-npAD vs. npAD cases, and correlations of the neuropathological lesions with all levels of CERAD and NIA-Reagan criteria.
| ROC Analyses (CERAD or NIA-Reagan Criteria) |
Spearman Correlations | |||
|---|---|---|---|---|
| AUC (SE) | Sensitivity | CERAD Criteria | NIA-Reagan Criteria | |
| Neocortical diffuse plaques | 0.91 (0.028) a | 0.879 | 0.712 a | 0.707 a |
| Limbic diffuse plaques | 0.86(0.039) a,b | 0.755 | 0.601 a,b | 0.604 a,b |
| Neocortical plaque burden | 0.76(0.050) b, c | 0.596 | 0.625 a,b, c | 0.627 a,b, c |
| Limbic plaque burden | 0.79(0.053) b, c, d | 0.693 | 0.578 a,b, c, d | 0.574 b, c, d |
| Neocortical neuritic plaques | 0.69(0.058) c, d, e | 0.530 | 0.465 b, d, e | 0.479 b, d, e |
| Limbic neuritic plaques | 0.73(0.054) c, d, e | 0.577 | 0.512 b, c, d, e | 0.509 b, c, d, e |
| Neocortical tangle density | 0.65(0.060) d, e | 0.543 | 0.259 e,f | 0.271 e,f |
| Limbic tangle density | 0.63(0.056) c, e | 0.337 | 0.237 f | 0.259 e,f |
Within a column, identical superscript letters indicate no significant differences between AUCs or correlations.
Specificity was fixed at 80% in the ROC analyses.
Abbreviations: ROC = Receiver Operating Characteristic Curve, AUC = Area Under the ROC Curve, NIA = National Institute on Aging, CERAD = Consortium to Establish a Registry for Alzheimer’s Disease, SE = Standard Error.
Note: the shaded values, presented for completeness, potentially are confounded by their use both in the classification of neuropathological AD and in the outcomes shown here.
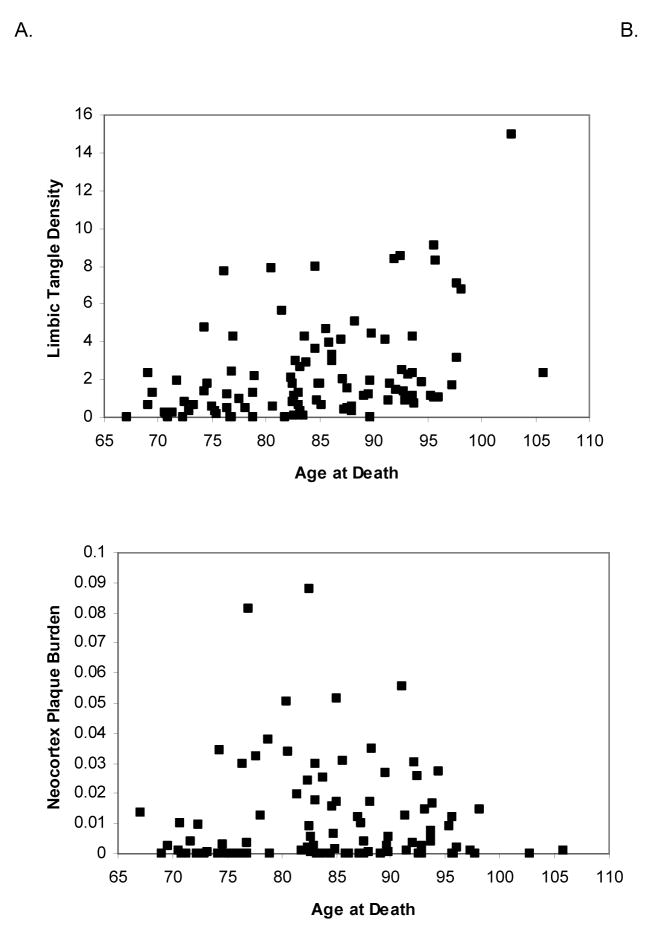
Correlation with Age
NFT density increased with age in limbic areas (entorhinal and perirhinal cortex, amygdala, and hippocampus field CA1) (Figure 4A; Spearman correlation=0.437, p<.001). In contrast, plaque burden was not significantly correlated with age (Figure 4B; Spearman correlation=0.089, p=0.40).
Figure 4.
The correlation of age with tangle density in limbic structures (A) and with plaque burden in neocortical areas (B). There is a significant increase in tangle density with age, but no age-related increase in plaque burden.
Relation of plaque burden and tangle density
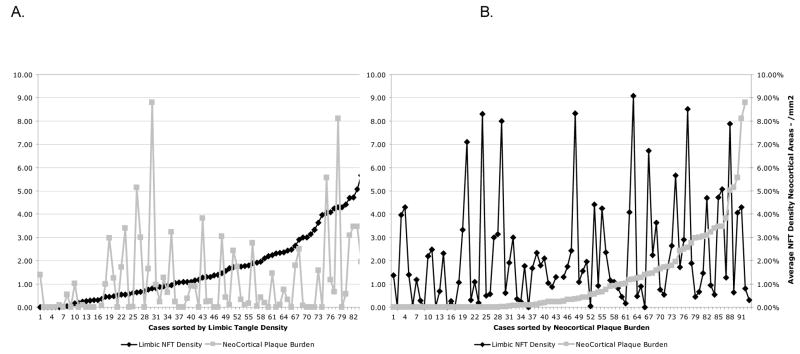
Neocortical plaque burden and limbic NFT density in nondemented cases were weakly correlated (Spearman correlation=0.196, p=.07, Figure 5).
Figure 5.
There is no relation between neocortical plaque burden and limbic NFT density for 97 nondemented cases, sorted by increasing NFT density (A) and by increasing plaque burden (B). Once present in the brain, both NFT density and plaque burden vary continuously from low to high values.
Correlation with psychometric tests
Although none of the participants in this study had clinically detectable dementia or cognitive impairment that was measurable on an individual basis, a significant correlation of some psychometric test scores was found with the density of AD lesions across the group, indicating that cognitive function in nondemented aging may decline with increasing numbers of NFTs or SPs, including diffuse SPs (Table 3). In all cases, greater pathology was associated with poorer cognitive performance at last assessment on measures of episodic memory (Logical Memory Delayed), semantic knowledge (Category Fluency), visuospatial ability (Trailmaking A), and executive function (Trailmaking B) as well as on a general measure, the MMSE. The correlations between the psychometric test scores and plaque burden, and between the test scores and diffuse SPs, were similar after adjustment for NFTs in the same brain region using partial correlation coefficients. Likewise, results were similar for the correlation of NFTs with the psychometric test scores after adjustment for plaque burden within the same region. Adjusting for age at death, all correlations reported in Table 3 remain significant except for that between limbic diffuse SPs and Trailmaking B (Spearman correlation=0.29, p=.16); conversely, the correlation become significant between limbic NFTs for Trailmaking A (Spearman correlation=−0.39, p=.003) and Digit Symbol (Spearman correlation=0.39, p=.04). Adjusting for education, all correlations reported in Table 3 still remain significant except for these between limbic diffuse SPs and Trailmaking B (Spearman correlation=0.36, p=.08) and between neocortical diffuse SPs and Logical Memory Delayed (Spearman correlation=−0.46, p=.06).
As noted in the methods section, the above analysis was limited to cases with lesions in the specified brain regions. Inclusion of all cases in the analyses, regardless of whether they demonstrated AD lesions, attenuated the correlations, as expected. Nonetheless, neocortical plaque burden remained significantly correlated with Logical Memory-Delayed (Spearman correlation=−0.42, p=0.03). Limbic NFTs also remained significantly correlated with MMSE (Spearman correlation=−0.38, p<.001), and neocortical NFTs were significantly correlated with MMSE (Spearman correlation=−0.26, p=0.03). Another limitation was missing data in some cases, although there were almost no statistical differences for age at death, years of education, and gender between participants missing data for the last psychometric tests before death and those completing the tests. The only exception was Trailmaking A, where the mean years of education was 16.0 for participants with data vs. 14.4 for participants with missing data (p=0.008). Interpretative caution still is indicated, however, because of the small numbers of individuals that had both neuropathological lesions and available test results for some measures.
Discussion
There are three major findings from this study. First, consistent with previous reports, a substantial proportion of nondemented older adults were found to have npAD, with the frequency ranging from about 20% (when criteria are restricted to Probable/Definite CERAD and Intermediate/High NIA-Reagan) to about 40% (all levels of criteria). Second, diffuse SPs are a prominent feature of npAD in nondemented cases and discriminate cases with and without npAD. Third, AD lesions (including diffuse SPs) in nondemented aging are associated with a deleterious effect on cognitive performance when examined across the group and hence are unlikely to be benign.
The finding that up to 40% of nondemented individuals with a mean age of death of 84 years have npAD corresponds with some estimates of the prevalence of clinically apparent DAT in this age group.(Hebert et al., 2003) Earlier neuropathological studies also reported similar frequency of npAD in nondemented cases, including one reporting that 37.3% of 134 cognitively normal older adults, autopsied at a mean age between 82–85 years, met “Intermediate/High” NIA-Reagan criteria for npAD.(Bennett et al., 2006) Although it is impossible to know whether autopsied cases with npAD would have developed dementia had they lived longer, these findings are consistent with the hypothesis that AD is characterized by a preclinical stage that is unaccompanied by sufficient cognitive impairment to yield a diagnosis of mild cognitive impairment, very mild dementia, or any other stage of clinical AD.(Price & Morris, 1999; Goldman et al., 2001; Galvin et al., 2005; Bennett et al., 2006) It is possible that factors such as pathological aging,(Dickson et al., 1992) cognitive and brain reserve,(Stern, 2006) or others yet to be identified may delay or mask the appearance of dementia symptoms in individuals with npAD. However, SPs and NFTs characterize the neuropathological substrate of AD and synaptic and neuronal loss developing from these or other lesions appear to be critical for the expression of cognitive symptoms.(Masliah et al., 2001; Price et al., 2001)
The individuals with npAD in this study did not differ in age from those without npAD, suggesting that age alone cannot account for the frequency of npAD. Neuronal loss in the entorhinal cortex or hippocampal field CA1 has been shown to be absent in preclinical npAD compared with substantial cell loss in cases with equivalent neuropathological lesion burden but with symptomatic very mild DAT (CDR = 0.5).(Gomez-Isla et al., 1996; Price et al., 2001; West et al., 2004) Presumably the absence of dementia in preclinical npAD reflects a delay between the appearance of Aβ and neurofibrillary lesions and the subsequent neuronal or synaptic degeneration that results in clinical symptoms of dementia. An apparent hypertrophy of cortical neurons has recently been described in “asymptomatic AD” that may represent an early reaction of the cells to amyloid or neurofibrillary changes prior to neuronal degeneration.(Riudavets et al., 2007; Iacono et al., 2008)
The results suggest several limitations of current criteria for diagnosing npAD, especially in nondemented individuals. Exclusion of diffuse SPs from CERAD and NIA-Reagan criteria may omit an important indicator of npAD. Diffuse SPs and consequently plaque burden appear initially in the neocortex and best discriminate npAD and no-npAD in nondemented aging. Senile plaques may not be ubiquitous with age;(Braak & Braak, 1997; den Dunnen et al., 2008) many of the nondemented older adults in this sample, including the eldest at age 106 years, had few if any SPs (Figure 4,B). This observation suggests the possibility that Aβ deposition in the cerebral cortex may represent a qualitative difference between cases with and without npAD.
In contrast, NFTs initially develop in limbic areas, and later extend into neocortical areas; they increase as a function of age even in the absence of SPs.(Price & Morris, 1999; Bennett et al., 2004) NFTs were present in almost all of the cases in this series, although 4 of the youngest cases had few if any (Braak Stage 0). Age-related neurofibrillary change may accelerate in the presence of Aβ lesions.(Price & Morris, 1999) Nondemented older adults have a wide range of Braak stages, even in individuals with high SP densities. Mismatches between NFT and SP densities are well-recognized(Dickson, 1995) and can present difficulties in categorization with NIA-Reagan criteria, which assume a strong correlation between NP density (reflected in the CERAD score) and NFT distribution (reflected in the Braak stage).(Geddes et al., 1997)
Once present in the brain, both SPs and NFTs occur in a continuous manner, without a clear boundary between “npAD” and “no-npAD” (Figure 5). The working group that formulated the NIA-Reagan criteria stated that the appearance of even one SP or NFT in the brain represents a pathological event, (National Institute on Aging & Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease, 1997) suggesting that any set of neuropathological criteria using quantitative levels of SPs and NFTs likely establishes an artificial diagnostic boundary. Given the potentially arbitrary distinction between npAD and no-npAD, neuropathological criteria for AD will need objective, data-driven assessments of their validity.
This is the first report, to our knowledge, that associates deficits in multiple cognitive domains, including episodic memory, semantic knowledge, visuospatial ability, and executive function with the neuropathological lesions of AD in nondemented aging. Although the small sample sizes impose interpretative caution, we found preliminary evidence for subtle cognitive impairment during the preclinical stage of npAD that correlates with NFT densities, plaque burden, and SP densities among individuals with those lesions. Although not detectable in individual subjects, and insufficient to produce dementia, these deficits nonetheless suggest that the neurofibrillary and Aβ lesions, including diffuse SPs, exert deleterious effects. Subtle cognitive deficits can be detected many years before AD diagnosis(Elias et al., 2000; Kawas et al., 2003; Saxton et al., 2004; Tierney et al., 2005) but have not previously been correlated with AD lesions in nondemented individuals. The pathobiological relevance of even diffuse SPs for AD is consistent with evidence that soluble Aβ dimers and oligomers, prior to the stage of SPs, have neurotoxic effects(Hung et al., 2008) and are associated with decreases in long term potentiation, dendritic spine density, and memory performance in experimental models.(Shankar et al., 2008)
This study had several strengths. First, it involved brain tissue obtained from several ADCs. Second, all participants had been carefully studied longitudinally with clinical and neuropsychological instruments to exclude contamination of the sample by individuals with unrecognized very mild dementia or mild cognitive impairment. Third, because there remains inter-laboratory variation in assessing AD lesions and consequently in neuropathological diagnosis,(Alafuzoff et al., 2006) the contributed sections were processed uniformly and interpreted at one center. Fourth, we compared 4 sets of neuropathological criteria for AD. Finally, the sample of 97 individuals was sufficiently large to permit preliminary analyses of the cognitive correlates of AD lesions in the absence of dementia.
Limitations of the study include the use of a convenience sample, inability to assess a genotype effect, small sample sizes for some of the cognitive associations, and variability in initial fixation and tissue processing at individual ADCs that may have influenced immunohistochemical staining and quantitative measurement of neuritic SPs. Another limitation is that SPs and NFTs may be downstream consequences of the pathophysiological processes responsible for the synaptic and neuronal degeneration that produces cognitive deficits in AD.(Shankar et al., 2008) This study did not address the early protein changes that lead to SPs and NFTs or the later synaptic or neuronal loss and other potential factors (e.g., vascular lesions; synucleinopathy) that could contribute to cognitive dysfunction; for example, SPs and NFTs may elicit a host response that contributes to the neurotoxicity of AD.(Castellani et al., 2008) An important caveat for the observations reported here is that they are from nondemented individuals and thus may not extrapolate to cases in which dementia already is manifest. For example, neurofibrillary lesions may have greater relevance for cognitive impairment once the clinical symptoms of AD are expressed.(Bennett et al., 2004)
The findings of this study are consistent with (but do not prove) the concept of preclinical AD. It may not be possible to recognize preclinical AD with standard clinical and cognitive measures, even in expert settings (e.g., ADCs). The detection of preclinical AD likely will require the development of validated biomarker, imaging, and other pre-mortem indicators of Alzheimer pathobiology.(Klunk et al., 2004; Mintun et al., 2006; Fagan et al., 2007; Ray et al., 2007; Ertekin-Taner et al., 2008; Fotenos et al., 2008) As effective treatments for AD become available, this may be increasingly important. Because there appears to be little neuronal degeneration in preclinical AD,(Gomez-Isla et al., 1996; Price et al., 2001; West et al., 2004) this stage of the illness could be an attractive target for treatment with agents developed to modify or arrest the AD process.
Acknowledgments
This study was supported by a National Institute of Aging (NIA) grant to the National Alzheimer Coordinating Center, (U01AG16976; PI, JC Morris), by NIA grants to individual Alzheimer Disease Centers (P50AG05681, P01AG03991, P50AG016574, P30AG028383, P30AG028377, P50AG005131, P30AG008017, and P30AG008665), by the Postdoctoral Program of 1UL1RR024992-01 from the National Center for Research Resources, and by the Charles and Joanne Knight Alzheimer Research Initiative of Washington University’s Alzheimer’s Disease Research Center (ADRC). At Washington University in St Louis, Hieu Van Luu, Javier Agraz, Jessica Church, Debra Carter and Kymberli Sykes provided excellent technical contributions, and Nigel Cairns PhD and James E. Galvin MD provided helpful comments on the manuscript. Geoffrey Murdoch MD, PhD of the University of Pittsburgh School of Medicine provided neuropathological assistance when he was at the Oregon Health Sciences University.
Footnotes
Disclosure Statement There are no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Bugiani O, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Hauw J, Kammphorts W, King A, Kopp N, Korkolopoulou P, Kovacs GG, Meyronet D, Parchi P, Patsouris E, Preusser M, Ravid R, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H. Interlaboratory comparison of assessments of Alzheimer disease-related lestions: A study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2006;65:740–757. doi: 10.1097/01.jnen.0000229986.17548.27. [DOI] [PubMed] [Google Scholar]
- Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psych Mono. 1946;60:1–48. [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, Kahle-Wrobleski K, Head E, Goodus M, Kim R, Kawas C. Dissociation of neuropathological findings and cognition; Case report of an Apolipoprotein E å2/å2 Genotype. Archives of Neurology. 2007;64:1193–1196. doi: 10.1001/archneur.64.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol. 2008;67:523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L. Clinico-pathologic studies in dementia: Nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- den Dunnen WFA, Brouwer WH, Bijlard E, Kamphuis J, Linschoten KV, Essens-Meijer E, Holstege G. No disease in the brain of a 115 year old woman. Neurobiol Aging. 2008;29:1127–1132. doi: 10.1016/j.neurobiolaging.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Dickson D, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Mismatch between plaques and tangles in staging Alzheimer pathology. Neurobiol Aging. 1995;16:283–284. [Google Scholar]
- Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of Alzheimer disease. A 22-year prospective study of the Famingham cohort. Archives of Neurology. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Ertekin-Taner N, Younkin LH, Yager DM, Parfitt F, Baker MC, Asthana S, Hutton ML, Younkin SG, Graff-Radford NR. Plasma amyloid beta protein is elevated in late-onset Alzheimer disease families. Neurology. 2008;70:596–606. doi: 10.1212/01.WNL.0000278386.00035.21. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relationship between socioeconomic status, preclinical Alzheimer’s disease, and reserve. Arch Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Powlishta KK, Wilkins K, McKeel D, Xiong C, Grant E, Storandt M, Morris JC. Predictors of preclinical Alzheimer’s disease and dementia: A clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- Geddes JW, Tekirian TL, Soultanian NS, Ashford JW, Davis DG, Markesbery WR. Comparison of neuropathologic criteria for the diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S99–S105. doi: 10.1016/s0197-4580(97)00063-8. [DOI] [PubMed] [Google Scholar]
- Goldman WP, Price JL, Storandt M, Grant EA, McKeel DW, Jr, Rubin EH, Morris JC. Absence of cognitive impairment or decline in preclinical Alzheimer’s disease. Neurology. 2001;56:361–367. doi: 10.1212/wnl.56.3.361. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Haroutunian V, Perl DP, Purohit DP, Marin D, Khalid K, Lantz M, Davis KL, Mohs RC. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Purohit DP, Perl DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol. 1999;56:713–718. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre NJ. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Hung LW, Ciccotosto G, Giannakis E, Tew DJ, Perez K, Masters CL, Cappai R, Wade JD, Barnham KJ. Amyloid-B peptide (AB) neurotoxicity is modulated by the rate of peptide aggregation: AB dimers and trimers correlate with neurotoxicity. The Journal of Neuroscience. 2008;28:11950–11958. doi: 10.1523/JNEUROSCI.3916-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono D, O’Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC. Neuronal hypertropy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:578–589. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zonderman AB, Arenberg D. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- McKeel DW, Price JL, Miller JP, Grant EA, Xiong C, Berg L, Morris JC. Neuropathologic criteria for diagnosing Alzheimer Disease in persons with pure dementia of Alzheimer type. J Neuropathol Exp Neurol. 2004;63:1028–1037. doi: 10.1093/jnen/63.10.1028. [DOI] [PubMed] [Google Scholar]
- Mintun MA, LaRossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C] PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Gearing M, McKeel DW, Jr, Crain BJ, Hughes JP, van Belle G, Heyman A. Interlaboratory comparison of neuropathology assessments in Alzheimer’s disease: A study of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) J Neuropathol Exp Neurol. 1994;53:303–315. doi: 10.1097/00005072-199405000-00012. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel DW, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L, CERAD neuropathologists participating The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Jr, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Riudavets MA, Iacono D, Resnick SM, O’Brien R, Zonderman AB, Martin LJ, Rudow G, Pletnikova O, Troncoso JC. Resistance to Alzheimer’s pathology is associated with nuclear hypertrophy in neurons. Neurobiol Aging. 2007;28:1484–1492. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L. Preclinical Alzheimer disease; neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. Preclinical AD revisited. Neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-B protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Medicine. 2008:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alz Dis Assoc Disord. 2006;20:112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Yao C, Kiss A, McDowell I. Neurospsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968;7:331–356. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Cataldo M, Nixon RA, Barnett JL, Lee MK, Checler F, Fowler DR, Smialek JE, Crain B, Martin LJ, Kawas CH. Neuropathology of preclinical and clinical late-onset Alzheimer’s disease. Ann Neurol. 1998;43:673–676. doi: 10.1002/ana.410430519. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Martin LJ, Dal Forno G, Kawas CH. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996;17:365–371. doi: 10.1016/0197-4580(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual: Wechsler Memory Scale-Revised. San Antonio, Texas: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Administration and scoring manual: Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Harcourt Brace; 1997. [Google Scholar]
- West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]