Abstract
Objective
Endothelial progenitor cells (EPCs) can differentiate into endothelial cells (ECs) and participate in post-natal vasculogenesis, but the mechanism of EPC differentiation remains largely unknown. We investigated the role of AMP-activated protein kinase (AMPK) in EPC differentiation and functions.
Methods and Results
Vascular endothelial growth factor caused the phosphorylation of AMPK, acetyl-CoA carboxylase (ACC), and eNOS in human cord blood-derived EPCs. The expression of EC markers, including VE-cadherin and ICAM-1, was also increased but blocked by Compound C, an AMPK inhibitor. AICAR, an AMPK agonist, increased the phosphorylation of ACC and eNOS and the expression of EC markers in a time- and dose-dependent manner, which reinforces the positive effect of AMPK on EPC differentiation. The effects of AICAR could be blocked by treatment with L-NAME, an eNOS inhibitor. Functionally, AICAR increased but Compound C decreased the angiogenesis of EPCs in vitro and in vivo. Furthermore, lovastatin promoted the activation of AMPK and eNOS, the expression of EC markers, tube formation, adhesion and in vivo vasculogenesis of EPCs, which could be blocked by treatment with Compound C.
Conclusion
The activation of eNOS by AMPK during EPC differentiation provides a novel mechanism for the pleiotropic effects of statins in benefiting the cardiovascular system.
Keywords: endothelial progenitor cells, differentiation, AMPK, eNOS, angiogenesis
Emerging evidence suggests that circulating endothelial progenitor cells (EPCs) contribute to postnatal neovascularization. These cells home to vascular injury sites, adopt the endothelial phenotype, and contribute to angiogenesis.1,2 Key determining factors of these cells are adhesion at the site of injury and differentiation into vascular endothelial cells (ECs). Although EPCs have clinical implications for therapeutic vasculogenesis, the identification of the involved cell populations and the mechanism by which they participate in vascular repair remains elusive.
AMP-activated protein kinase (AMPK) is a trimeric enzyme comprising a catalytic α subunit and regulatory β and γ subunits. AMPK is considered a “fuel gauge” to sense and regulate cellular energy homeostasis.3 The roles of AMPK in modulating the energy balance in skeletal muscles, liver, and adipocytes have been investigated extensively.3-5 Of note, AMPK phosphorylates endothelial nitric oxide synthase (eNOS) at Ser-1177 (bovine eNOS at Ser-1179), particularly in ECs.6,7 AMPK may exert beneficial effects on the vessel wall, since several recent studies, including ours, demonstrated that adiponectin, shear stress, and high-density lipoprotein activate AMPK in ECs with augmented NO production.8-10 Despite these findings, few reports exist of the involvement of AMPK in EPC differentiation and functions.
The clinical efficacy of HMG-CoA reductase inhibitors (i.e., statins) in decreasing cardiovascular mortality and morbidity is well recognized.11,12 Besides their ability to lower cholesterol, statins also have “pleiotropic” effects such as anti-inflammation, anti-oxidation, and improved NO bioavailability effects on the cardiovascular system.13-15 Various studies showed that bone marrow-derived EPCs mobilized by statins in animals and humans may contribute to vasculogenesis and reendothelialization.16-18 We recently reported that statins activate AMPK in ECs, leading to increased eNOS phosphorylation and NO bioavailability in vitro and in vivo.19 Because of the importance of AMPK in NO bioavailability and EPCs in therapeutic vasculogenesis, we tested the hypothesis that AMPK is crucial for the maturation and differentiation of bone marrow-derived EPCs. Further, we wondered whether the beneficial effects of statins on EPC-mediated vascular repair would be mediated, at least in part, by AMPK.
Materials and Methods
EPC isolation, culture, and identification
Human cord blood from umbilical cords of newborns was collected with the use of heparin (20 U/ml) from donors with their written agreement. The protocol was approved by Peking University Institutional Human Sample Use Committee. Mononuclear cells were isolated by density-gradient centrifugation with Ficoll (1.077 g/ml) and plated on plates coated with fibronectin (50 μg/ml; Sigma, St. Louis, MO). The culture media M199 (Gibico, Grand, NY) was supplemented with 20% fetal bovine serum (FBS; Hyclone, Logan, UT), human vascular endothelial growth factor (VEGF, 10 ng/ml; Sigma), human basic fibroblast growth factor (bFGF, 1 ng/ml; Sigma), human epidermal growth factor (EGF, 10 ng/ml; Sigma), insulin-like growth factor-II (IGF, 2 ng/ml; PetroTech, London, UK), and leukemia inhibitory factor (LIF, 10 ng/ml; Chemicon, Temecula, CA).
After culture for 2 to 3 weeks, clusters of EPC colonies grew at a high proliferative rate into a monolayer with a “cobblestone-like” morphology.20 This cell population appeared to be homogenous and maintained a similar morphology for several passages when cultured with growth factors and LIF. The population exhibited high levels of CD34 and VEGF receptor-2 (VEGFR-2; KDR) and had high capacity for taking up Dil-labeled acetylated LDL and binding to Ulex europaeus agglutinin-1 (UEA-1, Sigma).21-23 The methods and results for EPC identification are presented in Supplemental Fig. 1. EPCs within passages 2-6 after withdrawal of LIF were used in all studies.
Western blot analysis
Western blot analysis followed standard protocols. The primary antibodies were the polyclonal antibodies p-AMPK-Thr172, p-ACC-Ser79, and p-eNOS-Ser-1177 (all 1:1000; all Cell Signaling, Beverly, MA); anti-eNOS, vWF, ICAM-1, and VE-cadherin (all 1:1000; all Santa Cruz Biotechnology); and β-actin (Bioss, Beijing; 1:3000).
Quantitative Real-Time PCR
Total RNA was isolated from EPCs with use of TRIzol reagent (Invitrogen, Carlsbad, CA). The resulting cDNAs were used as templates for quantitative RT-PCR with the EVA Green fluorescent DNA stain (Biotium, Hayward, CA). 18S was used as an internal control. The nucleotide sequences of the primers were as follows: CD133: 5′-TTGTGGCAAATCACCAGGTA-3′ and 5′-TCAGATCTGTGAACGCCTTG-3′, CD34: 5′-GCAAGCCACCAGAGCTATTC-3′ and 5′-TCCACCGTTTTCCGTGTAAT-3′, vWF: 5′-TGCTGACACCAGAAAAGTGC-3′ and 5′-AGTCCCCAATGGACTCACAG-3′, CD31: 5′-ATGATGCCCAGTTTGAGGTC-3′ and 5′-GACGTCTTCAGTGGGGTTGT-3′, 18S: 5′-ACCGCAGCTAGGAATAATGGA and 5′-GCCTCAGTTCCGAAAACCA-3′.
Construction of adenoviruses and infection
AMPK-α1 knockdown was achieved by use of a Knockout Adenoviral RNAi System 2 (Clontech, Mountain View, CA) according to the manufacturer's instructions. The target sequence was 5′-AGTGAAGGTTGGCAAACAT-3′ (nt 126–144, accession number NM019142) for AMPK-α1 catalytic subunit. The forward and reverse synthetic 65-nt oligonucleotides were annealed and inserted into the BamHI/EcoRI sites of the vector RNAi-Ready pSIREN-DNR. The AMPK-α1 knockout recombinant adenovirus was then created by Cre-loxP site-specific recombination and referred to as Ad-AMPK-RNAi. Ad-AMPK-DN, a recombinant adenovirus expressing a dominant-negative mutant of AMPK, was described previously.9 Recombinant viruses were amplified and the titers were determined in HEK293 cells. Confluent EPCs were infected with recombinant adenoviruses (50 MOI) for 24 hr prior to further treatments. The parental adenoviral vector or Ad-GFP was used as an infection control.
Adhesion assays
The EPC adhesion assay and quantification are described in Supplemental Fig. 2.
In vitro angiogenesis assay
Matrigel (Becton Dickinson, Bedford, MA) at 300 μL/well was added to a 24-well plate, which was overlaid with 2×105 cells and then incubated in M199 medium supplemented with 20% FBS. After EPCs were treated with various stimuli, the formation of capillary tubes in Matrigel was examined by use of an inverted microscope equipped with a digital camera. The tube-like structures (those exceeding 6 cells in length) in 5 randomly selected fields of each well were counted by three investigators blinded to the treatment.24
In vivo vasculogenesis
The animal experimental protocol was approved by Peking University Institutional Animal Care and Use Committee. Male athymic nu/nu mice (6 weeks old) were fed with standard laboratory chow and tap water ad libitum. The isolated EPCs were pretreated with AICAR, Compound C or lovastatin in the absence or presence of Compound C for 12 hr, then 5×105 cells were resuspended in 100μL Matrigel (BD, Bedford, MA) on ice. The mixture was implanted on the flank of the athymic nu/nu mice (n=6) by subcutaneous injection with use of 25-gauge needle. After 7 days, Matrigel implants were removed, fixed in formalin, embedded in paraffin, and then sectioned. The histological sections were stained with hematoxylin and eosin (H&E) to examine the presence of luminal structure. For immunostaining of VE-cadherin and EPC nucleus, the sections were stained with anti-VE-cadherin (Santa Cruz Biotechnology, 1:50) or anti-human nuclei (Chemicon, 1:50) and horseradish peroxidase-conjugated secondary antibody. Diaminobenzidine tetrahydrochloride was used for the color development. Images were analyzed by use of an Olympus CKX41 microscope with OlympusMicro software. H&E-stained sections from each implant were used for quantification of microvessels. Microvessels with luminal structures containing erythrocytes were counted in 4 high-power views (40×) of each section.
Statistical analysis
The significance of variability was determined by ANOVA with post-hoc comparison by Student's t test for continuous variables and by chi-square or Fisher's exact test for nominal variables, as appropriate. All results are mean±SD from at least three independent experiments. A p<0.05 was considered statistically significant.
Results
VEGF activates AMPK and promotes EPC-EC differentiation
Because VEGF can increase the number of circulating EPCs in vivo and EPC differentiation in vitro,25 we first tested whether VEGF activates AMPK in cultured EPCs. Quiescent EPCs were treated with VEGF for 60 min. Western blot analysis showed that the phosphorylations of AMPK at Ser-172 and its target ACC at Ser-79 were increased by 5 or 25 ng/ml VEGF treatment (Fig. 1A). The phosphorylation of Ser-1177 of eNOS, also an AMPK target site, increased in a similar manner. VEGF treatment augmented the markers of mature ECs, including VE-cadherin and ICAM-1. These results indicate that AMPK phosphorylation is associated with the EPC-EC differentiation. We also confirmed the change of EPC to EC markers at the mRNA level. Quantitative real-time PCR analysis showed that the mRNA levels of CD133 and CD34 were decreased, whereas the levels of vWF and CD31 were increased in EPCs treated with 25 ng/ml VEGF for 4 hr (Fig. 1B).
Figure 1. VEGF activates AMPK and increases the expression of EC markers in EPCs.
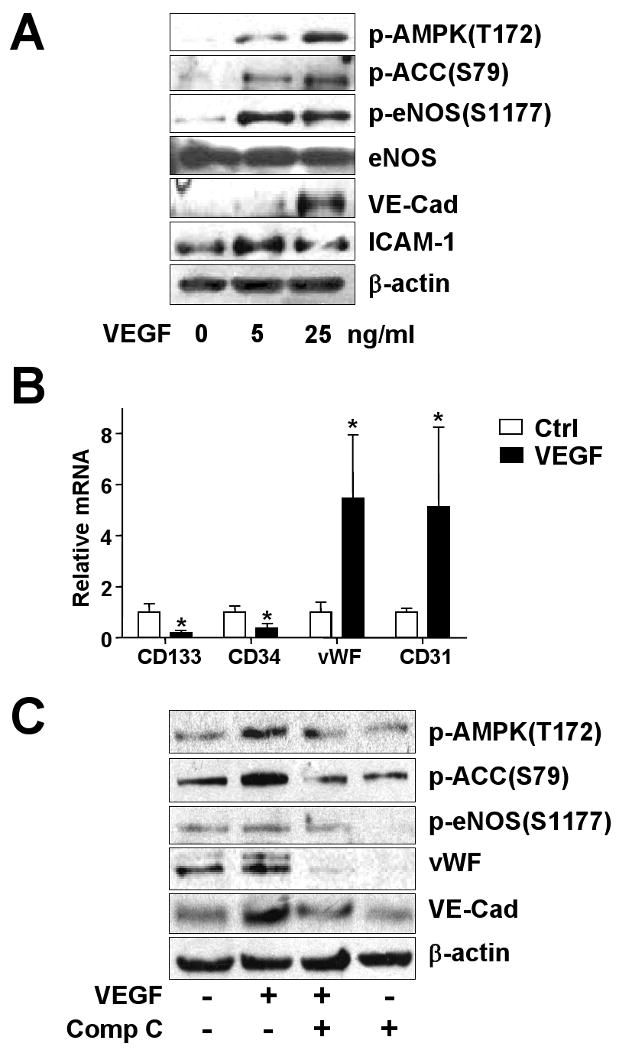
(A) Human EPCs were treated with 5 or 25 ng/ml recombinant VEGF or left untreated for 1 hr. (B) Human EPCs were treated with 25 ng/ml VEGF for 4 hr; the mRNA levels of CD133, CD34, vWF and CD31 were detected by quantitative real-time PCR. (C) EPCs were pretreated with Compound C (Comp C; 10 μM) for 30 min or left as untreated controls before the addition of VEGF (25 ng/ml). (A, C) Cells were lysed, and 50 μg of the cell lysates underwent 8% SDS-PAGE, followed by western blotting with various primary antibodies as indicated. The blots were visualized by a chemoluminescence-based detection system. Data are representative of 3 independent experiments.
AMPK is involved in the EPC-EC differentiation
To further investigate the involvement of AMPK in the EPC-EC differentiation, we pretreated cells with Compound C, an AMPK inhibitor, before stimulation with VEGF. Pretreatment with Compound C markedly attenuated the phosphorylation of AMPK, ACC, and eNOS responding to VEGF. In addition, the EPC-EC differentiation, indicated by levels of expressed VE-cadherin and ICAM-1, was also attenuated (Fig. 1C). To test whether AMPK is sufficient for EPC maturation, we treated EPCs with AICAR, an AMPK agonist. AICAR-treated cells showed increased AMPK activity, as indicated by augmented phosphorylation of ACC and eNOS (Fig. 2A). The result from a time-course study showed that the peak of eNOS phosphorylation/activation caused by AICAR in EPCs was 1.5-2 hr (data not shown). After 90-min treatment with AICAR, the levels of vWF, another EC maturation marker, VE-cadherin, and ICAM-1 increased in a dose-dependent manner. However, the AICAR-stimulated phosphorylation and maturation events were abolished in cells infected with Ad-AMPK-DN expressing a dominant-negative mutant of AMPK or with Ad-AMPK-RNAi to knock down endogenous AMPK-α1 (Fig. 2 B,C).
Figure 2. AICAR increases the phosphorylation of ACC and eNOS in EPCs.
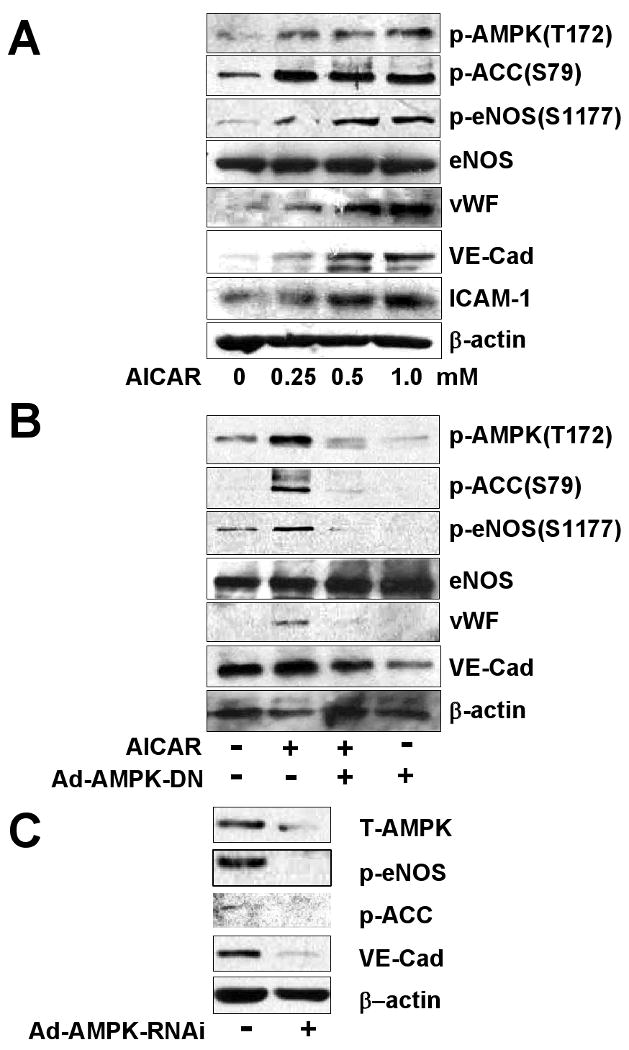
(A) EPCs were treated with various concentrations of AICAR for 90 min. (B) EPCs were infected without or with an adenovirus expressing a dominant-negative mutant of AMPK (Ad-AMPK-DN, 50 MOI) for 24 hr, then infected cells were treated without or with AICAR (1 mM) for 90 min. (C) EPCs were infected without or with an adenovirus knocking down AMPK (Ad-AMPK-RNAi; 50 MOI) for 24 hr. The phosphorylation of ACC, AMPK, and eNOS and expression of vWF, VE-cadherin, and ICAM-1 were analyzed by western blotting. Data are representative of 3 independent experiments.
AMPK enhances the in vitro angiogenesis of EPCs
Because EPCs can be recruited to sites of neovascularization where they differentiate into mature ECs in situ,1,26,27 we then investigated whether AMPK activation was increased in in vitro angiogenesis, namely, tube formation, of EPCs. AICAR enhanced the EPC tube formation in Matrigel in a dose-dependent manner (Fig. 3A,B). The tube formation was significantly attenuated on treatment with Compound C (Fig. 3C). These results suggest that AMPK is functionally involved in EPC-associated angiogenesis.
Figure 3. AMPK increases in vitro angiogenesis of EPCs.
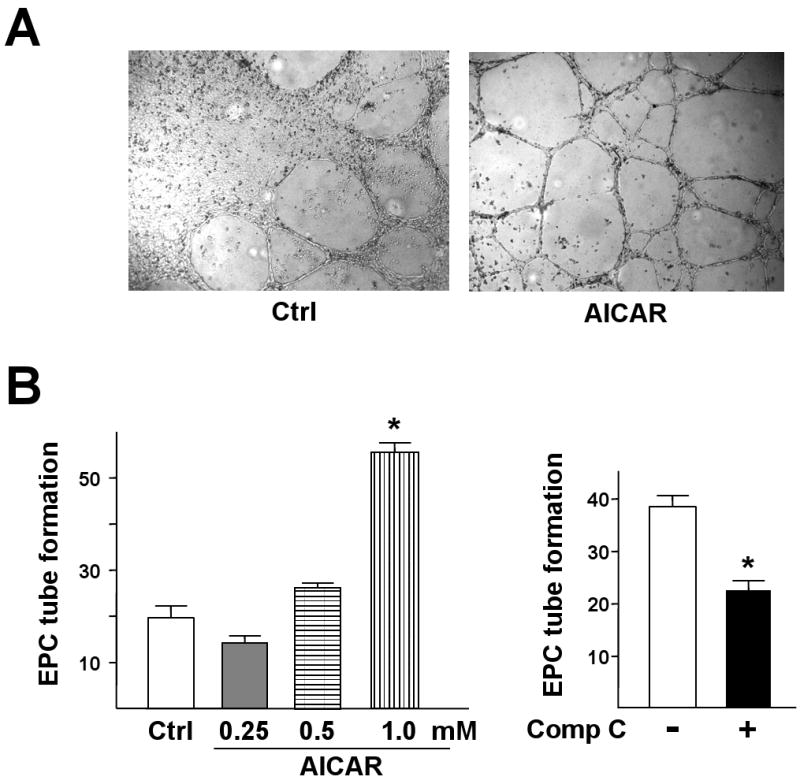
(A) Representative microscopic view of tube formation in EPCs without or with AICAR treatment (1 mM) for 24 hr. (B) EPCs were treated with different concentrations of AICAR or (C) Compound C (Comp C; 10 μM) for 24 hr. The tube formation was determined by counting the numbers of tubes in 5 randomly chosen low-power fields. Data are mean ± SD from 3 independent experiments, each performed in triplicate (*p<0.05).
Lovastatin induced EPC-EC differentiation depends on AMPK
Because statins promote the survival and differentiation of adult bone marrow-derived EPCs, with enhanced neovascularization,16,28 we studied whether statins regulate EPC differentiation via an AMPK-dependent pathway. EPCs were incubated with lovastatin at concentrations ranging from 2 to 40 μM. The phosphorylation of AMPK, ACC, and eNOS and expression of VE-cadherin and ICAM-1 were increased with lovastatin treatment, with maximal effect at 20 μM lovastatin (Fig. 4A). Notably, pre-treating EPCs with Compound C reduced the augmented phosphorylation and expression of EC markers in response to 10 μM lovastatin (Fig. 4B). In accordance with EPC differentiation, lovastatin, at concentrations up to 20 μM, also increased EPC adhesion to fibronectin (data not shown), a critical step during the process of EPC recruitment and/or homing.2 As well, inhibiting AMPK by Compound C treatment decreased 10 μM lovastatin-induced EPC adhesion to fibronectin (Supplemental Fig. 2) and tube formation (Fig. 4C). These results suggest a pivotal role of AMPK in EPC-EC differentiation and functions.
Figure 4. Lovastatin increases AMPK and eNOS phosphorylation and EPC-EC differentiation.
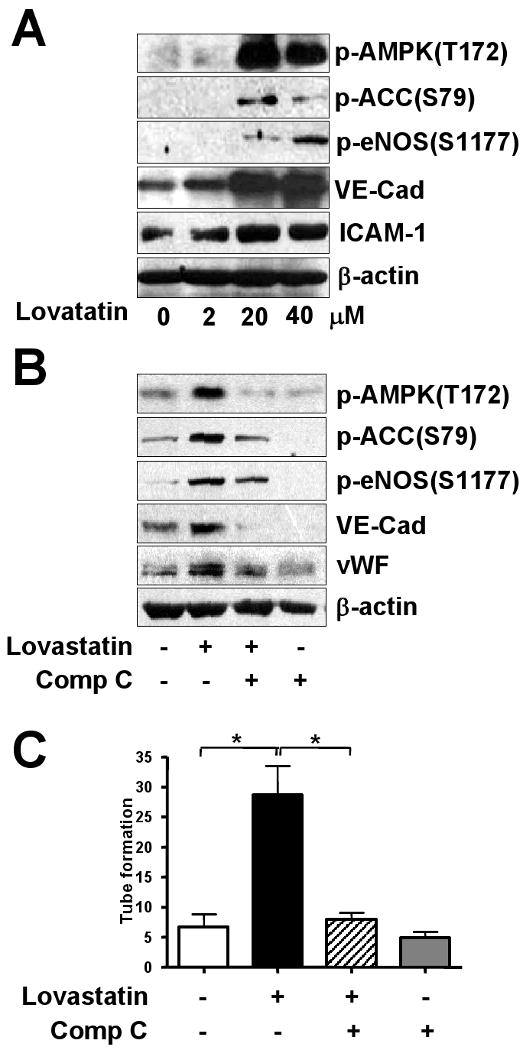
(A) EPCs were treated with various concentrations of lovastatin for 3 hr. (B) EPCs were pre-treated with or without Compound C (Comp C; 10 μM) for 30 min and then incubated further without or with lovastatin (10 μM) for 3 hr. Cell lysates (50 μg) underwent 8% SDS-PAGE followed by western blotting with various primary antibodies to detect the phosphorylation of AMPK, ACC, eNOS, and EC markers (i.e., VE-cadherin [VE-Cad] and ICAM-1). Blots are representative of 3 independent experiments. (C) EPCs were pretreated without or with Compound C (Comp C; 10 μM) and then incubated further without or with lovastatin (10 μM) for 24 hr. The numbers of formed tubes were counted. Results are mean ± S.D. from 3 independent experiments, each performed in triplicate (*p<0.05).
AMPK-induced EPC differentiation is mediated by NO bioavailability
AMPK can directly phosphorylate eNOS, whereas eNOS-derived NO bioavailability is essential for EPC differentiation.13 We investigated whether AMPK/eNOS/NO is involved in the EPC angiogenic process. EPCs were pretreated with L-NAME, an inhibitor of NO generation, prior to AICAR stimulation. As shown in Fig. 5A, L-NAME inhibited AICAR-induced vWF, VE-cadherin, and ICAM-1 expressio, but had little effect on the phosphorylation of AMPK, which indicates that the eNOS is an effector of AMPK signaling. L-NAME also reduced the basal and AICAR-induced tube formation of EPCs (Fig. 5B), which supports the critical role of the signaling cascade of AMPK-eNOS in the angiogenesis of EPCs.
Figure 5. AMPK-eNOS signaling is essential for EPC-EC differentiation.
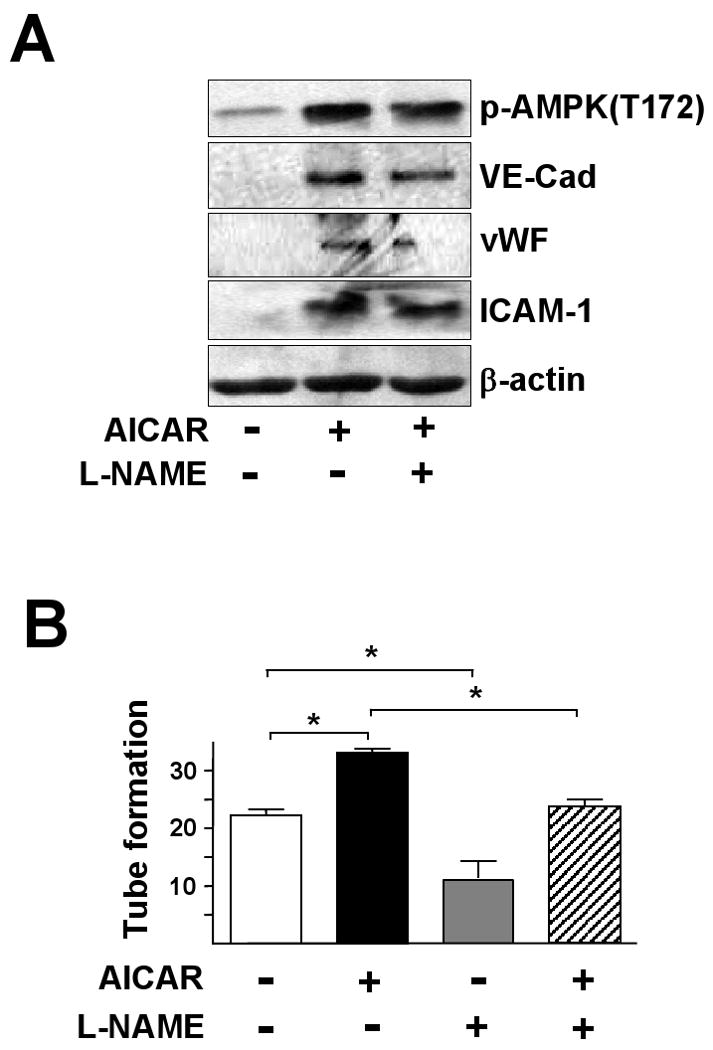
EPCs were pre-treated with L-NAME (1 mM) for 30 min, and then treated with AICAR (1 mM). (A) After 1.5 hr, cells were lysed and cell lysates underwent western blotting to detect phosphorylation of AMPK and levels of VE-Cadherin (VE-Cad), vWF and ICAM-1. (B) Cells were treated without or with L-NAME (1 mM) for 30 min, then without or with AICAR (1 mM) for 6 hr, then tube formation in Matrigel was assessed. Results are mean ± SD from 3 independent experiments, each performed in triplicate (*p<0.05).
AMPK enhances the vasculogenesis of EPCs in vivo
To correlate our in vitro results with EPC function and angiogensis in vivo, we observed capillary formation in Matrigel subcutaneously implanted in nude mice. This method has the advantage of reduced immuno-reaction. After AICAR or Compound C pretreatment, EPCs in Matrigel were injected subcutaneously into nude mice. After 7 days, the implants with EPCs pretreated with AICAR were more red in overall appearance than controls or those treated with Compound C (Fig. 6A). H&E staining revealed more luminal structure in implants with AICAR-treated EPCs than in untreated control or Compound C-pretreated EPCs (Fig. 6B,C, upper panel). To further characterize the differentiation of these EPCs, we stained the histological sections of the implants with anti-human VE-cadherin antibody. Immunostaining showed that many cells located in the luminal structure were positive for VE-cadherin (Fig. 6B,C, middle panel). An antibody specific for human nuclei also recognized transplanted EPCs in the formed luminal structure in AICAR-treated samples, which confirms that human EPCs injected into nude mice were directly involved in vasculogenesis in vivo (Fig. 6B,C, lower panel). Implants of Matrigel without cells as a control showed no cells (data not shown). As shown the quantitative data in Fig. 6D, the number of luminal structure in AICAR-treated samples was significantly higher than in untreated control. EPCs were also pretreated with 10 μM lovastatin in the presence or absence of Compound C, mixed with Matrigel, and then implanted into nude mice. Lovastatin increased the in vivo tube formation, and this effect could be inhibited by Compound C (Fig. 6), which suggests that lovastatin-enhanced EPC differentiation was mediated, at least in part, by AMPK.
Figure 6. AMPK increases neovasculogenesis of EPCs in vivo.
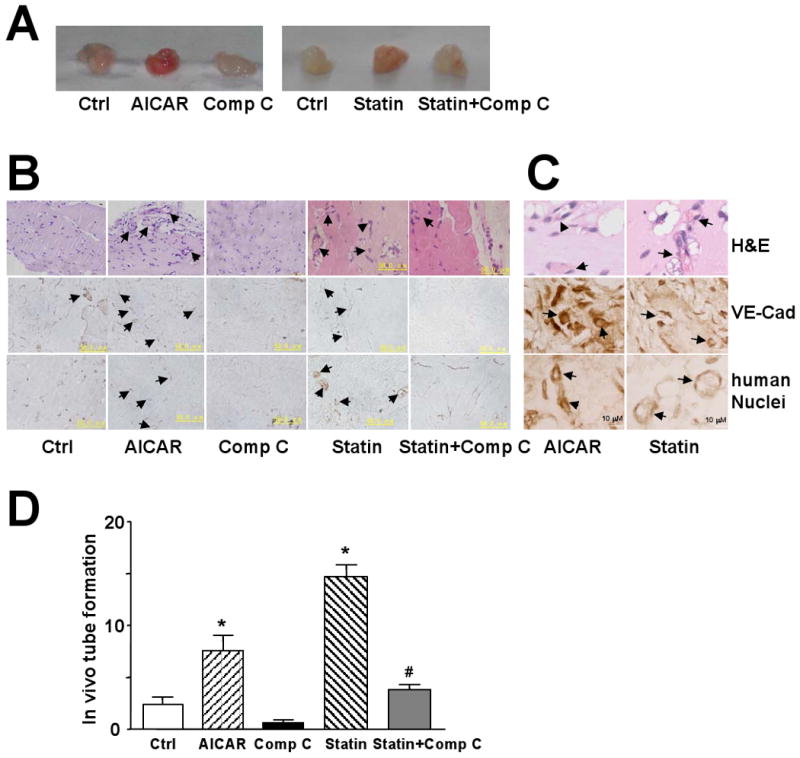
EPCs were pretreated with AICAR (1 mM), lovastatin (Statin; 10 μM), and without or with Compound C (Comp C; 10 μM) as indicated for 12 hr. After being mixed with Matrigel, the mixture was subcutaneously injected into nude mice and kept for 7 days. (A) Macroscopic view of the recovered Matrigel plugs under various conditions as shown. (B) The cross-sections of the implants underwent H&E staining and immunohistochemical staining with anti-VE-cadherin (VE-Cad) or anti-human nuclei antibody (400×, scale bar =50 μm). (C) Representative image of microvessels in AICAR- and lovastatin-treated groups under high-power magnification (1000×, scale bar =10 μm). The arrows indicate vessel-like structures. All images are representative of implants from 6 different animals. (D) Microvessel density in Matrigel implants was quantified by counting luminal structures containing erythrocytes. Results are mean ± SD microvessel density value determined from 3 different implants in 2 independent experiments (*p<0.05 vs. control; # p<0.05 vs. Statin group).
Discussion
In light of the importance of EPCs in therapeutic vasculogenesis and the involvement of AMPK in endothelial biology, we investigated the role of AMPK in the differentiation of primary human EPCs. The major findings are that (1) VEGF activated AMPK; (2) activated AMPK increased the eNOS phosphorylation, EPC-EC differentiation, and in vitro and in vivo angiogenesis; and (3) lovastatin-enhanced EPC differentiation was mediated, at least in part, by AMPK. Thus, these findings provide a novel mechanism underlying EPC differentiation.
Because NO bioavailability promotes EPC functions and maturation, previous studies by many others showed that the phosphorylation of eNOS Ser-1177 by PI3K-Akt is a key molecular event leading to eNOS-derived NO.16 eNOS Ser-1177 is also an AMPK targeting site, as shown by the direct phosphorylation of this site by activated AMPK immunoprecipitated from ECs.9 Using a dominant-negative mutant of AMPK, Ad-AMPK-RNAi, and a pharmacological agonist and antagonist of AMPK (i.e., AICAR and Compound C, respectively), we demonstrated that AMPK was necessary and sufficient for EPC-EC maturation, as evidenced by the increased expression of VE-cadherin, vWF, and ICAM-1, which are markers for ECs but not EPCs. Apparently, the augmentation in level of these markers in the AMPK-activated cells was through transcription and/or translation. Activation of AMPK can phosphorylate transcription factors altering gene expression29 such as the phosphorylation of elongation factor-2 leading to inhibition of the elongation step of translation30 and inhibition of translation by triggering the phosphorylation of p70S6 kinase and 4E-binding protein-1.31 These mechanisms are likely to be involved in EPC-EC differentiation. Because eNOS-derived NO can promote the differentiation of many cell types, including adipocytes, cardiac myocytes, and leukemia cells,32-34 AMPK is also likely involved in the maturation of these cell types.
Statins, similar to VEGF and exercise, have been shown to improve cardiovascular function in several animal models and initial clinical trials of patients with coronary artery diseases. One of the suggested mechanisms is increased circulating EPCs promoting neovascularization of the ischemic heart and homing to denuded vessels for reendothelization.35 At the cellular and molecular levels, neovascularization or reendothelization depends on the eNOS-derived NO bioavailability in ECs. The activation of eNOS by statins could be mainly contributed by the phosphorylation of eNOS Ser-1177 by Akt. Interestingly, statins (e.g., atorvastatin) can activate AMPK in vitro in cultured ECs and in vivo in the mouse aorta.19 In the current study, lovastatin activated AMPK in EPCs as well, which contributed to eNOS-derived NO bioavailability. The sufficiency of AMPK involved in this effect of statins is supported by results demonstrating that both AICAR and lovastatin increased angiogenesis of EPCs in Matrigel implants (Fig. 3 and 6). A recent study by Fisslthaler et al.36 suggested that NO is upstream of AMPK in reducing HMG CoA reductase activity in ECs responding to shear stress. However, we showed that L-NAME, although inhibiting endothelial marker expression and tube formation stimulated by AICAR, had little inhibitory effect on AMPK. These results suggest that AMPK is upstream of the eNOS-derived NO.
Studies by others demonstrated that adiponectin-, high-density-lipoprotein-, ApoAI-, and estradiol-activated AMPK in ECs is associated with enhanced eNOS phosphorylation and augmented NO production.10,37-38 AMPK-DN and/or Compound C can inhibit the adiponectin-, estradiol-, and metformin-activated eNOS and/or NO production.7,37-39 Although Akt has been considered the upstream kinase phosphorylating eNOS at Ser-1177, these results by others and ours illustrate that AMPK is at least as equally important as Akt in modulating eNOS-NO bioavailability in both EPCs and ECs. The cascade between AMPK, Akt, and eNOS in benefiting NO bioavailability may depend on the type of stimulation, which deserves further investigation.
In conclusion, our results suggest a newly defined role of AMPK in EPC-EC differentiation. This finding could be useful in developing tissue-engineered vessels derived from EPCs or clinical therapy in ischemic heart disease.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the National Natural Science Foundation of China (30600209, 30630032), the Major National Basic Research Grant of China (No. 2006BC503802), and the National Institutes of Health grant HL77448 (J.S.).
Contributor Information
Xiaoxia Li, Department of Physiology and Pathophysiology; Key Laboratory of Cardiovascular Science of Ministry of Education, Peking University Health Science Center, Beijing, 100083 China.
Yingying Han, Department of Physiology and Pathophysiology; Key Laboratory of Cardiovascular Science of Ministry of Education, Peking University Health Science Center, Beijing, 100083 China.
Wei Pang, Department of Physiology and Pathophysiology; Key Laboratory of Cardiovascular Science of Ministry of Education, Peking University Health Science Center, Beijing, 100083 China.
Chenghong Li, Department of Physiology and Pathophysiology; Key Laboratory of Cardiovascular Science of Ministry of Education, Peking University Health Science Center, Beijing, 100083 China.
Xuefen Xie, Department of Physiology and Pathophysiology; Key Laboratory of Cardiovascular Science of Ministry of Education, Peking University Health Science Center, Beijing, 100083 China.
Yi Zhu, Department of Physiology and Pathophysiology; Key Laboratory of Cardiovascular Science of Ministry of Education, Peking University Health Science Center, Beijing, 100083 China.
References
- 1.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 2.Jin H, Amy H, Su JM, Borgstrom P, Stupack D, Friedlander M, Varner J. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;116:652–62. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology. 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diebetes. 2002;51:2886–94. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 6.Morrow VA, Foufelle F, Connell JMC, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–39. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 7.Davis BJ, Xie ZL, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug Metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diebetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 8.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J Biol Chem. 2004;279:28670–4. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YJ, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–7. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 10.Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci USA. 2004;101:6999–7004. doi: 10.1073/pnas.0306266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sposito AC, Chapman MJ. Statin therapy in acute coronary syndromes: mechanistic insight into clinical benefit. Arterioscler Thromb Vasc Biol. 2002;22:1524–34. doi: 10.1161/01.atv.0000032033.39301.6a. [DOI] [PubMed] [Google Scholar]
- 12.Maycock CAA, Muhlestein JB, Horne BD, Carlquist JF, Bair TL, Pearson RR, Li QY, Anderson JL. Statin therapy is associated with reduced mortality across all age groups of individuals with significant coronary disease, including very elderly patients. J Am Coll Cardiol. 2002;40:1777–85. doi: 10.1016/s0735-1097(02)02477-4. [DOI] [PubMed] [Google Scholar]
- 13.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, Mueller M, Fuchs M, Hornig B, Haller H, Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–9. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 14.Kalinowski L, Dobrucki LW, Brovkovych V, Malinski T. Increased nitric oxide bioavailability in endothelial cells contributes to the pleiotropic effect of cerivastatin. Circulation. 2002;105:933–8. doi: 10.1161/hc0802.104283. [DOI] [PubMed] [Google Scholar]
- 15.Ni WH, Egashira K, Kataoka C, Kitamoto S, Koyanagi M, Inoue S, Takeshita A. Antiinflammatory and antiarteriosclerotic actions of HMG-CoA reductase inhibitors in a rat model of chronic inhibition of nitric oxide synthesis. Circ Res. 2001;89:415–21. doi: 10.1161/hh1701.096614. [DOI] [PubMed] [Google Scholar]
- 16.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 19.Sun W, Lee TS, Zhu MJ, Gu CA, Wang YS, Zhu Y, Shyy JY. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–62. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 20.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 21.Peichev M, Naiyer AJ, Pereira D, Zhu ZP, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MAS, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34 cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 22.Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schäfer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- 23.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–12. [PubMed] [Google Scholar]
- 24.Liu CS, Wang SH, Deb A, Nath KA, Katusic ZS, McConnell JP, Caplice NM. Proapoptotic, antimigratory, antiproliferative, and antiangiogenic effects of commercial C-reactive protein on various human endothelial cell types in vitro: implications of contaminating presence of sodium azide in commercial preparation. Circ Res. 2005;97:135–143. doi: 10.1161/01.RES.0000174612.90094.fd. [DOI] [PubMed] [Google Scholar]
- 25.Asahara T, Takahashi T, Masuda H, Kalka C, Chen DH, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Phsiol Cell Physiol. 2004;287:C572–C579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 27.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 28.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 29.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferré P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–11. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horman S, Browne GJ, Krause U, Patel JV, Vertommen D, Bertrand L, Lavolnne A, Hue L, Proud CG, Rider MH. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–23. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 31.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–80. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 32.Yan HY, Aziz E, Shillabeer G, Wong A, Shanghavi D, Kermouni A, Abdel-Hafez M, Lau DCW. Nitric oxide promotes differentiation of rat white preadipocytes in culture. J Lipid Res. 2002;43:2123–9. doi: 10.1194/jlr.m200305-jlr200. [DOI] [PubMed] [Google Scholar]
- 33.Gassanov N, Jankowski M, Danalache B, Wang DH, Grygorczyk R, Hoppe UC, Gutkowska J. Arginine vasopressin-mediated cardiac differentiation: Insights into the role of its receptors and nitric oxide signaling. J Biol Chem. 2007;282:11255–65. doi: 10.1074/jbc.M610769200. [DOI] [PubMed] [Google Scholar]
- 34.Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992;80:1880–4. [PubMed] [Google Scholar]
- 35.Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int. 2005;67:1672–9. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 36.Fisslthaler B, Fleming I, Keserü B, Walsh K, Busse R. Fluid shear stress and NO decrease the activity of the hydroxy-,methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ Res. 2007;100:e12–e21. doi: 10.1161/01.RES.0000257747.74358.1c. [DOI] [PubMed] [Google Scholar]
- 37.Schulz E, Anter E, Zou MH, Keaney JF. Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005;111:3473–80. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 39.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–9. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


