Abstract
Vocal deficits are prevalent and debilitating in Parkinson’s disease. These deficits may be related to the initial pathology of the nigrostriatal dopamine neurons and resulting dopamine depletion, which contributes to dysfunction of fine motor control in multiple functions. Although vocalization in animals and humans may differ in many respects, we evaluated complex (50-kHz) ultrasonic mate calls in two rat models of Parkinson’s disease, including unilateral infusions of 6-hydroxydopamine to the medial forebrain bundle and peripheral administration of a non-akinesia dose of the dopamine antagonist haloperidol. We examined the effects of these treatments on multiple aspects of the acoustic signal. The number of trill-like (frequency modulated) 50-kHz calls was significantly reduced, and appeared to be replaced by simpler (flat) calls. The bandwidth and maximum intensity of simple and frequency-modulated calls were significantly decreased, but call duration was not. Our findings suggest that the nigrostriatal dopamine pathway is involved to some extent in fine sensorimotor function that includes USV production and complexity.
Keywords: Parkinson’s disease, ultrasonic vocalization, sensorimotor, dopamine, speech, rat
Introduction
Voice and speech disorders (i.e. dysarthria) are frequently found in Parkinson’s disease, even in its early stages (Darley et al., 1969; Logemann et al., 1978; Hartelius & Svennson, 1994; Ho, Iansek, Marigliani, Bradshaw, & Gates, 1998; Fox et al., 2002). Unfortunately, dysarthria is often refractory to current pharmacological and surgical therapies. Levodopa therapy leads to changes in laryngeal muscle activity (Gallena, Smith, Zeffiro, & Ludlow, 2001) and mild improvement in fundamental frequency and perturbation measures (Goberman, Coelho, & Robb, 2002; Sanabria et al., 2001; Jiang, Lin, Wang, & Hanson, 1999). However, levodopa has not been shown to improve speech rate (DeLetter, Santens, DeBodt, Boon, & VanBorel, 2006), key functional parameters of voice and speech (Dromey, Kumar, Lang, & Lozano, 2000), or perceptual evaluation, prosody, articulation, and intensity (d’Alatri et al., 2007). Deep Brain Stimulation (DBS) improves limb motor symptoms of subjects (Carella et al., 2001; Brown, et al., 1999), but the effects on speech and voice are variable (Pinto et al., 2004; Klostermann et al., 2008), with reports of both improvement (d’Alatri et al., 2007) and exacerbation of dysarthria (Bejjani et al., 1999; Dromey, et al., 2000; Solomon, Robin, & Luschei, 2000).
There is evidence that degeneration of neurons occurs in lower brainstem structures, near the dorsal motor nucleus of the vagus (Braak, Rüb, Gai, & Del Tredici, 2003), prior to detectable degeneration of nigrostriatal neurons, and it progresses rostrally. This is not surprising given the ubiquitous nature, early onset, and severity of bulbar deficits in Parkinson’s disease, although research in this area is often overlooked. However, it is possible that dopamine terminal loss can also lead to degradation of at least some vocal function, as it does in other fine sensorimotor behaviors.
We began to address these issues in animal model by altering dopaminergic synaptic transmission and examining the effects on vocalization. Rats produce several types of ultrasonic vocalizations (USVs) that can be classified by frequency and complexity of the waveform. Generally, the peak energy of the calls falls into 2 main categories: 22-kilohertz (kHz) or 50-kHz frequency ranges (Brudzynski et al., 1993; Brudzynski & Ociepa, 1992). The 22-kHz calls generally occur as an alarm or sign of aversive conditions (Blanchard, et al., 1992) and are less complex than the 50-kHz calls, which are composed of either short constant frequency calls (flat calls) (Brudzynski & Pniak, 2002; Fu & Brudzynski, 1994; Wintik & Brudzynski, 2001) or frequency modulated (FM) calls (Ahrens, et al., 2008; Burgdorf et al., 2007). Male rat vocalizations of the 50-kHz type can be elicited by exposing sexually experienced male rats to an estrous female (McGinnis & Vakulenko, 2003; Bialy, Rydz, & Kaczmarek, 2000). 50-kHz calls are capable of changing the behavior of the signal recipient in several types of social contexts (Brudzynski, 2005; Wohr, Houx, Schwarting, & Spruijt, 2007). Although the mechanism of their production is unclear, it has been suggested that USVs are produced by a whistling mechanism by constriction and stabilization of vocal folds (Roberts, 1975; Sanders, et al., 2001). The extent to which rat USVs and human phonation share common neural anatomical substrates is unknown, but USVs nevertheless may be useful in the analysis of fine oro-motor control (Ciucci, et al., 2008).
In a previous preliminary study, rats sustaining either a unilateral infusion of the neurotoxin 6-hydroxydopamine (6-OHDA) to the medial forebrain bundle or a mild dose of the dopamine antagonist haloperidol showed reduced bandwidth in 50 kHz mate-calls of the frequency modulated (trill) type but not the number of calls (Ciucci, et al., 2007). The present study examined the effects of unilateral 6-OHDA and haloperidol on 50-kHz call bandwidth, frequency, intensity, and duration.
Method
Animals
A total of 16 male and 10 female Long-Evans rats were used in this study (Charles River). The female rats were used only to provide sexual experience and odor cues to elicit mate calling in all of the male rats. Four of the 10 males failed to mount the female or produce adequate numbers of calls for analysis and were therefore excluded from further analysis. The six male rats received both haloperidol and saline control on different days and were tested one-hour after injection for mounting latency and USVs. Six additional male rats received unilateral nigrostriatal infusions of 6-OHDA and were tested 5 weeks after surgery for mounting latency and USVs. The age of the rats at the time of testing ranged from 9–15 months, which reflects approximately the middle months of the rat lifespan (average lifespan is about 2.5 years). Animals were housed 2 per cage in standard polycarbonate cages with sawdust bedding, and food and water were provided ad libitum. Lights were maintained on a reverse 12:12h light:dark cycle, with all behavioral procedures occurring during the dark period of the cycle. Rats were handled for 7 days prior to behavioral testing, and habituated to the recording environment for 3 days prior to USV recording sessions. All procedures were approved by the University of Texas Institutional Animal Care and Use Committee.
Overview of Testing
All male rats were sexually experienced with female rats prior to testing. Testing included mounting latency and USV recording. Testing was performed one hour after control injections or haloperidol and 5 weeks after surgery for the 6-OHDA rats. Striatal dopamine content was estimated by behavioral measures in the 6-OHDA animals (which were carried out after USV recording), and with high pressure liquid chromatography after animal sacrifice. One 6-OHDA rat had only 12 % dopamine depletion and no behavioral asymmetries; therefore, its USV data were not included in the analysis (for 6-OHDA group, final n=5).
Dopaminergic Neurotoxin and Antagonist
Haloperidol
Rats in the haloperidol condition were injected with 0.1 mg/kg haloperidol (i.p.). Dose-response curves in our lab ranging from 0.05 – 0.5 mg/kg indicated that at 0.1 mg/kg, most rats continued to mount and vocalize within normal limits of baseline rates, with no hypokinesia seen in an open field.
6-OHDA
Moderate to severe degeneration of presynaptic striatal neurons is typically induced by unilateral infusion of 7 µg of 6-OHDA into the medial forebrain bundle (Fulceri et al., 2006; Marshall, 1979; Tillerson et al., 2001; Ungerstedt & Abuthnott, 1970). 6-OHDA treated rats were anesthetized with i.p. injections of 90 mg/kg ketamine and 10 mg/kg xylazine, and placed in a stereotaxic frame. All rats received unilateral infusions of 7 µg 6-OHDA hydrobromide (free base weight) dissolved in 3 µl artificial cerebrospinal fluid (composition: NaCl, KCl, CaCl2, MgCl2*6H20) containing 0.05% (w/v) ascorbic acid. Infusion coordinates were measured from bregma (−3.3 AP; ±1.7 ML; −8.0 DV from dural surface), and infusions were delivered at a rate of .3 µl/min for 10 minutes. Infusions were into the nigrostriatal projections in the hemisphere contralateral to the preferred forelimb, determined from baseline scores on a forelimb-use asymmetry test. Following surgery, animals were placed in a humidified incubator to prevent hypothermia, and upon waking were returned to their home cages.
Validation of Striatal Dopamine Degeneration
To estimate the degree of 6-OHDA induced degeneration, two behavioral tests were administered: forelimb-use asymmetry and apomorphine-induced rotation. At the completion of all experiments, striatal dopamine content was analyzed with high pressure liquid chromatography with electrochemical detection (HPLC-EC).
Behavioral Tests
Rats treated with 6-OHDA were tested for forelimb-use asymmetry on post-surgery days 7, 14, and 55 (Schallert & Tillerson, 2000; Schallert & Woodlee, 2005; Schallert et al., 2000) by placing them in an upright acrylic cylinder (diameter 20 cm) to encourage rearing and exploratory movements with the forepaws. The number of wall contacts made by either forelimb or by both forelimbs simultaneously was recorded. The percentage of contacts made by the non-impaired forelimb relative to the total number of contacts was calculated using the formula: (ipsilateral limb contacts + both (simultaneous or rapidly alternating) limb contacts)/ total number of contacts (limited to 20 per test day to prevent habituation). Scores significantly above 50% indicate a greater reliance on the ipsilateral limb for voluntary movement and have been well correlated with the degree of nigrostriatal dopamine depletion induced by 6-OHDA lesions (Schallert et al., 2000).
Apomorphine-induced rotational behavior was also examined. At 4 weeks post-surgery, animals were given 0.5 mg/kg apomorphine (s.c.), and the net number of contralateral quarter turns made during a 5 min trial was recorded (25 min post injection) (Herrera-Marschitz, Casas, & Ungerstedt, 1988).
HPLC
Rats were deeply anesthetized with halothane and decapitated. Brains were removed and the dorsal halves of the left and right striata were each dissected out over ice. Striata were then accurately weighed before being sonicated in 40 µl of cold mobile phase (see below) per mg of tissue. The sonicated suspension was then centrifuged at 13,500 RPM for 15 min at 4 °C and the supernatant was removed for analysis by HPLC-EC. Ten microliters of each sample were injected onto a BDS Hypersil C18 100 × 3 mm column (held at 40°C, and preceded by a 10 mm C18 guard column). T he mobile phase consisted of a15 mM sodium acetate / 20 mM citric acid buffer (pH 3.7) containing 70 µM disodium EDTA, 0.04% (w/v) sodium 1-heptanesulfonate, and 10% (v/v) methanol. This mobile phase was pumped through the system at 0.9 ml/min. A guard cell set at +450 mV was placed between the pump and sample injector to reduce electroactive impurities in the mobile phase. Eluates from the column were analyzed by an ESA Model 5011A analytical cell coupled to a Coulochem II controller. The first electrode was set at a potential of −100 mV and the second was maintained at +400 mV. The heights and retention times of current peaks recorded from the detecting (second) electrode were compared against those produced by a series of standards (obtained from Sigma) of known concentration to ultimately determine the striatal content of dopamine in terms of µg of substance per g of wet tissue weight. When samples fell below the limit of detection, a value halfway between zero and the detection limit was recorded for that sample. Lesion severity is expressed as percent dopamine loss in the lesioned hemisphere, relative to the intact hemisphere.
USV recording apparatus
The details of the recording apparatus that isolates male calls from the female calls but allows female estrous odor to permeate to the male rats have been published briefly elsewhere (see Ciucci, et al., 2007). A sound-isolated Plexiglas chamber (10 cm × 10 cm × 12 cm) was divided into upper (female rat) and lower (male rat) compartments by a Plexiglas panel containing an ultrasonic microphone with a frequency response range of 10–180-kHz and a flat frequency response of up to 150-kHz (CM16, Avisoft, Germany) directed at the lower compartment to record male USVs.
Behavioral Procedures
To maximize the number of USVs available for analysis, all males in this study were sexually experienced with estrous females prior to recording. Females were brought into estrous through s.c. injections of 10 µg estradiol benzoate and 500 µg progesterone, administered 48 hrs and 4 hrs prior to sexual encounters, respectively.
Prior to USV recording sessions, a receptive female was placed in the male’s homecage, and the male was allowed to mount twice without ejaculation before the female was removed. Mount latency was recorded to ensure that the unilateral dopamine depleted and haloperidol treated rats displayed normal appetitive/motivational behaviors. Rats that failed to mount twice within 5 minutes were excluded from the study. The male was then placed in the lower compartment of the recording chamber with homecage bedding and the female was placed in the upper compartment. USVs were recorded from the male for 5 minutes. The chamber was cleaned with ethanol between trials to remove the odors of male rats, although we have not found that latent male odors in this apparatus per se elicit USVs.
Data Recording and Analysis
Video recordings with a Panasonic PV-DV800 Infrared Camcorder were made with each session to ensure that behavior, rearing, and distance from the microphone were similar among all rats. However, mouth to microphone distance was not perfectly controlled. As such, selection of calls for analysis (discussed below) was designed to minimize the variability that may occur in analyzing intensity (loudness) as a result of slightly variable distances from the microphone.
USV recordings were collected on a computer and transferred to an external hard drive for storage and analysis. Analog recordings were digitized through a D/A card (National Instruments, USA) at 200-kHz sampling rate with 16 bit resolution. Recorded USVs were analyzed with Saslab Pro (Avisoft, Germany). Sonograms were generated under a 512 FFT-length and 75% overlap frame setup. A 300 s duration of vocalization recording was inspected after bypassing the initial 30 s of data collection to eliminate variability as the rats initially explored the chamber. Individual calls were then separated into single WAV audio file format for further parametric analysis. Calls were selected based on the quality of the acoustic signal (free from extraneous noise, sufficient energy in the signal), and all effort was applied to attempt to control for mouth to microphone distance. For the remaining dependent variables, 10% of each type of call and a minimum of 10 calls per animal were analyzed. However, not all rats made every type of call and not all calls were free from noise. Typically, the rats reared upwards toward the microphone while vocalizing. Because rats are different sizes and behave differently, the distance from their mouths to the microphone was not completely controlled. To offset this limitation, the ‘best’ calls were selected, meaning the calls that were the loudest (highest intensity on the spectrogram) and clearest upon visual inspection. This sampling technique models a human speech assessment method of analyzing the samples that have the same fundamental frequency and intensity level and was standard among all three groups in the experiment. Further, we analyzed the percent of time spent rearing to ensure that all three groups were behaving similarly.
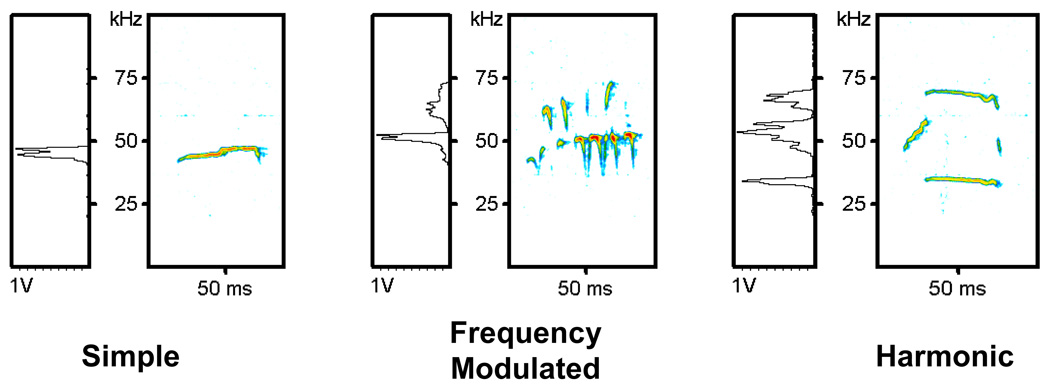
In this particular social paradigm, rats make three types of calls: Simple, Frequency Modulated (FM), and Harmonic (See Figure 1). Simple calls have a constant frequency, without frequency modulation. The number of calls made in each call category was counted. Several dependent variables for the acoustic signal were operationally defined and selected for offline spectral analysis:(1) Duration: offset of the signal minus the onset in seconds; (2) Bandwidth: maximum minus minimum frequency in Hertz (Hz); (3) Maximum frequency: highest frequency in kHz observed in a call of the same type, and (4) Maximum intensity: maximum intensity measured in decibels (dB). Note: Intensity in dB is measured against a reference point that is internal to the microphone. This reference is a negative value. Thus, the intensity measures are expressed in negative values and the less negative value in dB reflects a louder vocalization.
Figure 1.
Representative Sonograms of Simple, Frequency Modulated (FM), and Harmonic calls from a rat in the Control Condition. Left-hand boxes represent the amplitude spectrum of the call expressed in volts. Right handed boxes are the sonogram with time in milliseconds represented on the X-axis and frequency in kilohertz on the Y-axis. Relative amplitude is represented by color.
Statistical Analysis
Percentage of call type was analyzed with paired comparison t-tests. Duration, Bandwidth, Maximum Frequency, and Intensity were analyzed with a 3×2 Mixed ANOVA with dopamine condition (control, haloperidol, 6-OHDA) and call type (Simple, FM) as the factors; Control and haloperidol groups were within subject. Post-hoc testing was performed with a Tukey HSD. The Harmonic type calls were not included in this analysis as they did not comply with the selection criteria, especially due to the low intensity level. The Harmonic calls are also infrequently produced. A Bonferroni adjustment was made to the alpha level to control for the number of dependent variables in the parametric ANOVA (.05/4=.0125).
Results
Percent Call Type
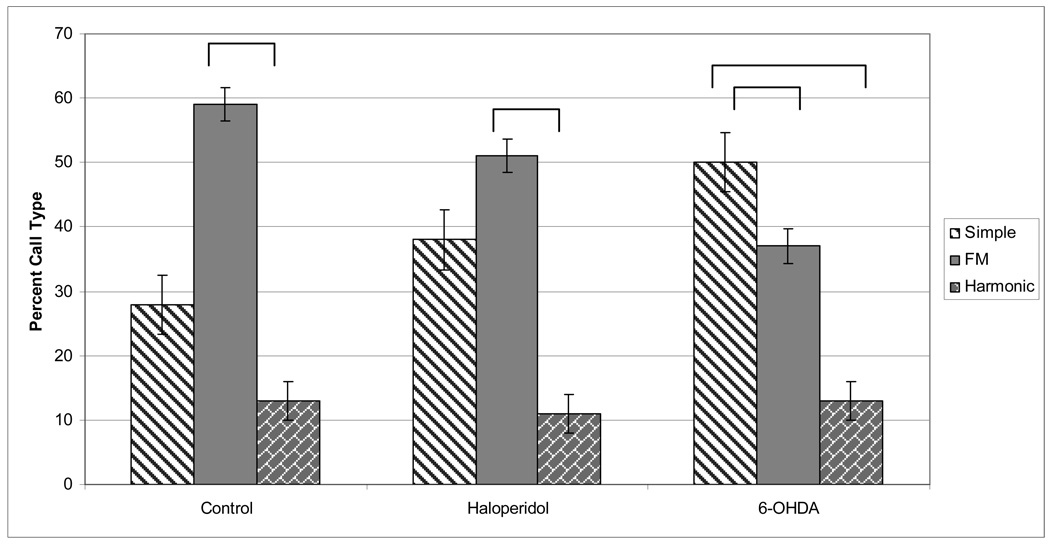
The type of call produced (Simple, FM, or Harmonic; Figure 1 and Figure 2) was expressed in percentage of total calls and compared among control, haloperidol and 6-OHDA animals using paired comparison t-tests. The total number of calls was not different between the control, haloperidol, and 6-OHDA groups. The percent of Simple calls was significantly greater than Harmonic in all groups (Control: t=4.587; p<.01 Haloperidol: t=7.252; p<.01; 6-OHDA: t=3.541; p<.05). Likewise, the percent of FM calls was significantly greater than harmonic for all groups (Control: t=3.331; p<.05, Haloperidol: t=4.246; p<01, 6-OHDA: t=4.207; p<.05), meaning that rats produce more Simple and FM calls than Harmonic calls regardless of dopamine depletion. The relative amount of call types produced revealed that the number of FM calls was greatest for the control (t=3.331; p< .05) and haloperidol groups (t=4.264; p<.01). FM represented the highest percentage of call type for the control and haloperidol animals, but not for the 6-OHDA animals. For the 6-OHDA animals, Simple was the most frequently observed versus other types of calls (F (2, 16)=3.876; p=.0457).
Figure 2.
The percent of each call type (Simple, FM, Harmonic) produced in the Control, Haloperidol, and 6-OHDA conditions. Overall, the most common Call Type produced is FM, followed by Simple and the least common is Harmonic. For the Control condition, the most common type of call is the FM call (p< .01). In the 6-OHDA condition, more Simple calls were produced than FM or Harmonic (p=.0457).
Duration
There were no significant main effects for duration (Figure 3) (F(2, 34)=2.485, p=.102) or interactions for duration, although Call Type approached significance (F(1, 33)=5.19, p=.031), in that the FM calls had a longer duration than the Simple calls. Duration was not an acoustic parameter affected by dopamine depletion.
Figure 3.
Means and standard error of the mean (SEM) for duration measured in seconds (s) in the Control, Haloperidol, and 6-OHDA conditions for the Simple and FM calls.
Bandwidth
There were significant main effects for bandwidth (Figure 4) for DA condition (F(2, 34)=64.671, p<.0001) and Call Type (F(1, 33)=8.079, p=.002) and no interactions. Post-hoc tests revealed that Bandwidth was significantly greater in the control condition versus the haloperidol (p=.004) and 6-OHDA (p=.006) conditions. The DA-altered conditions were not statistically significant from each other (p=.998).
Figure 4.
Means and SEM for bandwidth, measured in Hertz (Hz), in the Control, Haloperidol, and 6-OHDA conditions for the Simple and FM calls (p< .01).
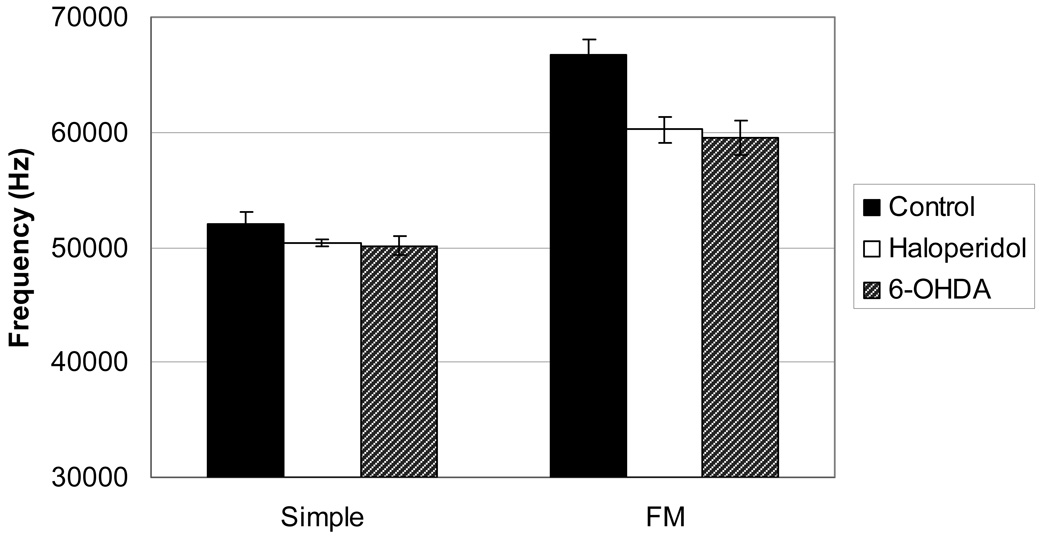
Maximum Frequency
There was a significant main effect for maximum frequency (Figure 5) for Call Type (F(1,33)=46.786, p<.001). DA condition approached significance (F(2,34)=3.763, p=.036) and there were no significant interactions. Overall, the control rats had a higher maximum frequency than the DA-altered rats for both the Simple and FM calls, but this was not statistically significant (p=.034) given our conservative p-values.
Figure 5.
Means and SEM of maximum frequency measured in Hertz (Hz) in the Control, Haloperidol, and 6-OHDA conditions for the Simple and FM calls.
Maximum Intensity
There were significant main effects for maximum intensity (Figure 6) for DA condition (F(2, 34)=6.140, p=.006) and Call Type (F(1, 33)=8.721, p=.006) and no significant interactions. Post-hoc tests revealed that intensity was significantly higher in the control condition versus the 6-OHDA condition (p=.008) and approached significance versus the haloperidol condition (p=.031). The DA-altered conditions were not statistically significant from each other (p=.766).
Figure 6.
Means and SEM for maximum intensity measured in dB in the Control, Haloperidol, and 6-OHDA conditions for the Simple and FM calls. A more negative value represents a call with less intensity (p< .01).
Striatal Dopamine Degeneration
Extent of Unilateral Striatal DA Depletion
The range of asymmetry scores in the test of forelimb use during exploration of the walls of a cylindrical enclosure for the 6-OHDA rats was 75 to 90% and, in the apomorphine rotation test, an average of 300+ 54 contra turns in 5/6 rats. The data suggested that there was an extensive depletion of dopamine in these 5 rats, which was confirmed by HPLC analysis (see below). The one rat that did not show motor asymmetry in either test had only a minimal (12%) depletion as indicated by HPLC analysis of DA content; therefore its data were excluded from analysis. Levels of dopamine (DA) in the dorsal striata of 6-OHDA-lesioned rats were assayed via HPLC-ED, as described above. DA levels in the DA-depleted striatum of all five animals were below the limit of detection for the assay (<194 ng DA/g wet tissue weight), indicating successful severe unilateral DA depletion. Unlesioned striata contained 9029 ± 1995 ng DA/g (mean ± SEM). High variability on the unlesioned side was due to one outlier who was observed to have a low DA level of only 1325 ng/g in the unlesioned striatum, for unknown reasons. Exclusion of this animal yielded a mean unlesioned-side DA content of 10955 ± 671 ng/g. Assuming a dopamine level halfway between 0 and the limit of detection (i.e., 97 ng/g) for the lesioned striata, the mean percentage depletion was calculated as 97.8% ± 1.3% DA loss compared to the intact side. The animal with a DA depletion on unlesioned side still demonstrated forelimb asymmetry, as the lesioned side was DA depleted to a greater degree.
Discussion
Complex 50-kHz ultrasonic calls were substantially degraded in a unilateral hemi-Parkinson rat model and after the DA antagonist antipsychotic drug, haloperidol. Adequate DA synaptic activity, therefore, may be required for normal 50-kHz vocalization. These data may be relevant to discussion of the role of nigrostriatal DA neurons vs. brainstem non-DA neurons on phonation deficits in the early stages of PD. It remains possible that midbrain DA projections at least modulate this complex oromotor function.
Parkinson’s disease leads to progressive phonatory sensorimotor deficits (Goberman & Blomgren, 2008; Baker, Ramig, Luschei, & Smith, 1998; Hanson, Gerratt, & Ward, 1984; Logemann et al., 1978). Vocalization deficits are amenable to targeted training (Pinto et al., 2004; Ramig et al., 2001a; Ramig et al., 2001b; Sapir, Ramig, & Fox, 2006;citations), but without training are often refractory to pharmacological (d’Alatri et al., 2007; Dromey, et al., 2000; Gallena, et al., 2001; Goberman, et al., 2002; Jiang, et al., 1999; Sanabria et al., 2001;) and surgical intervention (Pinto et al., 2004; Klostermann et al., 2008; Bejjani et al., 1999; Dromey, et al,, 2000; Solomon, et al., 2000).
The data confirm preliminary research suggesting that unilateral 6-OHDA lesions to the medial forebrain bundle or mild transient haloperidol administration reduce bandwidth in frequency modulated type USV (Ciucci, et al., 2007). Call intensity and bandwidth in both simple and frequency modulated calls of the 50-kHZ USV type were significantly reduced. Duration and maximum frequency were reduced but the extents of reduction were not statistically significant. The total number of calls in the 6-OHDA and haloperidol treated rats was not reduced. The average degree of dopamine depletion in the lesioned hemisphere was 97%, which is considered a severe depletion. The behavioral deficits were slightly less severe at the times of assessment. Relative to control, there was about an 80% decline in use of the contralateral forelimb for exploration in the rats with severe unilateral dopamine depletion. In these same rats, 50-kHz call bandwidth and intensity were reduced by about 40% and 23%, respectively. Given that the ipsilateral forelimb was not impaired, the overall decline in biforelimb function could be considered roughly similar to the reduction in USV bandwidth and somewhat more severe than the reduction in USV intensity. Future studies should include an exploration of the relationship between degree of dopamine depletion and acoustic impairment across a wide range of depletion, as well as the social implications of these deficits on mating behavior.
There are 3 main call types produced by sexually experienced male rats calling to estrous female rats: Simple, Frequency Modulated (FM) and Harmonic. In the control condition, the most common type produced was the FM call. However, the 6-OHDA treated animals produced more simple calls, which may be motorically less challenging. This was also true for haloperidol-treated rats, although this was not statistically significant (it should be noted that the p-values were conservative). Although it has been reported that rats with more experience in the testing conditions emit more Simple (‘flat’) type of calls (Wöhr, et al., 2007), all of our rats had similar amounts of experience. It is possible that FM calls, which are more acoustically complex, may require more sophisticated motor gestures to produce. Although the mechanism of ultrasonic vocalization is unclear, the sound source is thought to be a ‘whistle’ through a constricted larynx (Roberts, 1975, Sanders, et al., 2001) with modulations occurring further ‘upstream’ from the source, perhaps modified by the tongue and/or velopharynx. Impairment in fine motor control, rather than motivational or gross locomotor deficits, may have contributed to the reduction of trill-type calls in the 6-OHDA treated rats because there was no reduction in mounting latency or rearing up toward the female rat stimulus.
Dopamine depletion via unilateral 6-OHDA lesions produced more robust alterations in the acoustic properties of USVs than systemic administration of the dopamine antagonist haloperidol. This may be due to the use of a low dose of haloperidol, which may have produced variability in the occupancy of dopamine receptors.
To minimize variability produced by not strictly controlling mouth-microphone distance, we chose calls that occurred when the animal was 6–8cm from the microphone and were representative of each animal’s best performance (i.e., the loudest calls available in the sample). This sampling technique was standard among all three groups. In humans, a key early aspect of speech pathology communication assessment involves examination of the “best” performance. The percent of time spent rearing was not different (between 35–39% for all three groups). Additionally, we played back a call and analyzed sound pressure level at the microphone from several distances. The sound pressure differences between distances of 6 vs. 10 cm was less than the differences we found among groups. It is thus unlikely that the reduced intensity observed in the dopamine-altered groups was related to rearing height and distance from the microphone.
Conclusion
The reduced intensity and bandwidth of calls in 6-OHDA and haloperidol treated animals may have implications not only for research in Parkinson’s disease but also for schizophrenia, as drug therapy with dopamine antagonists is common in schizophrenia. From a motor control standpoint, adequate frequency modulation and intensity should require sufficient excursion of the vocalization organs. The reduction in bandwidth and intensity of mate calls and percentage of frequency modulated calls are reminiscent of some aspects of dysarthria in people with Parkinson’s disease. For example, patients with Parkinson’s disease may have a reduced working acoustic space, meaning fewer excursions of the articulatory structures (McRae at al., 2002). It is possible that deficits in the nigrostriatal dopamine pathway contribute to phonation deficits in Parkinson’s disease. In rat parkinsonian models, the quality of USV production may be useful for exploring phonatory deficits and treatment outcomes.
Acknowledgements
We would like to thank Dr. Nadine Connor for assistance in interpreting the results. This work is supported by the Davis Phinney Foundation, DARPA and grants NS 19608, HD 02023, and NS 042345.
References
- Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: Rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behavioural Brain Research. 2008 doi: 10.1016/j.bbr.2008.08.037. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal KR, Panksepp J. The neurobiology of 50-kHz ultrasonic vocalizations of rats: electrode mapping, lesion, and pharmacology studies. Behavioural Brain Research. 2007;18(2):274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Baker KK, Ramig LO, Luschei ES, Smith ME. Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology. 1998;51:1592–1598. doi: 10.1212/wnl.51.6.1592. [DOI] [PubMed] [Google Scholar]
- Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, Dornu P, Pidoux B, Samson Y, Agid Y. Transient acute depression induced by high-frequency deep-brain stimulation. New England Journal of Medicine. 1999;340:1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Procontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behavioral Neuroscience. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Agullana R, McGee L, Weiss S, Blanchard DC. Sex differences in the incidence and sonographic characteristics of antipredator ultrasonic cries in the laboratory rat (Rattus norvegicus) Journal of Comparative Psychology. 1992;106:270–277. doi: 10.1037/0735-7036.106.3.270. [DOI] [PubMed] [Google Scholar]
- Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. Journal of Neural Transmission. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Brown RG, Dowsey PL, Brown P, Jahanshahi M, Pollak P, Benabid AL, Rodriguez-Oroz MC, Obeso J, Rothwell JC. Impact of deep brain stimulation on upper limb akinesia in Parkinson’s disease. Annals of Neurology. 1999;45:473–488. doi: 10.1002/1531-8249(199904)45:4<473::aid-ana9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiology and Behavior. 1992;52:655–660. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Bihari F, Ociepa D, Fu XW. Analysis of 22 kHz ultrasonic vocalization in laboratory rats: long and short calls. Physiology and Behavior. 1993;54:215–221. doi: 10.1016/0031-9384(93)90102-l. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. Journal of Comparative Psychology. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behavior Genetics. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Carella F, Genitrini S, Bressanelli M, Soliveri P, Servello D, Broggi G, Piacentini S, Geminiani G, Girotti F. Acute effects of bilateral subthalamic nucleus stimulation on clinical and kinematic parameters in Parkinson's disease. Movement Disorders. 2001;16:651–655. doi: 10.1002/mds.1151. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behavioral Brain Research. 2007;182:284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T. Limb-use and complex ultrasonic vocalization in a rat model of Parkinson’s disease: Deficit targeted training. Parkinsonism and Related Disorders. 2008;14:S172–S175. doi: 10.1016/j.parkreldis.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of bilateral subthalamic nucleus stimulation and medication on parkinsonian speech impairment. Journal of Voice. 2007 doi: 10.1016/j.jvoice.2006.10.010. (in press). [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Research. 1969a;12:246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in dysarthrias. Journal of Speech and Hearing Research. 1969b;12:462–496. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- DeLetter M, Santens P, DeBodt M, Boon P, VanBorsel J. Levodopa-induced alterations in speech rate in advanced Parkinson’s disease. Acta Neurologica Belgica. 2006;106:19–22. [PubMed] [Google Scholar]
- Dromey C, Kumar R, Lang AE, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Movement Disorders. 2000;15:1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fox CM, Morrison CE, Ramig LO, Sapir S. Current perspectives on the Lee Silverman Voice Treatment (LSVT) for individuals with idiopathic Parkinson disease. American Journal of Speech-Language Pathology. 2002;27:309–319. [Google Scholar]
- Fu XW, Brudzynski SM. High-frequency ultrasonic vocalization induced by intracerebral glutamate in rats. Pharmacology, Biochemistry, and Behavior. 1994;49:835–841. doi: 10.1016/0091-3057(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Fulceri F, Biagioni F, Lenzi P, Falleni A, Gesi M, Ruggieri S, Forai F. Nigrostriatal damage with 6-OHDA: validation of routinely applied procedures. Annals of the New York Academy of Science. 2006;1074:344–348. doi: 10.1196/annals.1369.032. [DOI] [PubMed] [Google Scholar]
- Gallena S, Smith PJ, Zeffiro T, Ludlow CL. Effects of levodopa on laryngeal muscle activity for voice onset in Parkinson disease. Journal of Speech, Language, and Hearing Research. 2001;44:1284–1299. doi: 10.1044/1092-4388(2001/100). [DOI] [PubMed] [Google Scholar]
- Goberman A, Coelho C, Robb M. Phonatory characteristics of parkinsonian speech before and after morning medication: the ON and OFF states. Journal of Communication Disorders. 2002;35:217–239. doi: 10.1016/s0021-9924(01)00072-7. [DOI] [PubMed] [Google Scholar]
- Goberman AM, Blomgren M. Fundamental frequency change during offset and onset of voicing in individuals with Parkinson disease. Journal of Voice. 2008;22:178–191. doi: 10.1016/j.jvoice.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Hanson DG, Gerratt BR, Ward PH. Cinegraphic observations of laryngeal function in Parkinson’s disease. Laryngoscope. 1984;94:348–353. doi: 10.1288/00005537-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatrica Logopaedica. 1994;46:9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behavioural Neurology. 1998;11:131–137. [PubMed] [Google Scholar]
- Herrera-Marschitz M, Casas M, Ungerstedt U. Caffeine produces contralateral rotation in rats with unilateral dopamine denervation: comparisons with apomorphine-induced responses. Psychopharmacology. 1988;94:38–45. doi: 10.1007/BF00735878. [DOI] [PubMed] [Google Scholar]
- Jiang J, Lin E, Wang J, Hanson DG. Glottographic measures before and after levodopa treatment in Parkinson’s disease. Laryngoscope. 1999;109:1287–1294. doi: 10.1097/00005537-199908000-00019. [DOI] [PubMed] [Google Scholar]
- Klostermann F, Ehlen F, Vesper J, Nubel K, Gross M, Marzinzik F, Curio G, Sappok T. Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2008;79:522–529. doi: 10.1136/jnnp.2007.123323. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. Journal of Speech Hearing and Disorders. 1978;43:47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- Marshall JF. Somatosensory inattention after dopamine-depleting intracerebral 6-OHDA injections: spontaneous recovery and pharmacological control. Brain Research. 1979;177:311–324. doi: 10.1016/0006-8993(79)90782-0. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiology & Behavior. 2003;20:81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- McRae PA, Tjaden K, Schoonings B. Acoustic and perceptual consequences of articulatory rate change in Parkinson disease. Journal of Speech, Language, and Hearing Research. 2002;45:35–50. doi: 10.1044/1092-4388(2002/003). [DOI] [PubMed] [Google Scholar]
- Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzoum P. Treatments for dysarthria in Parkinson’s disease. Lancet Neurology. 2004;3:547–556. doi: 10.1016/S1474-4422(04)00854-3. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Gray S, Baker K, Corbin-Lewis K, Buder E, Luschei E, Coon H, Smith M. The aging voice: a review, treatment data and familial and genetic perspectives. Folia Phoniatrica Logopaedica. 2001a;53:252–265. doi: 10.1159/000052680. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O'Brien C, Hoehn M, Thompson LL. Intensive voice treatment (LSVT) for patients with Parkinson's disease: a 2 year follow up. Journal of Neurology, Neurosurgery and Psychiatry. 2001b;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria J, Ruiz PG, Gutierrez R, Marquez R, Marquez F, Escobar P, Gentil M, Cenjor C. The effect of levodopa on vocal function in Parkinson’s disease. Clinical Neuropharmacology. 2001;24:99–102. doi: 10.1097/00002826-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Roberts LH. The rodent ultrasound production mechanism. Ultrasonics. 1975;13:83–85. doi: 10.1016/0041-624x(75)90052-9. [DOI] [PubMed] [Google Scholar]
- Sanders I, Weisz DJ, Yang BY, Fung K, Amirali A. The mechanism of ultrasonic vocalization in the rat, Soc Neurosci Abstr. 2001 27: #88.19. [Google Scholar]
- Sapir S, Ramig LO, Fox C. The Lee Silverman Voice Treatment [LSVT] for voice, speech, and other orofacial disorders in people with Parkinson’s disease. Future Neurology. 2006;1:563–570. [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: optimizing behavioral assessment of outcome. In: Emerich DF, Dean RL, Sanberg PR, editors. Central Nervous System Diseases: Innovative Models of CNS Diseases from Molecule to Therapy. Totowa, NJ: Humana Press; 2000. pp. 131–151. [Google Scholar]
- Schallert T, Woodlee MT. Motor systems: orienting and placing. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat: A Hand Book with Tests. New York: Oxford University Press; 2005. pp. 129–140. [Google Scholar]
- Solomon NP, Robin DA, Luschei ES. Strength, endurance, and stability of the tongue and hand in Parkinson disease. Journal of Speech, Language, and Hearing Research. 2000;43:256–267. doi: 10.1044/jslhr.4301.256. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. Journal of Neuroscience. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott G. Quantitative recording of rotational behavior in rats after 6-OHDA lesions of the nigrostriatal dopamine system. Brain Research. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacology, Biochemistry, and Behavior. 2001;70:317–323. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiology and Behavior. 2007;93:766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]