Genetic testing now allows haemochromatosis to be diagnosed before symptoms emerge. Testing is potentially beneficial because early treatment of iron overload is the only way to prevent organ damage. Treatment by venesection is, however, lengthy and can have a detrimental effect on quality of life, and not all patients with the mutation will develop symptoms. A general practitioner and her patient describe their experience of treatment of asymptomatic haemochromatosis and ask whether it was really necessary. Their experiences are commented on by a general practitioner and a consultant haematologist.
The development of genetic testing for disease has raised the problem of whether to test asymptomatic individuals.1-1,1-2 Hereditary haemochromatosis is one such disease for which testing is possible. The associated gene, HFE (previously HLA-H), was identified in 1996 together with two mutations C282Y and H63D.1-3 The C282Y mutation is present in about 90% of known cases in the United Kingdom.1-4 The condition may be more prevalent (possibly one in 300) than previously thought. Patients do not usually develop symptoms until they are over 40 years old, by which time deposition of iron in organs such as the liver, pancreas, heart, and endocrine systems may have caused irreversible tissue damage.1-5–1-7 Repeated venesection is an effective treatment and can restore normal life expectancy in people with no evidence of organ damage. In women the onset may be later as menstruation provides physiological blood loss.
In 1997, MH was 67 and had had type 2 diabetes for five years. The disease was well controlled with an oral sulphonylurea. She took part in a study (which had ethical approval) to look at the genetic aetiology of diabetes and the role of haemochromatosis. She was found to be homozygous for the C282Y mutation. She was informed of this as she might have or develop complications from the condition. Subsequent tests confirmed the mutation status and showed a ferritin concentration of >1000 μg/l (normal range 12-110 μg/l). A review in the gastroenterology clinic showed no evidence of hepatic dysfunction and no symptoms attributable to haemochromatosis. She was advised to have venesection to prevent problems in the future. She and her general practitioner write about their experiences.
General practitioner's view
Before MH's case I knew little about haemochromatosis, having seen only a handful of cases of “bronzed diabetes” during my hospital training. I discussed the finding with my colleagues and, after liaison with the hospital team, contacted MH to explain that a potentially important finding had come from the study. MH came to the appointment with her family tree. Muscular dystrophy was already known in her family, and the tree was marked with carriers and affected individuals. I explained that a different condition had been found. It was arranged that MH should see a gastroenterologist and contact her immediate family regarding the need for testing. MH had no children. She had one brother and two sisters. One sister had a son and daughter. Only her unmarried sister is still being investigated. MH's brother and father also had diabetes so it is probable that MH's diabetes was unrelated to the haemochromatosis.
I initially had the impression that active treatment might not be needed, just monitoring of liver function. Regular venesection was, however, recommended to prevent complications of the disease. As she lived 16 miles from the district general hospital I was asked if she could have venesection at our local community hospital. I agreed to this as it made good use of the community hospital and saved MH travelling, although at the time I did not realise all it would entail or the time it would take.
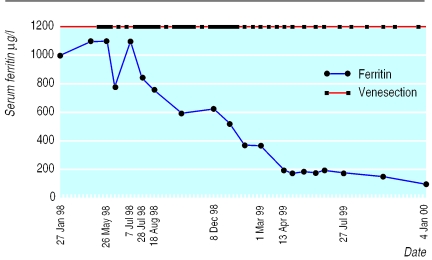
It soon became clear that we were not making much impact on MH's serum ferritin concentrations and that it would take many venesections to reach the normal range (figure). On further reading I discovered it could take a year, something I did not immediately share with MH. At times we both became dispirited, especially when because of holidays the concentration started to rise again. Fortunately we got on well so we were able to pass the time during the venesection in a reasonably pleasurable way. However, it was a lot more demanding than I had imagined. Venous access was not easy so I had to do most sessions.
Figure.
Serum ferritin concentration and venesection patterns (values over 1000 μg/l have been designated as 1100)
MH had to cancel her trip to Ascension Island, where she had worked for many years, because with even the slightest break from venesection her ferritin concentration rose and she did not want to risk being away for a few weeks at that time. The other problem was we forgot her diabetes. When she felt tired she put it down to the venesection, and it took a while to realise that it was in fact due to a deterioration in her diabetes. I was determined she should not miss her trip to New Zealand in early 1999. She was able to arrange venesection there and went armed with her computerised records and results.
It has taken a long time and many venesections (about 40) to get her serum ferritin concentration down. At the beginning of 2000 it was within normal range. We are now able to manage with venesection at 4-6 weekly intervals, and MH has resumed her travelling and her diabetic control has also improved. She has just celebrated her 70th birthday in New York.
Patient's view
March 1998
Diabetes has definitely affected my family. My father had to have insulin injections. It was a nerve racking experience which didn't help my parents' relationship, and in the end they parted. When I was first diagnosed I was angry and frightened, though I soon realised that matters might have been a lot worse. My brother has become a bit bowed down by his diabetes, but he is more seriously affected.
At present neither I nor my family have much idea what haemochromatosis entails so we are just intrigued. I should have a clearer picture once I have talked to the consultant. As a family we would be pleased to answer any questions.
July 1998
I have now been having blood taken regularly at Honiton Hospital by CS for two months. Afterwards I have around 48 hours of lethargy and find it hard to do my usual activities. I am sleeping longer, often 8-9 hours at night and for an hour or so in the afternoons. In mid-July we had to revert to weekly bloodletting after the blood test showed the iron count had gone up. I was too tired for the rest of the week to do anything else and feel I may have to reconsider my trip to Ascension Island in August. I went back to see the consultant, but unfortunately only saw a junior doctor who didn't give me much confidence.
At the beginning I could not perceive how haemochromatosis would affect me unduly as I felt so well. It has meant, however, that I lose two days a week because my normal energy levels are lowered. I have forgone my trip to Ascension Island, where I lived and worked for 17 years, in order not to jeopardise the treatment, and I will probably not be invited to do the job I was going to do there. Now I am worried about my trip to New Zealand and Australia in January 1999. If I cancel that I will lose a lot of money, which is worrying. I try not to think about it, but it is difficult.
I also feel that CS is getting a raw deal. I have really appreciated her accompanying me and taking the blood—I am a baby when it comes to hospitals, etc. However, I feel that she is acting over and beyond the call of duty and I realise she may have to hand me over. I just hope it will be to someone as skilled as she is.
I suppose that I am becoming weary of it all, now that it is affecting my life. What if it takes another six months or so before I've contained this condition and the iron count is at an acceptable level, and what happens after that?
Late 1998
The bloodletting continued, usually weekly, but occasionally two weekly. Although I cancelled my working holiday on Ascension Island because I didn't feel 100% after the bloodletting, I decided that come what may I was going to New Zealand. But how was I to do this and not return to square one as far as the ferritin count is concerned? CS suggested that I wrote to my friends in New Zealand to see if something could be arranged at their local hospital, and I received word that it could.
June 1999
I went to New Zealand in January and had four treatments at a hospital there. I had several blood tests, and they were kind and thorough and gave me results to bring home. I have had five sessions since my return, but the last have been poor with only about half a unit taken before the flow stopped. Still, the ferritin level has fallen to 180 or slightly below. The sad thing is that it does not look as though I will ever get back to Ascension as the booking system has changed and it will be too expensive.
It is over a year since I began this course, and I frankly feel sorry the condition was ever discovered—little did I realise how it would dominate my everyday life. I don't usually have any after effects these days, but even so my diary revolves around my visits to the hospital, and I grow weary of it. I am sure my doctors have similar feelings at times. Were it not for CS and all her support, so I feel I cannot let her down, I would have given up long ago. I was quite shocked to realise that this would go on for life—albeit less frequently, but still a drag. However, I have to count myself fortunate that it is not a life threatening condition and that knowing about it means that it can be monitored—alongside diabetes it is merely an irritant. I try to look at it all objectively.
Discussion
Population screening should ideally be promoted only when the natural course of the condition is known. Recent studies suggest that genetic screening for haemochromatosis will reveal many asymptomatic people,1-8 for whom the benefits of treatment are not yet clear.1-9,1-10 Although the condition is more common than previously realised, there may be differences in genetic expression, with some people less likely to be clinically affected.1-11,1-12
We hope that this account of how the treatment for asymptomatic haemochromatosis has affected MH has contributed to the discussion about whether to screen for haemochromatosis.1-13 In many ways MH feels she would rather not have known as she has seen no obvious benefit to herself or her family, and it has had a detrimental effect on the quality of her life and it constantly intrudes on her life. At present it is not possible for her medical advisers to quantify how treatment may have improved her long term health. We hope that we will prevent the complications of haemochromatosis, but we will never know now whether she would have developed them. For her general practitioner it has meant a lot of extra work that, although willingly undertaken, would not have been part of general medical services.
Acknowledgments
We thank MH's family for their willingness to be involved in genetic testing and agreeing to the story being told; David Seamark, who suggested the paper and provided constant encouragement; and the staff of Honiton Hospital who helped with the venesection. The Honiton Group Practice is an NHS Funded Research Practice.
Footnotes
Competing interests: None declared.
References
- 1-1.Gill M, Richards T. Meeting the challenge of genetic advance. BMJ. 1998;316:570. doi: 10.1136/bmj.316.7131.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1-2.Bell J. The new genetics in clinical practice. BMJ. 1998;316:618–620. doi: 10.1136/bmj.316.7131.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1-3.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature Genetics. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 1-4.UK Haemochromatosis Consortium. A simple genetic test identifies 90% of UK patients with haemochromatosis. Gut. 1997;41:841–844. doi: 10.1136/gut.41.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1-5.Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110:1107–1119. doi: 10.1053/gast.1996.v110.pm8613000. [DOI] [PubMed] [Google Scholar]
- 1-6.Powell LW, George K, McDonnell SM, Kowdley KV. Diagnosis of hemochromatosis. Ann Intern Med. 1998;129:925–931. doi: 10.7326/0003-4819-129-11_part_2-199812011-00002. [DOI] [PubMed] [Google Scholar]
- 1-7.Barton JC, McDonnell SM, Adams PC, Brissot P, Powell LW, Edwards CQ et al and Hemochromatosis Management Working Group. Management of hemochromatosis. Ann Intern Med. 1998;129:932–939. doi: 10.7326/0003-4819-129-11_part_2-199812011-00003. [DOI] [PubMed] [Google Scholar]
- 1-8.Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med. 1999;341:718–724. doi: 10.1056/NEJM199909023411002. [DOI] [PubMed] [Google Scholar]
- 1-9.Goldwurm S, Powell LW. Haemochromatosis after the discovery of HFE (“HLA-H”) Gut. 1997;41:855–856. doi: 10.1136/gut.41.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1-10.Davis JG. Population screening for hemochromatosis: the evolving role of the genetic analysis. Ann Intern Med. 1998;129:905–908. doi: 10.7326/0003-4819-129-11_part_1-199812010-00014. [DOI] [PubMed] [Google Scholar]
- 1-11.McDonnell SM, Phatak PD, Felitti V, Hover A, McLaren GD. Screening for hemochromatosis in primary care settings. Ann Intern Med. 1998;129:962–970. doi: 10.7326/0003-4819-129-11_part_2-199812011-00007. [DOI] [PubMed] [Google Scholar]
- 1-12.Olynyk JK. Hereditary haemochromatosis: diagnosis and management in the gene era. Liver. 1999;19:73–80. doi: 10.1111/j.1478-3231.1999.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 1-13.Haddow JE, Bradley LA. Hereditary haemochromatosis: to screen or not. BMJ. 1999;319:531–532. doi: 10.1136/bmj.319.7209.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
The whole enterprise of biomedical science can be seen as an attempt to refute the uncertainty of life. We will all die, but we cannot say where, when, or how. Almost all illness makes the future less certain and evokes fears of death. Both doctors and patients look to science to reassert certainty. The processes of science are used to fit a pattern of symptoms into a diagnostic category, which in turn posits a treatment and a prognosis. Uncertainty is diminished—or is it?
Much of the trial evidence on the effects of treatments on a whole range of diseases is conflicting, and each apparent answer raises a raft of new questions. Individual human variation makes the extrapolation of population data to an individual patient intrinsically and inevitably suspect. The more we recognise these limitations, the more the confidence and authority of science are undermined. Clinicians working with individual patients feel beleaguered by doubt and uncertainty. Simultaneously, health policy, driven by political imperative, pursues authority and certainty through the rhetoric of clinical guidelines and service frameworks. Margaret Hutchinson and Clare Seamark are trapped at the centre of this contradiction.
People with specialist knowledge of haemochromatosis have issued authoritative guidance on when to start treatment.2-1 Therapeutic phlebotomy should be initiated in men with serum ferritin concentrations of 300 μg/l or more and in women with concentrations of 200 μg/l or more, regardless of the presence or absence of symptoms.2-1 When MH is found to have a serum ferritin level of over 1000 μg/l, there seems little doubt about the appropriate course of action. And yet, not all individuals with hereditary haemochromatosis will develop serious clinical manifestations.2-2 Furthermore, MH has no symptoms of illness, her diabetes is thought to be unrelated to haemochromatosis, her liver seems to be working well, and the treatment is set to undermine her pleasure in life for more than a year.
Within a few months of starting her uncomfortable and debilitating treatment, she is beginning to express doubts about the wisdom of the decision to proceed. Her account raises questions which neither her general practitioner nor the authors of the clinical guidance can answer. How soon would she have become ill if she had declined treatment? At what age will it be safe to discontinue treatment? If the programme of treatment had been slower, with fewer venesections spread over a longer period, would she have been any worse off? How should she value her experience of life while she was ignorant of her iron overload compared with her experience informed by her new knowledge? Does the standard guidance overstate the benefit of treatment? Does biomedical science promise more than it can deliver?
Further research may provide some answers, but fundamental uncertainties will persist. We need to find ways of acknowledging the tentative nature of clinical guidance and adapting it to the values and aspirations of individual patients. Decision analysis offers some hope of achieving this by incorporating the utility value the individual patient attaches to the various likely outcomes of disease or its treatment.2-3 The technique feels cumbersome, however, and will need to be made much more accessible to both patients and clinicians.
Health is more than the absence of disease. It concerns the scope for autonomy and the ability to make choices about the structure and pattern of one's own life. When the capacity for self determination is eroded, health is compromised. It is too easy for the best of therapeutic intentions to “blind us to our patients' needs for space and stature.”2-4
Footnotes
Competing interests: None declared.
References
- 2-1.Barton JC, McDonell SM, Adams PC, Brissot P, Powell LW, Edwards CQ et al and the Hemochromatosis Management Working Group. Management of hemochromatosis. Ann Intern Med. 1998;129:932–939. doi: 10.7326/0003-4819-129-11_part_2-199812011-00003. [DOI] [PubMed] [Google Scholar]
- 2-2.Haddow JE, Bradley LA. Hereditary haemochromatosis: to screen or not. BMJ. 1999;319:531–532. doi: 10.1136/bmj.319.7209.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2-3.Lilford. R, Pauker SG, Braunholtz DA, Chard J. Decision analysis and the implementation of research findings. BMJ. 1998;317:405–409. doi: 10.1136/bmj.317.7155.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2-4.Metcalfe D. The crucible. J R Coll Gen Pract. 1986;36:349–354. [PMC free article] [PubMed] [Google Scholar]
Hereditary haemochromatosis is an autosomal recessive disorder of iron metabolism resulting in excess intestinal absorption and cellular deposition of iron. It is relatively common in people of northern European origin. The disease was found to be associated with the HLA-A3 allele, and over 83% of patients have now been discovered to have a mutated HFE gene, which is located on chromosome 6. Patients are homozygous for a single base change which substitutes tyrosine for cysteine at position 282 (C282Y). The HFE protein and the transferrin receptor are normally expressed in crypt enterocytes of the duodenum. The HFE protein is thought to modulate the uptake of transferrin bound iron from the plasma by crypt enterocytes and is part of the mechanism whereby the crypt enterocytes sense the level of body iron stores. The mutated HFE protein provides a paradoxical signal in crypt enterocytes, and the differentiating enterocytes are programmed to absorb more dietary iron when they mature into villus enterocytes.3-1
Patients with the disorder often present with non-specific complaints such as malaise, fatigue, arthralgia, sexual dysfunction, and abdominal pain. The classic “bronze diabetes” with hepatic fibrosis and cirrhosis, cardiomyopathy, endocrine dysfunction, and liver cancer presents only after prolonged iron loading when the condition is diagnosed late. A considerable proportion of patients with Vibrio vulnificus septicaemia have haemochromatosis as this organism thrives in an environment with abundant iron.3-2
If the condition is diagnosed early then treatment by venesection can restore a normal life expectancy, and most patients gain some improvement in symptoms.3-3 Transferrin saturation (serum iron/total iron binding capacity) is the most sensitive biochemical marker of iron overload. A transferrin saturation of >55% in men or >50% in women merits investigation for haemochromatosis. If a raised transferrin saturation is detected ferritin concentration should be monitored yearly and venesection initiated when it starts to rise. It is recommended that weekly phlebotomy is carried out until the serum ferritin concentration is 10 to 20 μg/l and then maintenance phlebotomy continued three or four times a year to maintain the serum ferritin at 50 μg/l. Liver biopsy should be considered when the serum ferritin concentration is greater than 400 μg/l in men and 200 μg/l in women to determine the amount of stainable iron and assess for injury.3-4
This disease is reversible if treated at an early stage but otherwise can result in severe organ damage. Although non-expression of disease in homozygotes is common, particularly in women,3-5 this patient clearly had iron overload and I find it impossible to argue for anything other than venesection in her case. The report illustrates the reality of subjecting patients to the recommended treatment. Perhaps a subcutaneous port may have avoided the problems with venous access. Further advice on adequate hydration, avoidance of excess physical activity for 24 hours, additional dietary protein, and even consideration of erythropoietin therapy may help with phlebotomy induced debility.3-4
The issue of screening for this condition has been widely discussed. This case demonstrates that when a case is detected early by screening, effective treatment is available.
Footnotes
Competing interests: None declared
References
- 3-1.Waheed A, Parkkila S, Saarnio J, Fleming RE, Zhou XY, Tomatsu S, Britton RS, Bacon BR, Sly S. Association of HFE protein with transferrin receptor in crypt enterocytes of human duodenum. Proc Natl Acad Sci U S A. 1999;96:1579–1584. doi: 10.1073/pnas.96.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3-2.Bullen JJ, Spalding PB, Ward CG, Gutteridge JMC. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–1609. [PubMed] [Google Scholar]
- 3-3.McDonnell SM, Preston BL, Jewell SA, Barton JC, Edwards CQ, Adams PC, et al. A survey of 2851 patients with hemochromatosis: symptoms and response to treatment. Am J Med. 1999;106:619–624. doi: 10.1016/s0002-9343(99)00120-5. [DOI] [PubMed] [Google Scholar]
- 3-4.Witte DL, Crosby WH, Edwards CQ, Fairbanks VF, Mitros FA. Hereditary hemochromatosis: practice guideline development task force of the college of American pathologists. Clin Chim Acta. 1996;245:139–200. doi: 10.1016/0009-8981(95)06212-2. [DOI] [PubMed] [Google Scholar]
- 3-5.Crawford DHG, Jazwinska EC, Cullen LM, Powell LW. Expression of HLA-linked hemochromatosis in subjects homozygous for the C282Y mutation. Gastroenterology. 1998;114:1003–1008. doi: 10.1016/s0016-5085(98)70320-8. [DOI] [PubMed] [Google Scholar]