Abstract
Recent evidence in animal models of Parkinson’s disease (PD) suggests that exercise and other forms of motor enhancement can be beneficial when applied during the degeneration of dopamine neurons. Behaviours that depend on adequate levels of striatal dopamine may provide particularly favourable targets for therapeutic motor interventions. Task-specific motor enrichment procedures have been used to improve functional and neural outcomes following unilateral infusions of 6-hydroxydopamine (6-OHDA) into the nigrostriatal pathway in rats. In contrast, forced non-use procedures can exaggerate the degree of degeneration. Limb-use akinesia and ultrasonic vocalization in the 50-kHz range may be useful behavioural indices of nigrostriatal integrity and may model common deficits found in PD. These deficits in movement initiation and fine sensorimotor control are potential targets for early training interventions.
1. Introduction
Parkinson’s disease (PD) is characterized primarily by progressive degeneration of nigrostriatal dopaminergic neurons and sensorimotor deficits [1]. Classic signs of PD, such as bradykinesia, postural instability and tremor at rest do not appear until well after neural degeneration has commenced, although other signs/symptoms may appear early in the disease, perhaps due to non-dopaminergic cell loss [2]. Early detection of ongoing degeneration may allow for therapeutic interventions that protect against cell loss. Neurotoxins that lead to the degeneration of dopamine cells in rats are useful for exploring sensorimotor deficits and neural and behavioural protection [3–5]. In animal models of neurotoxin exposure, severe motor signs such as akinesia emerge after approximately 80% of the neurons on the affected side have degenerated [6]. In unilateral (hemi-parkinsonian) dopamine depletion models, less severe levels of degeneration can be detected using sensitive behavioural methods [7]. A widely used technique for depleting dopamine unilaterally is micro-infusion of the neurotoxin 6-hydroxydopamine (6-OHDA) to the medial forebrain bundle, substantia nigra or striatum in one hemisphere. Behavioural enrichment methods, particularly those that target forelimb motor deficits and are applied before or during degeneration of dopamine terminals, have been shown to improve function and reduce the extent of dopamine terminal loss [8–12]. In this article, we review some of the behavioural tests used to determine the degree of impairment and rescue by forelimb-exercise interventions, as well as describe complex ultrasonic vocalization impairments that may model some of the voice and speech deficits commonly found in early PD.
2. Sensorimotor impairment and targeted training
Several behavioural tests have been developed or adapted from other neurological impairment models to elucidate the limb sensorimotor deficits associated with unilateral 6-OHDA-induced striatal dopamine depletion, and are correlated with the degree of dopaminergic degeneration (visit www.schallertlab.org for a video demonstration). The affected limb functions can be compared with that of the intact limb, thereby increasing the sensitivity and repeatability of assessment across days. Vibrissae-elicited forelimb placing involves gently stimulating the vibrissae on the edge of a tabletop to elicit placement of the corresponding (same side) limb [10,13,14]. Animals are held by their torsos with their forelimbs hanging freely to allow for unrestrained movement of the forelimb in response to sensory stimuli. The forelimb not being tested is gently restrained by placing the experimenter’s finger in front of that limb. This is done for 10 trials on the side contralateral to the lesion (impaired limb) and 10 trials on the side ipsilateral to the lesion (intact side). Scores are expressed as a ratio (percentage) of successful placing responses to total responses. Rats that have sustained a sufficiently severe loss of dopamine terminals (approximately 80% loss of striatal dopamine content) reliably show a placing deficit of the contralateral limb [15]. An intense regimen of repeated placing of the limb (hundreds of trials per day) before and after exposure to 6-OHDAcan ameliorate the placing deficit and protect against terminal degeneration [12].Forelimb-use asymmetry during spontaneous movement is measured while the rat rears and explores a vertically oriented transparent cylinder (diameter 20 cm, height 30 cm) with its forepaws. Rats with unilateral dopamine depletion preferentially use the unimpaired forelimb for support during exploration, and show little or no use of the impaired forelimb [8,9,15]. When the impaired forelimb is involved in vertical exploration, it typically is used simultaneously with the non-impaired forelimb, whereas the unimpaired forelimb is frequently used independently. If the dopamine depletion is not too severe, the degeneration and emergence of behavioural impairments are slow enough (5e7 days) for targeted motor therapy to be effective. For example, forcing the animal to use the limb impaired limb (by constraining the intact limb with a cast) can prevent the behavioural and neural deficits if this is done during the first week after neurotoxin exposure. This is possibly due to upregulation of neurotrophic factors and related mechanisms [11]. If the impaired forelimb is constrained instead, rats receiving even otherwise-subclinical doses of 6-OHDA will show detectable limb-use asymmetries [9]. Thus, motor therapy is protective and non-use is pro-degenerative. Because patients with early signs of PD (or even at subclinical stages) might self-motivate motor impoverishment during degeneration, these findings may have implications for early interventions that would enhance motor behaviours. Intense exercise and motor enrichment have been associated with a reduced risk of PD [16]. However, the participants in that study self-selected the degree of motor activity. Preclinical fatigue or related motivational factors may have influenced the findings. Animals, of course, can be randomly assigned to exercise regimens. Non-specific exercise in rodents, such as voluntary and treadmill running, has been associated with neurogenesis [17], angiogenesis [18], increased neurotrophic factors [19] and increased levels of dopamine [20]. However, although behavioural and/or neural outcomes were found to be improved with running wheel or treadmill activity [10,19,21], beneficial effects on specific skilled behaviour have not been demonstrated [20]. Thus, it appears that early interventions might be administered more effectively with task-specific targeted skill training. It remains unclear whether behavioural enrichment interventions may be more effective if applied while the animal is under the influence of anti-Parkinsonian drugs such as levodopa. In animal model experiments, exercise and related interventions are studied without levodopa and this does not reflect human treatment conditions. Physical therapies in PD patients are typically carried out while they are medicated. As dopamine synapses vacate, they may optimally encourage sprouting of other dopamine terminals to the appropriate receptors and the augmentation of dopamine by levodopa may interfere with the process. On the other hand, levodopa may allow more normal movements during physical therapy and may enhance motor learning, motivation and attention while reducing fatigue and bradykinesia. Thus, the concurrent effects and potential interactions of levodopa management and exercise should be explored specifically.
3. Voice and speech deficits and targeted sensorimotor training
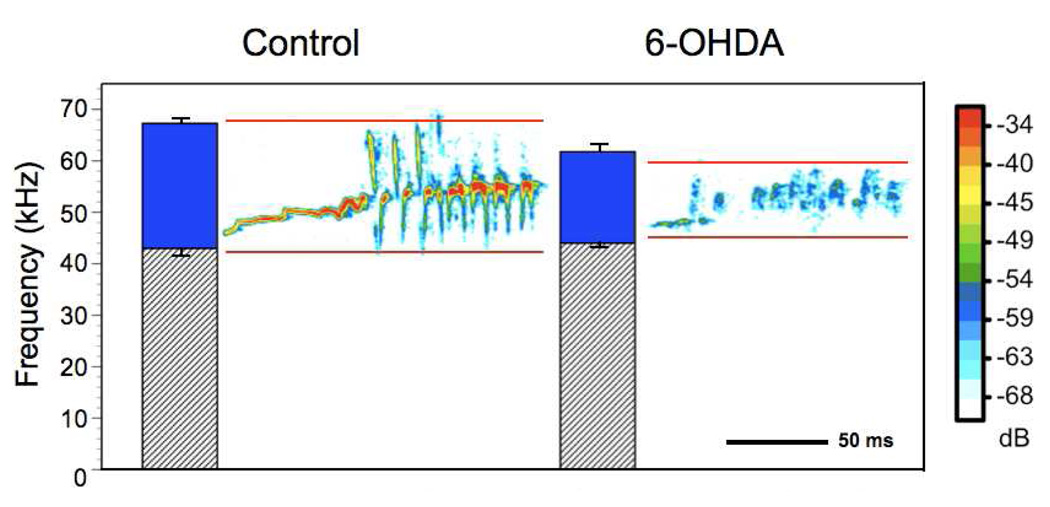
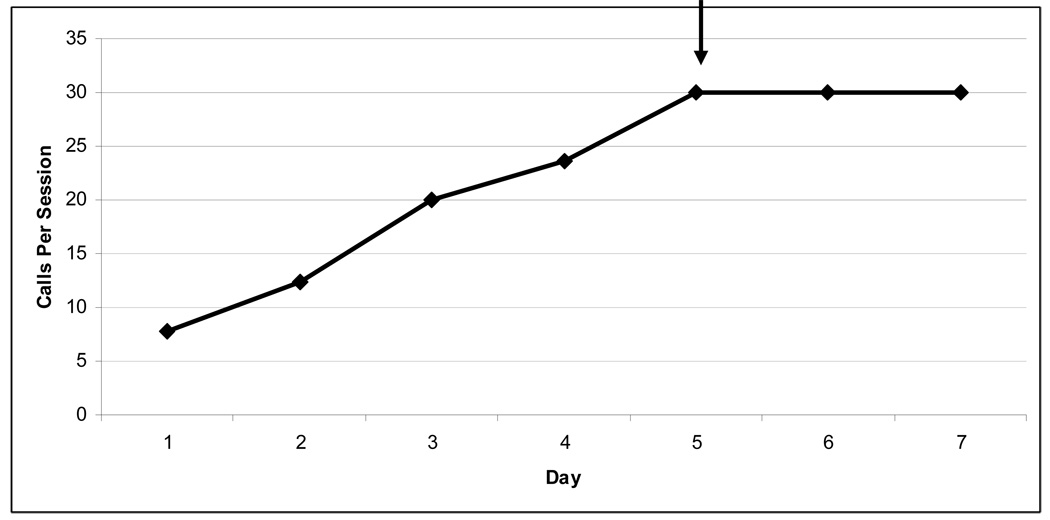
Voice and speech deficits are common complications of PD [22–26] and include decreased vocal loudness, decreased frequency variability (monotone), breathiness, hoarse voice quality and imprecise articulation [22,23], as well as degradation in speech intelligibility [27]. Similar to animal models of therapy, targeted exercise (speech/voice therapy) has been shown to improve specific deficits, such as communication and quality of life in patients with PD [28–30]. However, the underlying mechanisms of these behavioural, exercise-based interventions are not well understood. To systematically explore potential mechanisms, several factors need to be controlled, including: home environment, age post-onset, severity of lesion, and medications. These factors are virtually impossible to control in humans. Thus, an animal model is a useful tool to control for extraneous influences. It is unclear whether speech deficits in hemi-parkinsonian disease require at least partial loss of dopamine terminals in both hemispheres. In an animal model, we began to address this issue by depleting dopamine only in one hemisphere with the dopamine neurotoxin 6-OHDA and measuring complex ultrasonic vocalization (USV) [31]. Rats produce several types of USVs in the 50-kHz range. These calls carry semiotic value (have meaning), may have symbolic reference and are capable of changing the behaviour of the signal recipient [32]. Human speech/vocalizations and rat USVs share a semiotic/semantic nature and are produced by modifying egressive airflow. Thus, we suggest that USV may be a useful model to study phonatory and possibly communication deficits in a sensorimotor context. Details of this study are reported elsewhere [31]. Subjects were male Long-Evans rats, aged 6 months, housed in groups of two in standard polycarbonate cages with sawdust bedding on a reversed 12:12 h light: dark cycle with food and water available ad libitum. Prior to 6-OHDA infusion all animals were sexually experienced to ensure calling to female rats [33]. A novel apparatus was used to isolate and record only the male acoustic output. Female odors, but not vocalizations, were permitted to pass into the male’s chamber. An ultrasonic microphone with high directional properties (CM16, Avisoft, Germany) was used to record male USVs in the 50-kHz range. Avisoft, Germany). For this study, we analyzed the bandwidth of the “trill”-type call. Bandwidth and peak amplitude were markedly reduced in the 6-OHDAand haloperidol animals compared with the controls (Fig. 1) without altering the number of calls [31]. This study established the first rat model of communication deficits associated with PD and provided us with a platform to systematically test hypotheses regarding the onset and progression of communication deficits in early PD, as well as potential confounds of medication. Additionally, this model will allow us to explore behavioural and pharmacological treatment strategies as well as underlying mechanisms, which currently are unclear [34,35]. Currently, we are developing a training paradigm that systematically reinforces 50-kHz calls in rats as a targeted sensorimotor training. Our preliminary data show that rats can be trained to increase the number of calls in a training session (Fig. 2). It will be important to also reinforce the bandwidth, intensity, and other characteristics of calls as a target for returning the calls to their pre-lesion acoustic integrity. Thus, our model will parallel that in the limb, targeting skilled use of the sensorimotor system early in the disease process.
Figure 1.
Representative sonograms of the trill-type call for control and unilateral 6-OHDA infusion. Averages and standard errors of the maximum and minimum peak frequency are represented by the bar graphs. Solid color between the maximum and minimum peak frequency indicates the bandwidth of the call.
Figure 2.
Depicts average number of calls made by control rats during vocalization training. Rats were breifly exposed to estrous female to elicit calls after overnight water restriction. 50-kHZ calls were reinforced with water. Criteria of 30 calls per 5 minute session is indicated by arrow
4. Conclusions and future directions
Early motor training of dopamine-dependent movements appears to be beneficial when applied during early degeneration of dopamine neurons in an animal model of unilateral PD. Based on these studies, it appears that therapeutic exercise programmes should include targeted (task-specific) physical therapy and should be started early in the disease process. It may be important to consider that the body of animal research in PD does not consider the influence of levodopa management on exercise-related neuroprotective effects. That is, animals are generally studied without levodopa and this does not reflect human treatment conditions.
In humans, upon diagnosis, the primary form of intervention is the introduction of pharmacotherapy, primarily levodopa. However, in the early stages of PD, administration of levodopa is controversial [36]. Further, the effects of levodopa on the potential benefits of early exercise are unknown. Levodopa might be neurotoxic to aminergic cells [37,38] and this has raised concerns that chronic levodopa administration may advance disease progression [36]. On the other hand, dopamine replacement with levodopa has been shown to improve the quality of life and survival of patients [36]. Thus, it remains unclear whether levodopa is beneficial or harmful to disease-related plastic changes. In terms of sensorimotor control of vocalization, the clinical picture is more complicated, as voice and speech deficits are primarily resistant to levodopa therapy [39]. It is encouraging that the model of unilateral 6-OHDA lesions that has been useful for studying limb sensorimotor deficits also yields vocalization deficits that may be amenable to treatment with targeted training. This affords us an opportunity to explore the potential interactions of pharmaceutical and behavioural interventions.
Acknowledgements
This work is supported by the Davis Phinney Foundation and grant NS 19608, HD 02023, and NS 042345. We would like to thank E. Blake Windham for data collection and analysis. We would also like to thank Marty Woodlee, Larry Cormack, Cynthia Fox, and Lorraine Ramig for their ongoing expertise and consultation on rat vocalization.
References
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 3.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 4.Fleming SM, Delville Y, Schallert T. An intermittent, controlled-rate, slow progressive degeneration model of Parkinson’s disease: antiparkinson effects of Sinemet and protective effects of methylphenidate. Behav Brain Res. 2005;156:201–213. doi: 10.1016/j.bbr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Meredith GE, Kang UJ. Behavioral models of Parkinson's disease in rodents: a new look at an old problem. Mov Disord. 2006;21:1595–1606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- 6.Calne DB, Zigmond MJ. Compensatory mechanisms in degenerative neurologic diseases. Insights from parkinsonism. Arch Neurol. 1991;48(4):361–363. doi: 10.1001/archneur.1991.00530160025009. [DOI] [PubMed] [Google Scholar]
- 7.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 8.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced non-use in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 11.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85:299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 12.Anstrom KK, Schallert T, Woodlee MT, Shattuk A, Roberts DC. Repetitive vibrissae-elicited forelimb placing before and immediately after unilateral 6-hydroxydopamine improves outcome in a model of Parkinson's disease. Behav Brain Res. 2007;179:183–191. doi: 10.1016/j.bbr.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Woodlee MT, Schallert T. The interplay between behavior and neurodegeneration in rat models of Parkinson’s disease and stroke. Restor Neurol Neurosci. 2004;22:153–161. [PubMed] [Google Scholar]
- 14.Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, Dean RL III, Sanberg PR, editors. Central Nervous System Diseases: Innovative models of CNS diseases from molecule to therapy. Totowa, NJ: Humana Press; 2000. pp. 131–151. [Google Scholar]
- 15.Schallert T, Woodlee MT. Orienting and placing. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat. New York: Oxford University Press; 2005. pp. 129–140. [Google Scholar]
- 16.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson’s disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 17.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 19.Faherty CJ, Raviie Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res Mol Brain Res. 2005;134:170–179. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Foulton NP, Muir GD. Treadmill training ameliorates dopamine loss but not behavioral deficits in hemi-parkinson rats. Exp Neurol. 2005;193:181–197. doi: 10.1016/j.expneurol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Mabandla M, Kellaway L, Clair Gibson A, Russell VA. Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metab Brain Dis. 2004;19:43–50. doi: 10.1023/b:mebr.0000027416.13070.c3. [DOI] [PubMed] [Google Scholar]
- 22.Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res. 1969;12:246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- 23.Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. J Speech Hear Res. 1969;12:462–496. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- 24.Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J Speech Hear Disord. 1978;43:47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- 25.Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behav Neurol. 1998;11:131–137. [PubMed] [Google Scholar]
- 26.Fox CM, Morrison CE, Ramig LO, Sapir S. Current perspectives on the Lee Silverman Voice Treatment (LSVT) for individuals with idiopathic Parkinson disease. Amer J Speech Lang Pathol. 2002;11:111–123. [Google Scholar]
- 27.Miller N, Allcock L, Jones D, Noble E, Hildreth AJ, Burn DJ. Prevalence and pattern of perceived intelligibility changes in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78(11):1188–1190. doi: 10.1136/jnnp.2006.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, Thompson LL. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: a 2 year follow up. J Neurol Neurosurg Psychiatry. 2001;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramig LO, Sapir S, Fox C, Countryman S. Changes in vocal loudness following intensive voice treatment (LSVT®) in individuals with Parkinson’s disease: a comparison with untreated patients and normal age-matched controls. Mov Disord. 2001;16:79–83. doi: 10.1002/1531-8257(200101)16:1<79::aid-mds1013>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Sapir S, Ramig LO, Fox C. The Lee Silverman Voice Treatment (LSVT®) for voice, speech, and other orofacial disorders in people with Parkinson’s disease. Future Neurol. 2006;1:563–570. [Google Scholar]
- 31.Ciucci M, Ma TS, Fox C, Kane JR, Ramig L, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol. Behav Brain Res. 2007;182:284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- 33.Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- 34.Ackermann H, Konczak J, Hertrich I. The temporal control of repetitive articulatory movements in Parkinson’s disease. Brain Lang. 1997;56:312–319. doi: 10.1006/brln.1997.1851. [DOI] [PubMed] [Google Scholar]
- 35.Trail M, Fox C, Ramig LO, Sapir S, Howard J, Lai EC. Speech treatment for Parkinson’s disease. NeuroRehabilitation. 2005;20:205–221. [PubMed] [Google Scholar]
- 36.Fahn S. Parkinson Study Group. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252(Suppl 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 37.Fahn S. Parkinson disease, the effect of levodopa, and the ELLDOPA trial. Earlier vs Later L-DOPA. Arch Neurol. 1999;56(5):529–535. doi: 10.1001/archneur.56.5.529. [DOI] [PubMed] [Google Scholar]
- 38.Agid Y. Levodopa: is toxicity a myth? Neurology. 1998;50(4):858–863. doi: 10.1212/wnl.50.4.858. [DOI] [PubMed] [Google Scholar]
- 39.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]